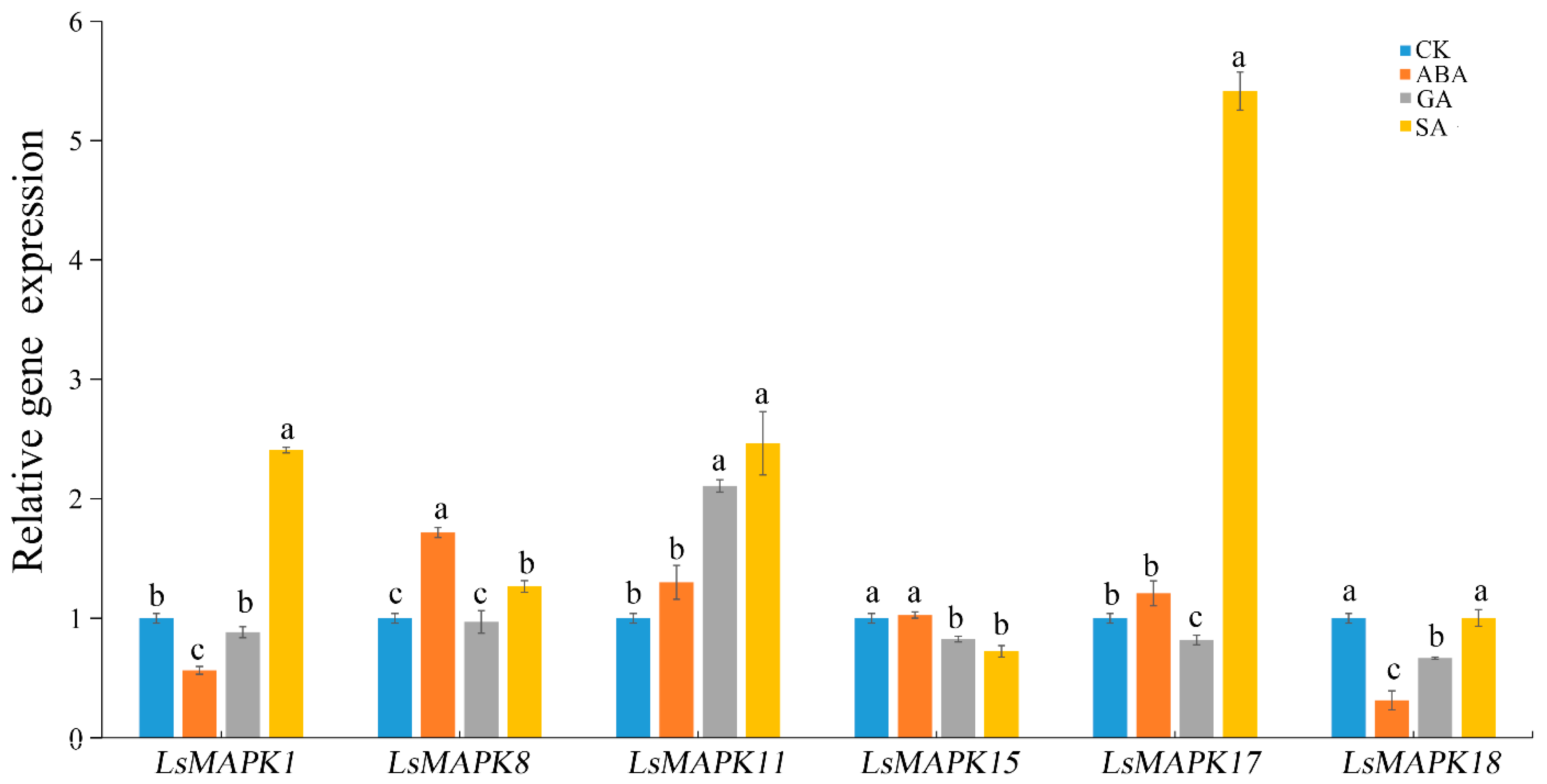Abstract
Mitogen-activated protein kinases (MAPKs) play essential roles in the process of stress response and plant growth and development. MAPK family genes have been identified in many plant species. In this study, 18 LsMAPK genes were identified in lettuce (Lactuca sativa). The LsMAPK members were divided into Group A, B, C, and D by phylogenetic tree analysis among Arabidopsis, rice, and lettuce. Cis-elements, which relate to abiotic stress, phytohormone response, and transcription factor binding site, were identified to exist in the promoter region of LsMAPK genes. Chromosomal location analysis showed the LsMAPK genes were distributed on eight chromosomes except chromosome 6. Interaction network analysis showed that LsMAPKs could interact with MAPK kinase (MAPKK), protein-tyrosine-phosphatase (PTP), and transcription factors (WRKY, bZIP). Quantitative reverse transcription PCR (qRT-PCR) showed that LsMAPK genes were induced by different abiotic stresses, hormone response, and stem enlargement. The comprehensive identification and characterization of LsMAPK genes in stem lettuce will lay a theoretical foundation for the functional analysis of LsMAPK genes and advance our knowledge of the regulatory mechanism of MAPK genes in plants.
1. Introduction
External stimuli, including abiotic stress (such as drought, salt, or extreme temperatures) and biotic stress (such as insect or pathogen infection), affect plant growth and development. Plants have developed certain defense mechanisms to counteract negative effects of extracellular stimuli [1,2]. The mitogen-activated protein kinase (MAPK) cascade pathway serves as the key pathway in eukaryotic signal transduction. It can regulate various cellular signaling cascades and participate in various fundamental biological processes. The MAPK cascade pathway consists of MAPK (MPK), MAPK kinase (MAPKK/MKK), and MAPKK kinase (MAPKKK/MEKK/MAP3K). The MAPK cascade pathway can be activated after stimulation of plant cells by adverse environmental factors [3,4].
Although at the bottom of the MAPK signaling cascade, MAPK genes are considered to be just some of the principal and highly conserved signaling molecular in eukaryotes [5]. As an important signal transduction mode, MAPK genes can be phosphorylated by activated MAPKKs and then modulate cellular response for normal growth and development by phosphorylating downstream target genes (transcription factor or other key proteins) [5,6,7]. Subsequently, the phosphorylated transcription factor could regulate target genes by binding the cis-elements existing in their promoter regions. Therefore, it is important to understand the process of signal transduction and regulation pathways in plants under different stresses. Thus, we focus on the analysis of the molecular mechanism of MAPKs involved in various biological processes.
MAPK, a kind of serine/threonine protein kinase, regulates eukaryotic cell signal transduction [8,9]. Eleven conserved motifs (I-XI) have been identified to exist in MAPKs. TXY motif, containing the phosphorylation sites, has been identified in subdomains VII and VIII. TXY motif is a key determinant of MAPK activity. The X residues in TXY motifs vary among different MAPKs. MAPKs are divided into A, B, C, and D subfamilies based on phylogenetic analysis and phosphorylation motifs. The TEY motif is the activation site of A, B, and C subfamily members, while the TDY phosphorylation motif is the activation site for members of the D subfamily [10,11]. Numerous MAPK genes have been identified and reported in different plants. Up to now, there are 20, 43, 16, 92, 56, 28, and 20 in Arabidopsis [12], strawberry [8], tartary buckwheat [13], Brassica napus [14], cotton [15], sunflower [16], and barley [17].
Large numbers of studies have shown that MAPK genes play critical roles in response to different stimuli. For example, Arabidopsis AtMPK3 and ZmMAPK1 genes positively regulated drought stress response [18,19]. Sorghum SbMPK14 gene improved drought hypersensitivity by promoting water loss [20]. Similar results were shown in Zea mays MAPK genes. ZmMPK3 and ZmSIMK1 enhanced plant growth by increasing tolerance to high salinity [21,22]. MAPK genes are also involved in plant development and physiological processes. AtMPK4 participated in photosynthesis regulation, plant growth, and immune defense [23]. AtMAPK3 and AtMAPK6 were required for another development [24]. MAPK proteins can participate in different biological processes through multiple regulation mechanisms. Numerous studies have shown that MAPK proteins can interact with other proteins such as transcription factors. For example, AtMAPK8 promoted seed germination by interaction with the TCP14 transcription factor [25]. Nicotiana tabacum WRKY transcription factors (WRKY4, WRKY6, and WRKY10) were able to interact with MAPK proteins to modulate plant defense against whiteflies [26]. Magnaporthe oryzae MAPK protein MoMps1 showed interaction with an APSES family transcription factor, and the interaction was required for hyphal and conidial morphogenesis, appressorial function and pathogenicity of M. oryzae [27].
Lettuce (Lactuca sativa L.) is a popular vegetable with several cultivars such as oil lettuce, leaf lettuce, and stem lettuce. Stem lettuce is a vegetable with low fat and high nutritional value. Studies on stem lettuce have mainly focused on its cultivation techniques and the effect of different fertilizers on yield and quality. The molecular mechanisms of stem lettuce growth and development remain unclear. Previous research has found that lettuce LsMAPK4 may be involved in high-temperature bolting in lettuce crops [28]. Here, we identified and analyzed the most important and highly conserved signaling molecular MAPK genes in lettuce. The exon–intron structure, phylogenetic relationships, motif compositions, collinearity analysis, and chromosome distribution of LsMAPK genes were identified. To investigate the possible function of LsMAPKs in different biological processes, the expression profiles of LsMAPK genes at different stages of stem expansion, abiotic stresses, and plant hormones were also conducted. Our results provide the basis for further research on the function of LsMAPK genes in stem expansion and stress response.
2. Materials and Methods
2.1. LsMAPK Genes Identification in Lettuce
The lettuce genome sequence used in this study was obtained from the Lettuce Genome Resource database (https://lgr.genomecenter.ucdavis.edu/, URL (accessed on 1 December 2021)). MAPK genes from Arabidopsis were used as the query sequence to identify the homologous genes of lettuce. The conserved domains within MAPK family genes were determined using the Pfam (http://pfam.xfam.org/, URL (accessed on 1 December 2021)) and NCBI Conserved Domain Database (CDD, https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi, URL (accessed on 1 December 2021)). All gene sequences encoding complete amino acid sequences with conserved domains were considered LsMAPK genes. Subsequently, the molecular weight (Mw) and isoelectric point (pI) of LsMAPKs were analyzed using the ExPASY online tool (https://web.expasy.org/protparam/, URL (accessed on 10 December 2021)). The subcellular localization analysis of LsMAPs was conducted using an online WOLF PSORT platform (https://www.genscript.com/wolf-psort.html/; URL (accessed on 10 December 2021)).
2.2. Characterization and Correlation Analysis of LsMAPKs in Lettuce
A phylogenetic tree among Arabidopsis, rice, and lettuce was constructed using the MEGA 7.0 software by the Neighbor-Joining method with 1000 bootstrap replicates. The MAPK amino acids of Arabidopsis and rice were obtained from the Arabidopsis Information Resource (https://www.arabidopsis.org/, URL (accessed on 10 December 2021)) and the Rice Genome Annotation Project (http://rice.plantbiology.msu.edu/, URL (accessed on 10 December 2021)), respectively. Conserved motif and chromosomal distribution of LsMAPKs were analyzed using the MEME online program (https://meme-suite.org/meme/, URL (accessed on 10 December 2021)) and MapChart software (version 2.32), respectively. The interaction network of LsMAPK proteins was predicted using STRING software (https://cn.string-db.org/, URL (accessed on 10 December 2021)) and visualized by Cytoscape software (version 3.9.1). The transcription start site (TSS) of LsMAPK genes was predicated on the website: http://www.fruitflfly.org/seq_tools/promoter.html, URL (accessed on 28 December 2021) [29]. The promoter region with 2000 bp of LsMAPK genes was extracted from the upstream of the TSS. Then, cis-element analysis of the promoter region was conducted by using the PLACE (https://www.dna.affrc.go.jp/PLACE/?action=newplace, URL (accessed on 28 December 2021)), PlantPAN 3.0 (http://plantpan.itps.ncku.edu.tw/, URL (accessed on 28 December 2021)), and PlanCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, URL (accessed on 28 December 2021)) online databases, respectively. Gene pair collinearity analysis among lettuce, Arabidopsis, and rice was determined by MCScanX software (http://chibba.pgml.uga.edu/mcscan2; URL (accessed on 28 December 2021)).
2.3. Plant Growth and Treatments
The seeds of stem lettuce cultivar ‘Yonganhong’ were sown in a controlled environment chamber for 12 h photoperiod at 22 and 18 °C (day vs. night) with a light intensity of 20,000 µmol/m²/s (lux) at Linyi University (Linyi, China). For plant hormone treatment, seedlings at the four-leaf stage were sprayed with 75 µmol/L abscisic acid (ABA), 50 µmol/L gibberellin (GA), 0.5 mmol/L salicylic acid (SA). Seedlings in the control group were sprayed with distilled water. The seedlings were collected and frozen under liquid nitrogen after spraying for 0 h and 12 h. For abiotic stress treatment, four-leaf stage seedlings were treated with 200 mmol/L NaCl (salt), 20% PEG6000 (drought), 4 °C (low temperature), and 37 °C (high temperature). The treated and untreated leaves were collected at 0 h and 12 h. Each treatment (plant hormone, abiotic stress) contained 15 seedlings and all the treatments were replicated three times and harvested after the treatments. The stem tissue of stem lettuce was also collected with three biological replicates at different stages of stem enlargement: S1 (transverse diameter length is 1 cm), S2 (transverse diameter length is 2 cm), S3 (transverse diameter length is 3 cm), and S4 (transverse diameter length is 4 cm).
2.4. Quantitative Reverse Transcription PCR (qRT-PCR) of LsMAPK Genes
To identify the differentially expressed genes, the four stem enlargement stages (S1, S2, S3, and S4) of ‘Yonganhong’ were chosen to conduct the RNA sequencing. The transcript abundance of LsMAPK genes at different stages of stem enlargement was obtained according to the RNA-Seq, which has been submitted to public transcriptome data (NCBI: PRJNA844256). The transcript abundance of LsMAPK genes at different developmental stages was counted by FPKM (fragments per kilobase exon per million fragments mapped). Total RNA was isolated from the samples using a plant total RNA isolation kit (Vazyme, Nanjing, China). For qRT-PCR, 6 LsMAPK genes involved in the process of stem enlargement were chosen to conduct expression pattern analysis. For qRT-PCR, 20 µL reaction system containing 10 µL SYBR qPCR master mix (Vazyme, Nanjing, China), 0.4 µL of each primer, 2 µL diluted cDNA and 7.2 µL deionized water was performed. The qRT-PCR was conducted using Roche LightCycler 96 with the following procedure: 95 °C for 30 s initially, followed by 40 cycles at 95 °C for 10 s, 60 °C for 30 s, and melting curve analysis at 95 °C for 15 s, 60 °C for 60 s, 95 °C for 15 s. The calculation of relative expression levels of LsMAPK genes used the 2−ΔΔCT methods based on the mean value of three technical repeats referred to previous research methods [30]. The expression levels of each LsMAPK gene were standardized and calculated by LsTIP41 (Lsat_1_v5_gn_5_116421) [31]. The experiments were repeated in three independent bio-replicates and tech-replicates. Primer Premier 6.0 was used to design the primer pairs used in the study (Supplemental Table S1). SPSS 17.0 software was used to analyze significant difference at 0.05 levels.
3. Results
3.1. The LsMAPK Genes in Lettuce
A total of 18 putative LsMAPK genes (denoted LsMAPK01-LsMAPK18) were identified in lettuce after homologous alignment and conservative domain verification with Arabidopsis MAPK genes. The nucleotide and amino acid sequences of LsMAPKs are shown in Supplemental Table S2. Sequence alignment showed the presence of TEY or TDY phosphorylation sites in the 18 LsMAPK genes (Supplemental Figure S1). As shown in Table 1, the amino acid length of 18 LsMAPKs ranged from 369 (LsMAPK8) to 761 (LsMAPK11). The pI and Mw of LsMAPK proteins varied from 4.99 (LsMAPK14) to 9.30 (LsMAPK10), and 42.416 kD (LsMAPK1) to 85.362 kD (LsMAPK11), respectively. According to the grand average of hydropathicity (GRAVY) values, which ranged from −0.174 (LsMAPK1) to −0.549 (LsMAPK15), all the LsMAPKs are hydrophilic proteins. Subcellular localization analysis showed that 12 LsMAPK proteins were located only in the cytoplasm, while two LsMAPK (LsMAPK13 and LsMAPK14) proteins were located in the cytoplasm and the cytoskeleton. LsMAPK9 was predicted to be located in the cytoskeleton. LsMAPK11 and LsMAPK18 were located in the chloroplast. LsMAPK15 was located both in the cytoplasm and the chloroplast.

Table 1.
The characteristic of LsMAPK genes.
3.2. Phylogenetic Analysis of LsMAPKs in Lettuce
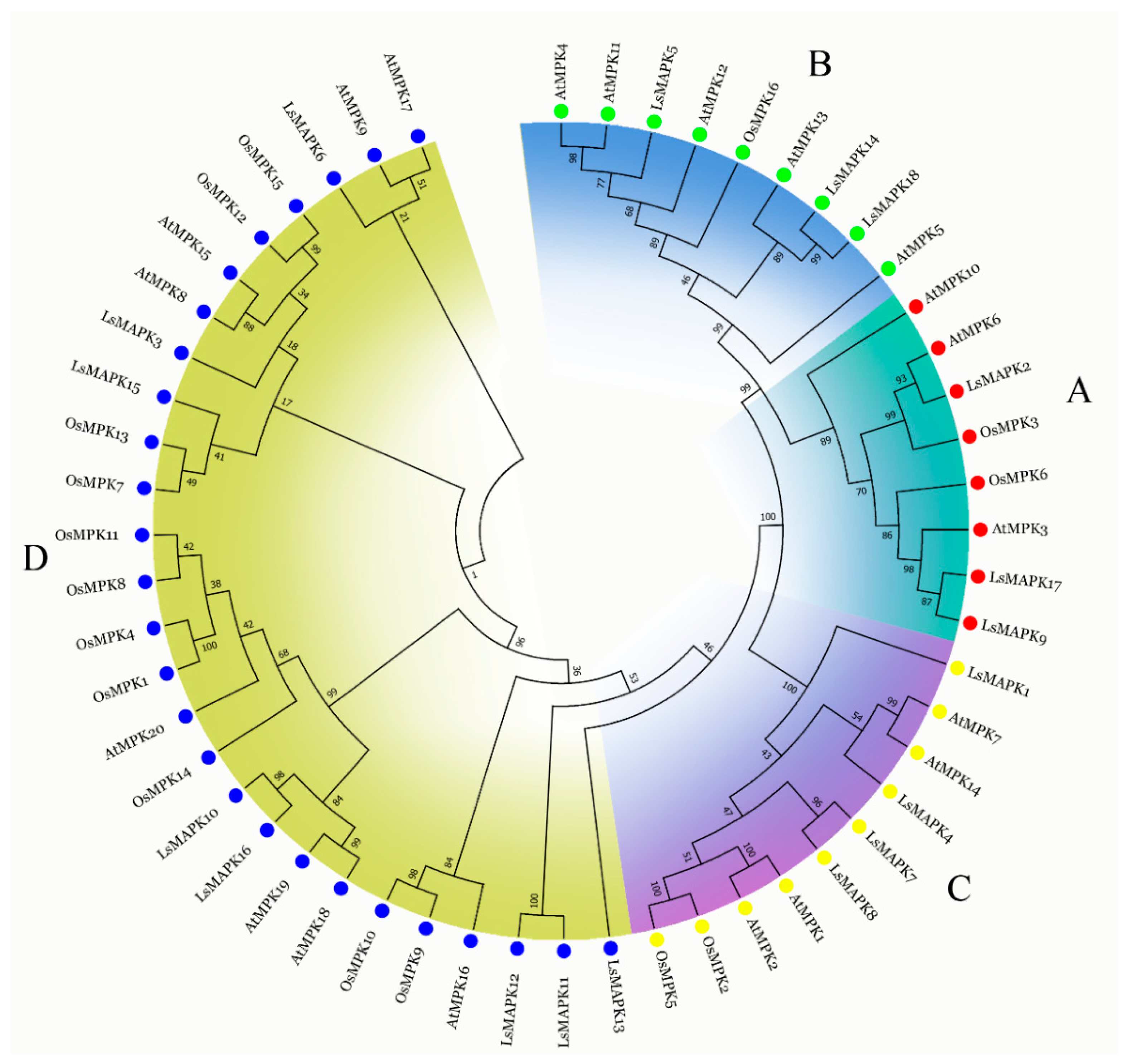
The amino acid sequences of 18 lettuce LsMAPKs, 20 Arabidopsis AtMPKs, and 16 rice OsMPKs were used to analyze the evolutionary relationships (Figure 1). As shown in Figure 1, 18 LsMAPKs were divided into A, B, C, and D subfamilies. The TEY motif existed in the A, B, and C subfamily LsMAPKs, while LsMAPKs in the D subfamily contained the TDY motif (Supplemental Figure S1). The D subfamily had the most LsMAPK members (eight), followed by the C subfamily, which had four members. The A and B subfamilies contained an equal number of LsMAPK members. LsMAPK2, LsMAPK9, and LsMAPK17 were classified into the A subfamily, which contained well-characterized AtMPK3, AtMPK6, AtMPK10, OsMPK3, and OsMPK6. LsMAPK5, LsMAPK14, and LsMAPK18 were classified into the B subfamily, which included AtMPK4, AtMPK11, AtMPK13, AtMPK12, AtMPK5, and OsMPK16.
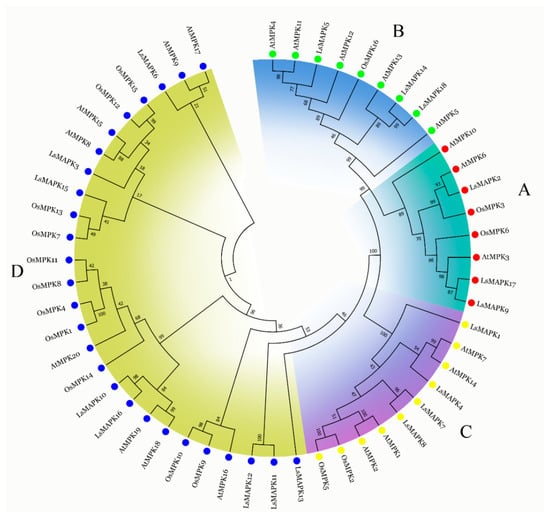
Figure 1.
Phylogenetic tree of MAPK proteins among lettuce, Arabidopsis, and rice. MAPK proteins were divided into four subfamilies (A, B, C, and D), with each color group representing a subfamily.
3.3. Analysis of Gene Structure and Motif of LsMAPK Genes
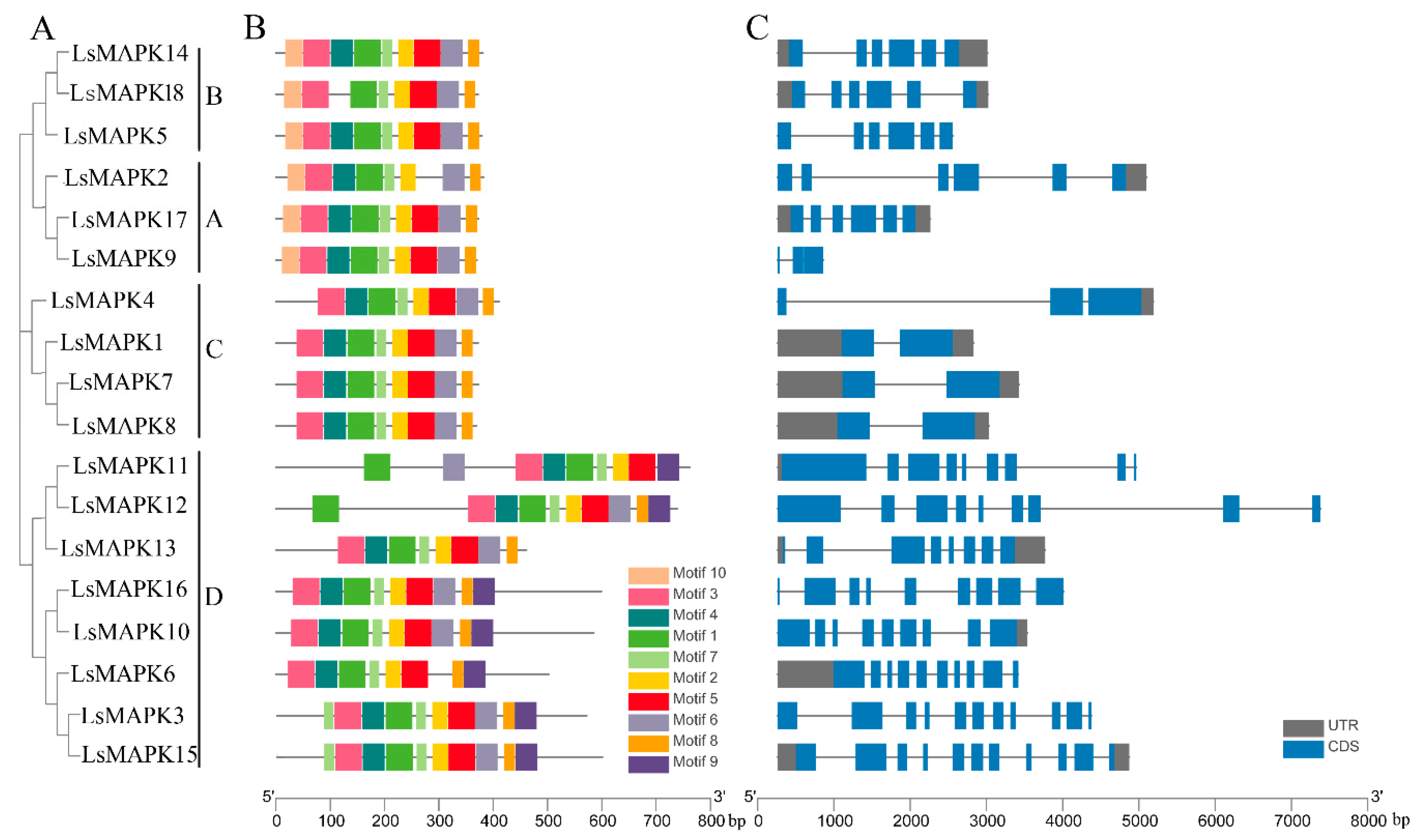
The LsMAPK protein structure was examined using the MEME online program. As shown in Figure 2B, ten motifs were identified. Motifs 1, 2, 7, and 3 existed in all LsMAPK proteins, while most LsMAPK proteins contained motifs 4, 5, 6, and 8. LsMAPKs in the same subfamily had similar motifs. For example, most LsMAPKs belonging to Group D except LsMAPK13 had specific motif 9. LsMAPK proteins belonging to Groups A and B contained motif 10.
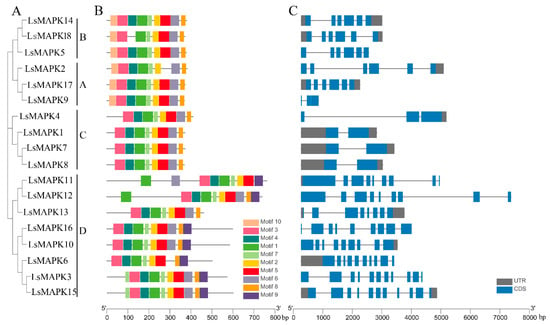
Figure 2.
Gene structure analysis of LsMAPK genes in lettuce. (A) Phylogenetic tree analysis created by MEGA 7.0. (B) Conserved motifs analysis. Different motifs were shown by different numbers of colored boxes. (C) Exon–intron structures analysis. Blue boxes and grey lines represent exons and introns, respectively; grey boxes represent the UTR; UTR: untranslated region, CDS: coding sequence.
The exon–intron structures of the identified LsMAPK genes were analyzed (Figure 2C). LsMAPK genes belonging to the same subfamily had conserved exon–intron structure. For instance, the LsMAPKs identified in Groups A and B, except LsMAPK9, had six exons, while Group C LsMAPK genes had two to three exons. The LsMAPK4 in Group C had three exons, while LsMAPK1, LsMAPK7, and LsMAPK8 each had two exons. Group D LsMAPK genes had eight to eleven exons. LsMAPK3 and LsMAPK15 had the highest number of exons (eleven).
3.4. Cis-Element Analysis of LsMAPK Genes
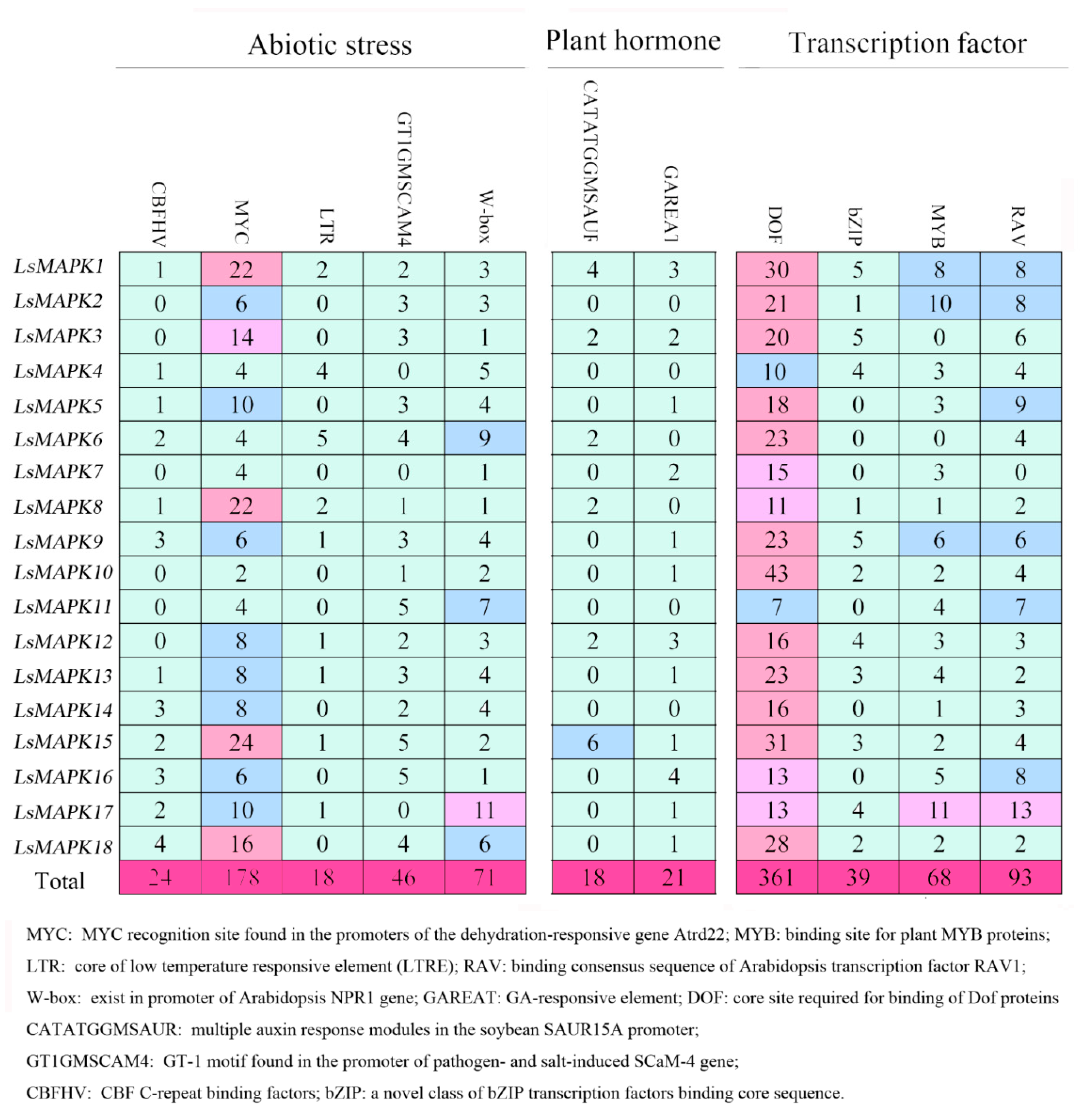
To better understand the function of LsMAPKs involved in different biological processes, we analyzed the cis-elements existing in the promoter regions of LsMAPK genes by using PLCAE (Figure 3), PlantCARE database (Supplement Table S3), and PlantPAN 3.0 (Supplement Table S4), respectively. As shown in Figure 3, the identified cis-elements were related to abiotic stress, phytohormone responses, and transcription factors. Abiotic- related elements contained five cis-elements such as low-temperature responsiveness element (LTR), drought inducibility element, salt induced element, CBF, and W-box. The promoter region of nine LsMAPK genes contained the LTR element, which was essential for the low temperature responsive. W-box, CBFHV, and GT1GMSCAM4 motifs appeared 71, 24, and 46 times, respectively. All LsMAPK genes contained the wound response element W-box. According to the analysis by the PLACE and PlantCARE database, cis-elements related to phytohormone responses also existed in LsMAPK genes, including GA, auxin, ABRE, ERE, TCA-element, and CGTCA/TGACG motif (Figure 3 and Supplemental Table S3). Six LsMAPK genes contained the auxin-responsive element. Twelve out of eighteen LsMAPK genes contained a GA responsive element (GAREAT) in their promoter region. Apart from stress-related and plant hormone-related cis-elements, some cis-elements, belonging to the binding sites of transcription factors (Dof, MYB, RAV, and bZIP), were also identified by the analysis of the PLACE and PlantPAN database (Figure 3 and Supplemental Table S4). Dof transcription factor binding sites existed in the promoter region of all the LsMAPK genes. All LsMAPK genes except LsMAPK7 contained the RAV transcription factor binding site. Cis-elements of the MYB transcription factor binding site were identified to exist in the promoter region of 16 LsMAPKs, except LsMAPK3 and LsMAPK6.
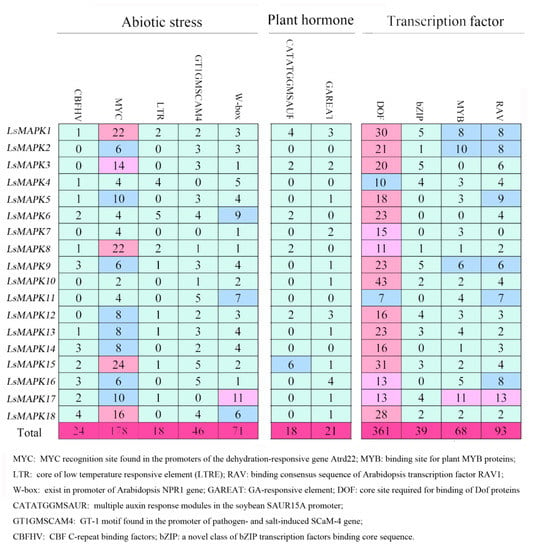
Figure 3.
Cis-element analysis of LsMAPK genes in lettuce by PLACE database. Boxes with different numbers represent each cis-element identified by the PLACE database. Different colors represent different number ranges of identified cis-element.
3.5. Chromosomal Location of LsMAPK Genes
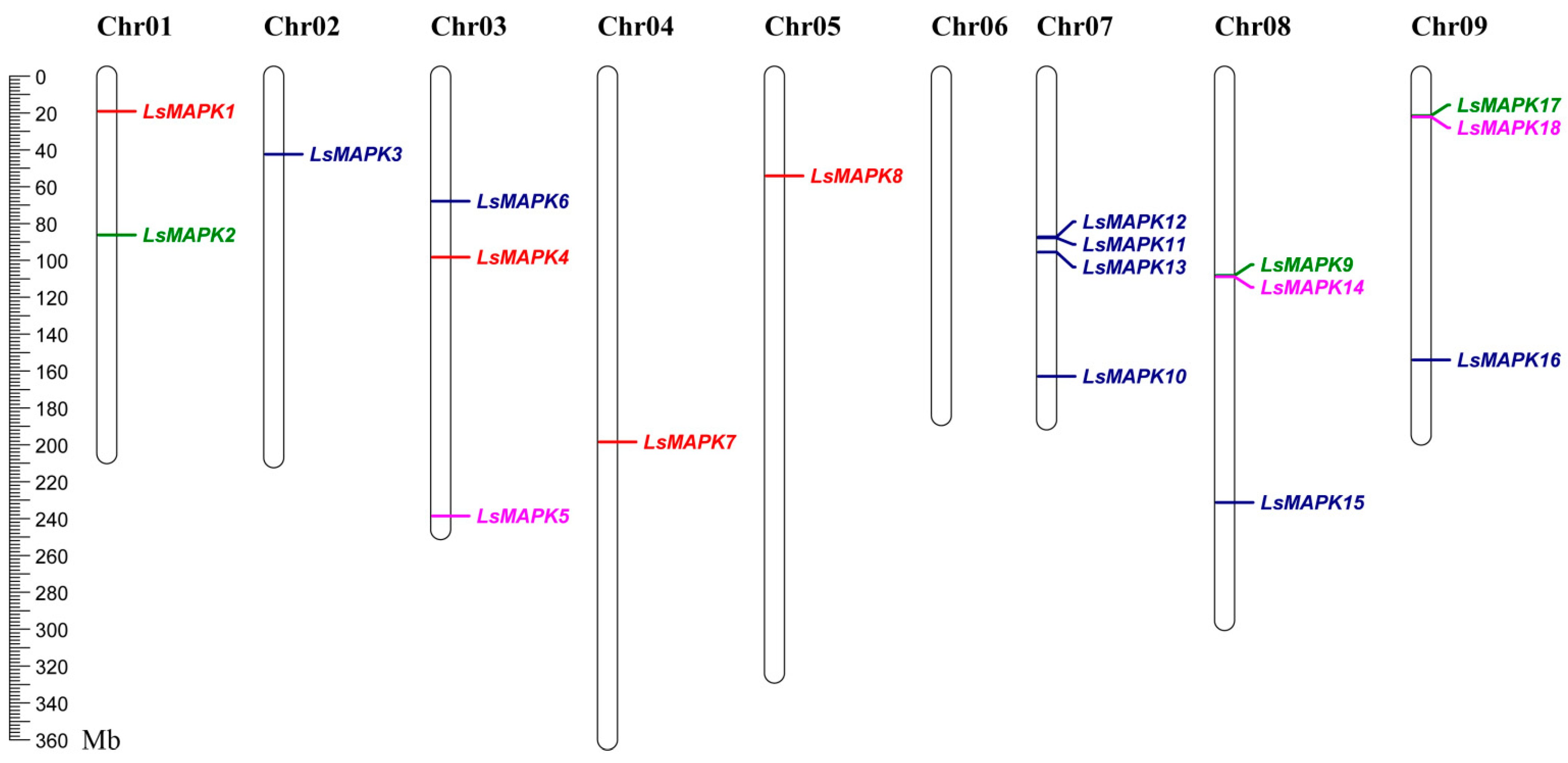
As shown in Figure 4, the chromosomal distribution of 18 LsMAPK genes was investigated. The 18 LsMAPK genes were mapped on eight chromosomes except chromosome 6, which contained zero MAPK genes. Chromosomes 2, 4, and 5 had only one LsMAPK, i.e., LsMAPK3, LsMAPK7, and LsMAPK8, respectively. Chromosome 1 contained two LsMAPK genes. LsMAPK1 and LsMAPK2 were mapped on chromosomes 1. In addition, three LsMAPK genes were found on chromosomes 3, 8, and 9. Chromosome 7 had the largest number of LsMAPK genes, including LsMAPK10, LsMAPK11, LsMAPK12, and LsMAPK13. Interesting, LsMAPK genes belonging to the same subfamily were not distributed on the same chromosomes. For example, LsMAPK5, LsMAPK14, and LsMAPK18, which both belong to the Group B subfamily, were mapped on chromosomes 3, 8, and 9, respectively. LsMAPK genes (LsMAPK2, LsMAPK9, and LsMAPK17) belonging to Group A were divided into three different chromosomes (1, 8, and 9).
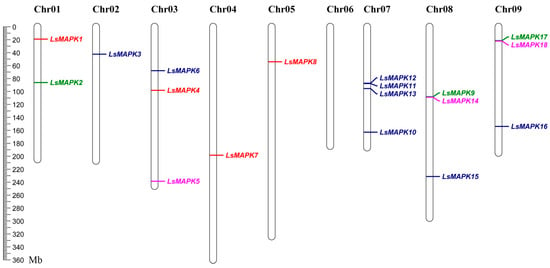
Figure 4.
Chromosomal distribution of LsMAPK genes in lettuce chromosomes. Different colors represent different subfamily LsMAPK genes.
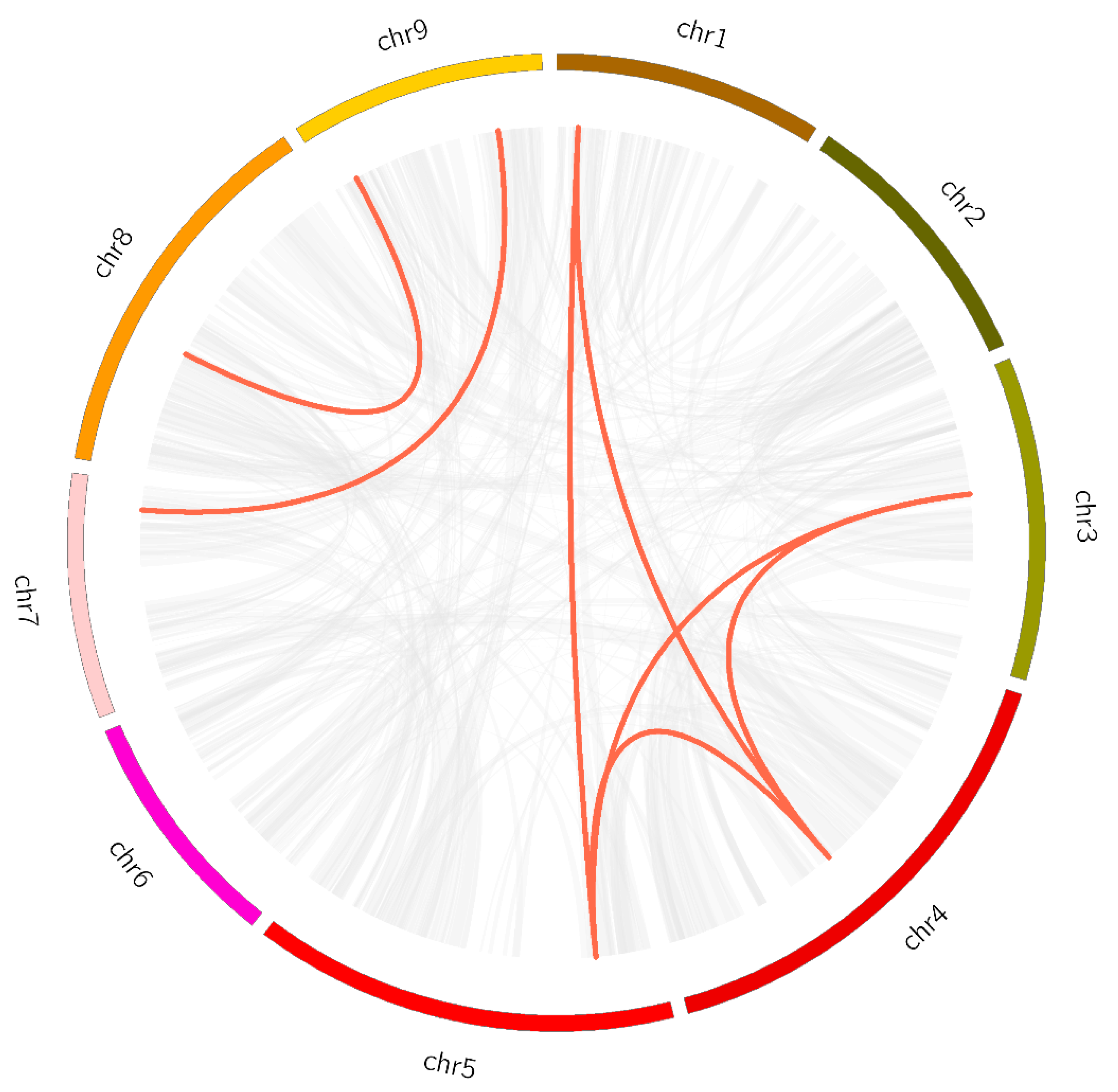
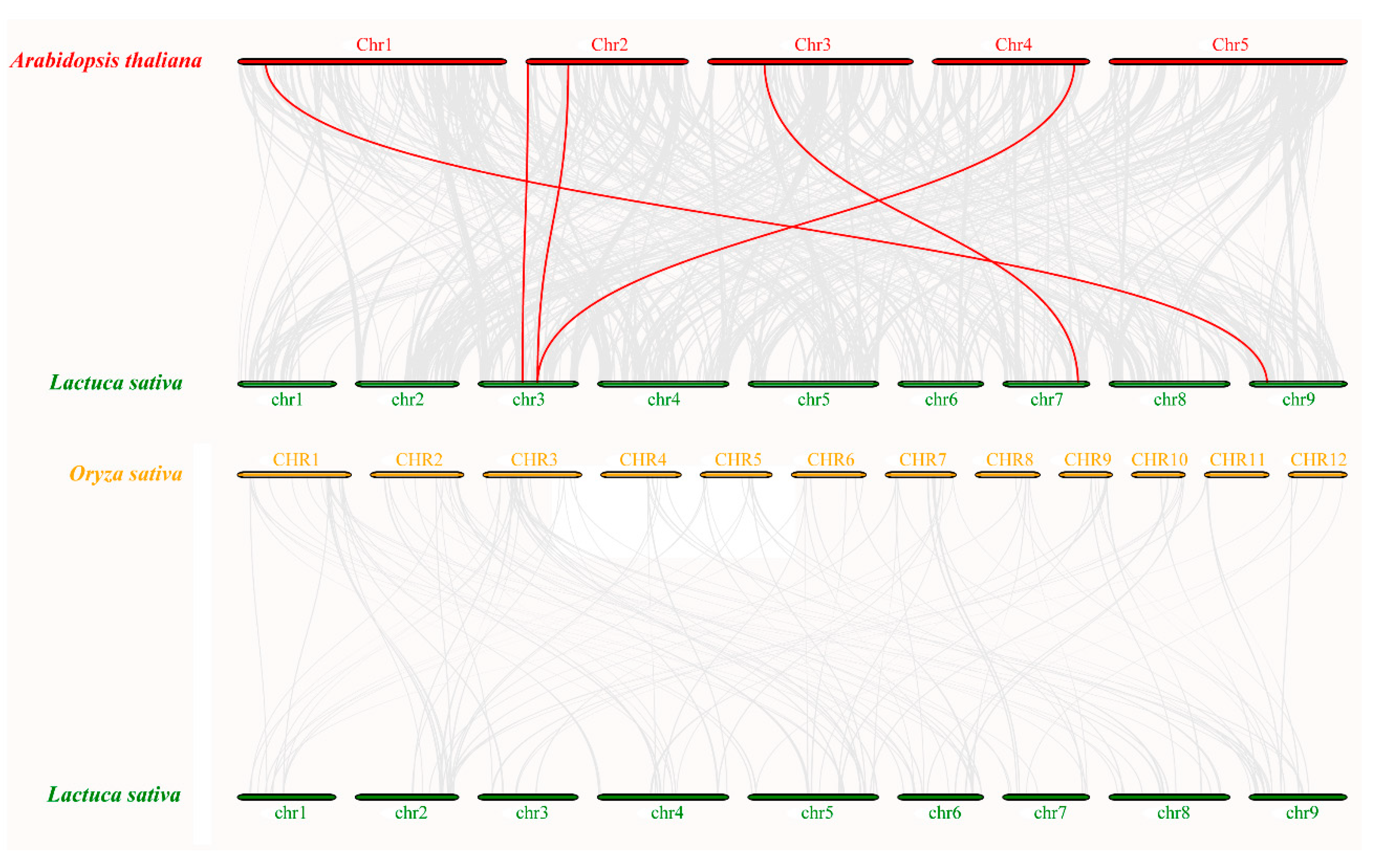
3.6. Synteny Analysis of MAPK Genes
As shown in Figure 5, seven LsMAPK gene pairs in lettuce chromosome were identified as collinear pairs by collinearity analysis. The collinear pairs of LsMAPK genes in lettuce belonged to the same subfamily, for example, LsMAPK1 and LsMAPK7, LsMAPK10 and LsMAPK16. To further determine the evolutionary relationship, MAPK genes of Arabidopsis and rice were chosen for synteny analysis with lettuce. Five pairs of orthologous MAPK genes were identified between Arabidopsis and lettuce. The collinear pairs between Arabidopsis and lettuce were clustered on the same branch, such as LsMAPK4 and AtMPK7, LsMAPK6 and AtMPK17, LsMAPK10 and AtMPK19 (Figure 6, Supplemental Table S5). However, no collinear pairs of MAPK genes were identified between rice and lettuce, indicating the genetic relationship between lettuce and Arabidopsis was more advanced than that of rice, and the MAPK genes were conserved in the evolution of dicotyledons, not in the evolution of monocotyledon.
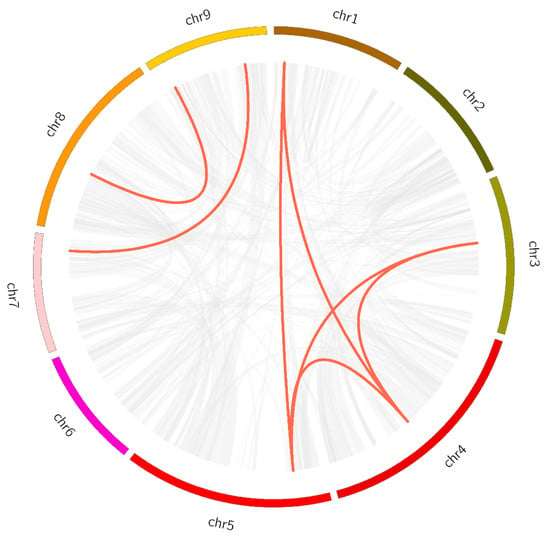
Figure 5.
Synteny analysis of LsMAPK genes in lettuce. Colored circular rectangles represent the chromosomes (1–9) of lettuce. Grey and red curves represent the identified collinear blocks with the genomes and the collinear with LsMAPK genes, respectively.
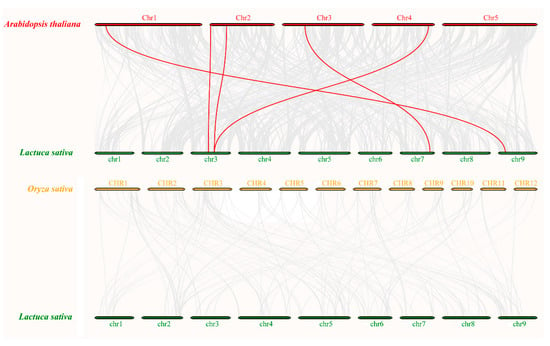
Figure 6.
Comparative analysis of synteny among lettuce, Arabidopsis, and rice. The chromosomes of three plants are shown by colored circular rectangles. Grey and red curves represent the identified collinear blocks with the genomes and the collinear with MAPK genes, respectively.
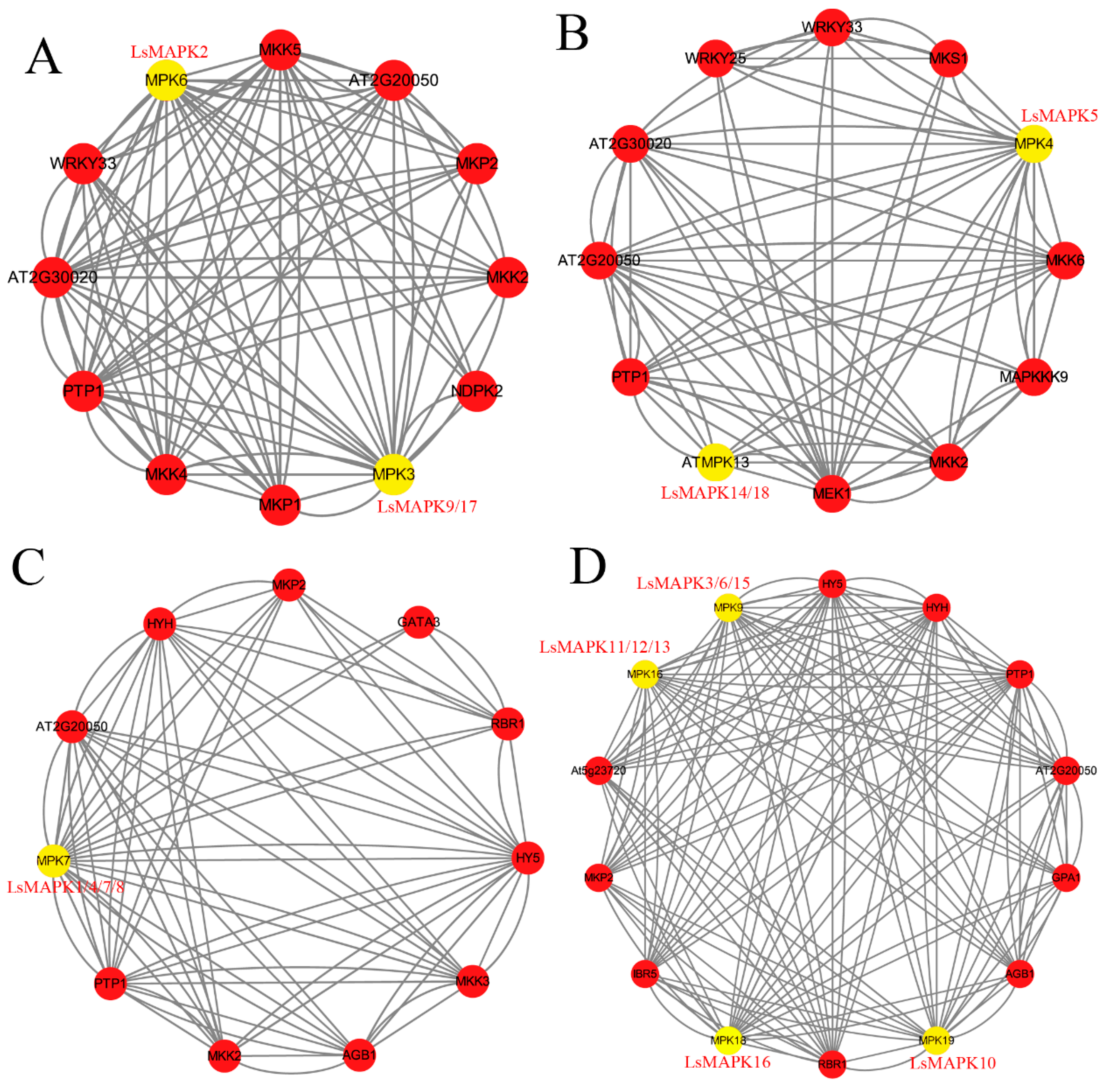
3.7. Interaction Network Analysis of LsMAPK Proteins
In order to identify the interaction relationship of LsMAPK proteins with other proteins in lettuce, an interaction network analysis was conducted based on orthologous genes in A. thaliana, using STRING software. As shown in Figure 7, LsMAPK proteins in Groups A, B, C, and D showed complex interaction relationships with other proteins. For Group A MAPK proteins LsMAPK2 (a homolog of Arabidopsis MPK6) and LsMAPK9/17 (a homolog of Arabidopsis MPK3), both showed interaction with MKK2/4/5, WRKY33, PP2C (protein phosphatase 2C family protein), and protein-tyrosine-phosphatase (PTP1). For Group B MAPK proteins, MPK4 (LsMAPK5) and ATMAPK13 (LsMAPK14/18) were able to interact with MKK proteins (MKK2, MKK6), PP2C, and PTP1. Furthermore, LsMAPK5 also showed an interaction relationship with WRKY transcription factors (WRKY33 and WRKY25). For Group C MAPK proteins, LsMAPK1/4/7/8 proteins, the Arabidopsis MPK7 homologs, showed the same interaction networks. They both showed complex interaction with PP2C, MKK, and bZIP transcription factor. For Group D MAPK proteins, MPK9 (LsMAPK3/6/15), MPK16 (LsMAPK11/12/13), MPK18 (LsMAPK16), and MPK19 (LsMAPK10) showed ten interaction relationships with other proteins, including PTP1, PP2C, and bZIP transcription factor (HY5, HYH) (Supplemental Table S6).
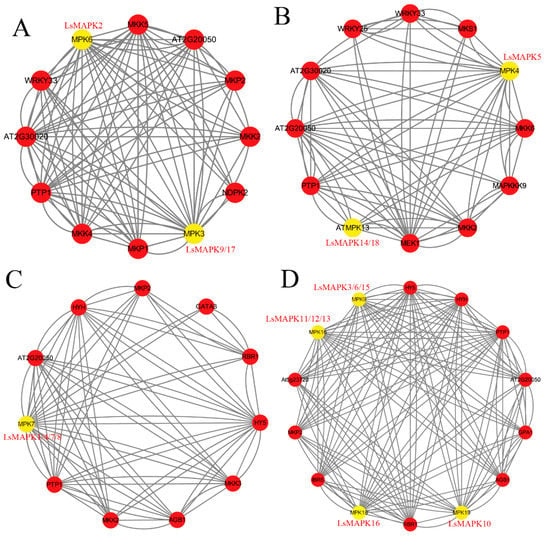
Figure 7.
An interaction network analysis of LsMAPK proteins. (A) Interaction proteins of LsMAPK proteins belonging to the Group A subfamily. (B) Interaction proteins of LsMAPK proteins belonging to the Group B subfamily. (C) Interaction proteins of LsMAPK proteins belonging to the Group C subfamily. (D) Interaction proteins of LsMAPK proteins belonging to the Group D subfamily.
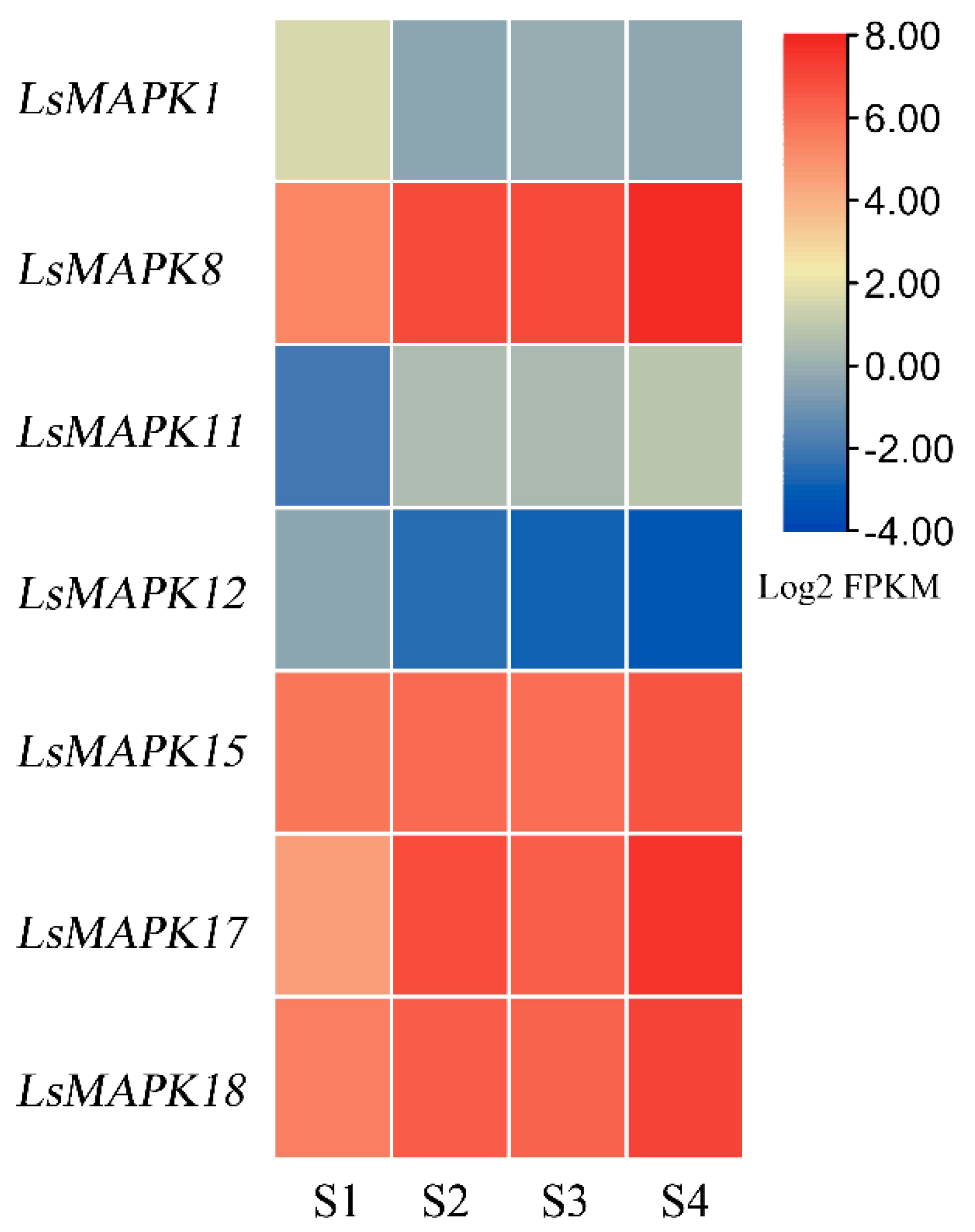
3.8. Expression Patterns of LsMAPK Genes in Response to Stem Enlargement
Numerous studies have shown that MAPK genes can participate in the process of plant growth and development. In this study, the roles of LsMAPKs involved in the stem enlargement process of stem lettuce were evaluated by RNA-Seq (Supplemental Table S7). As shown in Figure 8, seven LsMAPK genes, including LsMAPK1, LsMAPK8, LsMAPK11, LsMAPK12, LsMAPK15, LsMAPK17, and LsMAPK18, showed different expression during four stem enlargement stages (S1, S2, S3, and S4). LsMAPK8, LsMAPK11, LsMAPK17, and LsMAPK18 showed increased expression levels during the process of stem enlargement, while LsMAPK1 and LsMAPK12 showed decreased expression levels at S2-S4 stages compared with the S1 stage. Expression of LsMAPK15 showed no significant change during the process of stem enlargement.
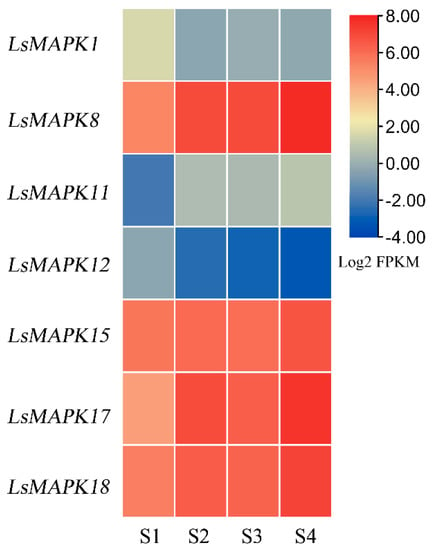
Figure 8.
Expression profiles of LsMAPK genes by transcriptome data analysis at different lettuce stem enlargement periods. S1: diameter length is 1 cm; S2: diameter length is 2 cm; S3: diameter length is 3 cm; S4: diameter length is 4 cm. Different color represented different expression levels of LsMAPK genes identified by RNA-Seq.
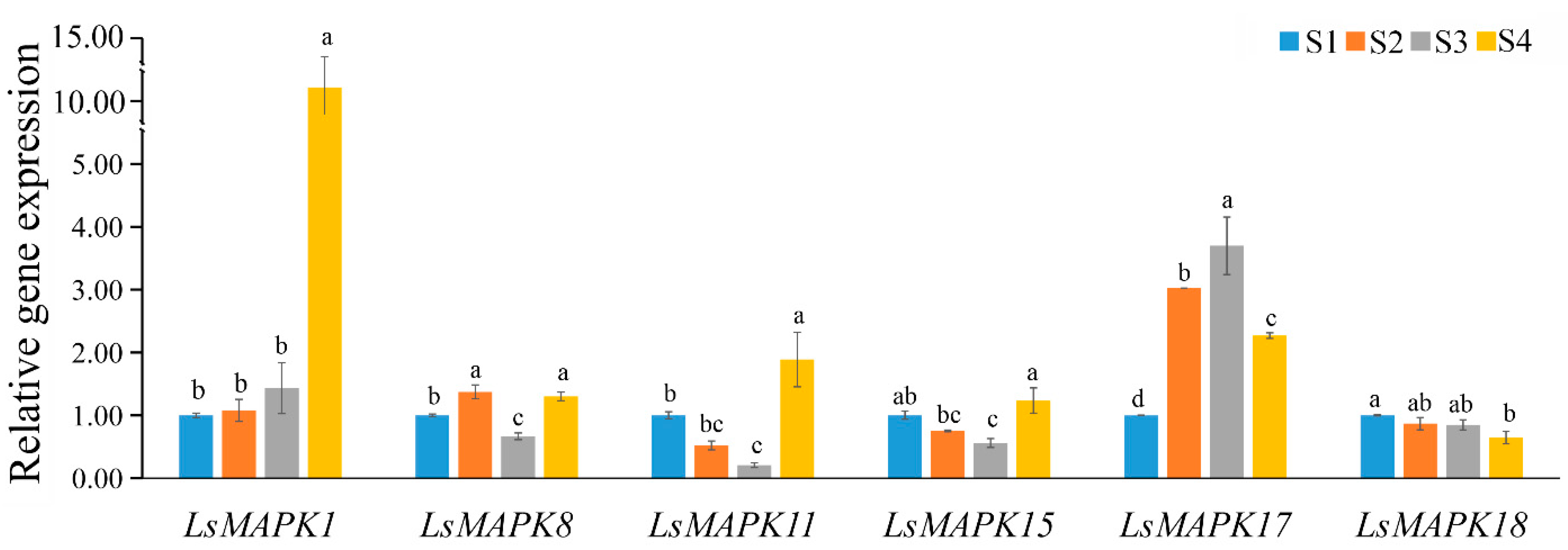
The accuracy of the transcriptome profiles was validated by qRT-PCR analysis. The expression profiles of six LsMAPK genes (LsMAPK1, LsMAPK8, LsMAPK11, LsMAPK15, LsMAPK17, and LsMAPK18) were quantified at different stem enlargement stages (S1, S2, S3, and S4). As shown in Figure 9, these six genes showed differential expression patterns. For instance, LsMAPK1 peaked at the S4 stage. The expression levels of LsMAPK17 increased significantly during the process of stem enlargement and peaked at the S3 stage. Compared with the S1 stage, the expression profiles of LsMAPK11 and LsMAPK15 decreased at the S2 and S3 stages but increased at the S4 stage. LsMAPK18 showed decreased expression levels. Overall, the RNA-Seq and qRT-PCR analysis results of most LsMAPK genes were consistent, suggesting that the LsMAPK genes may be involved in the stem enlargement process.
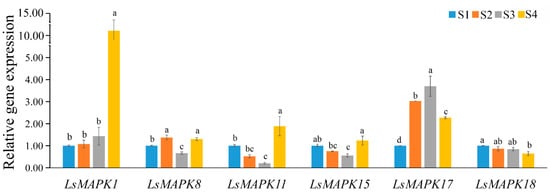
Figure 9.
Expression profiles of LsMAPK genes at different lettuce stem enlargement periods. S1: diameter length is 1 cm; S2: diameter length is 2 cm; S3: diameter length is 3 cm; S4: diameter length is 4 cm. Bars with different lowercase letters (a, ab, bc, b, c) represented significantly different by Duncan’s multiple range tests at the 0.05 levels.
3.9. Expression Levels of LsMAPK Genes Involved in Abiotic Stresses
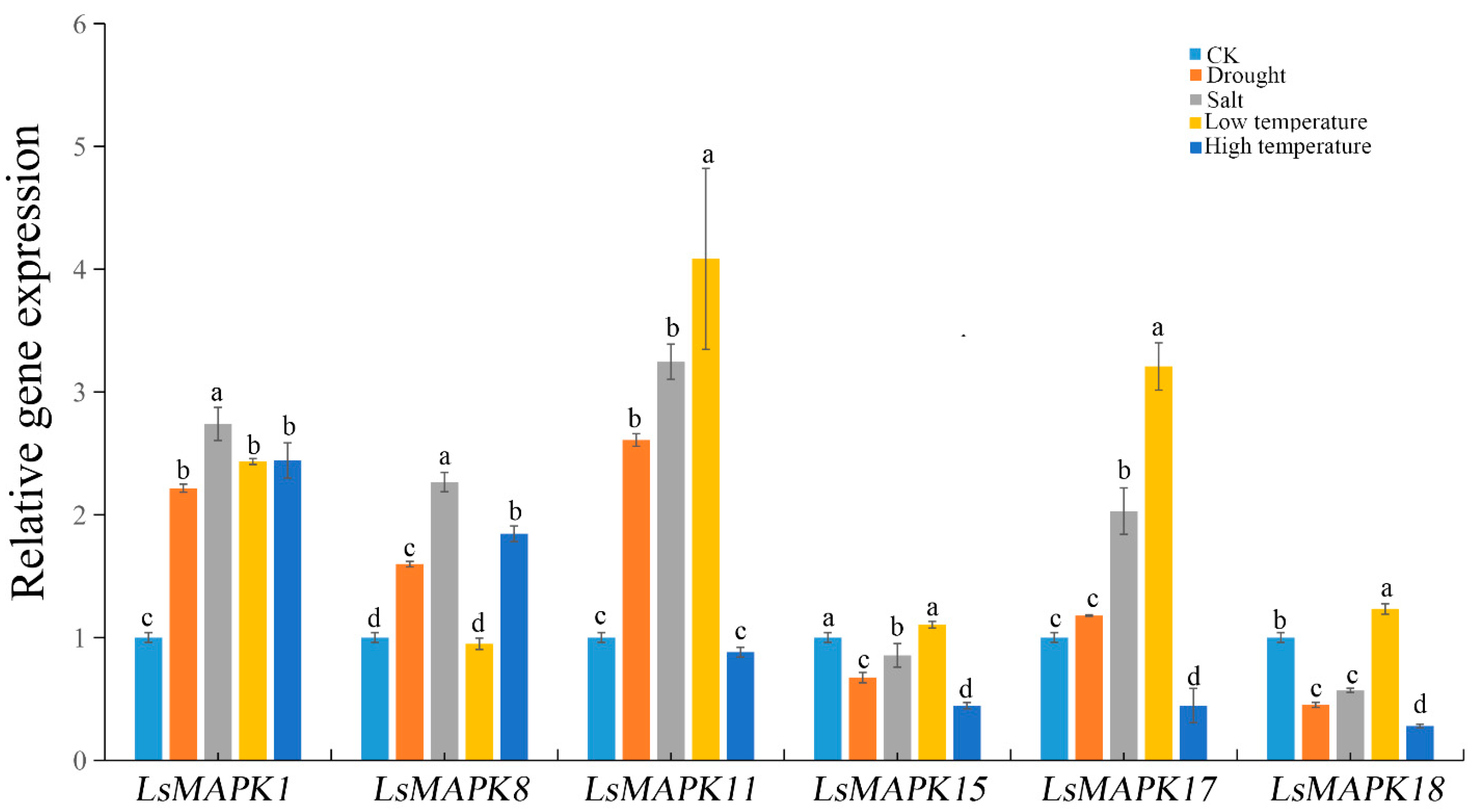
MAPK genes not only participated in plant growth and development, but were also involved in various abiotic stresses such as drought, salt, and extreme temperature. Analysis of cis-elements showed that LsMAPK genes contained various abiotic stresses including low-temperature-responsive element LTR, and drought-induced MYB binding site (Figure 3). For a preliminary investigation of the potential role of LsMAPK genes under abiotic stresses, six LsMAPK genes (LsMAPK1, LsMAPK8, LsMAPK11, LsMAPK15, LsMAPK17, and LsMAPK18) which responded to stem enlargement periods were selected to determine the roles of LsMAPK genes in abiotic stress response (drought, salt, low temperature, and high temperature) by qRT-PCR. As shown in Figure 10, the expression profiles of the six LsMAPK genes differed after drought treatment for 12 h. The expression of three genes, including LsMAPK1, LsMAPK8, and LsMAPK11, increased. The expression levels of LsMAPK1 and LsMAPK11 both increased twice as much as CK. However, the expression levels of LsMAPK15 and LsMAPK18 decreased after drought treatment. There was no significant change in the expression of LsMAPK17 under drought treatment. For salt treatment, the expression patterns of these six LsMAPK genes were similar to drought treatment. Four LsMAPK genes showed increased expression levels, including LsMAPK1, LsMAPK8, LsMAPK11, and LsMAPK17; however, the expression levels of LsMAPK15 and LsMAPK18 under salt treatment decreased by 0.86-fold and 0.57-fold, respectively. Low temperature also induced the expression of four LsMAPK genes. The expression levels of LsMAPK1, LsMAPK11, LsMAPK17, and LsMAPK18 increased by 2.5-fold, 4.0-fold, 3.21-fold, and 1.32-fold, respectively, whereas LsMAPK8 and LsMPAK15 were insensitive to low temperature. For high-temperature treatment, only LsMAPK1 and LsMAPK8, which belong to Group C, showed significantly increased expression; the expression levels of LsMAPK1 and LsMAPK8 increased by about 2.5-fold and 2-fold, respectively. In contrast, the expression patterns of LsMAPK15, LsMAPK17, and LsMAPK18 decreased under high temperatures. LsMAPK11 showed no significant change in expression level compared to the control under high temperatures.
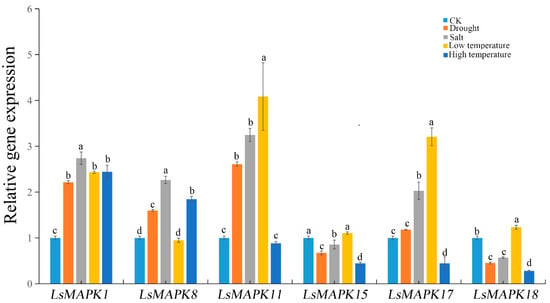
Figure 10.
Relative expression of LsMAPK genes under salt, drought, low temperature and high temperature at 12 h. Bars with different lowercase letters (a, b, c) represented significantly different by Duncan’s multiple range tests at the 0.05 levels.
3.10. LsMAPK Genes Expression Patterns in Response to Hormone Stresses
MAPK genes have been identified as participating in regulating plant hormone signal transduction. To investigate whether LsMAPK genes participate in hormone signal transduction, expression levels of LsMAPK genes under different plant hormone treatments were investigated using qRT-PCR. As shown in Figure 11, SA induced the expression of four genes (LsMAPK1, LsMAPK8, LsMAPK11, and LsMAPK17) except for LsMAPK15 and LsMAPK18. The expression levels of LsMAPK17, LsMAPK1, and LsMAPK11 increased by about 5.5-fold, 2.5-fold, and 2.5-fold, respectively, under SA treatment. For GA treatment, three LsMAPK genes—LsMAPK15, LsMAPK17, and LsMAPK18—showed decreased expression levels. Only the expression profile of LsMAPK11 was up-regulated under GA treatment; the expression level of LsMAPK11 increased 2-fold. For ABA treatment, the expression level of LsMAPK8 increased about 2-fold, while LsMAPK1 and LsMAPK18 expression decreased. The expression patterns of three genes, LsMAPK11, LsMAPK15, and LsMAPK17, showed no significant change under ABA treatment.
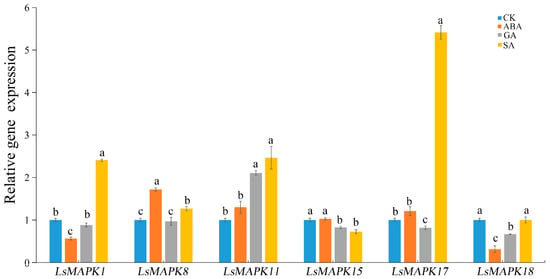
Figure 11.
Relative expression of LsMAPK genes under SA (salicylic acid, 0.5 mmol/L), ABA (abscisic acid, 75 µmol/L), GA (gibberellin, 50 µmol/L) and untreated control (CK) at 12 h. Bars with different lowercase letters (a, b, c) represented significantly different by Duncan’s multiple range tests at the 0.05 levels.
4. Discussion
During their lifetime, plants encounter various abiotic stresses which can seriously affect their growth and development, and change the distribution of plant species [32]. Higher plants adapt to various adverse environmental factors by developing complex signal transduction pathways. The MAPK cascade is one of the important signal transduction pathways in all eukaryotes and can provide developmental and environmental clues on intracellular responses [33]. Studies have shown that MAPK genes play critical roles in modulating various abiotic stress (temperature, drought, salinity, UV, heavy metal), biotic stress (pathogen infection, wounding), plant hormone response, and plant growth and development [34,35,36]. The possible function of gene family can be predicated by phylogenetic, evolutionary, and structural analysis. To predicate the roles of LsMAPK genes in lettuce, the gene structure of LsMAPK genes was analyzed. As shown in Figure 2, the exon–intron numbers in the same group of lettuce LsMAPKs were similar. The LsMAPKs in group C contained two or three exons, while, LsMAPKs in group D contained eight or eleven exons. Similar results were also shown in other plant species, such as Arabidopsis, poplar, and tomato [37,38,39]. The results showed that the evolution of exon–intron structure among different species was highly conserved.
Previous research has shown that there were 17 LsMAPK family genes in lettuce [40]. In the study, 18 LsMAPK genes were identified to exist in lettuce. There were some differences in the results of our study and those of Wang et al. [40]. For example, the LsMAPK16 (Lsat_1_v5_gn_9_101600), which was not identified in 2022 by Wang et al. [40], was identified as a member of the MAPK family in our study. This difference may be related to the different e values used when searching the genes by BLASTP. Phylogenetic tree analysis showed the lettuce LsMAPKs were classified into A, B, C, and D groups based on the TE(D)Y motif. Research demonstrates that group D has the largest number of MAPK members than other groups, which indicates that group D has undergone significant expansion in the evolution of MAPKs [8]. In the study, the number of LsMAPK genes in different groups varied, and group D contained the largest number of members (8). Phylogenetic tree analysis also showed that 18 LsMAPKs in lettuce were more closely clustered with Arabidopsis than with rice, indicating the evolution relationship between lettuce and Arabidopsis was closer than with rice. The results were consistent with the results of synteny analysis. As shown in Figure 6, the collinear gene pairs among lettuce, Arabidopsis, and rice were performed. Five collinear gene pairs existed between lettuce and Arabidopsis, while no collinear gene pairs existed between lettuce and rice. The LsMAPK genes and their corresponding AtMPKs were clustered on the same branch, which indicated a similar function among these genes [41].
Plants have developed complex mechanisms to protect themselves from various abiotic stresses (extreme temperature, salinity, drought, and UV). MAPK proteins participate in plant abiotic stress response [42,43]. For instance, the PtrMAPK-overexpressing transgenic tobacco lines showed improved drought tolerance than wild-type (WT) varieties [44]. BnMAPK1 from Brassica napus enhanced drought resistance by increasing root activity and cell water retention [45]. In our study, a large number of cis-elements related to abiotic stresses were found in the promoter region of LsMAPK genes, which indicated the possible function of LsMAPK genes in response to abiotic stress. The expression patterns of six LsMAPK genes were determined by qRT-PCR analysis. LsMAPK1 and LsMAPK8 were significantly induced by drought, salt, and high temperature. The function analysis of Arabidopsis MPK3, MPK4, and MPK6 was comprehensive and thorough. MPK3 and MPK4, which belong to group A, participated in plant salinity response through a complex regulation network [46]. MPK3 and MPK4 promoted salt tolerance by interacting with and phosphorylating key cytokinin signaling components, ARR genes, or heat shock factor HSFA4A [46,47]. In the study, the expression of LsMAPK17, a homolog of AtMPK3, was significantly induced by salt treatment and low temperature. Rice group A MAPK gene OsMPK3 positively regulated low-temperature response by phosphorylating OsICE1 genes, which directly targeted OsTPP1, the key enzyme in the trehalose biosynthetic pathway, to improve cold tolerance [48]. Similar, tomato SlMPK3 enhanced low-temperature tolerance by improving antioxidant enzyme activity; however, SlMPK1 served as a negative regulator in response to high temperature [49,50]. Arabidopsis MPK3/MPK6 played a negative role in response to low temperature, while the cold tolerance in mutation MPK3, MPK6, or both lines was improved compared with WT lines [51]. These results indicated that MAPK genes classified in the same group may play similar functions in response to abiotic stresses.
Although the roles of MAPK proteins in plant stress response are characterized, their functions in diverse signaling networks, including plant development such as pollen development and plant ovule development, are unclear [52,53]. In the study, the expression patterns of LsMAPK genes involved in stem enlargement process were detected. The expression levels of several LsMAPK genes, including LsMAPK1 and LsMAPK17, were significantly increased in the stem enlargement process. In Arabidopsis, AtMPK3, AtMPK4, and AtMPK6 were involved in flower development, including early pollen development, anther, and ovule integument development [54,55]. As the homolog of AtMPK3, the expression levels of LsMAPK17 increased continuously in the process of stem expansion, implying they may participate in regulating this process. However, the important roles of LsMAPK genes in the stem enlargement process need to be explored further.
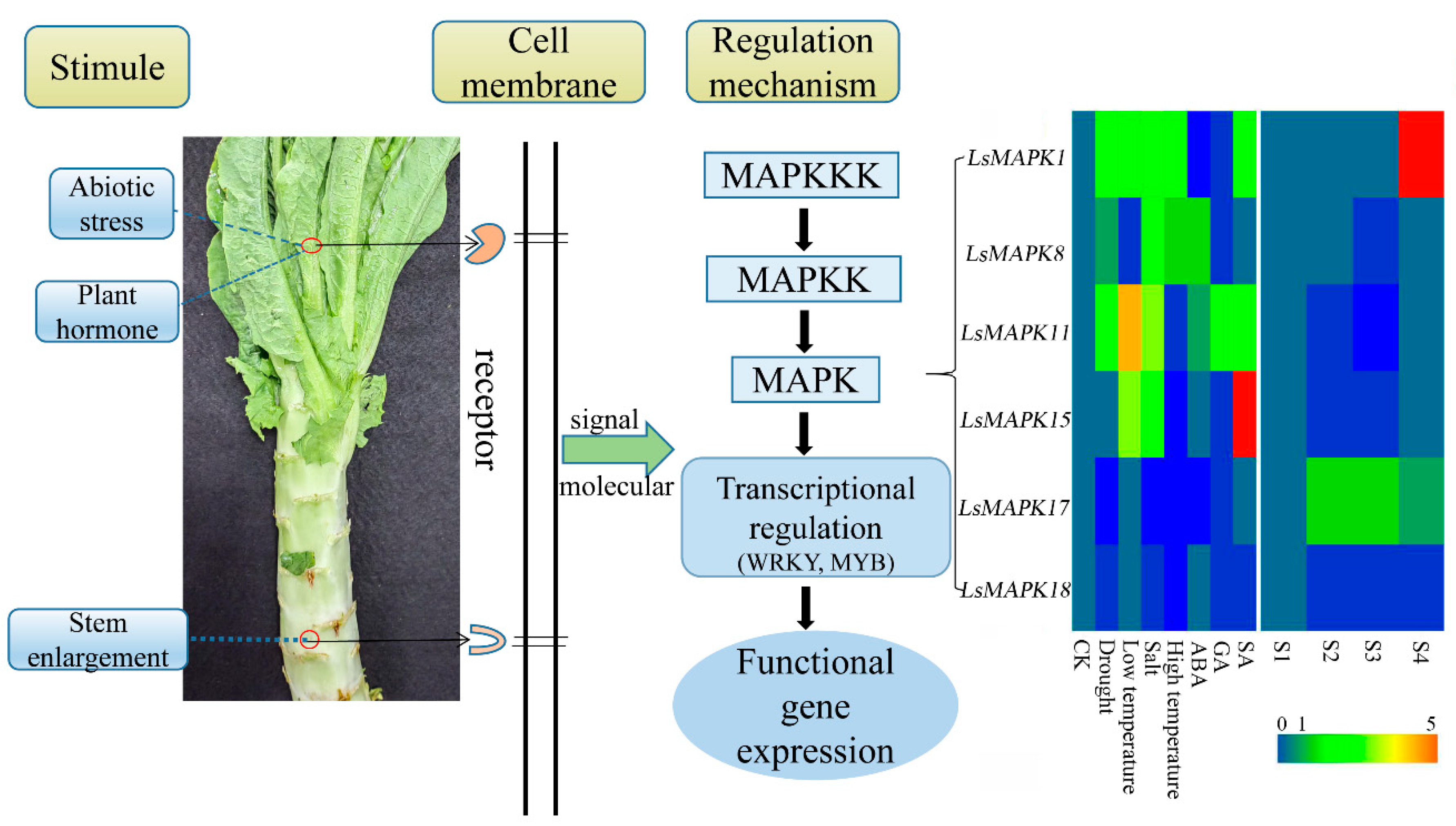
Some studies have confirmed the interconnections between MAPK signaling and plant hormones. ABA enhanced plants’ resistance to various unfavorable environmental conditions and was needed for plant growth and development. Studies have shown that MAPK genes are involved in ABA signaling through different molecular biology techniques. Arabidopsis MPK3 negatively regulated the ABA signaling pathway. The MPK3-overexpressed Arabidopsis seedlings were stunted even under ABA treatment [56]. Interestingly, MPK3 also participated in ABA-inhibited stomatal opening [57]. In addition, Arabidopsis MPK9 and MPK12 were involved in ABA signaling [58]. Arabidopsis MPK3 and MPK6 were able to disrupt normal plant growth and development by inducing SA accumulation [59]. LsMAPK17, the homolog of AtMPK3, was significantly induced under SA treatment, and the expression level increased about 6-fold. The roles of MAPK genes in JA, auxin, and the ethylene signaling pathway have also been identified in plants including Arabidopsis, tomato, and tobacco [36]. Here, different expression patterns under ABA, GA and SA existed in the examined LsMAPK genes, indicating the potential different functions of these genes in lettuce hormone signaling pathways. In summary, when stem lettuce is threatened by different adverse environments, various stimuli (abiotic stress, plant hormone, and plant growth and development) will be transmitted to the MAPK cascades through signaling molecular on the cell membrane. MAPK cascades can phosphorylate downstream target genes such as transcription factor, thereby inducing the expression of downstream functional genes (Figure 12). These results will provide the information for the function analysis of lettuce MAPK genes.
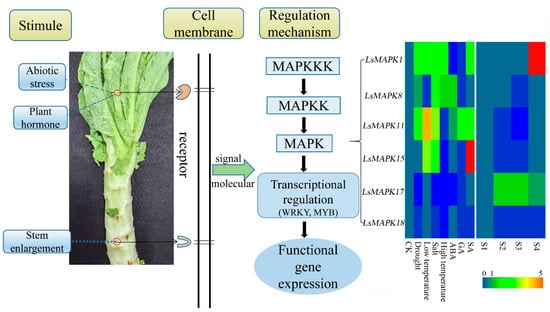
Figure 12.
Possible model of stem lettuce LsMAPK genes involved in different stimuli.
5. Conclusions
In our study, 18 LsMAPK genes were identified in lettuce. The systematic analysis and identification of LsMAPK genes were conducted by the analysis of exon–intron structure, motif compositions, collinearity analysis, phylogenetic relationships, chromosome distribution, and expression patterns. Our study provides important information about the evolution and diversity of the MAPK gene family in lettuce. These findings can provide a basis for further analysis of the function of MAPK genes in plants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8111087/s1, Supplemental Tables: Table S1. Primer sequences for qRT-PCR in the study. Table S2. The nucleotide and amino acid sequences of LsMAPK genes. Table S3. Cis-elements identified in the promoter region of LsMAPK genes by PlantCARE. Table S4. Cis-elements identified in the promoter region of LsMAPK genes by PlantPAN. Table S5. The paralogs and orthologs genes of MAPK genes between lettuce and Arabidopsis. Table S6. Interaction proteins between LsMAPK proteins and other proteins. Table S7. Expression profiles of LsMAPK genes by transcriptome data analysis at different lettuce stem enlargement periods. with 1, 2, and 3 representing three biological repeats. Supplemental Figure S1. The amino acid sequence alignment of LsMAPK proteins.
Author Contributions
Conceptualization, Y.H. and M.L.; investigation, Y.H., Y.L. and Z.L.; resources, J.D. and Y.L.; data curation, X.C. and Z.L.; writing—original draft preparation, Y.H. and J.D.; writing—review and editing, Y.H. and M.L.; funding acquisition, Y.H. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (32102405, 32002027), Natural Science Foundation of Shandong Province (ZR2020QC156, ZR2020QC063), the Innovation Team of Youth Technology Project of High School in Shandong Province: 2021KJ055.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The RNA-seq data were downloaded from SRA database of NCBI (Accession ID: PRJNA844256; Link: https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA844256, accessed on 1 July 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kissoudis, C.; van de Wiel, C.; Visser, R.G.; van der Linden, G. Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front. Plant Sci. 2014, 5, 207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.C.; Petersen, M.; Mundy, J. Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 2010, 61, 621–649. [Google Scholar] [PubMed]
- He, X.; Wang, C.; Wang, H.; Li, L.; Wang, C. The function of MAPK cascades in response to various stresses in horticultural plants. Front. Plant Sci. 2020, 11, 952. [Google Scholar] [CrossRef]
- Sharma, D.; Verma, N.; Pandey, C.; Verma, D.; Bhagat, P.K.; Noryang, S.; Tayyeba, S.; Banerjee, G.; Sinha, A.K. MAP kinase as regulators for stress responses in plants: An overview. In Protein Kinases and Stress Signaling in Plants; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020. [Google Scholar]
- Wang, C.; Guo, H.; He, X.; Zhang, S.; Wang, J.; Wang, L.; Guo, D.; Guo, X. Scaffold protein GhMORG1 enhances the resistance of cotton to Fusarium oxysporum by facilitating the MKK6-MPK4 cascade. Plant Biotechnol. J. 2020, 18, 1421–1433. [Google Scholar] [CrossRef]
- Stone, J.M.; Walker, J.C. Plant protein kinase families and signal transduction. Plant Physiol. 1995, 108, 451–457. [Google Scholar] [CrossRef]
- Li, M.; Li, B.; Yang, M.; Wang, L.; Hou, G.; Lin, Y.; Zhang, Y.; Zhang, Y.; Chen, Q.; Wang, Y.; et al. Genome-wide identification and expression of MAPK gene family in cultivated strawberry and their involvement in fruit developing and ripening. Int. J. Mol. Sci. 2022, 23, 5201. [Google Scholar] [CrossRef]
- Wang, G.; Wang, T.; Jia, Z.H.; Xuan, J.P.; Pan, D.L.; Guo, Z.R.; Zhang, J.Y. Genome-wide bioinformatics analysis of MAPK gene family in kiwifruit (Actinidia chinensis). Int. J. Mol. Sci. 2018, 19, 2510. [Google Scholar] [CrossRef]
- Andreasson, E.; Ellis, B. Convergence and specificity in the Arabidopsis MAPK nexus. Trends Plant Sci. 2010, 15, 106–113. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z.; Schwarz, E.M.; Lin, A.; Guan, K.; Ulevitch, R.J.; Han, J. Structure-function studies of p38 mitogen-activated protein kinase. Loop 12 influences substrate specificity and autophosphorylation, but not upstream kinase selection. J. Biol. Chem. 1997, 272, 11096–11102. [Google Scholar] [CrossRef]
- Colcombet, J.; Hirt, H. Arabidopsis MAPKs: A complex signalling network involved in multiple biological processes. Biochem. J. 2008, 413, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhao, H.; Sun, L.; Wu, W.; Li, C.; Wu, Q. Genome-wide identification of MAPK gene family members in Fagopyrum tataricum and their expression during development and stress responses. BMC Genom. 2022, 23, 96. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wan, Y.; Meng, X.; Zhang, X.; Yao, M.; Miu, W.; Zhu, D.; Yuan, D.; Lu, K.; Li, J.; et al. Genome-wide identification and analysis of MKK and MAPK gene families in Brassica species and response to stress in Brassica napus. Int. J. Mol. Sci. 2021, 22, 544. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wang, N.N.; Zhang, J.B.; Zheng, Y.; Li, X.B. Genome-wide identification of the mitogen-activated protein kinase (MAPK) family in cotton (Gossypium hirsutum) reveals GhMPK6 involved in fiber elongation. Plant Mol. Biol. 2020, 103, 391–407. [Google Scholar] [CrossRef]
- Neupane, S.; Schweitzer, S.E.; Neupane, A.; Andersen, E.J.; Fennell, A.; Zhou, R.; Nepal, M.P. Identification and characterization of mitogen-activated protein kinase (MAPK) genes in sunflower (Helianthus annuus L.). Plants 2019, 8, 28. [Google Scholar] [CrossRef]
- Cui, L.; Yang, G.; Yan, J.; Pan, Y.; Nie, X. Genome-wide identification, expression profiles and regulatory network of MAPK cascade gene family in barley. BMC Genom. 2019, 20, 750. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Irie, K.; Hirayama, T.; Hayashida, N.; Yamaguchi-Shinozaki, K.; Matsumoto, K.; Shinozaki, K. A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold, and water stress in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1996, 93, 765–769. [Google Scholar] [CrossRef]
- Wu, L.; Zu, X.; Zhang, H.; Wu, L.; Xi, Z.; Chen, Y. Overexpression of ZmMAPK1 enhances drought and heat stress in transgenic Arabidopsis thaliana. Plant Mol. Biol. 2015, 88, 429–443. [Google Scholar] [CrossRef]
- Zhou, M.; Zhao, B.; Li, H.; Ren, W.; Zhang, Q.; Liu, Y.; Zhao, J. Comprehensive analysis of MAPK cascade genes in sorghum (Sorghum bicolor L.) reveals SbMPK14 as a potential target for drought sensitivity regulation. Genomics 2022, 114, 110311. [Google Scholar] [CrossRef]
- Ding, H.D.; Zhang, X.H.; Xu, S.C.; Sun, L.L.; Jiang, M.Y.; Zhang, A.Y.; Jin, Y.G. Induction of protection against paraquat-induced oxidative damage by abscisic acid in maize leaves is mediated through mitogen-activated protein kinase. J. Integr. Plant Biol. 2009, 51, 961–972. [Google Scholar] [CrossRef]
- Gu, L.; Liu, Y.; Zong, X.; Liu, L.; Li, D.P.; Li, D.Q. Overexpression of maize mitogen-activated protein kinase gene, ZmSIMK1 in Arabidopsis increases tolerance to salt stress. Mol. Biol. Rep. 2010, 37, 4067–4073. [Google Scholar] [CrossRef]
- Gawroński, P.; Witoń, D.; Vashutina, K.; Bederska, M.; Betliński, B.; Rusaczonek, A.; Karpiński, S. Mitogen-activated protein kinase 4 is a salicylic acid-independent regulator of growth but not of photosynthesis in Arabidopsis. Mol. Plant. 2014, 7, 1151–1166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zheng, Y.F.; Zeng, T.; Sun, R.; Yang, J.Y.; Li, Y.; Ren, D.T.; Ma, H.; Xu, Z.H.; Bai, S.N. Phosphorylation of SPOROCYTELESS/NOZZLE by the MPK3/6 kinase is required for another development. Plant Physiol. 2017, 173, 2265–2277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cochet, F.; Ponnaiah, M.; Lebreton, S.; Matheron, L.; Pionneau, C.; Boudsocq, M.; Resentini, F.; Huguet, S.; Blázquez, M.Á.; et al. The MPK8-TCP14 pathway promotes seed germination in Arabidopsis. Plant J. 2019, 100, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.M.; Zou, C.; Shu, Y.N.; Liu, S.S. WRKY transcription factors in Nicotiana tabacum modulate plant immunity against whitefly via interacting with MAPK cascade pathways. Insects 2020, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Wang, Q.; Dou, X.; Wang, W.; Zhao, Q.; Lv, R.; Zhang, H.; Zheng, X.; Wang, P.; Zhang, Z. MoSwi6, an APSES family transcription factor, interacts with MoMps1 and is required for hyphal and conidial morphogenesis, appressorial function and pathogenicity of Magnaporthe oryzae. Mol. Plant Pathol. 2012, 13, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hao, J.H.; Qi, Z.Y.; Liu, W.H.; Lu, C.J.; Han, Y.Y.; Fan, S.X. Cloning and expression of mitogen-activated protein kinase 4 (MAPK4) in response to high temperature in lettuce (Lactuca sativa). Intl. J. Agric. Biol. 2019, 21, 54–60. [Google Scholar]
- Sun, Z.; Li, Z.; Huang, J.; Zheng, B.; Zhang, L.; Wang, Z. Genome-wide comparative analysis of LEAFY promoter sequence in angiosperms. Physiol. Mol. Biol. Plants. 2017, 23, 23–33. [Google Scholar] [CrossRef]
- Pfafflfl, M.W. A new mathematical model for relative quantification in realtime RT-PCR. Nucleic. Acids. Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Borowski, J.M.; Galli, V.; Messias Rda, S.; Perin, E.C.; Buss, J.H.; dos Anjos e Silva, S.D.; Rombaldi, C.V. Selection of candidate reference genes for real-time PCR studies in lettuce under abiotic stresses. Planta 2014, 239, 1187–1200. [Google Scholar] [CrossRef]
- Wang, Y.; Frei, M. Stressed food-The impact of abiotic environmental stresses on crop quality. Agric. Ecosyst. Environ. 2011, 141, 271–286. [Google Scholar] [CrossRef]
- Sinha, A.K.; Jaggi, M.; Raghuram, B.; Tuteja, N. Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signal. Behav. 2011, 6, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, X.; Lu, Z.; Qiu, W.; Yu, M.; Li, H.; He, Z.; Zhuo, R. MAPK cascades and transcriptional factors: Regulation of heavy metal tolerance in plants. Int. J. Mol. Sci. 2022, 23, 4463. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Z.; Liao, W. The involvement of gaseous signaling molecules in plant MAPK cascades: Function and signal transduction. Planta 2021, 254, 127. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S. Mitogen-activated protein kinase cascades in plant signaling. J. Integr. Plant Biol. 2022, 64, 301–341. [Google Scholar] [CrossRef]
- Hamel, L.P.; Nicole, M.C.; Sritubtim, S.; Morency, M.J.; Ellis, M.; Ehlting, J.; Beaudoin, N.; Barbazuk, B.; Klessig, D. Ancient signals: Comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci. 2006, 11, 192–198. [Google Scholar] [CrossRef]
- Pitzschke, A.; Schikora, A.; Hirt, H. MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol. 2009, 12, 421–426. [Google Scholar] [CrossRef]
- Kong, F.; Wang, J.; Cheng, L.; Liu, S.; Wu, J.; Peng, Z.; Lu, G. Genome-wide analysis of the mitogen-activated protein kinase gene family in Solanum lycopersicum. Gene 2012, 499, 108–120. [Google Scholar] [CrossRef]
- Wang, T.; Liu, M.; Wu, Y.; Tian, Y.; Han, Y.; Liu, C.; Hao, J.; Fan, S. Genome-wide identification and expression analysis of MAPK gene family in lettuce (Lactuca sativa L.) and functional analysis of LsMAPK4 in high-temperature-induced bolting. Int. J. Mol. Sci. 2022, 23, 11129. [Google Scholar] [CrossRef]
- Li, M.; He, Q.; Huang, Y.; Luo, Y.; Zhang, Y.; Chen, Q.; Wang, Y.; Lin, Y.; Zhang, Y.; Liu, Z.; et al. Sucrose synthase gene family in Brassica juncea: Genomic organization, evolutionary comparisons, and expression regulation. PeerJ 2021, 9, e10878. [Google Scholar] [CrossRef]
- Wang, J.; Pan, C.; Wang, Y.; Ye, L.; Wu, J.; Chen, L.; Zou, T.; Lu, G. Genome-wide identification of MAPK, MAPKK, and MAPKKK gene families and transcriptional profiling analysis during development and stress response in cucumber. BMC Genom. 2015, 16, 386. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Liu, X.; Long, D.; Guo, Q.; Fang, Y.; Bian, C.; Zhang, D.; Zeng, Q.; Xiang, Z.; Zhao, A. Molecular cloning and expression analysis of mulberry MAPK gene family. Plant Physiol. Biochem. 2014, 77, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.S.; Luo, T.; Fu, X.Z.; Fan, Q.J.; Liu, J.H. Cloning and molecular characterization of a mitogen-activated protein kinase gene from Poncirus trifoliata whose ectopic expression confers dehydration/drought tolerance in transgenic tobacco. J. Exp. Bot. 2011, 62, 5191–5206. [Google Scholar] [CrossRef]
- Weng, C.M.; Lu, J.X.; Wan, H.F.; Wang, S.W.; Wang, Z.; Kun, L.U.; Liang, Y. Over-expression of BnMAPK1 in Brassica napus enhances tolerance to drought stress. J. Integr. Agric. 2014, 13, 2407–2415. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, J.; Wang, F.; Xie, C.; Lv, B.; Yu, Z.; Dai, S.; Liu, X.; Xia, G.; Tian, H.; et al. MPK3/6-induced degradation of ARR1/10/12 promotes salt tolerance in Arabidopsis. EMBO Rep. 2021, 22, e52457. [Google Scholar] [CrossRef]
- Andrási, N.; Rigó, G.; Zsigmond, L.; Pérez-Salamó, I.; Papdi, C.; Klement, E.; Pettkó-Szandtner, A.; Baba, A.I.; Ayaydin, F.; Dasari, R.; et al. The mitogen-activated protein kinase 4-phosphorylated heat shock factor A4A regulates responses to combined salt and heat stresses. J. Exp. Bot. 2019, 70, 4903–4918. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Li, F.; Liu, H.; Yang, W.; Chong, K.; Xu, Y. OsMAPK3 phosphorylates OsbHLH002/OsICE1 and inhibits its ubiquitination to activate OsTPP1 and enhances rice chilling tolerance. Dev. Cell. 2017, 43, 731–743. [Google Scholar] [CrossRef]
- Ding, H.; He, J.; Wu, Y.; Wu, X.; Ge, C.; Wang, Y.; Zhong, S.; Peiter, E.; Liang, J.; Xu, W. The tomato mitogen-activated protein kinase SlMPK1 is as a negative regulator of the high-temperature stress response. Plant Physiol. 2018, 177, 633–651. [Google Scholar] [CrossRef]
- Yu, L.; Yan, J.; Yang, Y.; Zhu, W. Overexpression of tomato mitogen-activated protein kinase SlMPK3 in tobacco increases tolerance to low temperature stress. Plant Cell. Tiss. Org. 2014, 121, 21–34. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, P.; Si, T.; Hsu, C.C.; Wang, L.; Zayed, O.; Yu, Z.; Zhu, Y.; Dong, J.; Tao, W.A.; et al. MAP kinase cascades regulate the cold response by modulating ICE1 protein stability. Dev. Cell. 2017, 43, 618–629. [Google Scholar] [CrossRef]
- Chen, L.; Guan, X.; Qin, L.; Zou, T.; Zhang, Y.; Wang, J.; Wang, Y.; Pan, C.; Lu, G. Downregulation of the mitogen-activated protein kinase SlMAPK7 gene results in pollen abortion in tomato. Plant Cell. Tiss. Org. 2016, 126, 79–92. [Google Scholar] [CrossRef]
- Chai, L.; Tudor, R.L.; Poulter, N.S.; Wilkins, K.A.; Eaves, D.J.; Franklin, F.C.H.; Franklin-Tong, V.E. MAP kinase PrMPK9-1 contributes to the self-incompatibility response. Plant Physiol. 2017, 174, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Hord, C.L.; Sun, Y.J.; Pillitteri, L.J.; Torii, K.U.; Wang, H.; Zhang, S.; Ma, H. Regulation of Arabidopsis early anther development by the mitogen-activated protein kinases, MPK3 and MPK6, and the ERECTA and related receptor-like kinases. Mol. Plant. 2008, 1, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Chen, J.G.; Ellis, B.E. AtMPK4 is required for male-specific meiotic cytokinesis in Arabidopsis. Plant J. 2011, 67, 895–906. [Google Scholar] [CrossRef]
- Lu, C.; Han, M.H.; Guevara-Garcia, A.; Fedoroff, N.V. Mitogen-activated protein kinase signaling in postgermination arrest of development by abscisic acid. Proc. Natl. Acad. Sci. USA 2002, 99, 15812–15817. [Google Scholar] [CrossRef]
- Gudesblat, G.E.; Iusem, N.D.; Morris, P.C. Guard cell-specific inhibition of Arabidopsis MPK3 expression causes abnormal stomatal responses to abscisic acid and hydrogen peroxide. New. Phytol. 2007, 173, 713–721. [Google Scholar] [CrossRef]
- Jammes, F.; Song, C.; Shin, D.; Munemasa, S.; Takeda, K.; Gu, D.; Cho, D.; Lee, S.; Giordo, R.; Sritubtim, S.; et al. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 20520–20525. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, S. Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci. 2015, 20, 56–64. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).