Calcium Ascorbate Coating Improves Postharvest Quality and Storability of Fresh-Cut Slices of Coscia and Abate Fétel Pears (Pyrus communis L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
- (1)
- distilled water (500 mL) with 1% hydroxypropyl methylcellulose (HPMC) and 50 mL of glycerol used as plasticizer;
- (2)
- distilled water (500 mL) with 2% calcium ascorbate (ASC) and 50 mL of glycerol used as plasticizer;
- (3)
- distilled water (500 mL) with 3% Xanthan gum (XAN) and 50 mL of glycerol used as plasticizer.
2.3. Fresh-Cut Slice Analysis
2.3.1. Firmness
2.3.2. Weight Loss
2.3.3. Soluble Solids and Titratable Acidity
2.3.4. Color
2.3.5. Antioxidant Activity
2.3.6. Total Phenols Content
2.3.7. Sensory Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Solid Soluble, Titratable Acid, Weight Loss, and Firmness
3.2. Color Loss and Browning Index
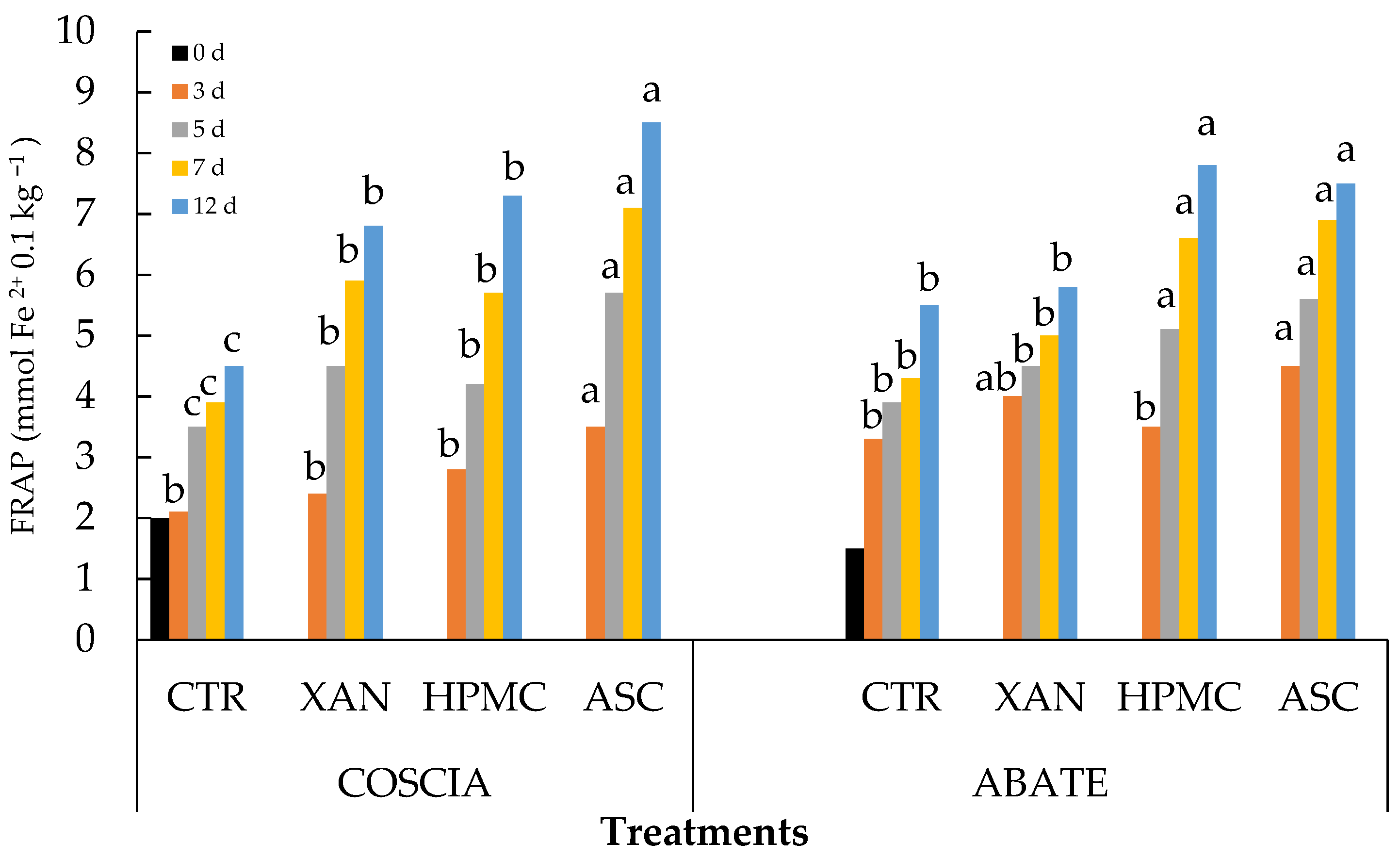
3.3. ABTS, DPPH, FRAP and Phenols Content
3.4. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hodges, D.M.; Toivonen, P.M.A. Quality of Fresh-Cut Fruits and Vegetables as Affected by Exposure to Abiotic Stress. Postharvest Biol. Technol. 2008, 48, 155–162. [Google Scholar] [CrossRef]
- Amiot, M.J.; Tacchini, M.; Aubert, S.Y.; Oleszek, W. Influence of Cultivar, Maturity Stage, and Storage Conditions on Phenolic Composition and Enzymic Browning of Pear Fruits. J. Agric. Food Chem. 1995, 43, 1132–1137. [Google Scholar] [CrossRef]
- Gorny, J.R.; Gil, M.I.; Kader, A.A. Postharvest physiology and quality maintenance of fresh-cut pears. Acta Hortic. 1998, 464, 231–236. [Google Scholar] [CrossRef]
- Sapers, G.M.; Miller, R.L. Browning Inhibition in Fresh-Cut Pears. J. Food Sci. 1998, 63, 342–346. [Google Scholar] [CrossRef]
- Gorny, J.R.; Hess-Pierce, B.; Cifuentes, R.A.; Kader, A.A. Quality Changes in Fresh-Cut Pear Slices as Affected by Controlled Atmospheres and Chemical Preservatives. Postharvest Biol. Technol. 2002, 24, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible Coatings with Antibrowning Agents to Maintain Sensory Quality and Antioxidant Properties of Fresh-Cut Pears. Postharvest Biol. Technol. 2008, 50, 87–94. [Google Scholar] [CrossRef]
- Abreu, M.; Beirao-da-Costa, S.; Gonçalves, E.M.; Beirão-da-Costa, M.L.; Moldão-Martins, M. Use of Mild Heat Pre-Treatments for Quality Retention of Fresh-Cut ‘Rocha’Pear. Postharvest Biol. Technol. 2003, 30, 153–160. [Google Scholar] [CrossRef]
- Aguayo, E.; Requejo-Jackman, C.; Stanley, R.; Woolf, A. Effects of Calcium Ascorbate Treatments and Storage Atmosphere on Antioxidant Activity and Quality of Fresh-Cut Apple Slices. Postharvest Biol. Technol. 2010, 57, 52–60. [Google Scholar] [CrossRef]
- Rosen, J.C.; Kader, A.A. Postharvest Physiology and Quality Maintenance of Sliced Pear and Strawberry Fruits. J. Food Sci. 1989, 54, 656–659. [Google Scholar] [CrossRef]
- Allegra, A.; Sortino, G.; Farina, V.; Inglese, P. Effect of Passive Atmosphere and Chemical Treatment on Fresh Cut of White-Flesh Peach Cultivar’Settembrina Di Bivona’. Acta Hort. 2013, 1084, 765–770. [Google Scholar]
- Poovaiah, B.W. Role of Calcium in Prolonging Storage Life of Fruits and Vegetables. Food Technol. 1986, 40, 86–89. [Google Scholar]
- Zudaire, L.; Viñas, I.; Lafarga, T.; Plaza, L.; Echevarria, G.; Gloria, B.; Altisent, R.; Aguiló-Aguayo, I. Effect of Calcium Salts and Antioxidant Treatment on the Storage Quality of Fresh-Cut Conference Pears. Int. J. Agric. For. Life Sci. 2019, 3, 331–344. [Google Scholar]
- Soliva-Fortuny, R.; Martín-Belloso, O. Fresh-Cut Fruits: Apples and Pears. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce; Elsevier: Amsterdam, The Netherlands, 2020; pp. 487–494. [Google Scholar]
- Passafiume, R.; Gugliuzza, G.; Gaglio, R.; Busetta, G.; Tinebra, I.; Sortino, G.; Farina, V. Aloe-Based Edible Coating to Maintain Quality of Fresh-Cut Italian Pears (Pyrus communis L.) during Cold Storage. Horticulturae 2021, 7, 581. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Nisperos-Carriedo, M.O.; Baker, R.A. Use of Edible Coatings to Preserve Quality of Lightly (and Slightly) Processed Products. Crit. Rev. Food Sci. Nutr. 1995, 35, 509–524. [Google Scholar] [CrossRef]
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel Materials in the Preparation of Edible Films and Coatings—A Review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- FDA (Food and Drug Administration). Title 21 Food and Drugs Section 172: Food Additives Permitted for Direct Addition to Food for Human Consumption. Code of Federal Regulations, 2013. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-172 (accessed on 3 March 2021).
- Freitas, I.R.; Cortez-Vega, W.R.; Pizato, S.; Prentice-Hernández, C.; Borges, C.D. Xanthan Gum as a Carrier of Preservative Agents and Calcium Chloride Applied on Fresh-Cut Apple. J. Food Saf. 2013, 33, 229–238. [Google Scholar] [CrossRef]
- Sharma, S.; Rao, T.R. Xanthan Gum Based Edible Coating Enriched with Cinnamic Acid Prevents Browning and Extends the Shelf-Life of Fresh-Cut Pears. LWT-Food Sci. Technol. 2015, 62, 791–800. [Google Scholar] [CrossRef]
- Dave, R.K.; Ramana Rao, T.V.; Nandane, A.S. Improvement of Post-Harvest Quality of Pear Fruit with Optimized Composite Edible Coating Formulations. J. Food Sci. Technol. 2017, 54, 3917–3927. [Google Scholar] [CrossRef]
- Gol, N.B.; Patel, P.R.; Rao, T.R. Improvement of Quality and Shelf-Life of Strawberries with Edible Coatings Enriched with Chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Choi, W.S.; Singh, S.; Lee, Y.S. Characterization of Edible Film Containing Essential Oils in Hydroxypropyl Methylcellulose and Its Effect on Quality Attributes of ‘Formosa’ Plum (Prunus salicina L.). LWT 2016, 70, 213–222. [Google Scholar] [CrossRef]
- Predieri, S.; Gatti, E. Effects of Cold Storage and Shelf-Life on Sensory Quality and Consumer Acceptance of ‘Abate Fétel’ Pears. Postharvest Biol. Technol. 2009, 51, 342–348. [Google Scholar] [CrossRef]
- Arias, E.; González, J.; López-Buesa, P.; Oria, R. Optimization of Processing of Fresh-Cut Pear. J. Sci. Food Agric. 2008, 88, 1755–1763. [Google Scholar] [CrossRef]
- Ruangchakpet, A.; Sajjaanantakul, T. Effect of Browning on Total Phenolic, Flavonoid Content and Antioxidant Activity in Indian Gooseberry (Phyllanthus emblica Linn.). Agric. Nat. Resour. 2007, 41, 331–337. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Sricharoen, P.; Techawongstein, S.; Chanthai, S. A High Correlation Indicating for an Evaluation of Antioxidant Activity and Total Phenolics Content of Various Chilli Varieties. J. Food Sci. Technol. 2015, 52, 8077–8085. [Google Scholar] [CrossRef] [Green Version]
- Sortino, G.; Allegra, A.; Farina, V.; De Chiara, M.L.V.; Inglese, P. Genotype Influence on Shelf Life Behaviour of Minimal Processed Loquat (Eriobotrya japonica (Thunb.) Lindl.) Fruit: The Role of Sugar, Acid Organics and Phenolic Compounds. Chem. Biol. Technol. Agric. 2022, 9, 1–18. [Google Scholar] [CrossRef]
- Miceli, C.d.; Infante, R.; Inglese, P. Instrumental and Sensory Evaluation of Eating Quality of Peaches and Nectarines. Eur. J. Hortic. Sci. 2010, 75, 97–102. [Google Scholar] [CrossRef]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of Chitosan Coatings on the Physicochemical Characteristics of Eksotika II Papaya (Carica papaya L.) Fruit during Cold Storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef]
- Benítez, S.; Achaerandio, I.; Sepulcre, F.; Pujolà, M. Aloe Vera Based Edible Coatings Improve the Quality of Minimally Processed ‘Hayward’ Kiwifruit. Postharvest Biol. Technol. 2013, 81, 29–36. [Google Scholar] [CrossRef]
- Chironi, S.; Sortino, G.; Allegra, A.; Saletta, F.; Caviglia, V.; Ingrassia, M. Consumer Assessment on Sensory Attributes of Fresh Table Grapes Cv’Italia’and’Red Globe’after Long Cold Storage Treatment. Chem. Eng. Trans. 2017, 58, 421–426. [Google Scholar]
- Plotto, A.; Narciso, J.A.; Rattanapanone, N.; Baldwin, E.A. Surface Treatments and Coatings to Maintain Fresh-Cut Mango Quality in Storage. J. Sci. Food Agric. 2010, 90, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Bico, S.L.S.; Raposo, M.F.J.; Morais, R.M.S.C.; Morais, A.M.M.B. Combined Effects of Chemical Dip and/or Carrageenan Coating and/or Controlled Atmosphere on Quality of Fresh-Cut Banana. Food Control 2009, 20, 508–514. [Google Scholar] [CrossRef]
- Allegra, A.; Gallotta, A.; Carimi, F.; Mercati, F.; Inglese, P.; Martinelli, F. Metabolic Profiling and Post-Harvest Behavior of “Dottato” Fig (Ficus carica L.) Fruit Covered with an Edible Coating from O. Ficus-indica. Front. Plant. Sci. 2018, 9, 1321. [Google Scholar] [CrossRef] [PubMed]
- Nxumalo, K.A.; Matsuane, C.; Masarirambi, M.T. Calcium-Related Post-Harvest Physiological Disorders of Fruits and Vegetables in Eswatini: A Review. Curr. J. Appl. Sci. Technol. 2019, 33, 1–10. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Elgammal, R.E.; Alhaithloul, H.A.S.; Alghanem, S.M.; Fikry, M.; Abdein, M.A.; Hikal, D.M. Enhance Fruit Ripening Uniformity and Accelerate the Rutab Stage by Using ATP in ‘Zaghloul’ Dates during the Shelf Life. Foods 2021, 10, 2641. [Google Scholar] [CrossRef]
- Li-Qin, Z.; Jie, Z.; Shu-Hua, Z.; Lai-Hui, G. Inhibition of Browning on the Surface of Peach Slices by Short-Term Exposure to Nitric Oxide and Ascorbic Acid. Food Chem. 2009, 114, 174–179. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-Talk between Signaling Pathways: The Link between Plant Secondary Metabolite Production and Wounding Stress Response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Long, Q.; Gao, F.; Han, C.; Jin, P.; Zheng, Y. Effect of Cutting Styles on Quality and Antioxidant Activity in Fresh-Cut Pitaya Fruit. Postharvest Biol. Technol. 2017, 124, 1–7. [Google Scholar] [CrossRef]
- Klaiber, R.G.; Baur, S.; Koblo, A.; Carle, R. Influence of Washing Treatment and Storage Atmosphere on Phenylalanine Ammonia-Lyase Activity and Phenolic Acid Content of Minimally Processed Carrot Sticks. J. Agric. Food Chem. 2005, 53, 1065–1072. [Google Scholar] [CrossRef]
- Amodio, M.L.; Derossi, A.; Colelli, G. Modeling Phenolic Content during Storage of Cut Fruit and Vegetables: A Consecutive Reaction Mechanism. J. Food Eng. 2014, 140, 1–8. [Google Scholar] [CrossRef]
- Salta, J.; Martins, A.; Santos, R.G.; Neng, N.R.; Nogueira, J.M.; Justino, J.; Rauter, A.P. Phenolic Composition and Antioxidant Activity of Rocha Pear and Other Pear Cultivars–A Comparative Study. J. Funct. Foods 2010, 2, 153–157. [Google Scholar] [CrossRef]
- Ingrassia, M.; Columba, P.; Chironi, S.; Allegra, A.; Sortino, G. Study on Consumer Preferences for Quality Attributes of Fig Fruit after Storage at Room Temperature. Acta Hort. 2021, 1310, 135–140. [Google Scholar] [CrossRef]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit Softening: Revisiting the Role of Pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Naser, F.; Rabiei, V.; Razavi, F.; Khademi, O. Effect of Calcium Lactate in Combination with Hot Water Treatment on the Nutritional Quality of Persimmon Fruit during Cold Storage. Sci. Hortic. 2018, 233, 114–123. [Google Scholar] [CrossRef]











| Cultivar | |||
|---|---|---|---|
| Time of Storage (Days) | Treatment | Coscia | Abate |
| 0 | 11.9 ± 0.37 | 13.4 ± 0.4 | |
| 3 | CTR | 12.7 ± 0.1 ns | 13.7 ± 0.2 ns |
| XAN | 12.0 ± 0.2 | 13.1 ± 0.1 | |
| HPMC | 12.2 ± 0.2 | 13.5 ± 0.2 | |
| ASC | 12.1 ± 0.3 | 13.2 ± 0.2 | |
| 5 | CTR | 12.9 ± 0.2 ns | 14.0 ± 0.3 ns |
| XAN | 12.3 ± 0.4 | 13.5 ± 0.3 | |
| HPMC | 12.5 ± 0.1 | 13.8 ± 0.2 | |
| ASC | 12.3 ± 0.1 | 13.6 ± 0.2 | |
| 7 | CTR | 13.5 ± 0.5 a | 15.2 ± 0.5 a |
| XAN | 12.5 ± 0.2 b | 14.5 ± 0.1 a | |
| HPMC | 12.8 ± 0.1 b | 14.5 ± 0.1 a | |
| ASC | 12.4 ± 0.2 b | 13.9 ± 0.1 b | |
| 12 | CTR | 14.5 ± 0.2 a | 15.2 ± 0.4 a |
| XAN | 12.8 ± 0.05 b | 14.5 ± 0.1 a | |
| HPMC | 13.1 ± 0.2 b | 14.8 ± 0.2 a | |
| ASC | 12.6 ± 0.2 b | 14.2 ± 0.3 b | |
| Cultivar | |||
|---|---|---|---|
| Time of Storage (Days) | Treatment | Coscia | Abate |
| 0 | 1.9 ± 0.01 | 2.1 ± 0.02 | |
| 3 | CTR | 1.8 ± 0.1 ns | 2.0 ± 0.2 a |
| XAN | 1.7 ± 0.1 | 1.9 ± 0.1 a | |
| HPMC | 1.7 ± 0.1 | 1.6 ± 0.3 b | |
| ASC | 1.7 ± 0.1 | 2.0 ± 0.3 a | |
| 5 | CTR | 1.8 ± 0.4 ns | 1.9 ± 0.1 a |
| XAN | 1.7 ± 0.2 | 1.8 ± 0.4 a | |
| HPMC | 1.7 ± 0.3 | 1.6 ± 0.4 b | |
| ASC | 1.7 ± 0.3 | 2.0 ± 0.1 a | |
| 7 | CTR | 1.6 ± 0.3 ns | 1.9 ± 0.1 a |
| XAN | 1.6 ± 0.4 | 1.8 ± 0.1 a | |
| HPMC | 1.6 ± 0.5 | 1.6 ± 0.6 b | |
| ASC | 1.6 ± 0.6 | 1.9 ± 0.1 a | |
| 12 | CTR | 1.5 ± 0.1 ns | 1.8 ± 0.4 a |
| XAN | 1.6 ± 0.7 | 1.7 ± 0.5 a | |
| HPMC | 1.6 ± 0.4 | 1.4 ± 0.1 b | |
| ASC | 1.6 ± 0.4 | 1.6 ± 0.3 a | |
| Cultivar | |||
|---|---|---|---|
| Time of Storage (Days) | Treatment | Coscia | Abate |
| 0 | - | - | |
| 3 | CTR | 0.97 ± 0.15 a | 0.6 ± 0.19 a |
| XAN | 0.73 ± 0.12 b | 0.10 ± 0.04 b | |
| HPMC | 0.33 ± 0.15 c | 0.8 ± 0.34 a | |
| ASC | 0.35 ± 0.28 c | 0.2 ± 0.29 b | |
| 5 | CTR | 1.33. ± 0.15 a | 0.8 ± 0.14 a |
| XAN | 0.75 ± 0.2 b | 0.29 ± 0.08 b | |
| HPMC | 0.90 ± 0.11 b | 1.01 ± 0.24 a | |
| ASC | 0.69 ± 0.12 b | 0.4 ± 0.19 b | |
| 7 | CTR | 1.85 ± 0.15 a | 1.3 ± 0.17 a |
| XAN | 1.0 ± 0.2 b | 0.58 ± 0.03 ab | |
| HPMC | 0.90 ± 0.15 b | 1.12 ± 0.19 ab | |
| ASC | 0.81 ± 0.15 b | 0.6 ± 0.39 b | |
| 12 | CTR | 3.80 ± 0.19 a | 2.3 ± 0.51 a |
| XAN | 2.0 ± 0.12 b | 1.28 ± 0.02 b | |
| HPMC | 1.9 ± 0.12 b | 1.42 ± 0.15 b | |
| ASC | 1.81 ± 0.19 b | 1.6 ± 0.21 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allegra, A.; Inglese, P.; Guccione, E.; Farina, V.; Sortino, G. Calcium Ascorbate Coating Improves Postharvest Quality and Storability of Fresh-Cut Slices of Coscia and Abate Fétel Pears (Pyrus communis L.). Horticulturae 2022, 8, 227. https://doi.org/10.3390/horticulturae8030227
Allegra A, Inglese P, Guccione E, Farina V, Sortino G. Calcium Ascorbate Coating Improves Postharvest Quality and Storability of Fresh-Cut Slices of Coscia and Abate Fétel Pears (Pyrus communis L.). Horticulturae. 2022; 8(3):227. https://doi.org/10.3390/horticulturae8030227
Chicago/Turabian StyleAllegra, Alessio, Paolo Inglese, Eugenia Guccione, Vittorio Farina, and Giuseppe Sortino. 2022. "Calcium Ascorbate Coating Improves Postharvest Quality and Storability of Fresh-Cut Slices of Coscia and Abate Fétel Pears (Pyrus communis L.)" Horticulturae 8, no. 3: 227. https://doi.org/10.3390/horticulturae8030227
APA StyleAllegra, A., Inglese, P., Guccione, E., Farina, V., & Sortino, G. (2022). Calcium Ascorbate Coating Improves Postharvest Quality and Storability of Fresh-Cut Slices of Coscia and Abate Fétel Pears (Pyrus communis L.). Horticulturae, 8(3), 227. https://doi.org/10.3390/horticulturae8030227










