Chemotypes of Species of the Genus Thymus L. in Carpathians Region of Ukraine—Their Essential Oil Qualitative and Quantitative Characteristics and Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of the Plant Material
2.2. Thymus Oil Distillation
2.3. Analyses of the Essential Oils
2.4. Method for the Testing of the Antimicrobial Activity
2.5. Statistical Analyses of the Data
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Najar, B.; Pistelli, L.; Ferri, B.; Angelini, L.G.; Tavarini, S. Crop Yield and Essential Oil Composition of Two Thymus vulgaris Chemotypes along Three Years of Organic Cultivation in a Hilly Area of Central Italy. Molecules 2021, 26, 5109. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; Bruno, M.; Formisano, C.; De Feo, V.; Napolitano, F.; Rosselli, S.; Senatore, F. Chemical Composition and Antimicrobial Activity of the Essential Oils from Two Species of Thymus Growing Wild in Southern Italy. Molecules 2009, 14, 4614–4624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, R.S.; Lu, R.; Chanotiya, C.S.; Verma, R.K.; Singh, A.; Yadav, A.; Chauhan, A.; Yadav, A.K.; Singh, A.K. Essential oil composition of Thymus serpyllum cultivated in the Kumaon region of western Himalaya, India. Nat. Prod. Commun. 2009, 4, 1934578X0900400723. [Google Scholar] [CrossRef] [Green Version]
- Mohan, M.; Seth, R.; Singh, P.; Lohani, H.; Gupta, S. Composition of the Volatiles of Hyssopus officinalis (L.) and Thymus serpyllum (L.) from Uttarakhand Himalaya. Natl. Acad. Sci. Lett. 2012, 35, 445–448. [Google Scholar] [CrossRef]
- Varga, E.; Bardocz, A.; Belak, A.; Maraz, A.; Boros, B.; Felinger, A.; Böszörményi, A.; Horváth, G. Antimicrobial activity and chemical composition of thyme essential oils and the polyphenolic content of different Thymus extracts. Farmacia 2015, 6, 357–361. [Google Scholar]
- Ložionė, K.; Venskutonis, R.P. Chemical Composition of the Essential Oil of Thymus serpyllum L. ssp. serpyllum Growing Wild in Lithuania. J. Essent. Oil Res. 2006, 18, 206–211. [Google Scholar] [CrossRef]
- Paaver, U.; Orav, A.; Arak, E.; Mäeorg, U.; Raal, A. Phytochemical analysis of the essential oil of Thymus serpyllum L. growing wild in Estonia. Nat. Prod. Res. 2008, 22, 108–115. [Google Scholar] [CrossRef]
- Hrytsyna, M.R.; Kryvtsova, M.V.; Salamon, I.; Skybitska, M.I. Promising ex situ essential oil from Thymus camphoratus (Lamiaceae). Regul. Mech. Biosyst. 2020, 11, 310–314. [Google Scholar] [CrossRef]
- Ani, R.; Josip, M. Essential Oil and Glycosidically Bound Volatiles of Thymus pulegioides L. growing Wild in Croatia. Croat. Chem. Acta 2008, 81, 599–606. [Google Scholar]
- Kirillov, V.; Stikhareva, T.; Mukanov, B.; Chebotko, N.; Ryazantsev, O.; Atazhanova, G.; Adekenov, S. Composition of the Essential Oil of Thymus serpyllum L. from Northern Kazakhstan. J. Essent. Oil Bear. Plants 2016, 19, 212–222. [Google Scholar] [CrossRef]
- Sárosi, S.; Bernáth, J.; Bertoli, A.; Pistelli, L.; Benvenuti, S. Essential oil polymorphism of Thymus pulegioides collected in Monti Pisani, Italy. Acta Hortic. 2012, 955, 59–64. [Google Scholar] [CrossRef]
- Nikolić, B.; Matović, M.; Mladenović, K.; Todosijević, M.; Stanković, J.; Đorđević, I.; Marin, D.P.; Tešević, V. Volatiles of Thymus serpyllum Obtained by Three Different Methods. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Mariconda, A.; Vassallo, A.; Bonomo, M.G.; Calabrone, L.; Salzano, G.; Claps, M.; Sinicropi, M.S.; Capasso, A.; Saturnino, C. Herbal formulations of Thymus Serpyllum L. and Hypericum Perforatum L. from Southern Italy: Preparationand Chemical Characterization. PharmacologyOnLine 2020, 1, 1–10. Available online: https://pharmacologyonline.silae.it/front/archives_2020_1 (accessed on 16 December 2022).
- Milevskaya, V.; Temerdashev, Z.A.; Butyl’Skaya, T.S.; Kiseleva, N.V. Determination of phenolic compounds in medicinal plants from the Lamiaceae family. J. Anal. Chem. 2017, 72, 342–348. [Google Scholar] [CrossRef]
- Niculae, M.; Hanganu, D.; Oniga, I.; Benedec, D.; Ielciu, I.; Giupana, R.; Sandru, C.D.; Ciocârlan, N.; Spinu, M. Phytochemical Profile and Antimicrobial Potential of Extracts Obtained from Thymus marschallianus Willd. Molecules 2019, 24, 3101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhumakanova, B.; Korona-Głowniak, I.; Skalicka-Woźniak, K.; Ludwiczuk, A.; Baj, T.; Wojtanowski, K.; Józefczyk, A.; Zhaparkulova, K.; Sakipova, Z.; Malm, A. Phytochemical Fingerprinting and In Vitro Antimicrobial and Antioxidant Activity of the Aerial Parts of Thymus marschallianus Willd. and Thymus seravschanicus Klokov Growing Widely in Southern Kazakhstan. Molecules 2021, 26, 3193. [Google Scholar] [CrossRef]
- Pluhár, Z.; Kocsis, M.; Kuczmog, A.; Csete, S.; Simkó, H.; Sárosi, S.; Molnár, P.; Horváth, G. Essential oil composition and preliminary molecular study of four Hungarian Thymus species. Acta Biol. Hung. 2012, 63, 81–96. [Google Scholar] [CrossRef]
- Council of Europe European Directorate for the Quality of Medicines & Healthcare. European Pharmacopoeia 8.0: Published in Accordance with the Convention on the Elaboration of a European Pharmacopoeia, 8th ed.; European Directorate for the Quality of Medicines & Healthcare Council of Europe: Strasbourg, France, 14 February 2013. [Google Scholar]
- State Pharmacopoeia of Ukraine. In State enterprise “Ukrainian Scientific Pharmacopoeial Center for Quality of Medicines”, 2nd ed.; Suppl. 3, State Enterprise “Ukrainian Scientific Pharmacopoeial Center for the Quality of Medicines”: Kharkiv, Ukraine, 2014; Volume 3, p. 732.
- Atlas of the Lviv Region. Available online: https://geoknigi.com/view_map.php?id=28 (accessed on 16 December 2022).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Vaičiulytė, V.; Butkienė, R.; Ložienė, K. Effects of meteorological conditions and plant growth stage on the accumulation of carvacrol and its precursors in Thymus pulegioides. Phytochemistry 2016, 128, 20–26. [Google Scholar] [CrossRef]
- Wester, P.; Möseler, B.M.; Knöss, W. Intra-population terpene polymorphism of Thymus pulegioides L.: Evidence for seven chemotypes in a German limestone grassland. Biochem. Syst. Ecol. 2020, 93, 104173. [Google Scholar] [CrossRef]
- Senatore, F. Influence of Harvesting Time on Yield and Composition of the Essential Oil of a Thyme (Thymus pulegioides L.) Growing Wild in Campania (Southern Italy). J. Agric. Food Chem. 1996, 44, 1327–1332. [Google Scholar] [CrossRef]
- Gluschenko, L.A. Ecological, cenotic and resource characteristic of the species of genus Thymus L. at the territory of Left-bank Forest-Steppe—Manuscript. Thesis on a degree of the candidate of the biological sciences by specialty 03.00.05–botany. M.M.. Ph.D. Thesis, Gryshko National Botanical Garden of the National Ukrainian Academy of Sciences, Kyiv, Ukraine, 2005. [Google Scholar]
- Tymchenko, I.À.; Minarchenko, V.M.; Glushchenko, L.A.; Ànishchenko, Ì.M.; Gurinovich, N.V. Resources monitoring of Thymus L. in Ukraine. Ukr. Botan. J. 2007, 64, 78–87. [Google Scholar]
- Penkovska, L. Analysis of Ontogenic Structure of Thymus Serpyllum L. Emend. Mill. and Thymus x Polessicus Klokov (Lamiaceae) Coenopopulations Under the Conditions of the Ympil District, Sumy Region (Ukraine). Lesya Ukr. East. Eur. Natl. Univ. Sci. Bulletin. Series Biol. Sci. 2019, 3, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Penkovska, L. Dimensional features of Thymus serpyllum L. Emend. Mill. and Thymus x polessicus Klokov (Lamiaceae) in the conditions of different phytocenoses in the Shostka geobotanical district, Sumy region. Bull. Cherkasy University. Series Biol. Sci. 2020, 1, 53–61. [Google Scholar] [CrossRef]
- Mockute, D.; Bernotiene, G. 1,8-Cineole—Caryophyllene Oxide Chemotype of Essential Oil of Thymus serpyllum L. Growing Wild in Vilnius (Lithuania). J. Essent. Oil Res. 2004, 16, 236–238. [Google Scholar] [CrossRef]
- Mockute, D.; Bernotiene, G. The Main Citral–Geraniol and Carvacrol Chemotypes of the Essential Oil of Thymus pulegioides L. Growing Wild in Vilnius District (Lithuania). J. Agric. Food Chem. 1999, 47, 3787–3790. [Google Scholar] [CrossRef] [PubMed]
- Mockute, D.; Bernotiene, G. The α-terpenyl acetate chemotype of essential oil of Thymus pulegioides L. Biochem. Syst. Ecol. 2001, 29, 69–76. [Google Scholar] [CrossRef]
- Vaičiulytė, V.; Ložienė, K.; Švedienė, J.; Raudonienė, V.; Paškevičius, A. α-Terpinyl Acetate: Occurrence in Essential Oils Bearing Thymus Pulegioides, Phytotoxicity, and Antimicrobial Effects. Molecules 2021, 26, 1065. [Google Scholar] [CrossRef]
- Svydenko, L.V.; Hlushchenko, L.A. Component composition of essential oil in the forms of species creeping thyme (Thymus serpylum L.) and broad-leaved thyme (Thymus pulgioides L.) in Kherson region. Agroecol. J. 2016, 2, 129–134. [Google Scholar]
- Ložienė, K.; Venskutonis, P. Influence of environmental and genetic factors on the stability of essential oil composition of Thymus pulegioides. Biochem. Syst. Ecol. 2005, 33, 517–525. [Google Scholar] [CrossRef]
- StakelienĖ, V.; Ložienė, K. Gynodioecy in Thymus pulegioides L., T. serpyllum L., and their hybrid T. × oblongifolius Opiz (Lamiaceae): Flower size dimorphism, female frequency, and effect of environmental factors. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2013, 148, 49–57. [Google Scholar] [CrossRef]
- Al-Fekaiki, D.F.; Niamah, A.K.; Al-Sahlany, S.T.G. Extraction and identification of essential oil from Cinnamomum zeylanicum barks and study the antibacterial activity. J. Microbiol. Biotechnol. Food Sci. 2017, 7, 312–316. [Google Scholar] [CrossRef]
- Hao, R.; Roy, K.; Pan, J.; Shah, B.R.; Mraz, J. Critical review on the use of essential oils against spoilage in chilled stored fish: A quantitative meta-analyses. Trends Food Sci. Technol. 2021, 111, 175–190. [Google Scholar] [CrossRef]
- Tekorienė, R.; Ložienė, K. Disinfecting capacity of essential oil of Thymus pulegioides L. (lamiaceae) chemotypes against phytopathogenic Pseudomonas species. Acta Aliment. 2012, 41, 257–264. [Google Scholar] [CrossRef]
- Pinto, E.; Pina-Vaz, C.; Salgueiro, L.; Gonçalves, M.J.; Costa-De-Oliveira, S.; Cavaleiro, C.; Palmeira, A.; Rodrigues, A.G.; de Oliveira, J.M. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2006, 55, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Steshenko, Y.M.; Mazulin, O.V.; Polishchuk, N.M. Study of the antimicrobial and fungicidal activity of the essential oil Thymus × citriodorus (Pers.) Schreb. var. “Silver Queen”. Curr. Issues Pharm. Med. Sci. Pr. 2021, 14, 211–214. [Google Scholar] [CrossRef]
- Momin, S.; Irfan, S.; Ahmad, S.; Manzoor, M.; Irfan, H. Comparison of antimicrobial activities, herb and essential oil production of Thymus vulgaris L. and T. serpyllum L. in Balochistan, Pakistan. Pak. J. Weed Sci. Res. 2017, 23, 119–125. [Google Scholar]
- Kačániová, M.; Terentjeva, M.; Arpášová, H.; Hleba, L.; Ivanišová, E.; Petrová, J.; Kántor, A.; Čuboň, J.; Haščík, P. Effect of Thymus Serpyllum and Ocimum Basilicum Essential Oils on the Shelf-Life of Chicken’s Meat during Refrigerated Storage. Sci. Pap. Anim. Sci. Biotechnol. 2016, 49, 103–108. [Google Scholar]
- Sokolić-Mihalak, D.; Frece, J.; Slavica, A.; Delaš, F.; Pavlović, H.; Markov, K. The effects of wild thyme (Thymus serpyllum L.) essential oil components against ochratoxin-producing Aspergilli. Arh. Hig. Rada. Toksikol. 2012, 63, 457–462. [Google Scholar] [CrossRef]
 High-altitude meadows combined with thickets of huckleberries and blueberry bushes.
High-altitude meadows combined with thickets of huckleberries and blueberry bushes.  Carpathian spruce, fir, beech–spruce, and beech–fir forests from European spruce, white fir, and ordinary beech.
Carpathian spruce, fir, beech–spruce, and beech–fir forests from European spruce, white fir, and ordinary beech.  Post-forest meadows are largely plowed.
Post-forest meadows are largely plowed.  Pine, oak–pine, beech–pine, and broadleaf-pine forests of ordinary pine, ordinary oak, ordinary beech, ordinary hornbeam, and heart-leaved linden.
Pine, oak–pine, beech–pine, and broadleaf-pine forests of ordinary pine, ordinary oak, ordinary beech, ordinary hornbeam, and heart-leaved linden.  Agricultural land (meadow, arable land) in the place of pine and broadleaf-pine forests.
Agricultural land (meadow, arable land) in the place of pine and broadleaf-pine forests.  Beech forests from ordinary beech with an admixture of conifers (spruce and fir) and other broad-leaved species (sycamore maple, hornbeam, and ordinary oak).
Beech forests from ordinary beech with an admixture of conifers (spruce and fir) and other broad-leaved species (sycamore maple, hornbeam, and ordinary oak).  Oak forests from ordinary oak with admixture of other broad-leaved species (heart-leaved linden, sharp-leaf maple, ordinary ash, and bare elm).
Oak forests from ordinary oak with admixture of other broad-leaved species (heart-leaved linden, sharp-leaf maple, ordinary ash, and bare elm).  Agricultural land in the place of oak and beech forests.
Agricultural land in the place of oak and beech forests.  Polissja forest of black alder coarse-grass and forest-steppe grass swamps, sometimes with fragments of black alder forests and agricultural land in place of drained swamps.
Polissja forest of black alder coarse-grass and forest-steppe grass swamps, sometimes with fragments of black alder forests and agricultural land in place of drained swamps.  Floodplain meadows with fragments of willow and black alder forests in combination with lowland swamps.
Floodplain meadows with fragments of willow and black alder forests in combination with lowland swamps.
 High-altitude meadows combined with thickets of huckleberries and blueberry bushes.
High-altitude meadows combined with thickets of huckleberries and blueberry bushes.  Carpathian spruce, fir, beech–spruce, and beech–fir forests from European spruce, white fir, and ordinary beech.
Carpathian spruce, fir, beech–spruce, and beech–fir forests from European spruce, white fir, and ordinary beech.  Post-forest meadows are largely plowed.
Post-forest meadows are largely plowed.  Pine, oak–pine, beech–pine, and broadleaf-pine forests of ordinary pine, ordinary oak, ordinary beech, ordinary hornbeam, and heart-leaved linden.
Pine, oak–pine, beech–pine, and broadleaf-pine forests of ordinary pine, ordinary oak, ordinary beech, ordinary hornbeam, and heart-leaved linden.  Agricultural land (meadow, arable land) in the place of pine and broadleaf-pine forests.
Agricultural land (meadow, arable land) in the place of pine and broadleaf-pine forests.  Beech forests from ordinary beech with an admixture of conifers (spruce and fir) and other broad-leaved species (sycamore maple, hornbeam, and ordinary oak).
Beech forests from ordinary beech with an admixture of conifers (spruce and fir) and other broad-leaved species (sycamore maple, hornbeam, and ordinary oak).  Oak forests from ordinary oak with admixture of other broad-leaved species (heart-leaved linden, sharp-leaf maple, ordinary ash, and bare elm).
Oak forests from ordinary oak with admixture of other broad-leaved species (heart-leaved linden, sharp-leaf maple, ordinary ash, and bare elm).  Agricultural land in the place of oak and beech forests.
Agricultural land in the place of oak and beech forests.  Polissja forest of black alder coarse-grass and forest-steppe grass swamps, sometimes with fragments of black alder forests and agricultural land in place of drained swamps.
Polissja forest of black alder coarse-grass and forest-steppe grass swamps, sometimes with fragments of black alder forests and agricultural land in place of drained swamps.  Floodplain meadows with fragments of willow and black alder forests in combination with lowland swamps.
Floodplain meadows with fragments of willow and black alder forests in combination with lowland swamps.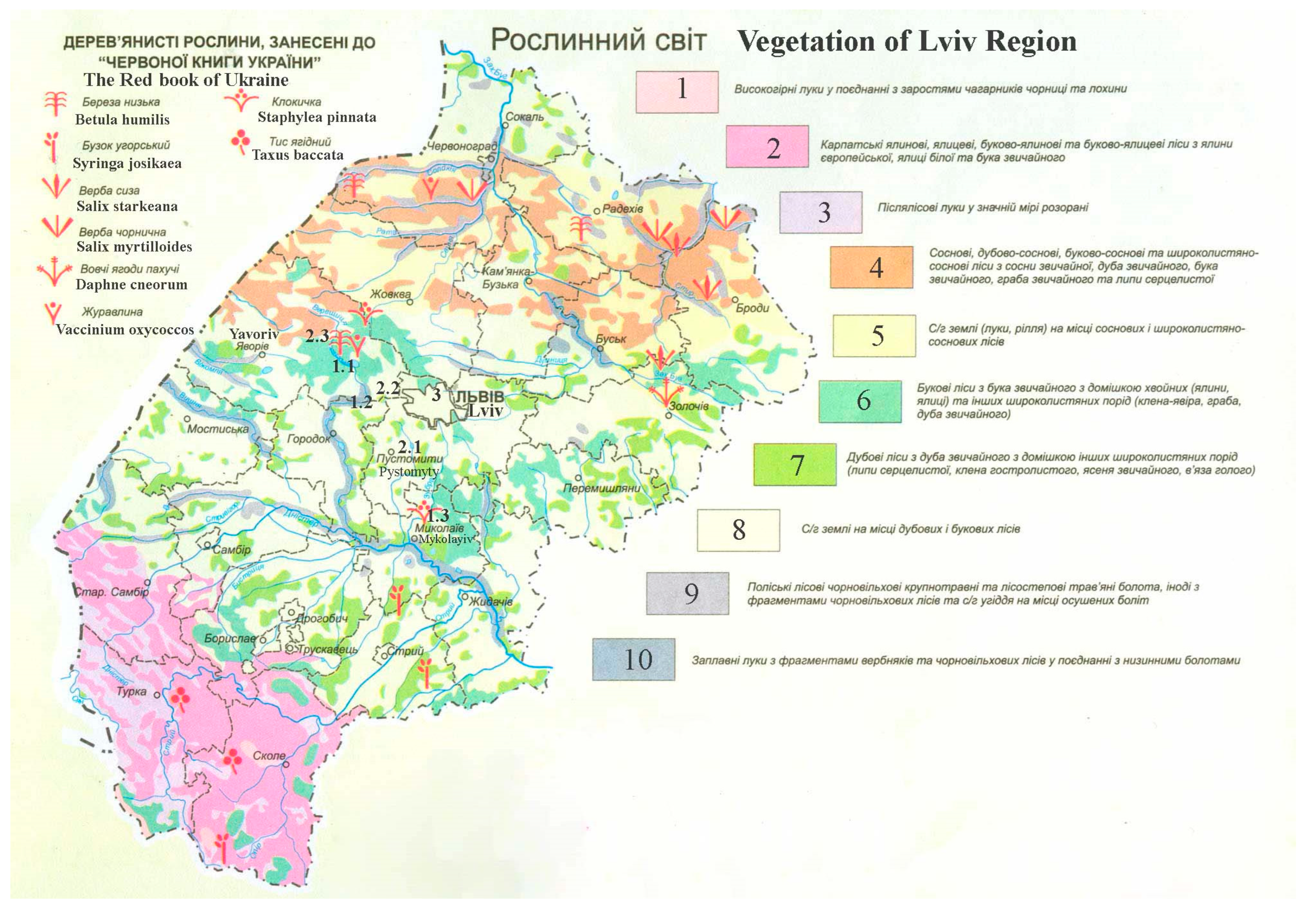


| Population | 1.1 | 1.2 | 1.3 | ||||
|---|---|---|---|---|---|---|---|
| EO Content (%, v/w, Expressed in Dry Weight) | 0.90 ± 0.05 | 0.70 ± 0.05 | 0.80 ± 0.05 | ||||
| GC Analysis (%) | RT (min) | 1* | 2* | 1* | 2* | 1* | 2* |
| β-myrcene | 12.84 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| 1,8-cineole | 14.18 | trace ** | trace | 0.1 ± 0.1 | 0.1 ± 0.1 | trace | trace |
| limonene | 14.74 | trace | trace | trace | trace | trace | trace |
| α-terpinene | 16.0 | 3.0 ± 0.5 | 2.5 ± 0.5 | 4.0 ± 0.5 | 3.5 ± 0.5 | 4.0 ± 0.5 | 3.2 ± 0.5 |
| p-cymene | 16.77 | 2.0 ± 0.5 | 2.0 ± 0.5 | 10.0 ± 1.0 | 9.1 ± 1.0 | 1.6 ± 0.2 | 1.6 ± 0.2 |
| terpinolene | 17.91 | trace | trace | trace | trace | trace | trace |
| α-thujone | 22.5 | 0.1 ± 0.1 | 0.1 ± 0.1 | 1.6 ± 0.2 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| linalool | 24.54 | 63.0 ± 2.0 | 63.0 ± 2.0 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| bornyl acetate | 25.26 | 0.5 ± 0.1 | 0.3 ± 0.1 | 4.5 ± 0.5 | 3.5 ± 0.5 | 1.4 ± 0.2 | 1.0 ± 0.2 |
| β-caryophyllene | 26.51 | 2.5 ± 0.5 | 2.0 ± 0.5 | 3.5 ± 0.5 | 3.0 ± 0.5 | 4.0 ± 0.5 | 5.5 ± 0.5 |
| borneol | 29.25 | 1.3 ± 0.2 | 1.7 ± 0.2 | 7.5 ± 0.5 | 7.5 ± 0.5 | 8.0 ± 0.5 | 9.5 ± 1.0 |
| α- terpineol | 29.92 | 2.5 ± 0.5 | 2.2 ± 0.5 | 20.2 ± 1.0 | 20.0 ± 1.0 | 17.0 ± 1.0 | 18.3 ± 1.0 |
| fenchol | 32.09 | 0.2 ± 0.1 | 0.2 ± 0.1 | 2.0 ± 0.5 | 2.0 ± 0.5 | 4.0 ± 0.5 | 3.0 ± 0.5 |
| geraniol | 33.19 | 5.0 ± 0.5 | 4.5 ± 0.5 | 15.0 ± 1.0 | 15.1 ± 1.0 | 35.0 ± 2.0 | 35.0 ± 2.0 |
| thymol | 42.65 | 0.6 ± 0.1 | 0.8 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | trace |
| carvacrol | 45.53 | 6.0 ± 0.5 | 6.0 ± 0.5 | 20.0 ± 1.0 | 18.0 ± 1.0 | 6.0 ± 0.5 | 6.5 ± 0.5 |
| Content of the main EOs (%) | 87.0 | 85.6 | 89.5 | 83.5 | 82.7 | 85.1 | |
| Type | Th. Pulegoides | Th. Marschtallianus | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Population | 2.1 | 2.2 | 2.3 | ||||||
| EO Content (%, v/w, Expressed in Dry Weight) | 0.90 ± 0.05 | 0.85 ± 0.05 | 0.80 ± 0.05 | 0.35 ± 0.05 | |||||
| GC Analysis (%) | RT (min) | 1* | 2* | 1* | 2* | 1* | 2* | 1* | 2* |
| β-myrcene | 12.84 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 3.5 ± 0.5 | 3.5 ± 0.5 |
| 1,8-cineole | 14.18 | 0.1 ± 0.1 | 0.1 ± 0.1 | trace | trace | trace | trace | 0.4 ± 0.1 | 0.3 ± 0.1 |
| limonene | 14.74 | trace ** | trace | trace | trace | trace | trace | 0.9 ± 0.1 | 0.8 ± 0.1 |
| α-terpinene | 16.0 | 8.0 ± 0.5 | 7.5 ± 0.5 | 1.6 ± 0.2 | 1.5 ± 0.2 | 2.5 ± 0.5 | 2.0 ± 0.5 | 4.0 ± 0.5 | 4.0 ± 0.5 |
| p-cymene | 16.77 | 6.0 ± 0.5 | 6.0 ± 0.5 | 3.0 ± 0.5 | 3.2 ± 0.5 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| terpinolene | 17.91 | trace | trace | trace | trace | 1.8 ± 0.2 | 2.0 ± 0.5 | 2.2 ± 0.2 | 2.4 ± 0.2 |
| α-thujone | 22.5 | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 |
| linalool | 24.54 | 0.6 ± 0.1 | 0.6 ± 0.1 | trace | trace | 13.0 ± 1.0 | 12.0 ± 1.0 | 4.5 ± 0.5 | 3.5 ± 0.5 |
| bornyl acetate | 25.26 | 3.0 ± 0.5 | 3.0 ± 0.5 | 1.0 ± 0.2 | 1.0 ± 0.2 | 0,3 ± 0.1 | 0.4 ± 0.1 | 4.0 ± 0.5 | 3.0 ± 0.5 |
| β-caryophyllene | 26.51 | 2.5 ± 0.5 | 3.5 ± 0.5 | 4.0 ± 0.5 | 5.0 ± 0.5 | 3.0 ± 0.5 | 4.0 ± 0.5 | 2.0 ± 0.2 | 1.0 ± 0.2 |
| borneol | 29.25 | 7.0 ± 0.5 | 8.0 ± 0.5 | 10.0 ± 1.0 | 11.0 ± 1.0 | 6.0 ± 0.5 | 7.5 ± 0.5 | 2.5 ± 0.5 | 2.5 ± 0.5 |
| α-terpineol | 29.92 | 13.0 ± 1.0 | 11.0 ± 1.0 | 20.0 ± 2.0 | 21.0 ± 2.0 | 15.0 ± 1.0 | 15.0 ± 1.0 | 28.0 ± 1.0 | 30.1 ± 1.0 |
| fenchol | 32.09 | 3.5 ± 0.5 | 3.5 ± 0.5 | 4.0 ± 0.5 | 4.0 ± 0.5 | 2.5 ± 0.5 | 2.5 ± 0.5 | 1.2 ± 0.2 | 1.2 ± 0.2 |
| geraniol | 33.19 | 22.0 ± 2.0 | 22.0 ± 2.0 | 33.0 ± 2.0 | 33.0 ± 2.0 | 15.0 ± 1.0 | 15.0 ± 1.0 | 3.5 ± 0.5 | 2.5 ± 0.5 |
| thymol | 42.65 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 2.0 ± 0.5 | 2.0 ± 0.5 | 2.2 ± 0.2 | 2.2 ± 0.2 |
| carvacrol | 45.53 | 17.0 ± 1.0 | 18.0 ± 1.0 | 9.5 ± 0.5 | 9.5 ± 0.5 | 3.5 ± 0.5 | 4.0 ± 0.5 | 6.5 ± 0.5 | 9.0 ± 0.5 |
| Content of the main EOs (%) | 84.8 | 85.3 | 87.7 | 90.6 | 67.7 | 69.3 | 67.2 | 67.7 | |
| Population | |||
|---|---|---|---|
| 1.1 | 1.2 | 1.3 | |
| Test microorganisms | Inhibition zone, mm, M ± m | ||
| Staphylococcus aureus ATCC 25923 | 11.17 ± 0.29 | 13.17 ± 0.29 | 11.50 ± 0.50 |
| Escherichia coli ATCC 25922 | 15.66 ± 0.58 | 19.83 ± 0.76 | 9.66 ± 0.58 |
| Streptococcus pyogenes ATCC 19615 | 11.67 ± 0.58 | 14.00 ± 0.25 | 18.50 ± 0.87 |
| Candida albicans ATCC 885-653 | 11.17 ± 0.29 | 29.66 ± 1.52 | 22.66 ± 0.58 |
| Th. Pulegoides (Population) | Th. Marschtallianus | |||
|---|---|---|---|---|
| 2.1 | 2.2. | 2.3 | 3 | |
| Test microorganisms | Inhibition zone, mm, M ± m | |||
| Staphylococcus aureus ATCC 25923 | 14.50 ± 0.50 | 13.50 ± 0.50 | 13.50 ± 0.50 | 8.67 ± 0.58 |
| Escherichia coli ATCC 25922 | 14.50 ± 0.50 | 15.33 ± 0.58 | 10.83 ± 0.76 | 0 |
| Streptococcus pyogenes ATCC 19615 | 15.83 ± 0.29 | 13.33 ± 0.58 | 15.50 ± 0.50 | 18.83 ± 0.76 |
| Candida albicans ATCC 885-653 | 41.00 ± 1.00 | 29.00 ± 1.00 | 11.66 ± 0.58 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryvtsova, M.; Hrytsyna, M.; Salamon, I.; Skybitska, M.; Novykevuch, O. Chemotypes of Species of the Genus Thymus L. in Carpathians Region of Ukraine—Their Essential Oil Qualitative and Quantitative Characteristics and Antimicrobial Activity. Horticulturae 2022, 8, 1218. https://doi.org/10.3390/horticulturae8121218
Kryvtsova M, Hrytsyna M, Salamon I, Skybitska M, Novykevuch O. Chemotypes of Species of the Genus Thymus L. in Carpathians Region of Ukraine—Their Essential Oil Qualitative and Quantitative Characteristics and Antimicrobial Activity. Horticulturae. 2022; 8(12):1218. https://doi.org/10.3390/horticulturae8121218
Chicago/Turabian StyleKryvtsova, Maryna, Myroslava Hrytsyna, Ivan Salamon, Maria Skybitska, and Olha Novykevuch. 2022. "Chemotypes of Species of the Genus Thymus L. in Carpathians Region of Ukraine—Their Essential Oil Qualitative and Quantitative Characteristics and Antimicrobial Activity" Horticulturae 8, no. 12: 1218. https://doi.org/10.3390/horticulturae8121218
APA StyleKryvtsova, M., Hrytsyna, M., Salamon, I., Skybitska, M., & Novykevuch, O. (2022). Chemotypes of Species of the Genus Thymus L. in Carpathians Region of Ukraine—Their Essential Oil Qualitative and Quantitative Characteristics and Antimicrobial Activity. Horticulturae, 8(12), 1218. https://doi.org/10.3390/horticulturae8121218







