Flower Thinning Improves Fruit Quality and Oil Composition in Camellia oleifera Abel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Determination of Flower and Fruit Parameters
2.3.1. Flower Bud and Fruit Set Rates
2.3.2. Fruit Growth Parameters
2.3.3. Accumulation of Nutrients and Fatty Acids in Fruits
2.3.4. Endogenous IAA and ABA in the Peel
2.3.5. Yield and Oil Production per Tree
2.4. Statistical Analysis
3. Results
3.1. Effect of the Number of Flowers per Tree on Flowering and Fruit Set in ‘Ganshi 83-4′
3.2. Effects of the Number of Flowers per Tree on Fresh Seed Rate and Dry Kernel Rate of C. oleifera Fresh Fruits
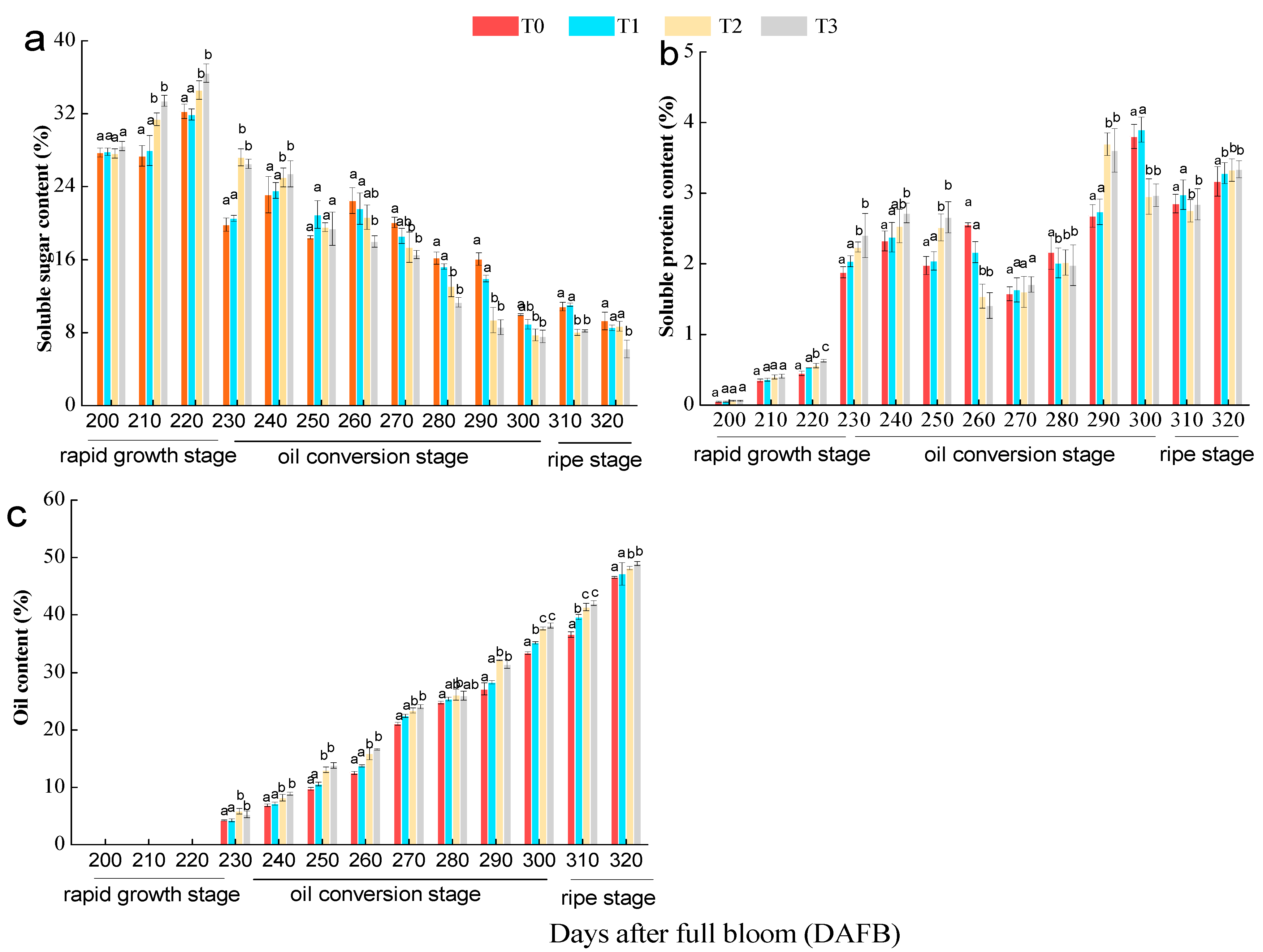
3.3. Effect of the Number of Flowers per Tree on Oil, Soluble Sugar, and Soluble Protein Contents of C. oleifera Seeds
3.4. Correlation between Kernel Nutrients at Various Stages of C. oleifera Fruit Development
3.5. Effect of the Number of Flowers per Tree on the Content of Oil Components in C. oleifera Seed Kernels
3.6. Effect of the Number of Flowers per Tree on Economic Indicators of C. oleifera Fruit at Maturity
3.7. Effects of the Number of Flowers per Tree on Fruit IAA and ABA Content during the Late Developmental Stage of C. oleifera Fruit
3.8. Correlation between Hormones and Quality Traits of Fruit at Oil Conversion and Fruit Ripening Stages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quan, W.; Wang, A.; Gao, C.; Li, C. Applications of Chinese Camellia oleifera and Its By-Products: A Review. Front. Chem. 2022, 10, 921246. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ye, H.; Rui, Y.; Chen, G.; Zhang, N. Fatty Acid Composition of Camellia oleifera Oil. J. Verbrauch. Leb. 2011, 6, 9–12. [Google Scholar] [CrossRef]
- Perona, J.S.; Botham, K.M. Olive Oil as a Functional Food: Nutritional and Health Benefits. Handb. Olive Oil Anal. Prop. 2013, 60, 677–714. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Q.; Verardo, V.; Contreras, M.M. Fatty Acid and Sterol Composition of Tea Seed Oils: Their Comparison by the “FancyTiles” Approach. Food Chem. 2017, 233, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Endo, Y. Effects of Cultivars and Geography in China on the Lipid Characteristics of Camellia oleifera Seeds. J. Oleo Sci. 2019, 68, 1051–1061. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Li, X.; Huang, G.; Li, X. Camellia oleifera Fresh Fruit Harvesting in China. In Proceedings of the 2020 5th International Conference on Mechanical, Control and Computer Engineering (ICMCCE), Harbin, China, 25–27 December 2020; pp. 699–702. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, H.; Wang, H.; Zhang, Y. Fruit Detection and Positioning Technology for a Camellia oleifera. Abel Orchard Based on Improved YOLOv4-Tiny Model and Binocular Stereo Vision. Expert Syst. Appl. 2022, 211, 118573. [Google Scholar] [CrossRef]
- Wang, D.; Tang, J.Y.; Fan, Z.Y.; Qu, Z. Design and Test of a Vibratory Camellia oleifera Fruit Harvester. For. Mach. Woodwork. Equip. 2020, 48, 4–7. [Google Scholar] [CrossRef]
- Peifer, L.; Ottnad, S.; Kunz, A.; Damerow, L.; Blanke, M. Effect of Non-Chemical Crop Load Regulation on Apple Fruit Quality, Assessed by the DA-Meter. Sci. Hortic. 2018, 233, 526–531. [Google Scholar] [CrossRef]
- Haouari, A.; Van Labeke, M.C.; Steppe, K.; Mariem, F.B.; Braham, M.; Chaieb, M. Fruit Thinning Affects Photosynthetic Activity, Carbohydrate Levels, and Shoot and Fruit Development of Olive Trees Grown under Semiarid Conditions. Funct. Plant Biol. 2013, 40, 1179–1186. [Google Scholar] [CrossRef]
- Castro, D.C.; ÁLvarez, N.; Gabriel, P.; Micheloud, N.; Buyatti, M.; Gariglio, N. Crop Loading Studies on ‘Caricia’ and ‘Eva’ Apples Grown in a Mild Winter Area. Sci. Agric. 2015, 72, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Celton, J.M.; Kelner, J.J.; Martinez, S.; Bechti, A.; Touhami, A.K.; James, M.J.; Durel, C.E.; Laurens, F.; Costes, E. Fruit Self-Thinning: A Trait to Consider for Genetic Improvement of Apple Tree. PLoS ONE 2014, 9, e91016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, W.; Xie, S.; Huang, H.; Jiang, Z.; Wang, D. Fruit Dropping Regularity of Cenruan-3 Camellia oleifera and Dynamic Change of Nutrient Element Contents in Leaves. Sci. Guangxi For. 2020, 49, 42–48. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, K.Q.; Yin, B.Q.; Wang, Z.H.; Li, L.H.; Cun, M. Comparative Analysis of Main Mineral Elements in Branches and Leaves of “On and Off-Year” Camellia oleifera. For. Invent. Plan. 2021, 46, 17–20. [Google Scholar]
- Beyá-marshall, V.; Fichet, T. Crop Load Regulates the next Season’s Crop Potential and Fruit Components in Frantoio Olive Trees (Olea europaea L.). Sci. Hortic. 2017, 215, 149–156. [Google Scholar] [CrossRef]
- Smith, H.M.; Samach, A. Constraints to Obtaining Consistent Annual Yields in Perennial Tree Crops. I: Heavy Fruit Load Dominates over Vegetative Growth. Plant Sci. 2013, 207, 158–167. [Google Scholar] [CrossRef]
- Wang, W.; Liang, Y.Y.; Quan, G.; Wang, X.; Xi, Z. Thinning of Cluster Improves Berry Composition and Sugar Accumulation in Syrah Grapes. Sci. Hortic. 2022, 297, 110966. [Google Scholar] [CrossRef]
- Piernas, J.; Giménez, M.J.; Noguera-Artiaga, L.; García-Pastor, M.E.; García-Martínez, S.; Zapata, P.J. Influence of Bunch Compactness and Berry Thinning Methods on Wine Grape Quality and Sensory Attributes of Wine in Vitis vinifera L. cv. ‘Monastrell’. Agronomy 2022, 12, 680. [Google Scholar] [CrossRef]
- Li, T.S.; Du, G.G.; Diao, S.F.; Han, W.J.; Fu, J.M.; Yang, S.B.; Li, F.D. Crop Load Influences the Components of Sugar, Acid and Flavor in Prunus domestica × Armeniaca ‘Weidi’. J. China Agric. Univ. 2017, 22, 39–48. [Google Scholar]
- Yuan, J.; Shi, B.; Wu, Z.L.; Tan, X.F. Response of Fruit Quality and Leaf Photosynthesis to Different Sink-Source Relationships in Camellia oleifera. Plant Physiol. J. 2015, 51, 1287–1292. [Google Scholar] [CrossRef]
- Song, Q.; Ji, K.; Mo, W.; Wang, L.; Chen, L.; Gao, L.; Gong, W.; Yuan, D. Dynamics of Sugars, Endogenous Hormones, and Oil Content during the Development of Camellia oleifera Fruit. Botany 2021, 99, 515–529. [Google Scholar] [CrossRef]
- Yang, C.; Liu, X.; Chen, Z.; Lin, Y.; Wang, S. Comparison of Oil Content and Fatty Acid Profile of Ten New Camellia oleifera Cultivars. J. Lipids 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yao, X.; Ren, H.; Wang, K. Changes in Contents of Endogenous Hormones and Main Mineral Elements in Oil-Tea Camellia Fruit during Maturation. J. Beijing For. Univ. 2015, 37, 76–81. [Google Scholar]
- Xiao, A.H.; Chen, F.; Jia, Z.; Sang, Z.; Zhu, Z.; Ma, L.Y. Determination of 4 Plant Hormones in Magnolia wufengensis by Gradient Elution High Performance Liquid Chromatography. Chin. J. Anal. Lab. 2020, 39, 249–254. [Google Scholar]
- Ding, N.; Chen, Q.; Zhu, Z.; Peng, L.; Ge, S.; Jiang, Y. Effects of Crop Load on Distribution and Utilization of 13C and 15N and Fruit Quality for Dwarf Apple Trees. Sci. Rep. 2017, 7, 14172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mészáros, M.; Hnátková, H.; Čonka, P.; Náměstek, J. Linking Mineral Nutrition and Fruit Quality to Growth Intensity and Crop Load in Apple. Agronomy 2021, 11, 506. [Google Scholar] [CrossRef]
- Fernández, F.J.; Ladux, J.L.; Hammami, S.B.M.; Rapoport, H.F.; Searles, P.S. Fruit, Mesocarp, and Endocarp Responses to Crop Load and to Different Estimates of Source: Sink Ratio in Olive (cv. Arauco) at Final Harvest. Sci. Hortic. 2018, 234, 49–57. [Google Scholar] [CrossRef]
- Choi, K.O.; Im, D.; Park, S.J.; Lee, D.H.; Kim, S.J.; Hur, Y.Y. Effects of Berry Thinning on the Physicochemical, Aromatic, and Sensory Properties of Shine Muscat Grapes. Horticulturae 2021, 7, 487. [Google Scholar] [CrossRef]
- Delgado, J.A.; Obis, E.; Yuste, V. Effects of Fruit Thinning on Fruit and Seed Features of Cistus Ladanifer. Plant Ecol. 2010, 211, 297–303. [Google Scholar] [CrossRef]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L. Source-to-Sink Transport of Sugar and Regulation by Environmental Factors. Front. Plant Sci. 2013, 4, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Zhang, Y.; Wang, J.; Liu, X.; Zhao, C. Seed Development Lipid Accumulation and Its Relationship with Carbohydrates and Protein in Xanthoceras Sorbifolia Bunge. Bull. Bot. Res. 2015, 35, 140–145. [Google Scholar]
- Fang, L.S.; Liu, Z.; Liu, Z.; Geng, X.D.; Cai, Q.W.; Zhai, W.J.; Zhou, H. Changes of Contents Endogenous Hormones and Photosynthetic Characteristics of Idesia polocarpa Fruit at Different Developmental. Sci. Silvae Sin. 2020, 56, 41–45. [Google Scholar]
- Zhang, L.Y.; Wang, X.Y.; Cao, Y.B. Analysis on the Relationship between Sugar Content, Metabolism Related Enzyme Activity and Oil Accumulation in Camellia oleifera Fruits. J. Beijing For. Univ. 2013, 35, 18–23. [Google Scholar]
- King, S.P.; Lunn, J.E.; Furbank, R.T. Carbohydrate Content and Enzyme Metabolism in Developing Canola siliques. Plant Physiol. 1997, 114, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Jiang, L.; Zhang, L.; Kang, R.; Yu, Z. Dynamic Changes in Proteins during Apple (Malus x Domestica) Fruit Ripening and Storage. Horti. Res. 2014, 1, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Wu, M.C.; Hu, H.T.; Yang, L. Proteomic Analysis of Up-Accumulated Proteins Associated with Fruit Quality during Autumn Olive (Elaeagnus umbellata) Fruit Ripening. J. Agric. Food Chem. 2011, 59, 577–583. [Google Scholar] [CrossRef]
- Leng, X.M.; Wang, D. Changes of Soluble Sugar and Protein Contents in Different Parts of Jin jiujuble in North Shananxi. J. Northwest For. Univ. 2019, 105–108. [Google Scholar]
- Shi, T.; Wu, G.; Jin, Q.; Wang, X. Camellia Oil Authentication: A Comparative Analysis and Recent Analytical Techniques Developed for Its Assessment. A Review. Trends Food Sci. Technol. 2020, 97, 88–99. [Google Scholar] [CrossRef]
- Micha, R.; Darisush, M. Saturated Fat and Cardiomentabolic Risk Factors, Coronary Heart Disease, Stroke and Diabetes: A Fresh Look at the Evidence. Lipids 2010, 45, 893–905. [Google Scholar] [CrossRef] [Green Version]
- Gottrand, F. Polyunsaturated Fatty Acids and Immunity. Arch. Pediatr. 2007, 14, 5–7. [Google Scholar] [CrossRef]
- Wu, M.Y.; Du, M.H.; Wen, H.; Wang, W.Q.; Tang, J.; Shen, L.R. Effects of N-6 PUFA-Rich Soybean Oil, MUFA-Rich Olive Oil and Camellia Seed Oil on Weight and Cardiometabolic Profiles among Chinese Women: A 3-Month Double-Blind Randomized Controlled-Feeding Trial. Food Funct. 2022, 13, 4375–4383. [Google Scholar] [CrossRef]
- Joris, P.J.; Mensink, R.P. Role of Cis-Monounsaturated Fatty Acids in the Prevention of Coronary Heart Disease. Curr. Atheroscler. Rep. 2016, 18, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shokri, Z.; Ashouri, M.; Doroudian, H.R.; Roshan, N.M.; Sadeghi, S.M. Evaluating the Yield, Fatty Acid Composition, and the Seed Micronutrients Content in Peanut Cultivars under the Influence of Various Rates of Sulfur and Zinc Application. J. Nuts 2022, 13, 183–197. [Google Scholar] [CrossRef]
- Harun, M. Fatty Acid Composition of Sunflower in 31 Inbreed and 28 Hybrid. Biomed. J. Sci. Tech. Res. 2019, 16, 12032–12038. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; Zhang, S.; Azam, M.; Shaibu, A.S.; Feng, Y.; Li, Y.; Tian, Y.; Hong, H.; Li, B.; Sun, J. Profiling of Seed Fatty Acid Composition in 1025 Chinese Soybean Accessions from Diverse Ecoregions. Crop J. 2020, 8, 635–644. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Pavadhgul, P.; Kalpravidh, R.W. Camellia Oil-Enriched Diet Attenuates Oxidative Stress and Inflammatory Markers in Hypercholesterolemic Subjects. J. Med. Food 2016, 19, 895–898. [Google Scholar] [CrossRef]
- Kou, X.; Feng, Y.; Yuan, S.; Zhao, X.; Wu, C.; Wang, C.; Xue, Z. Different Regulatory Mechanisms of Plant Hormones in the Ripening of Climacteric and Non—Climacteric Fruits: A Review. Plant Mol. Biol. 2021, 107, 477–497. [Google Scholar] [CrossRef]
- Zhang, A.; Xu, Y.; Pan, H.; Zhang, J.; Gao, Z.; Shu, B.; Guo, C. Dynamic Changes of Endogenous Hormone Content in Dangshansu Pear during Fruit Development. Agric. Sci. Technol. 2011, 12, 940–942. [Google Scholar] [CrossRef]
- Sutton, M.; Doyle, J.; Chavez, D.; Malladi, A. Optimizing Fruit-Thinning Strategies in Peach (Prunus persica) Production. Horticulturae 2020, 6, 41. [Google Scholar] [CrossRef]
- Ren, J.; Chen, P.; Dai, S.J.; Li, P.; Li, Q.; Ji, K.; Wang, Y.P.; Leng, P. Role of Abscisic Acid and Ethylene in Sweet Cherry Fruit Maturation: Molecular Aspects. N. Z. J. Crop Hortic. Sci. 2011, 39, 161–174. [Google Scholar] [CrossRef]
- Luo, Y.; Ge, C.; Ling, Y.; Mo, F.; Yang, M.; Jiang, L.; Chen, Q.; Lin, Y.; Sun, B.; Zhang, Y. ABA and Sucrose Co-Regulate Strawberry Fruit Ripening and Show Inhibition of Glycolysis. Mol. Genet. Genom. 2020, 295, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Lafuente, M.T. Abscisic Acid Deficiency Alters Epicuticular Wax Metabolism and Morphology That Leads to Increased Cuticle Permeability during Sweet Orange (Citrus sinensis) Fruit Ripening. Front. Plant Sci. 2020, 11, 594184. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, C.; Boss, P.K.; Davies, C. Delaying Riesling Grape Berry Ripening with a Synthetic Auxin Affects Malic Acid Metabolism and Sugar Accumulation, and Alters Wine Sensory Characters. Funct. Plant Biol. 2012, 39, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Sun, Y.; Li, Z.; Zhang, X.; Yin, B.; Zhou, S.; Xu, J. Crop Load Influences Growth and Hormone Changes in the Roots of “Red Fuji” Apple. Front. Plant Sci. 2020, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Mcatee, P.; Karim, S.; Schaffer, R.; David, K. A Dynamic Interplay between Phytohormones Is Required for Fruit Development, Maturation, and Ripening. Front. Plant Sci. 2013, 4, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, J.; Wang, J.; An, J.; Liu, L.; Lin, Z.; Wang, R.; Wang, L.; Ma, C.; Shi, L.; Lin, S. Integrated MRNA and MiRNA Transcriptome Reveal a Cross-Talk between Developing Response and Hormone Signaling for the Seed Kernels of Siberian Apricot. Sci. Rep. 2016, 6, 35675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seehuber, C.; Damerow, L.; Blanke, M. Regulation of Source: Sink Relationship, Fruit Set, Fruit Growth and Fruit Quality in European Plum (Prunus domestica L.)—Using Thinning for Crop Load Management. Plant Growth Regul. 2011, 65, 335–341. [Google Scholar] [CrossRef]
- Hehnen, D.; Hanrahan, I.; Lewis, K.; Mcferson, J.; Blanke, M. Mechanical Flower Thinning Improves Fruit Quality of Apples and Promotes Consistent Bearing. Sci. Hortic. 2012, 134, 241–244. [Google Scholar] [CrossRef]
- Samant, A.P.; Warang, O.S.; Kulkarni, M.M.; Bhuwad, A.V.; Bhingarde, R.T.; Salvi, B.R.; Haldankar, P.M.; Parulekar, Y.R. Relationship between Plant Canopy Volume, Leaf Area Index and Yield in Mango (Mangifera indica L.) cv. Alphonso. Int. J. Curr. Microbiol. Appl. Sci. 2021, 11, 859–863. [Google Scholar]
- Jia, T.T.; Su, S.; Ma, L.Y.; Su, Q.W. Effects of Different Sink Source Relationships on Flower Bud Differentiation of Camellia oleifera. J. Northeast For. Univ. 2018, 46, 19–23. [Google Scholar] [CrossRef]
- You, L.; Yu, S.; Liu, H.; Wang, C.; Zhou, Z.; Zhang, L.; Hu, D. Effects of Biogas Slurry Fertilization on Fruit Economic Traits and Soil Nutrients of Camellia oleifera Abel. PLoS ONE 2019, 14, e0208289. [Google Scholar] [CrossRef] [PubMed]




| Treatment | Flower Number per Tree | Fruit Numbers | Fruit Set (%) | Flower Bud Rate (%) | ||
|---|---|---|---|---|---|---|
| 60 DAFB | 320 DAFB | 60 DAFB | 320 DAFB | |||
| T0 | 2350 | 863 ± 54.5 a | 357 ± 8.2 a | 38.5 ± 0.3 a | 16.3 ± 1.1 a | 23.1 ± 1.8 a |
| T1 | 1800 | 646 ± 47.9 b | 339 ± 15.8 a | 38.7 ± 1.2 a | 20.3 ± 1.5 ab | 25.3 ± 1.9 b |
| T2 | 1360 | 537 ± 50.5 bc | 321 ± 12.4 b | 44.0 ± 2.3 b | 24.3 ± 2.4 bc | 28.8 ± 2.7 c |
| T3 | 920 | 435 ± 10.5 c | 293 ± 17 b | 47.0 ± 1.2 b | 28.0 ± 2.7 c | 33.3 ± 2.9 d |
| Fruit Development Stage | Oil and Soluble Sugar Content | Oil and Soluble Protein |
|---|---|---|
| Rapid growth | 0.601 * | 0.458 |
| Oil conversion stage | −0.944 ** | 0.553 * |
| Ripe stage | −0.697 | 0.872 ** |
| Treatments | Fruit Yield (kg/plant) | Dry Kernel Yield (kg/plant) | Kernel Oil Content (%) | Oil Yield (kg/plant) | Unsaturated Fatty Acids (%) |
|---|---|---|---|---|---|
| T0 | 14.64 ± 0.37 A | 2.23 ± 0.06 a | 46.95 ± 0.16 a | 1.05 ± 0.03 a | 88.17 ± 0.01 a |
| T1 | 14.49 ± 0.26 A | 2.26 ± 0.04 a | 47.95 ± 1.06 a | 1.09 ± 0.02 a | 88.39 ± 0.02 ab |
| T2 | 12.94 ± 0.63 B | 2.11 ± 0.11 ab | 48.16 ± 0.65 b | 1.03 ± 0.05 a | 88.45 ± 0.12 b |
| T3 | 10.53 ± 0.11 C | 1.97 ± 0.01 b | 48.95 ± 0.81 b | 0.96 ± 0.01 a | 88.71 ± 0.10 c |
| Factors | Oil Conversion Period | Fruit Ripening Period | ||||
|---|---|---|---|---|---|---|
| OC | PC | SC | OC | PC | SC | |
| IAA | 0.520 * | 0.285 | −0.519 * | −0.944 ** | −0.902 * | 0.676 |
| ABA | 0.717 ** | 0.388 | −0.318 | 0.909 ** | 0.773 * | −0.689 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, T.; Liu, X.; Liang, X.; Zhu, X.; Bai, Q.; Su, S. Flower Thinning Improves Fruit Quality and Oil Composition in Camellia oleifera Abel. Horticulturae 2022, 8, 1077. https://doi.org/10.3390/horticulturae8111077
Ye T, Liu X, Liang X, Zhu X, Bai Q, Su S. Flower Thinning Improves Fruit Quality and Oil Composition in Camellia oleifera Abel. Horticulturae. 2022; 8(11):1077. https://doi.org/10.3390/horticulturae8111077
Chicago/Turabian StyleYe, Tiantian, Xue Liu, Xiaojie Liang, Xueyan Zhu, Qian Bai, and Shuchai Su. 2022. "Flower Thinning Improves Fruit Quality and Oil Composition in Camellia oleifera Abel" Horticulturae 8, no. 11: 1077. https://doi.org/10.3390/horticulturae8111077
APA StyleYe, T., Liu, X., Liang, X., Zhu, X., Bai, Q., & Su, S. (2022). Flower Thinning Improves Fruit Quality and Oil Composition in Camellia oleifera Abel. Horticulturae, 8(11), 1077. https://doi.org/10.3390/horticulturae8111077






