Growth Performance of Guava Trees after the Exogenous Application of Amino Acids Glutamic Acid, Arginine, and Glycine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vegetative Growth Parameters
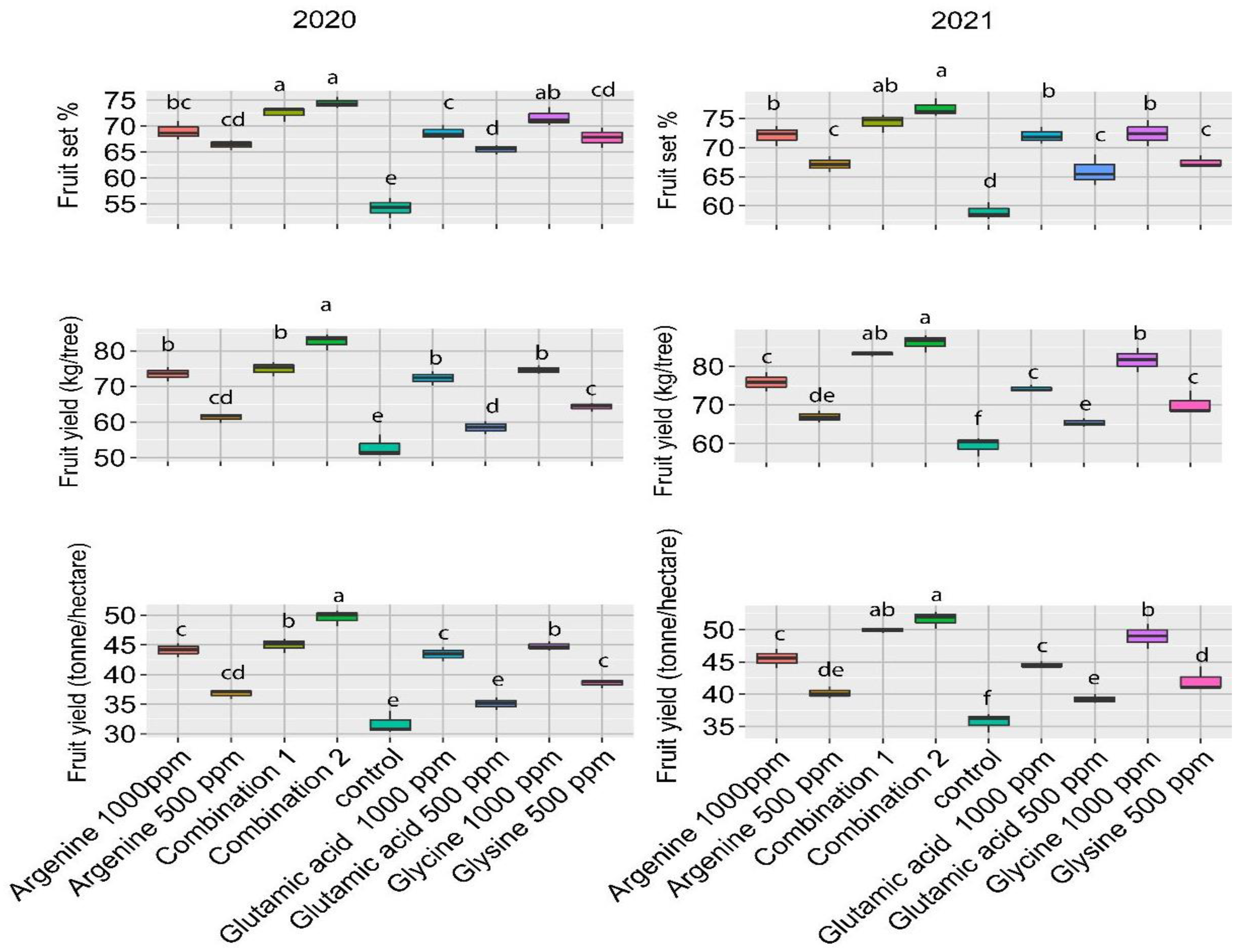
2.2. Fruit Set Percentage, Fruit Yield
2.3. Fruit Quality
2.3.1. Fruit Physical Characteristics
2.3.2. Fruit Chemical Characteristics
2.4. Nutritional Status
2.5. Statistical Analysis
3. Results
3.1. Vegetative Growth Parameters
3.2. Fruit Set and Yield
3.3. Fruit Quality
3.3.1. Fruit Physical Quality Characteristics
3.3.2. Fruit Chemical Quality Characteristics
3.4. Nutritional Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Omayio, D.G.; Abong, G.O.; Okoth, M.W.; Gachuiri, C.K.; Mwang’ombe, A.W. Current status of guava (Psidium guajava L.) production, utilization, processing and preservation in Kenya: A review. Curr. Agric. Res. J. 2019, 7, 318–331. [Google Scholar] [CrossRef]
- Esitken, A.; Ercisli, S.; Karlidag, H.; Sahin, F. Potential use of plant growth promoting rhizobacteria (PGPR) in organic apricot production. In Proceedings of the International Scientific Conference: Environmentally Friendly Fruit Growing, Polli, Estonia, 7–9 September 2005; pp. 90–97. [Google Scholar]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Mohammadi, P.; Khoshgoftarmanesh, A.H. The effectiveness of synthetic zinc (Zn)-amino chelates in supplying Zn and alleviating salt-induced damages on hydroponically grown lettuce. Sci. Hortic. 2014, 172, 117–123. [Google Scholar] [CrossRef]
- Mohammadipour, N.; Souri, M.K. Beneficial effects of glycine on growth and leaf nutrient concentrations of coriander (Coriandrum sativum) plants. J. Plant Nutr. 2019, 42, 1637–1644. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, J.; Li, R.; Wang, H.; Wang, J.; Soil. Effect of exogenous amino acids on Cu uptake and translocation in maize seedlings. Plant Biotechnol. J. 2007, 292, 105–117. [Google Scholar] [CrossRef]
- Garcia, A.; Madrid, R.; Gimeno, V.; Rodriguez-Ortega, W.; Nicolas, N.; Garcia-Sanchez, F. The effects of amino acids fertilization incorporated to the nutrient solution on mineral composition and growth in tomato seedlings. Span. J. Agric. Res. 2011, 9, 852–861. [Google Scholar] [CrossRef] [Green Version]
- Noroozlo, Y.A.; Souri, M.K.; Delshad, M. Stimulation effects of foliar applied glycine and glutamine amino acids on lettuce growth. Open Agric. 2019, 4, 164–172. [Google Scholar] [CrossRef]
- Sh Sadak, M.; Abdelhamid, M.T.; Schmidhalter, U. Effect of foliar application of aminoacids on plant yield and some physiological parameters in bean plants irrigated with seawater. Acta Biológica Colomb. 2015, 20, 141–152. [Google Scholar]
- Souri, M.K.; Hatamian, M. Aminochelates in plant nutrition: A review. J. Plant Nut. 2019, 42, 67–78. [Google Scholar] [CrossRef]
- Galili, G.; Amir, R. Fortifying plants with the essential amino acids lysine and methionine to improve nutritional quality. Plant Biotechnol. J. 2013, 11, 211–222. [Google Scholar] [CrossRef]
- Cerdan, M.; Sanchez-Sanchez, A.; Jordan, J.D.; Juarez, M.; Sanchez-Andreu, J. Effect of commercial amino acids on iron nutrition of tomato plants grown under lime--induced iron deficiency. J. Plant Nut. Soil Sci. 2013, 176, 859–866. [Google Scholar] [CrossRef]
- D’Mello, J. Delivering innovative solutions and paradigms for a changing environment. In Amino Acids in Higher Plants; CAB International: Wallingford, UK, 2015; pp. 538–583. [Google Scholar]
- García García, J.; García García, B. Econometric model of viability/profitability of octopus (Octopus vulgaris) ongrowing in sea cages. Aquac. Int. 2011, 19, 1177–1191. [Google Scholar] [CrossRef]
- Zhou, X.-B.; Chen, C.; Li, Z.-C.; Zou, X.-Y. Using Chou’s amphiphilic pseudo-amino acid composition and support vector machine for prediction of enzyme subfamily classes. J. Theor. Biol. 2007, 248, 546–551. [Google Scholar] [CrossRef]
- Kauffman, G.L.; Kneivel, D.P.; Watschke, T.L. Effects of a biostimulant on the heat tolerance associated with photosynthetic capacity, membrane thermostability, and polyphenol production of perennial ryegrass. Crop Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Parađiković, N.; Vinković, T.; Vinković Vrček, I.; Žuntar, I.; Bojić, M.; Medić-Šarić, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef]
- Claussen, W. Proline as a measure of stress in tomato plants. Plant Sci. 2005, 168, 241–248. [Google Scholar] [CrossRef]
- Nur, D.; Selcuk, G.; Yuksel, T. Effect of organic manure application and solariziation of soil microbial biomass and enzyme activities under greenhouse conditions. Biol. Agric. Hortic. 2006, 23, 305–320. [Google Scholar]
- El–Bassiouny, H.M.S.; Mostafa, H.A.; El–Khawas, S.A.; Hassanein, R.A.; Khalil, S.I.; Abd El–Monem, A.A. Physiological responses of wheat plant to foliar treatments with arginine or putrescine. Austr. J. Basic Appl. Sci. 2008, 2, 1390–1403. [Google Scholar]
- Jerry, A.N.; AL-Jarah, T.M. Effect of foliar application of two amino acids” arginine and cysteine” and potassium nitrate on the growth and yield of the tomato plants grown in plastic houses. Kufa J. Agric. Sci. 2015, 7, 16–35. [Google Scholar]
- Liepman, A.H.; Olsen, L.J. Genomic analysis of aminotransferases in Arabidopsis thaliana. Crit. Rev. Plant Sci. 2004, 23, 73–89. [Google Scholar] [CrossRef]
- Forde, B.; Lea, P. Glutamate in plants: Metabolism, regulation, and signalling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef]
- Yaronskaya, E.; Vershilovskaya, I.; Poers, Y.; Alawady, A.E.; Averina, N.; Grimm, B. Cytokinin effects on tetrapyrrole biosynthesis and photosynthetic activity in barley seedlings. Planta 2006, 224, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Walch-Liu, P.; Liu, L.-H.; Remans, T.; Tester, M.; Forde, B.G. Evidence that L-glutamate can act as an exogenous signal to modulate root growth and branching in Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 1045–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walch-Liu, P.; Forde, B.G. L-Glutamate as a novel modifier of root growth and branching what’s the sensor? Plant Signal Behav. 2017, 2, 284–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, A.A.; Gharib, F.A.; El-Awadi, M.; Rashad, E.-S.M. Physiological response of onion plants to foliar application of putrescine and glutamine. Sci. Hortic. 2011, 129, 353–360. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2011. [Google Scholar]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Umburanas, R.C.; Reichardt, K.; Neto, D.D. Foliar and seed application of amino acids affects the antioxidant metabolism of the soybean crop. Front. Plant Sci. 2017, 8, 327. [Google Scholar] [CrossRef] [Green Version]
- Taiz, L.; Zeiger, E.; Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2010; ISBN 978-0-87893-866-7. [Google Scholar]
- Brosnan, J.; Brosnan, M.E. Glutamate: A truly functional amino acid. Amino Acids 2013, 45, 413–418. [Google Scholar] [CrossRef]
- Reiner, A.; Levitz, J. Glutamatergic signaling in the central nervous system: Ionotropic and metabotropic receptors in concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Chakrabarty, S. Gibberellic acid in plant: Still a mystery unresolved. Plant Signal. Behav. 2013, 8, e25504. [Google Scholar] [CrossRef] [Green Version]
- Walch-Liu, P.; Forde, B.G. Nitrate signalling mediated by the NRT1. 1 nitrate transporter antagonises L-glutamate-induced changes in root architecture. Plant J. 2008, 54, 820–828. [Google Scholar] [CrossRef]
- Dennison, K.L.; Spalding, E.P. Glutamate-gated calcium fluxes in Arabidopsis. Plant Physiol. Biochem. 2000, 124, 1511–1514. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Gul, B.; Weber, D.J. Action of plant growth regulators and salinity on seed germination of Ceratoides lanata. Can. J. Bot. 2004, 82, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Haghighi, M.; Teixeira Da Silva, J.A. Amendment of hydroponic nutrient solution with humic acid and glutamic acid in tomato (Lycopersicon esculentum Mill.) culture. Soil Sci. Plant Nutr. 2013, 59, 642–648. [Google Scholar] [CrossRef]
- Cheng, D.; Yadav, N.; King, R.W.; Swanson, M.S.; Weinstein, E.J.; Bedford, M.T. Small molecule regulators of protein arginine methyltransferases. J. Biol. Chem. 2004, 279, 23892–23899. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.-H.; Nada, K.; Honda, C.; Kitashiba, H.; Wen, X.-P.; Pang, X.-M.; Moriguchi, T. Polyamine biosynthesis of apple callus under salt stress: Importance of the arginine decarboxylase pathway in stress response. J. Exp. Bot. 2006, 57, 2589–2599. [Google Scholar] [CrossRef]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef]
- Öhlund, J.; Näsholm, T. Growth of conifer seedlings on organic and inorganic nitrogen sources. Tree Physiol. 2001, 21, 1319–1326. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Yao, P.; Chu, X.; Hao, L.; Guo, X.; Xu, B. Isolation of arginine kinase from Apis cerana cerana and its possible involvement in response to adverse stress. Cell Stress Chaperones 2015, 20, 169–183. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 2011, 29, 300–311. [Google Scholar] [CrossRef]
- Won, Y.-W.; Kim, K.-M.; An, S.S.; Lee, M.; Ha, Y.; Kim, Y.-H. Suicide gene therapy using reducible poly (oligo-D-arginine) for the treatment of spinal cord tumors. Biomaterials 2011, 32, 9766–9775. [Google Scholar] [CrossRef]
- Winter, G.; Todd, C.D.; Trovato, M.; Forlani, G.; Funck, D. Physiological implications of arginine metabolism in plants. Front. Plant Sci. 2015, 6, 534. [Google Scholar] [CrossRef] [Green Version]
- Mohseni, F.; Pakkish, Z.; Panahi, B. Arginine impact on yield and fruit qualitative characteristics of strawberry. Agric. Conspec. Sci. 2017, 82, 19–26. [Google Scholar]
- Cheng, L.; Ma, F.; Ranwala, D. Nitrogen storage and its interaction with carbohydrates of young apple trees in response to nitrogen supply. Tree Physiol. 2004, 24, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekhon, B.S. Chelates for micronutrients nutrition among crops. Resonance 2003, 8, 46–53. [Google Scholar] [CrossRef]
- Liu, J.; Wisniewski, M.; Droby, S.; Vero, S.; Tian, S.; Hershkovitz, V. Glycine betaine improves oxidative stress tolerance and biocontrol efficacy of the antagonistic yeast Cystofilobasidium infirmominiatum. Int. J. Food Microbiol. 2011, 146, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Jin, P.; Zhang, Y.; Huang, Y.; Wang, X.; Zheng, Y. Exogenous glycine betaine treatment enhances chilling tolerance of peach fruit during cold storage. Postharvest Biol. Technol. 2016, 114, 104–110. [Google Scholar] [CrossRef]
- Jiang, J.; Fan, X.; Zhang, Y.; Tang, X.; Li, X.; Liu, C.; Zhang, Z. Construction of a high-density genetic map and mapping of firmness in grapes (Vitis vinifera L.) based on whole-genome resequencing. Int. J. Mol. Sci. 2020, 21, 797. [Google Scholar] [CrossRef] [Green Version]
- Lo’ay, A.; Doaa, M. The potential of vine rootstocks impacts on ‘Flame Seedless’ bunches behavior under cold storage and antioxidant enzyme activity performance. Sci. Hortic. 2020, 260, 108844. [Google Scholar] [CrossRef]
- Mosa, W.F.; Salem, M.Z.; Al-Huqail, A.A.; Ali, H.M. Application of glycine, folic Acid, and moringa extract as bio-stimulants for enhancing the production of’Flame Seedless’ grape cultivar. Bioresources 2021, 16, 3391–3410. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis, Part 3: Chemical Methods; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Demirsoy, H.J.F. Leaf area estimation in some species of fruit tree by using models as a non-destructive method. Fruits 2009, 64, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.S. Phenol-sulfuric acid method for total carbohydrates. In Food Analysis Laboratory Manual; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; pp. 47–53. [Google Scholar]
- Association of Official Analytical Chemist (AOAC). Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Nielsen, S.S. Vitamin C determination by indophenol method. In Food Analysis Laboratory Manual; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2017; pp. 143–146. [Google Scholar]
- Arrobas, M.; Afonso, S.; Rodrigues, M.Â. Diagnosing the nutritional condition of chestnut groves by soil and leaf analyses. Sci. Hortic. 2018, 228, 113–121. [Google Scholar] [CrossRef]
- Wang, H.; Pampati, N.; McCormick, W.M.; Bhattacharyya, L. Protein nitrogen determination by Kjeldahl digestion and ion chromatography. J. Pharm. Sci. 2016, 105, 1851–1857. [Google Scholar] [CrossRef]
- Weiwei, C.; Jinrong, L.; Fang, X.; Jing, L. Improvement to the determination of activated phosphorus in water and wastewater by yellow vanadomolybdate method. Ind. Water Treat. 2017, 37, 95–97. [Google Scholar]
- Ott, R.L.; Longnecker, M.T.; Ott, R.L.; Longnecker, M.T. An Introduction to Statistical Methods and Data Analysis; Cengage Learning: Boston, MA, USA, 2015. [Google Scholar]
- Lv, D.; Yu, C.; Yang, L.; Qin, S.; Ma, H.; Du, G.; Liu, G.; Khanizadeh, S. Effects of foliar-applied L-glutamic acid on the diurnal variations of leaf gas exchange and chlorophyll fluorescence parameters in hawthorn (Crataegus pinnatifida Bge.). Eur. J. Hortic. Sci. 2009, 74, 204. [Google Scholar]
- Suharja, S.; Sutarno, S. Biomass, chlorophyll and nitrogen content of leaves of two chili pepper varieties (Capsicum annum) in different fertilization treatments. Nus. Biosci 2009, 1, 9–16. [Google Scholar]
- Tsang, E.W.; Yang, J.; Chang, Q.; Nowak, G.; Kolenovsky, A.; McGregor, D.I.; Keller, W.A. Chlorophyll reduction in the seed of Brassica napus with a glutamate 1-semialdehyde aminotransferase antisense gene. Plant Mol. Biol. 2003, 51, 191–201. [Google Scholar] [CrossRef]
- Septiyana, E.; Setiari, N.; Darmanti, S. Glutamic acid application for enhancement of growth and productivity of okra plant (Abelmoschus esculentus L. Moench). Biogenesis J. Ilm. Biol. 2019, 7, 124–131. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, J.S.; Lee, S.G.; Kim, S.K.; Mun, B.; Choi, C.S. Glutamic acid foliar application enhances antioxidant enzyme activities in kimchi cabbages leaves treated with low air temperature. Hortic. Sci. Technol. 2017, 700–706. [Google Scholar] [CrossRef] [Green Version]
- El-Shiekh, A.; Umaharan, P. Effect of gibberellic acid, glutamic acid and pollen grains extract on yield, quality and marketability of ’khalas’date palm fruits. Acta Hortic. 2014, 1047, 93–97. [Google Scholar] [CrossRef]
- Price, M.B.; Jelesko, J.; Okumoto, S. Glutamate receptor homologs in plants: Functions and evolutionary origins. Front. Plant Sci. 2012, 3, 235. [Google Scholar] [CrossRef] [Green Version]
- Forde, B.G.; Roberts, M.R. Glutamate receptor-like channels in plants: A role as amino acid sensors in plant defence? F1000prime Rep. 2014, 6, 37. [Google Scholar] [CrossRef]
- Weiland, M.; Mancuso, S.; Baluska, F. Signalling via glutamate and GLRs in Arabidopsis thaliana. Funct. Plant Biol. 2015, 43, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumoto, S.; Funck, D.; Trovato, M.; Forlani, G. Amino acids of the glutamate family: Functions beyond primary metabolism. Front. Plant Sci. 2016, 7, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbrin, E.d.S.; Mógor, Á.; Margoti, G.; Fowler, J.; Bettoni, M. Purple chicory’Palla Rossa’seedlings growth according to the foliar application of L-glutamic acid. Sci. Agrar. 2013, 14, 91–94. [Google Scholar]
- Röder, C.; Mógor, Á.F.; Szilagyi-Zecchin, V.J.; Gemin, L.G.; Mógor, G. Potato yield and metabolic changes by use of biofertilizer containing L-glutamic acid. Comun. Sci. 2018, 9, 211–218. [Google Scholar] [CrossRef]
- Yang, C.; Ko, B.; Hensley, C.T.; Jiang, L.; Wasti, A.T.; Kim, J.; Sudderth, J.; Calvaruso, M.A.; Lumata, L.; Mitsche, M. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol. Cell 2014, 56, 414–424. [Google Scholar] [CrossRef] [Green Version]
- Abou-Zaid, E.A.A.; Eissa, M.A. Thompson seedless grapevines growth and quality as affected by glutamic acid, vitamin b, and algae. J. Soil Sci. Plant Nut. 2019, 19, 725–733. [Google Scholar] [CrossRef]
- Morris Jr, S.M. Arginine: Beyond protein. Am. J. Clin. Nutr. 2006, 83, 508S–512S. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Yamaji, N.; Che, J.; Shen, R.F.; Ma, J.F. Normal root elongation requires arginine produced by argininosuccinate lyase in rice. Plant J. 2014, 78, 215–226. [Google Scholar] [CrossRef]
- Petridis, A.; van der Kaay, J.; Chrysanthou, E.; McCallum, S.; Graham, J.; Hancock, R.D. Photosynthetic limitation as a factor influencing yield in highbush blueberries (Vaccinium corymbosum) grown in a northern European environment. J. Exp. Bot. 2018, 69, 3069–3080. [Google Scholar] [CrossRef]
- Babalar, M.; Pirzad, F.; Sarcheshmeh, M.A.A.; Talaei, A.; Lessani, H. Arginine treatment attenuates chilling injury of pomegranate fruit during cold storage by enhancing antioxidant system activity. Postharvest Biol. Technol. 2018, 137, 31–37. [Google Scholar] [CrossRef]
- Shu, P.; Min, D.; Ai, W.; Li, J.; Zhou, J.; Li, Z.; Zhang, X.; Shi, Z.; Sun, Y.; Jiang, Y. L-Arginine treatment attenuates postharvest decay and maintains quality of strawberry fruit by promoting nitric oxide synthase pathway. Postharvest Biol. Technol. 2020, 168, 111253. [Google Scholar] [CrossRef]
- Eslami, M.; Nasibi, F.; Manouchehri Kalantari, K.; Khezri, M.; Oloumi, H. Effect of exogenous application of l-arginine and sodium nitroprusside on fruit abscission and physiological disorders of pistachio (Pistacia vera L.) Scions. Int. J. Hortic. Sci.Tech. 2019, 6, 51–62. [Google Scholar]
- Li, X.; Wang, C.; Jiang, H.; Luo, C. A patent review of arginine methyltransferase inhibitors (2010–2018). Expert Opin. Ther. Pat. 2019, 29, 97–114. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Z.; Tsai, H.-i.; Liu, Y.; Gao, J.; Wang, M.; Song, L.; Cao, X.; Xu, Z.; Chen, H. Branched-chain amino acid aminotransferase 2 regulates ferroptotic cell death in cancer cells. Cell Death Differ. 2021, 28, 1222–1236. [Google Scholar] [CrossRef]
- Mohseni, M.; Hamidoghli, A.; Bai, S.C. Organic and inorganic dietary zinc in beluga sturgeon (Huso huso): Effects on growth, hematology, tissue concertation and oxidative capacity. Aquac. Int. 2021, 539, 736672. [Google Scholar] [CrossRef]
- Pakkish, Z.; Mohammadrezakhani, S. Quality characteristics and antioxidant activity of the mango (Mangifera indica) fruit under arginine treatment. J. Plant Physiol. 2021, 11, 63–74. [Google Scholar]
- Yagi, M.; Abdulkareem, S. Effects of exogenous arginine and uric acid on Eruca sativa mill grown under saline conditions. J. Sci. Technol. 2006, 7, 1–11. [Google Scholar]
- Ali, M.H.; Khan, A.S.; Jaskani, M.J.; Anwar, R.; Ali, S.; Malik, A.U.; Hasan, M.U.; Rehman, R.N.U.; Ayyub, S. Pre-storage application of L-arginine mitigates chilling injury and maintains quality of Sandhuri guava fruit. J. Food Process. Preserv. 2022, 46, e16405. [Google Scholar] [CrossRef]
- Abdul-Qados, A. Effect of arginine on growth, yield and chemical constituents of wheat grown under salinity condition. Acad. J. Plant Sci. 2009, 2, 267–278. [Google Scholar]
- Xiaochuang, C.; Chu, Z.; Lianfeng, Z.; Junhua, Z.; Hussain, S.; Lianghuan, W.; Qianyu, J. Glycine increases cold tolerance in rice via the regulation of N uptake, physiological characteristics, and photosynthesis. Plant Physiol. Biochem. 2017, 112, 251–260. [Google Scholar] [CrossRef]
- Zargar Shooshtari, F.; Souri, M.K.; Hasandokht, M.R.; Jari, S.K. Glycine mitigates fertilizer requirements of agricultural crops: Case study with cucumber as a high fertilizer demanding crop. Chem. Biol. Technol. Agric. 2020, 7, 19. [Google Scholar] [CrossRef]
- Yang, N.; Wang, C.-L.; He, W.-P.; Qu, Y.-Z.; Li, Y.-S. Photosynthetic characteristics and effects of exogenous glycine of Chorispora bungeana under drought stress. Photosynthetica 2016, 54, 459–467. [Google Scholar] [CrossRef]
- Ge, T.; Song, S.; Roberts, P.; Jones, D.; Huang, D.; Iwasaki, K. Amino acids as a nitrogen source for tomato seedlings: The use of dual-labeled (13C, 15N) glycine to test for direct uptake by tomato seedlings. Environ. Exp. Bot. 2009, 66, 357–361. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Q.; Xu, Q.; Zhang, C.; Li, Y.; Fan, X.; Xie, X.; Chen, N. Systems metabolic engineering strategies for the production of amino acids. Synth. Syst. Biotechnol. 2017, 2, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Cao, X.; Wu, L.; Mi, W.; Feng, Y. Light intensity affects the uptake and metabolism of glycine by pakchoi (Brassica chinensis L.). Sci. Rep. 2016, 6, 21200. [Google Scholar] [CrossRef] [Green Version]
- Forsum, O.; Svennerstam, H.; Ganeteg, U.; Näsholm, T. Capacities and constraints of amino acid utilization in Arabidopsis. New Phytologist 2008, 179, 1058–1069. [Google Scholar] [CrossRef]
- Ghasemi, S.; Khoshgoftarmanesh, A.H.; Afyuni, M.; Hadadzadeh, H. The effectiveness of foliar applications of synthesized zinc-amino acid chelates in comparison with zinc sulfate to increase yield and grain nutritional quality of wheat. Eur. J. Agron. 2013, 45, 68–74. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M. Is dietary taurine supplementation beneficial for farmed fish and shrimp? A comprehensive review. Rev. Aquac. 2014, 6, 241–255. [Google Scholar] [CrossRef]
- Sooraki, F.Y.; Moghadamyar, M. Growth and quality of cucumber, tomato, and green bean under foliar and soil applications of an aminochelate fertilizer. Hortic. Environ. Biotechnol. 2017, 58, 530–536. [Google Scholar]
- Kurepin, L.V.; Ivanov, A.G.; Zaman, M.; Pharis, R.P.; Hurry, V.; Hüner, N. Interaction of glycine betaine and plant hormones: Protection of the photosynthetic apparatus during abiotic stress. In Photosynthesis: Structures, Mechanisms, and Applications; Springer: Berlin/Heidelberg, Germany, 2017; pp. 185–202. [Google Scholar]
- Souri, M.K.; Naiji, M.; Kianmehr, M.H. Nitrogen release dynamics of a slow release urea pellet and its effect on growth, yield, and nutrient uptake of sweet basil (Ocimum basilicum L.). J. Plant Nutr. 2019, 42, 604–614. [Google Scholar] [CrossRef]
- Mosa, W.F.; Ali, H.M.; Abdelsalam, N.R. The utilization of tryptophan and glycine amino acids as safe alternatives to chemical fertilizers in apple orchards. Environ. Sci. Pollut. Res. 2021, 28, 1983–1991. [Google Scholar] [CrossRef]

| Depth | pH | EC dS/m | O.M % | Textural Class | Sand % | Silt % | Clay % | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–60 | 7.4 | 1.94 | 1.72 | Clay | 9.12 | 20.78 | 70.1 | |||
| Nutrients (mg/kg Soil) | Soluble Anions(meq/L) | Soluble Cations(meq/L) | ||||||||
| N | P | K | CaCo3 % | HCO3− | Cl− | SO42− | Ca2+ | Mg2+ | Na+ | K+ |
| 142 | 21 | 789 | 1.27 | 6.96 | 5.45 | 5.65 | 6.10 | 3.87 | 5.55 | 2.78 |
| Treatment | Shoot Thickness (cm) | Shoot Length (cm) | Leaf Area (cm2) | Total Chlorophyll (SPAD) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| Control | 0 | 2.24 f | 2.30 g | 26.06 d | 24.63 d | 34.77 f | 37.84 e | 41.11 e | 43.20 e |
| Glutamic acid | 500 ppm | 2.78 e | 2.77 f | 27.30 cd | 24.7 d | 42.3 e | 46.28 d | 45.05 d | 45.41 d |
| 1000 ppm | 2.96 d | 3.41 d | 32.08 ab | 28.66 c | 46.21 bcd | 50.12 bc | 50.39 c | 51.73 c | |
| Arginine | 500 ppm | 2.78 e | 3.16 e | 27.71 cd | 27.73 c | 42.66 de | 46.54 d | 46.32 d | 45.85 d |
| 1000 ppm | 3.58 c | 3.61 c | 32.33 ab | 32.11 b | 46.82 bc | 51.96 b | 51.39 bc | 52.01 c | |
| Glycine | 500 ppm | 2.79 e | 3.26 e | 30.24 bc | 28.00 c | 43.36 cde | 47.87 cd | 47.07 d | 46.23 d |
| 1000 ppm | 3.73 b | 3.76 b | 33.71 ab | 32.33 b | 47.28 b | 56.70 a | 52.89 abc | 53.26 bc | |
| Combination | 1 | 3.8 ab | 4.16 a | 34.03 ab | 36.17 a | 48.89 b | 57.25 a | 54.29 ab | 54.70 b |
| 2 | 3.58 a | 4.16 a | 35.91 a | 37.27 a | 53.78 a | 58.21 a | 55.03 a | 57.03 a | |
| LSD0.05 | 0.11 | 0.13 | 3.69 | 2.68 | 3.50 | 3.03 | 2.84 | 1.84 | |
| Treatment | Fruit Weight (g) | Fruit Size (cm3) | Fruit Length (cm) | Fruit Diameter (cm) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| Control | 0 | 148.24 e | 152.81 g | 159.24 d | 165.28 f | 7.42 g | 7.65 e | 4.40 e | 4.43 f |
| Glutamic acid | 500 ppm | 155.43 d | 156.82 ef | 167.67 c | 170.28 de | 8.18 f | 8.20 d | 5.09 d | 5.10 e |
| 1000 ppm | 162.58 c | 164.34 d | 173.25 b | 178.14 c | 8.62 cd | 8.65 bc | 5.54 b | 5.60 bc | |
| Arginine | 500 ppm | 156.33 d | 155.04 f | 167.09 c | 168.51 ef | 8.41 e | 8.54 c | 5.22 c | 5.35 d |
| 1000 ppm | 164.22 bc | 165.06 cd | 175.55 b | 178.53 c | 8.63 c | 8.68 bc | 5.55 b | 5.63 bc | |
| Glycine | 500 ppm | 158.01 d | 158.37 e | 167.68 c | 172.83 d | 8.53 d | 8.57 bc | 5.50 b | 5.52 c |
| 1000 ppm | 165.56 bc | 166.63 bc | 175.56 b | 179.43 bc | 8.69 c | 8.71 b | 5.58 b | 5.66 b | |
| Combination | 1 | 167.46 b | 168.37 b | 176.46 b | 182.51 b | 8.84 b | 8.95 a | 5.81 a | 6.18 a |
| 2 | 172.73 a | 176.17 a | 183.73 a | 189.30 a | 8.95 a | 8.97 a | 5.89 a | 6.21 a | |
| LSD0.05 | 3.15 | 2.15 | 3.04 | 3.34 | 0.09 | 0.14 | 0.08 | 0.10 | |
| Treatment | Seed Weight (g) | Pulp Weight (g) | Juice (g) | Fruit Firmness (Ib/inch2) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| Control | 0 | 21.70 a | 21.76 a | 126.54 g | 131.06 g | 86.29 f | 86.65 e | 5.29 e | 5.30 f |
| Glutamic acid | 500 ppm | 20.08 b | 21.10 ab | 135.35 f | 135.72 ef | 88.62 ef | 89.55 de | 6.01 d | 5.96 e |
| 1000 ppm | 18.79 bcd | 19.89 cd | 143.79 d | 144.45 d | 91.61 cde | 93.09 bc | 6.33 c | 6.14 de | |
| Arginine | 500 ppm | 19.74 bc | 20.49 bc | 136.60 ef | 134.55 f | 89.15 def | 90.62 cd | 5.94 d | 6.08 e |
| 1000 ppm | 18.63 cd | 19.51 cde | 145.58 cd | 145.55 cd | 92.30 bc | 93.30 bc | 6.47 c | 6.38 cd | |
| Glycine | 500 ppm | 18.99 bc | 20.10 bcd | 139.02 e | 138.26 e | 90.46 de | 90.81 cd | 5.99 d | 6.13 de |
| 1000 ppm | 17.62 d | 19.02 def | 147.95 bc | 147.61 bc | 94.59 bcd | 95.05 b | 6.45 c | 6.60 c | |
| Combination | 1 | 17.52 d | 18.51 ef | 149.94 b | 149.87 b | 94.85 ab | 95.58 b | 7.47 b | 7.60 b |
| 2 | 17.45 d | 18.22 f | 155.28 a | 157.95 a | 97.01 a | 103.88 a | 8.00 a | 8.17a | |
| LSD0.05 | 1.09 | 1.09 | 3.28 | 2.91 | 2.93 | 2.91 | 0.25 | 0.27 | |
| Treatment | TSS % | Total acidity % | TSS-Acidity Ratio | VC (mg/100 mL) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| Control | 0 | 8.63 f | 9.27 e | 0.50 a | 0.53 a | 17.30 f | 17.49 f | 176.53 d | 176.58 d |
| Glutamic acid | 500 ppm | 9.80 e | 10.30 d | 0.49 a | 0.50 a | 20.50 ef | 20.54 f | 178.22 cd | 181.33 cd |
| 1000 ppm | 12.50 b | 12.53 b | 0.36 cd | 0.37 c | 35.07 c | 34.21 d | 187.20 b | 185.90 c | |
| Arginine | 500 ppm | 10.50 d | 11.60 c | 0.44 ab | 0.44 b | 24.01 de | 26.09 e | 183.77 bcd | 181.84 cd |
| 1000 ppm | 12.57 b | 12.87 b | 0.35 cd | 0.33 cd | 36.35 c | 39.45 c | 191.80 b | 197.00 b | |
| Glycine | 500 ppm | 11.30 c | 11.19 c | 0.40 bc | 0.41 b | 28.57 d | 27.43 e | 184.88 bc | 183.80 c |
| 1000 ppm | 12.63 b | 13.77 a | 0.34 cd | 0.32 de | 37.54 c | 43.49 b | 199.87 a | 197.33 b | |
| Combination | 1 | 13.63 a | 14.10 a | 0.32 d | 0.31 de | 42.76 b | 46.27 b | 200.09 a | 205.00 a |
| 2 | 14.06 a | 14.20 a | 0.29 d | 0.28 e | 47.97 a | 51.58 a | 207.63 a | 208.11 a | |
| LSD0.05 | 0.49 | 0.64 | 0.06 | 0.04 | 5.18 | 3.78 | 7.64 | 5.38 | |
| Treatment | N % | P % | K % | ||||
|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| Control | 0 | 2.06 e | 2.11 d | 0.32 d | 0.36 d | 2.32 f | 2.51 f |
| Glutamic acid | 500 ppm | 2.12 e | 2.19 d | 0.34 cd | 0.36 d | 2.52 e | 2.72 e |
| 1000 ppm | 2.72 c | 2.79 b | 0.44 ab | 0.45 abc | 3.53 c | 3.97 b | |
| Arginine | 500 ppm | 2.25 d | 2.29 c | 0.35 cd | 0.38 cd | 2.71 d | 2.82 de |
| 1000 ppm | 2.68 c | 2.72 b | 0.40 bc | 0.45 abc | 3.48 c | 3.58 c | |
| Glycine | 500 ppm | 2.26 d | 2.34 c | 0.38 bcd | 0.40 bcd | 2.72 d | 3.00 d |
| 1000 ppm | 2.86 b | 2.80 b | 0.45 ab | 0.47 ab | 3.88 b | 3.99 b | |
| Combination | 1 | 2.94 ab | 2.81 b | 0.45 ab | 0.49 a | 3.99 ab | 4.12 ab |
| 2 | 3.04 a | 3.11 a | 0.49 a | 0.53 a | 4.14 a | 4.20 a | |
| LSD0.05 | 0.12 | 0.10 | 0.06 | 0.07 | 0.17 | 0.19 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almutairi, K.F.; Saleh, A.A.; Ali, M.M.; Sas-Paszt, L.; Abada, H.S.; Mosa, W.F.A. Growth Performance of Guava Trees after the Exogenous Application of Amino Acids Glutamic Acid, Arginine, and Glycine. Horticulturae 2022, 8, 1110. https://doi.org/10.3390/horticulturae8121110
Almutairi KF, Saleh AA, Ali MM, Sas-Paszt L, Abada HS, Mosa WFA. Growth Performance of Guava Trees after the Exogenous Application of Amino Acids Glutamic Acid, Arginine, and Glycine. Horticulturae. 2022; 8(12):1110. https://doi.org/10.3390/horticulturae8121110
Chicago/Turabian StyleAlmutairi, Khalid F., Abaidalah A. Saleh, Muhammad Moaaz Ali, Lidia Sas-Paszt, Hesham S. Abada, and Walid F. A. Mosa. 2022. "Growth Performance of Guava Trees after the Exogenous Application of Amino Acids Glutamic Acid, Arginine, and Glycine" Horticulturae 8, no. 12: 1110. https://doi.org/10.3390/horticulturae8121110
APA StyleAlmutairi, K. F., Saleh, A. A., Ali, M. M., Sas-Paszt, L., Abada, H. S., & Mosa, W. F. A. (2022). Growth Performance of Guava Trees after the Exogenous Application of Amino Acids Glutamic Acid, Arginine, and Glycine. Horticulturae, 8(12), 1110. https://doi.org/10.3390/horticulturae8121110











