Microalga Biofertilizer Triggers Metabolic Changes Improving Onion Growth and Yield
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalga Source and Treatments
2.2. Study Area, Cultivars, and Seedling Production
2.3. Pot Experiment
2.4. Field Experiment
2.5. Biochemical Analysis
2.6. Statistical Treatment
3. Results
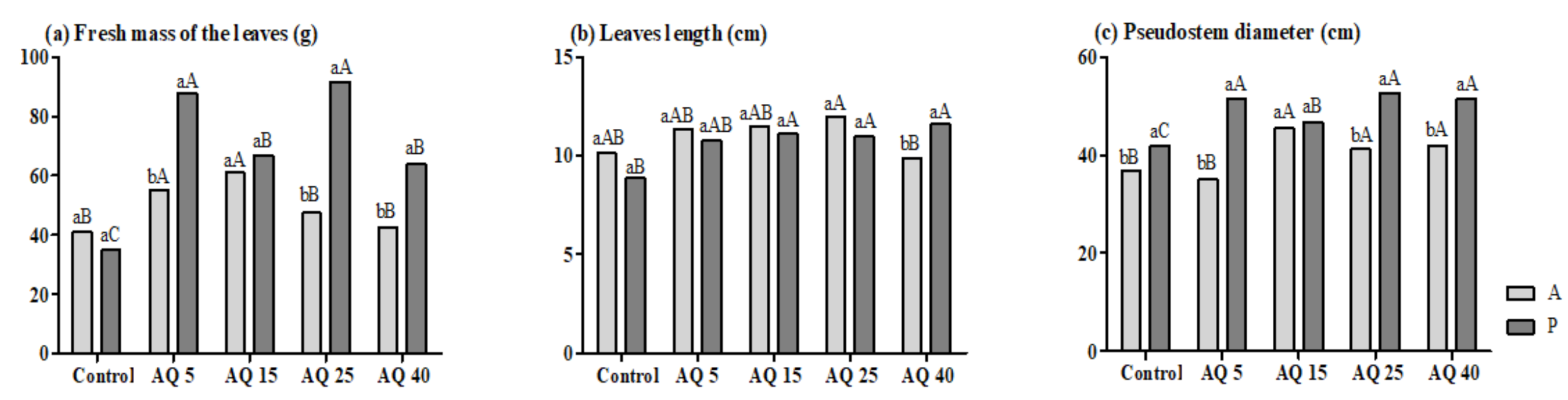
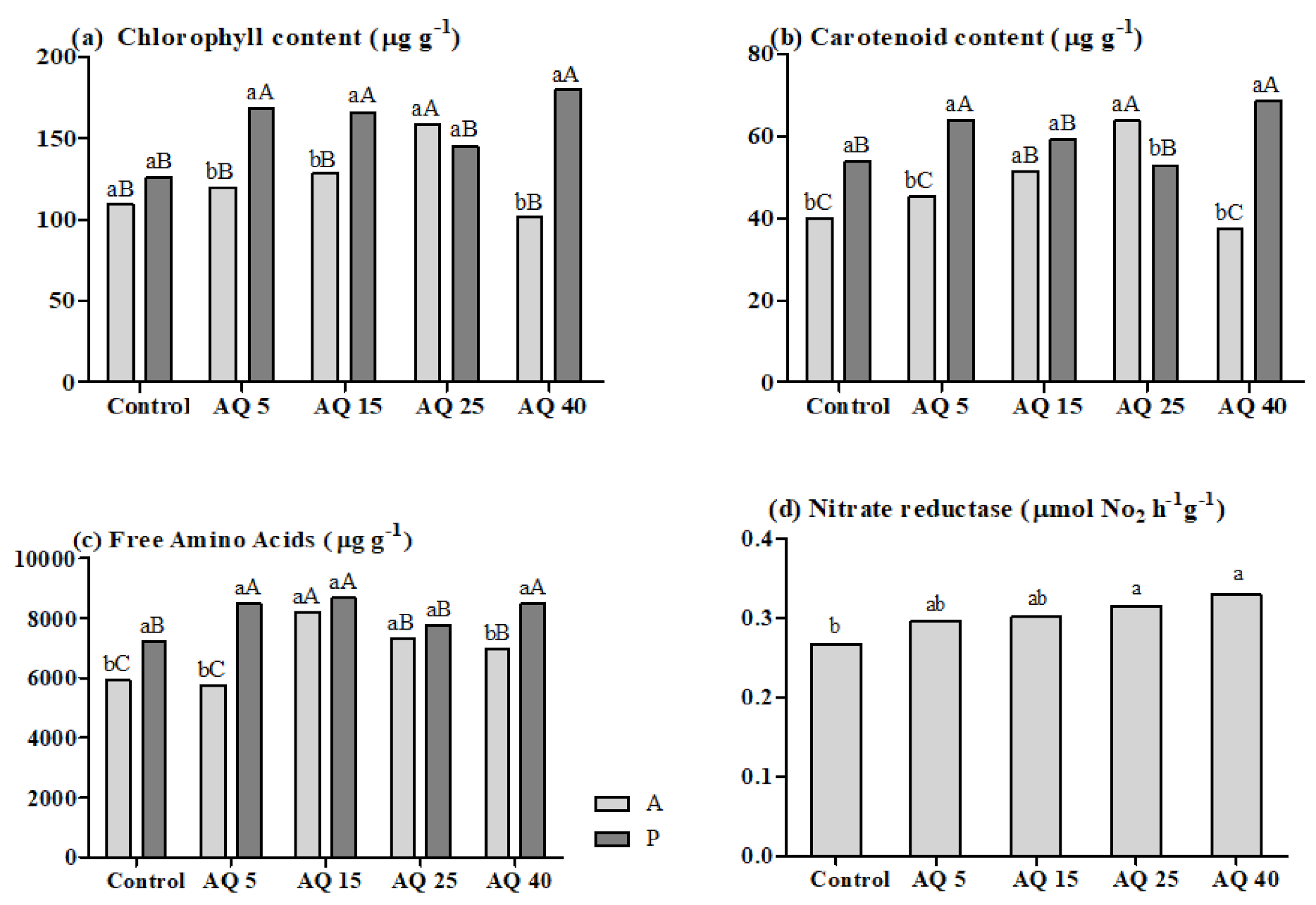
3.1. Pot Experiment
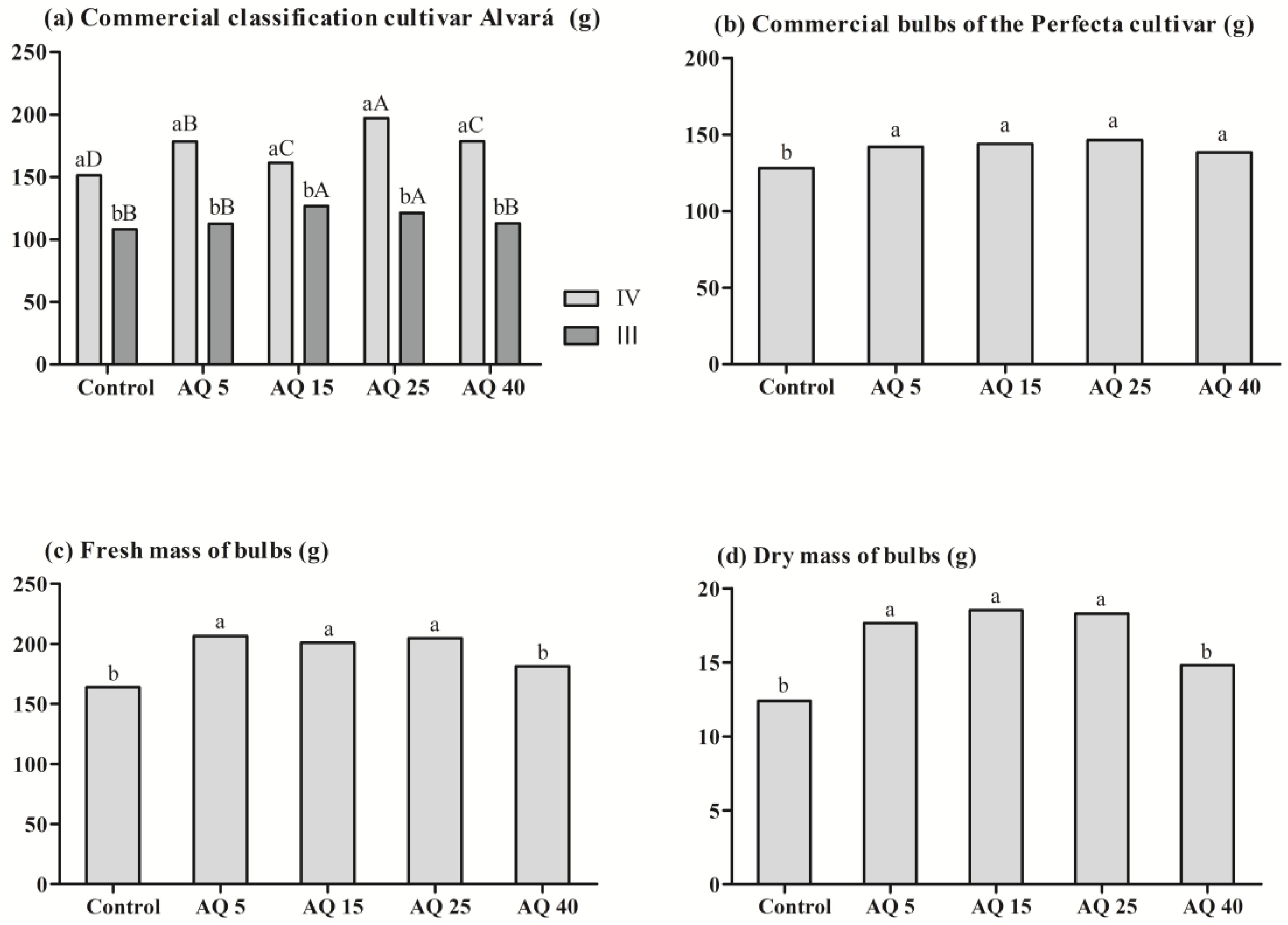
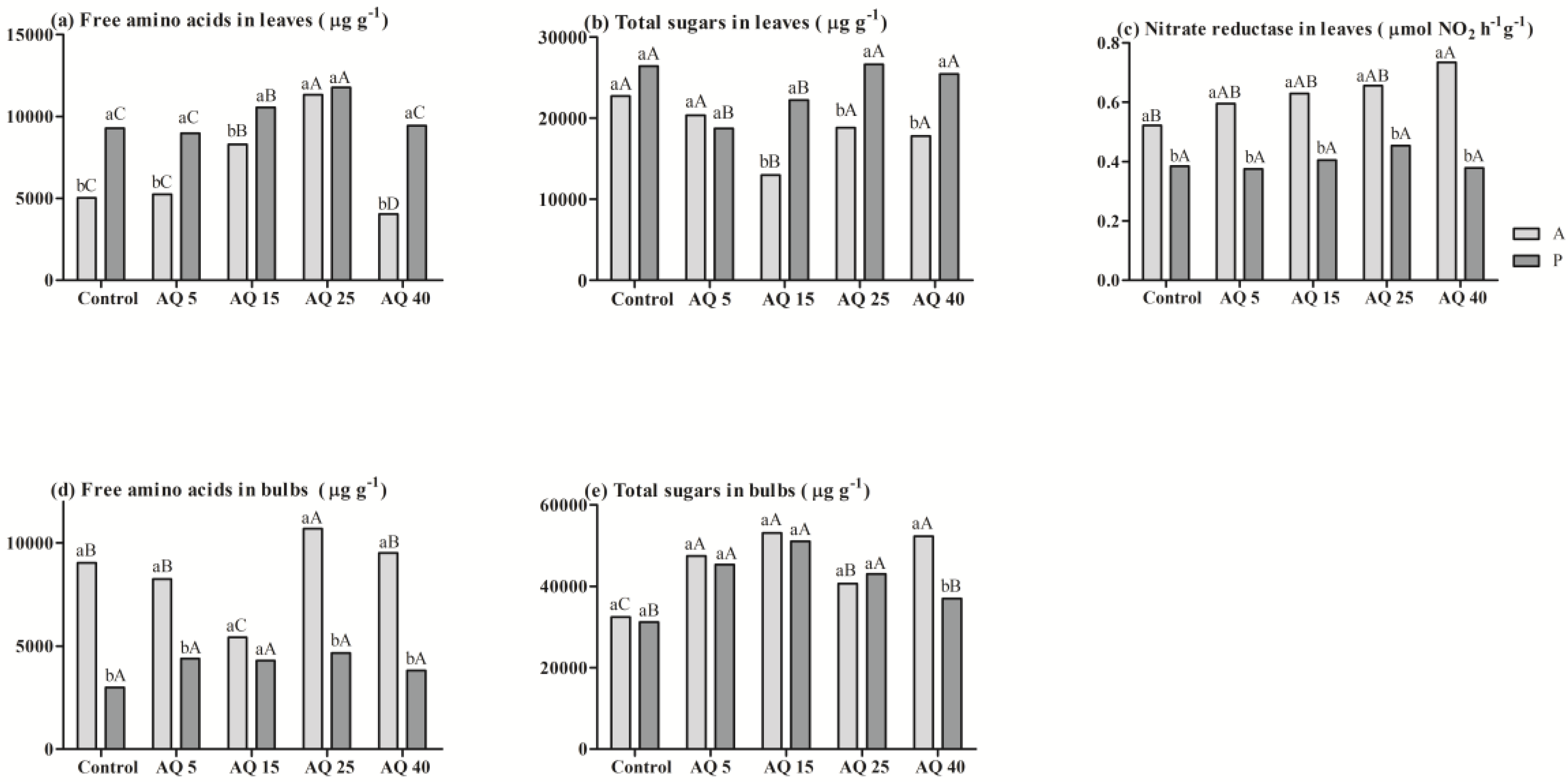
3.2. Field Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abou Chehade, L.; Al Chami, Z.; De Pascali, S.A.; Cavoski, I.; Fanizzi, F.P. Biostimulants from food processing by-products: Agronomic, quality and metabolic impacts on organic tomato (Solanum lycopersicum L.). J. Sci. Food Agri. 2018, 98, 1426–1436. [Google Scholar] [CrossRef]
- Singh, M.; Dotaniya, M.L.; Mishra, A.; Dotaniya, C.K.; Regar, K.L.; Lata, M. Role of biofertilizers in conservation agriculture. In Conservation Agriculture; Springer: Singapore, 2016; pp. 113–134. [Google Scholar]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as multifunctional options in modern agriculture: Current trends, prospects and challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef] [PubMed]
- Gemin, L.G.; Mógor, A.F.; Amatussi, J.O.; Mógor, G. Microalgae associated to humic acid as a novel biostimulant improving onion growth and yield. Sci. Hortic. 2019, 256, 108560. [Google Scholar] [CrossRef]
- Ishaq, A.G.; Matias-Peralta, H.M.; Basri, H. Bioactive compounds from green microalga-Scenedesmus and its potential applications: A brief review. Pertanika J. Trop. Agric. Sci. 2016, 39, 1–15. [Google Scholar]
- Mógor, A.F.; Ördög, V.; Lima, G.P.P.; Molnár, Z.; Mógor, G. Biostimulant properties of cyanobacterial hydrolysate related to polyamines. J. Appl. Phycol. 2017, 30, 453–460. [Google Scholar] [CrossRef]
- Mógor, A.F.; Amatussi, J.O.; Mógor, G.; Lara, G.B. Bioactivity of cyanobacterial biomass related to amino acids induces growth and metabolic changes on seedlings and yield gains of organic red beet. Am. J. Plant Sci. 2018, 9, 966–978. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Mazepa, E.; Malburg, B.V.; Mógor, G.; de Oliveira, A.C.; Amatussi, J.O.; Corrêa, D.O.; Lemos, J.S.; Ducatti, D.R.B.; Duarte, M.E.R.; Mógor, A.F.; et al. Plant growth biostimulant activity of the green microalga Desmodesmus subspicatus. Algal Res. 2021, 59, 102434. [Google Scholar] [CrossRef]
- Schreiber, C.; Schiedung, H.; Harrison, L.; Briese, C.; Ackermann, B.; Kant, J.; Schrey, S.D.; Hofmann, D.; Singh, D.; Ebenhoh, O.; et al. Evaluating potential of green alga Chlorella vulgaris to accumulate phosphorus and to fertilize nutrient-poor soil substrates for crop plants. J. Appl. Phycol. 2018, 30, 2827–2836. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Subramanian, J.; Gopalsamy, J.; Jayasingam, P.; Arumugam, A.; Kannadasan, S.; Sampathkumar, P. The impact of using microalgae as biofertilizer in maize (Zea mays L.). Waste Biomass Valor. 2019, 10, 1101–1110. [Google Scholar] [CrossRef]
- Faheed, F.A.; Abd-El Fattah, Z. Effect of Chlorella vulgaris as bio-fertilizer on growth parameters and metabolic aspects of lettuce plant. J. Agric. Soc. Sci. 2008, 4, 165–169. [Google Scholar]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.P.; Khattar, J.S.; Rajput, A.; Chaudhary, R.; Singh, R. High production of carotenoids by the green microalga Asterarcys quadricellulare PUMCC 5.1.1 under optimized culture conditions. PLoS ONE 2019, 14, e0221930. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.N.; Bhowmick, T.K.; Gayen, K. Effect of macronutrient supplements on growth and biochemical compositions in photoautotrophic cultivation of isolated Asterarcys sp. (BTA9034). Energy Convers. Manag. 2017, 149, 39–51. [Google Scholar] [CrossRef]
- Faostat. Food and Agriculture Organization of the United Nations. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 14 November 2021).
- Winters, A.L.; Lloyd, J.D.; Jones, R.; Merry, R.J. Evaluation of a rapid method for estimating free amino acids in silages. Anim. Feed. Sci. Technol. 2002, 99, 177–187. [Google Scholar] [CrossRef]
- Magné, C.; Larher, F. High sugar content interferes with colorimetric determinationof amino acids and free proline. Anal. Biochem. 1992, 200, 115–118. [Google Scholar] [CrossRef]
- Empresa Brasileira de Pesquisa Agropecuária—EMBRAPA. Sistema Brasileiro de Classificação de Solos, 3rd ed.; Embrapa: Brasília, Brazil, 2013; 353p. (In Portuguese) [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids:Measurement and characterization by UV–VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Maldonade, I.R.; Carvalho, P.G.B.; Ferreira, N.A. Protocolo para a Determinação de Açúcares Totais em Hortaliças Pelo Método de DNS; Embrapa: Brasília, Brazil, 2013; pp. 1–4. (In Portuguese) [Google Scholar]
- Jaworski, E.K. Nitrate reductase assay in intact plant tissues. Biochem. Biophys. Res. Commun. 1971, 43, 1274–1279. [Google Scholar] [CrossRef]
- Silva, F.A.S.; Azevedo, C.A.V. The Assistat Software Version 7.7 and its use in the analysis of experimental data. Afr. J. Agric. Res. 2016, 11, 3733–3740. [Google Scholar] [CrossRef] [Green Version]
- Bettoni, M.M.; Mógor, A.F.; Kogerastki, J.F.; Pauletti, V. Onion (Allium cepa L.) Seedling Growth using Humic Substances. Idesia 2016, 34, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Rachidi, F.; Benhima, R.; Sbabou, L.; El Arroussi, H. Microalgae polysaccharides bio-stimulating effect on tomato plants: Growth and metabolic distribution. Biotechnol. Rep. 2020, 25, e00426. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Zhao, X.; Ho, S.H.; Ma, R.; Xie, Y.; Chen, J. Biorefining and the Functional Properties of Proteins from Lipid and Pigment Extract Residue of Chlorella pyrenoidosa. Mar. Drugs 2019, 17, 454. [Google Scholar] [CrossRef] [Green Version]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signaling. J. Exper. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef] [PubMed]
- Röder, C.; Mógor, A.F.; Szilagyi-Zecchin, V.J.; Gemin, L.G.; Mógor, G. Potato yield and metabolic changes by use of biofertilizer containing L-glutamic acid. Com. Sci. 2018, 9, 211–218. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Fisiologia e Desenvolvimento Vegetal; Artmed Editora: Brasília, Brazil, 2017; 888p. (In Portuguese) [Google Scholar]
- Kholssi, R.; Marks, E.A.N.; Miñón, J.; Montero, O.; Debdoubi, A.; Rad, C. Biofertilizing Effect of Chlorella sorokiniana Suspensions on Wheat Growth. J. Plant Growth Regul. 2018, 38, 644–649. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Zhan, Z.; Liu, B.; Chen, Z.; Liang, Y. Transcriptome analysis of sucrose metabolism during bulb swelling and development in onion (Allium cepa L.). Front. Plant Sci. 2016, 7, 1425. [Google Scholar] [CrossRef] [Green Version]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Hilderandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [Green Version]
- Pratelli, R.; Pilot, G. Regulation of amino acid metabolic enzymes and transporters in plants. J. Exp. Bot. 2014, 65, 5535–5556. [Google Scholar] [CrossRef]
- Zhou, Y.; Kishchenko, O.; Stepanenko, A.; Chen, G.; Wang, W.; Zhou, J.; Pan, C.; Borisjuk, N. The Dynamics of NO3− and NH4+ Uptake in Duckweed Are Coordinated with the Expression of Major Nitrogen Assimilation Genes. Plants 2022, 11, 11. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef]
- Barone, V.; Baglieri, A.; Stevanato Broccanello, C.; Bertoldo, G.; Bertaggia, M.; Cagnin, M.; Pizzeghello, D.; Moliterni, V.M.C.; Mandolino, G.; Fornasier, F.; et al. Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.). J. Appl. Phycol. 2018, 30, 1061–1071. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordeiro, E.C.N.; Mógor, Á.F.; de Oliveira Amatussi, J.; Mógor, G.; de Lara, G.B.; Marques, H.M.C. Microalga Biofertilizer Triggers Metabolic Changes Improving Onion Growth and Yield. Horticulturae 2022, 8, 223. https://doi.org/10.3390/horticulturae8030223
Cordeiro ECN, Mógor ÁF, de Oliveira Amatussi J, Mógor G, de Lara GB, Marques HMC. Microalga Biofertilizer Triggers Metabolic Changes Improving Onion Growth and Yield. Horticulturae. 2022; 8(3):223. https://doi.org/10.3390/horticulturae8030223
Chicago/Turabian StyleCordeiro, Ely Cristina Negrelli, Átila Francisco Mógor, Juliana de Oliveira Amatussi, Gilda Mógor, Gabriel Bocchetti de Lara, and Harielly Marianne Costa Marques. 2022. "Microalga Biofertilizer Triggers Metabolic Changes Improving Onion Growth and Yield" Horticulturae 8, no. 3: 223. https://doi.org/10.3390/horticulturae8030223
APA StyleCordeiro, E. C. N., Mógor, Á. F., de Oliveira Amatussi, J., Mógor, G., de Lara, G. B., & Marques, H. M. C. (2022). Microalga Biofertilizer Triggers Metabolic Changes Improving Onion Growth and Yield. Horticulturae, 8(3), 223. https://doi.org/10.3390/horticulturae8030223





