Effects of Azorhizobium caulinodans and Piriformospora indica Co-Inoculation on Growth and Fruit Quality of Tomato (Solanum lycopersicum L.) under Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. P. indica and A. caulinodans Cultures
2.1.1. Tomato Growth, Microbial Inoculation, and Salt Treatments
2.1.2. Micrography
2.1.3. Determination of Height and Stem Base Diameters of Tomato Plants
2.1.4. Determination of Chlorophyll Fluorescence
2.1.5. Plant Harvesting and Shoot Biomass Determination
2.1.6. Determination of Photosynthetic Pigment Concentrations
2.1.7. Determination of Soluble Sugars and Proteins
2.1.8. Assay of Enzymes
2.2. Assay of Reduced and Oxidized Glutathione
2.3. Determination of Ascorbate Concentrations
2.4. Determination of Malondialdehyde (MDA) Levels
2.5. Determination of Total Proanthocyanidin Concentrations
2.6. Statistical Analysis
3. Results
3.1. Colonization of A. caulinodans and P. indica
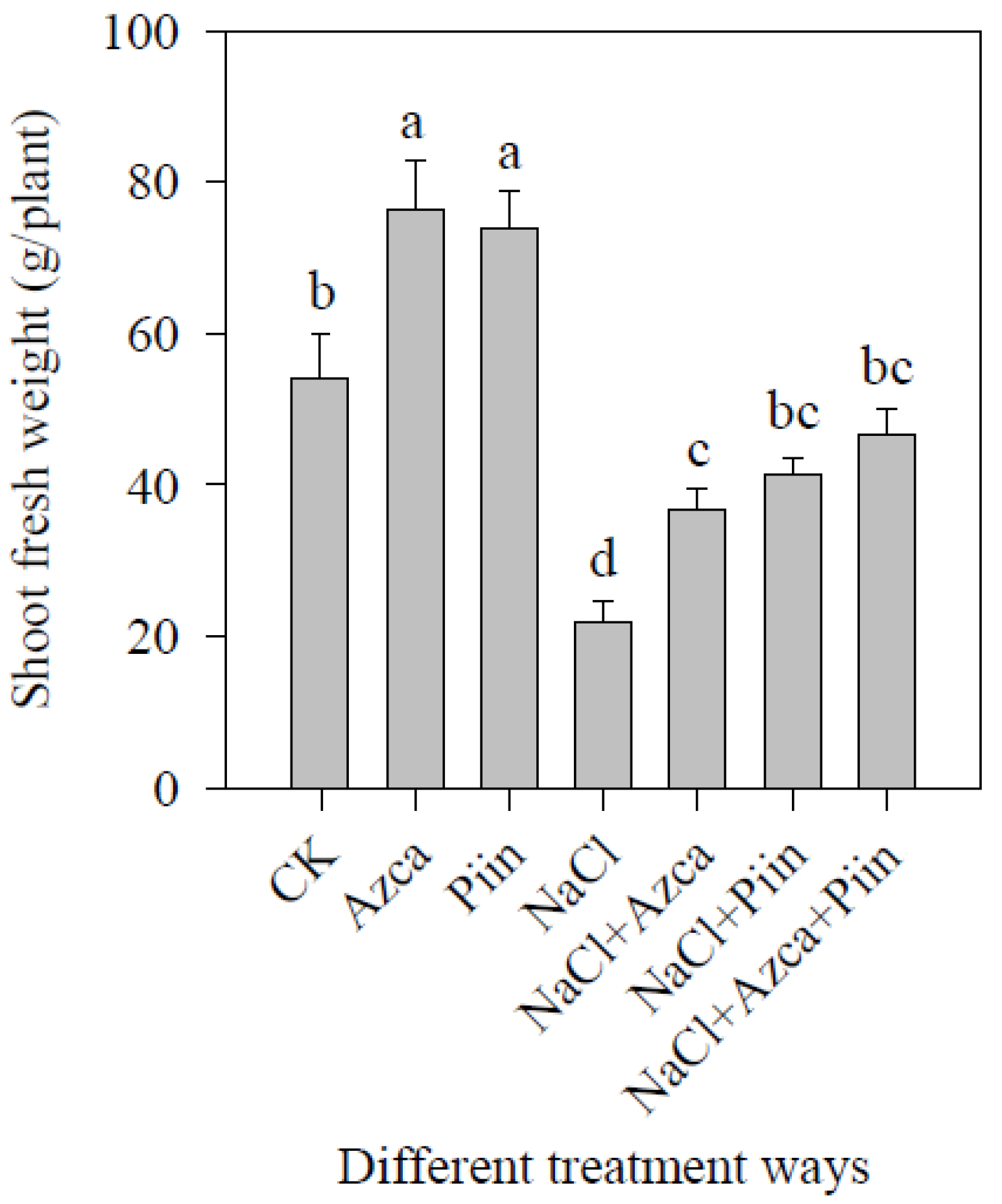
3.2. Effects of Inoculations on Tomato Plant Growth under Different Treatments
3.3. Effects of Inoculations on the Photosynthetic Pigments
3.4. Effects of Inoculations on the Chlorophyll Fluorescence Parameters
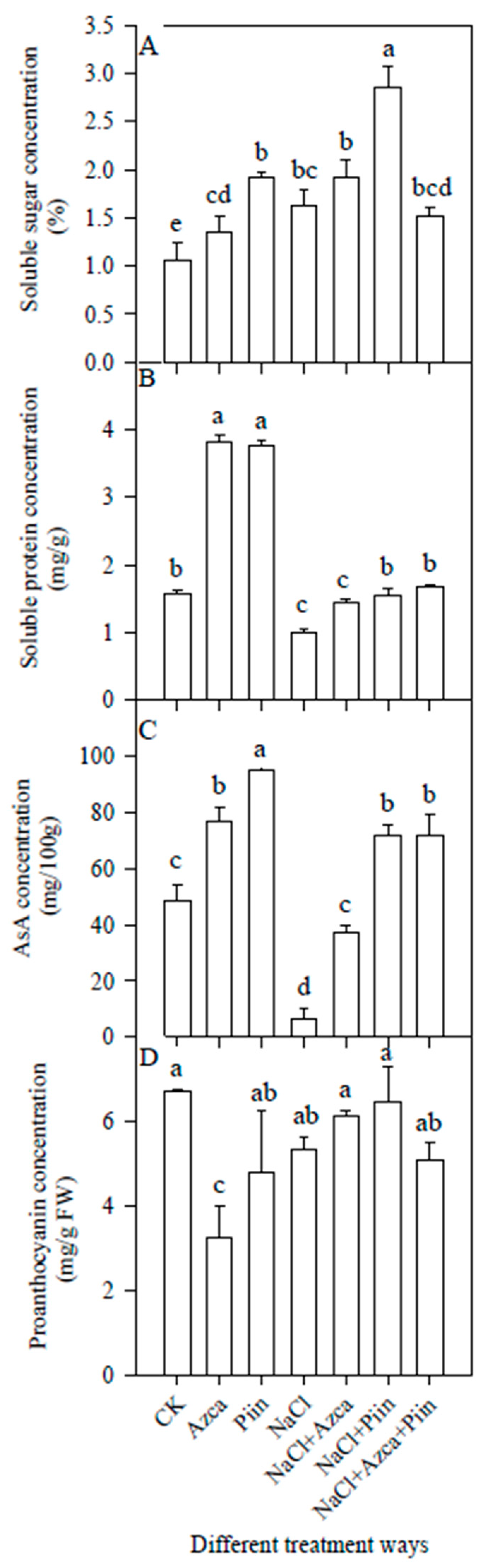
3.5. Effects of Inoculations on the Soluble Sugars and Proteins
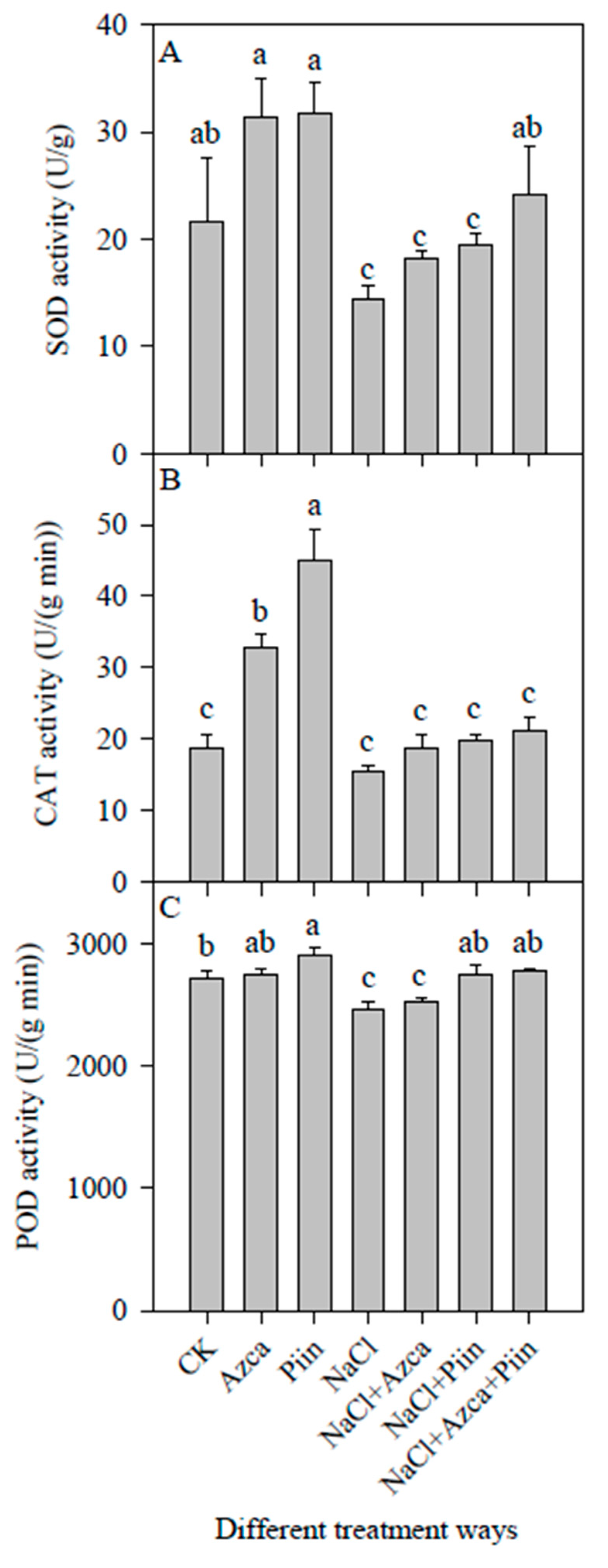
3.6. Effects of Inoculations on the Antioxidant Enzyme Activities
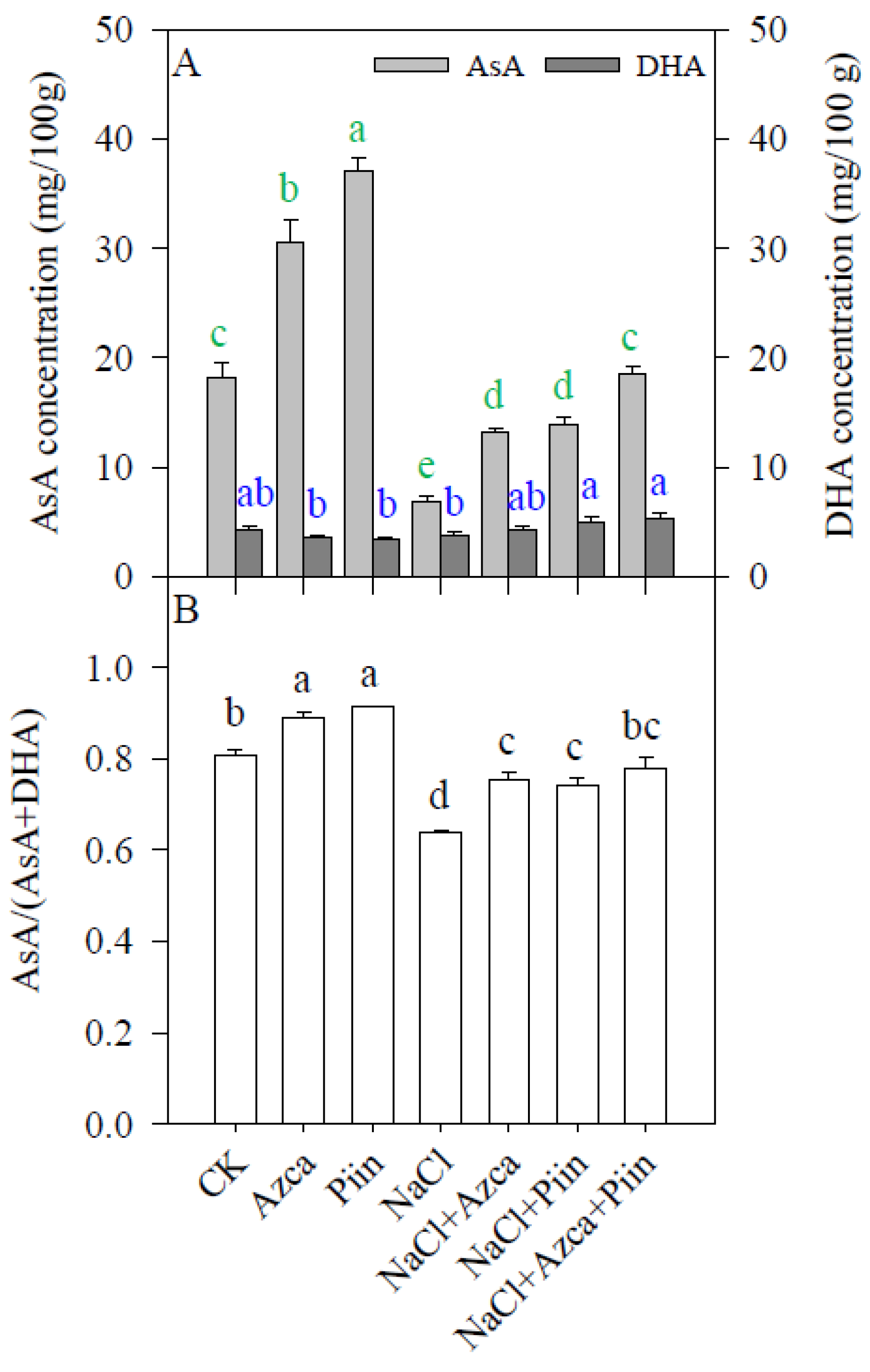
3.7. Effects of Inoculations on the Antioxidants
3.8. Effect of Inoculations on the Malondialdehyde Concentrations
3.9. Effects of Inoculations on the Fruit Quality
4. Discussion
4.1. Co-Inoculation of A. caulinodans and P. indica Promotes Growth and Fruit Quality
4.2. Co-Inoculation of A. caulinodans and P. indica Enhances Salt Tolerance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouhibi, C.; Attia, H.; Rebah, F.; Msilini, N.; Chebbi, M.; Aarrouf, J.; Urban, L.; Lachaal, M. Salt stress mitigation by seed priming with UV-C in lettuce plants: Growth, antioxidant activity and phenolic compounds. Plant Physiol. Biochem. 2014, 83, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, A.; Takeda, S.; Nick, P. Life and death under salt stress: Same players, different timing? J. Exp. Bot. 2014, 65, 2963–2979. [Google Scholar] [CrossRef] [PubMed]
- Bo, C.; Chen, H.; Luo, G.; Li, W.; Zhang, X.; Ma, Q.; Cheng, B.; Cai, R. Maize WRKY114 gene negatively regulates salt-stress tolerance in transgenic rice. Plant Cell Rep. 2020, 39, 135–148. [Google Scholar] [CrossRef]
- Jini, D.; Joseph, B. Physiological mechanism of salicylic acid for alleviation of salt stress in rice. Rice Sci. 2017, 24, 97–108. [Google Scholar] [CrossRef]
- Karan, R.; DeLeon, T.; Biradar, H.; Subudhi, P.K. Salt stress induced variation in DNA methylation pattern and its influence on gene expression in contrasting rice genotypes. PLoS ONE 2012, 7, e40203. [Google Scholar] [CrossRef]
- Niu, C.F.; Wei, W.; Zhou, E.I.; Tian, Q.Y.; Hao, A.G.; Zhang, Y.J.; Zhang, W.K.; Chen, S.Y. Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Environ. 2012, 35, 1156–1170. [Google Scholar] [CrossRef]
- Oyiga, B.C.; Sharma, R.C.; Shen, J.; Baum, M.; Ogbonnaya, F.C.; Léon, J.; Ballvora, A. Identification and characterization of salt tolerance of wheat germplasm using a multivariable screening approach. J. Agron. Crop Sci. 2016, 202, 472–485. [Google Scholar] [CrossRef]
- Ghorbani, A.; Razavi, S.M.; Ghasemi Omran, V.O.; Pirdashti, H. Piriformospora indica inoculation alleviates the adverse effect of NaCl stress on growth, gas exchange and chlorophyll fluorescence in tomato (Solanum lycopersicum L.). Plant Biol. 2018, 20, 729–736. [Google Scholar] [CrossRef]
- Keshishian, E.A.; Hallmark, H.T.; Ramaraj, T.; Plačková, L.; Sundararajan, A.; Schilkey, F.; Novák, O.; Rashotteet, A.M. Salt and oxidative stresses uniquely regulate tomato cytokinin levels and transcriptomic response. Plant Direct 2018, 2, e00071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Wang, N.; Ji, D.; Zhang, W.; Wang, Y.; Yu, Y.; Zhao, S.; Lyu, M.; You, J.; Zhang, Y.; et al. A GmSIN1/GmNCED3s/GmRbohBs feed-forward loop acts as a signal amplifier that regulates root growth in soybean exposed to salt stress. Plant Cell 2019, 31, 2107–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.G.; Han, X.; Yang, T.; Cui, W.H.; Wu, A.M.; Fu, C.X.; Wang, B.C.; Liu, L.J. Genome-wide transcriptional adaptation to salt stress in Populus. BMC Plant Biol. 2019, 19, 367. [Google Scholar] [CrossRef] [PubMed]
- Çakir Aydemir, B.; Yüksel Özmen, C.; Kibar, U.; Mutaf, F.; Büyük, P.B.; Bakır, M.; Ergül, A. Salt stress induces endoplasmic reticulum stress-responsive genes in a grapevine rootstock. PLoS ONE 2020, 15, e0236424. [Google Scholar] [CrossRef]
- Shah, A.N.; Tanveer, M.; Abbas, A.; Fahad, S.; Baloch, M.S.; Ahmad, M.I.; Saud, S.; Song, Y. Targeting salt stress coping mechanisms for stress tolerance in Brassica: A research perspective. Plant Physiol. Biochem. 2021, 158, 53–64. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant. Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Kim, W.Y.; Yun, D.J. A new insight of salt stress signaling in plant. Mol. Cells 2016, 39, 447–459. [Google Scholar] [CrossRef]
- Lou, L.; Yu, F.; Tian, M.; Liu, G.; Wu, Y.; Wu, Y.; Xia, R.; Pardo, J.M.; Guo, Y.; Xie, Q. ESCRT-I component VPS23A sustains salt tolerance by strengthening the SOS module in Arabidopsis. Mol. Plant 2020, 13, 1134–1148. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, J.; Li, X.; Li, Y. E2 conjugases UBC1 and UBC2 regulate MYB42-mediated SOS pathway in response to salt stress in Arabidopsis. New Phytol. 2020, 227, 455–472. [Google Scholar] [CrossRef] [PubMed]
- Kiegerl, S.; Cardinale, F.; Siligan, C.; Gross, A.; Baudouin, E.; Liwosz, A.; Eklöf, S.; Till, S.; Bögre, L.; Hirt, H.; et al. SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress-induced MAPK, SIMK. Plant Cell 2000, 12, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Cao, X.; Zhang, Y.; Jiang, J.; Qiao, D.; Xu, H.; Cao, Y. Two splice variants of the DsMEK1 mitogen-activated protein kinase kinase (MAPKK) are involved in salt stress regulation in Dunaliella salina in different ways. Biotechnol. Biofuels 2020, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, K.P.; Polkowska-Kowalczyk, L.; Lichocka, M.; Maszkowska, J.; Dobrowolska, G. SNF1-Related Protein Kinases SnRK2.4 and SnRK2.10 modulate ROS homeostasis in plant response to salt stress. Int. J. Mol. Sci. 2019, 20, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, M.; Li, Y.; Wu, A.; Huang, J. Effects of arbuscular mycorrhizal fungi on growth and nitrogen uptake of Chrysanthemum morifolium under salt stress. PLoS ONE 2018, 13, e0196408. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, N.; Meng, S.; Wu, F.; Liu, T. Arbuscular mycorrhizal fungi (AMF) enhance the tolerance of Euonymus maackii Rupr. at a moderate level of salinity. PLoS ONE 2020, 15, e0231497. [Google Scholar] [CrossRef]
- Tisarum, R.; Theerawitaya, C.; Samphumphuang, T.; Polispitak, K.; Thongpoem, P.; Singh, H.P.; Cha-Um, S. Alleviation of salt stress in upland rice (Oryza sativa L. ssp. indica cv. Leum Pua) using arbuscular mycorrhizal fungi inoculation. Front. Plant Sci. 2020, 11, 348. [Google Scholar] [CrossRef] [Green Version]
- Thiem, D.; Piernik, A.; Hrynkiewicz, K. Ectomycorrhizal and endophytic fungi associated with Alnus glutinosa growing in a saline area of central Poland. Symbiosis 2018, 75, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Galán, C.; Calvo-Polanco, M.; Zimmermann, S.D. Ectomycorrhizal symbiosis helps plants to challenge salt stress conditions. Mycorrhiza 2019, 29, 291–301. [Google Scholar] [CrossRef]
- Zwiazek, J.J.; Equiza, M.A.; Karst, J.; Senorans, J.; Wartenbe, M.; Calvo-Polanco, M. Role of urban ectomycorrhizal fungi in improving the tolerance of lodgepole pine (Pinus contorta) seedlings to salt stress. Mycorrhiza 2019, 29, 303–312. [Google Scholar] [CrossRef]
- Fadaei, S.; Vaziriyeganeh, M.; Young, M.; Sherr, I.; Zwiazek, J.J. Ericoid mycorrhizal fungi enhance salt tolerance in ericaceous plants. Mycorrhiza 2020, 30, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Kord, H.; Fakheri, B.; Ghabooli, M.; Solouki, M.; Emamjomeh, A.; Khatabi, B.; Sepehri, M.; Salekdeh, G.H.; Ghaffari, M.R. Salinity-associated microRNAs and their potential roles in mediating salt tolerance in rice colonized by the endophytic root fungus Piriformospora indica. Funct. Integr. Genom. 2019, 19, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Farias, G.C.; Nunes, K.G.; Soares, M.A.; de Siqueira, K.A.; Lima, W.C.; Neves, A.L.R.; de Lacerda, C.F.; Filho, E.G. Dark septate endophytic fungi mitigate the effects of salt stress on cowpea plants. Braz. J. Microbiol. 2020, 51, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Bouzouina, M.; Kouadria, R.; Lotmani, B. Fungal endophytes alleviate salt stress in wheat in terms of growth, ion homeostasis and osmoregulation. J. Appl. Microbiol. 2021, 130, 913–925. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Lata, C.; Tiwari, S.; Chauhan, A.S.; Mishra, S.K.; Agrawal, L.; Chakrabarty, D.; Nautiyal, C.S. Transcriptional alterations reveal Bacillus amyloliquefaciens-rice cooperation under salt stress. Sci. Rep. 2019, 9, 11912. [Google Scholar] [CrossRef]
- Alexander, A.; Singh, V.K.; Mishra, A. Halotolerant PGPR Stenotrophomonas maltophilia BJ01 induces salt tolerance by modulating physiology and biochemical activities of Arachis hypogaea. Front. Microbiol. 2020, 11, 568289. [Google Scholar] [CrossRef]
- Sultana, S.; Paul, S.C.; Parveen, S.; Alam, S.; Rahman, N.; Jannat, B.; Hoque, S.; Rahman, M.T.; Karim, M.M. Isolation and identification of salt-tolerant plant-growth-promoting rhizobacteria and their application for rice cultivation under salt stress. Can. J. Microbiol. 2020, 66, 144–160. [Google Scholar] [CrossRef]
- Li, J.; Bao, S.; Zhang, Y.; Ma, X.; Mishra-Knyrim, M.; Sun, J.; Sa, G.; Shen, X.; Polle, A.; Chen, S. Paxillus involutus strains MAJ and NAU mediate K+/Na+ homeostasis in ectomycorrhizal Populus × canescens under sodium chloride stress. Plant Physiol. 2012, 159, 1771–1786. [Google Scholar] [CrossRef] [Green Version]
- Sa, G.; Yao, J.; Deng, C.; Liu, J.; Zhang, Y.; Zhu, Z.; Zhang, Y.; Ma, X.; Zhao, R.; Lin, S.; et al. Amelioration of nitrate uptake under salt stress by ectomycorrhiza with and without a Hartig net. New Phytol. 2019, 222, 1951–1964. [Google Scholar] [CrossRef]
- Lee, S.H.; Calvo-Polanco, M.; Chung, G.C.; Zwiazek, J.J. Role of aquaporins in root water transport of ectomycorrhizal jack pine (Pinus banksiana) seedlings exposed to NaCl and fluoride. Plant Cell Environ. 2010, 33, 69–80. [Google Scholar] [CrossRef]
- Bois, G.; Bertrand, A.; Piché, Y.; Fung, M.; Khasa, D.P. Growth, compatible solute and salt accumulation of five mycorrhizal fungal species grown over a range of NaCl concentrations. Mycorrhiza 2006, 16, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Bois, G.; Bigras, F.J.; Bertrand, A.; Piché, Y.; Fung, M.Y.; Khasa, D.P. Ectomycorrhizal fungi affect the physiological responses of Picea glauca and Pinus banksiana seedlings exposed to an NaCl gradient. Tree Physiol. 2006, 26, 1185–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.; Calvo Polanco, M.; Zwiazek, J.J. Gas exchange and growth responses of ectomycorrhizal Picea mariana, Picea glauca, and Pinus banksiana seedlings to NaCl and Na2SO4. Plant Biol. 2006, 8, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, B.; Garcia, L.L.; Gillis, M. Characterization of Azorhizobium caulinodans gen. nov., sp. nov., a stem-nodulating nitrogen-fixing bacterium isolated from Sesbania rostrata. Int. J. Syst. Bacteriol. 1988, 38, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Cocking, E.C. Xylem colonization of tomato by Azorhizobium caulinodans ORS571. Acta Biol. Hung. 2001, 52, 189–194. [Google Scholar] [CrossRef]
- Buvana, R.; Kannaiyan, S. Influence of cell wall degrading enzymes on colonization of N2 fixing bacterium, Azorhizobium caulinodans in rice. Indian J. Exp. Biol. 2002, 40, 369–372. [Google Scholar]
- Webster, G.; Jain, V.; Davey, M.R.; Gough, C.; Vasse, J.; Denarie, J.; Cocking, E.C. The flavonoid naringenin stimulates the intercellular colonization of wheat roots by Azorhizobium caulinodans. Plant Cell Environ. 1998, 21, 373–383. [Google Scholar] [CrossRef]
- Qiu, L.; Li, Q.; Zhang, J.; Chen, Y.; Lin, X.; Sun, C.; Wang, W.; Liu, H.; Zhang, B. Migration of endophytic diazotroph Azorhizobium caulinodans ORS571 inside wheat (Triticum aestivum L.) and its effect on microRNAs. Funct. Integr. Genom. 2017, 17, 311–319. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Liu, X.; Dang, X.; Dong, X.; Xie, Z. Azorhizobium caulinodans c-di-GMP phosphodiesterase Chp1 involved in motility, EPS production, and nodulation of the host plant. Appl. Microbiol. Biotechnol. 2020, 104, 2715–2729. [Google Scholar] [CrossRef]
- Liu, W.; Bai, X.; Li, Y.; Min, J.; Kong, Y.; Hu, X. CheY1 and CheY2 of Azorhizobium caulinodans ORS571 regulate chemotaxis and competitive colonization with the host plant. Appl. Environ. Microbiol. 2020, 86, e00599-20. [Google Scholar] [CrossRef]
- Si, Y.; Guo, D.; Deng, S.; Lu, X.; Zhu, J.; Rao, B.; Cao, Y.; Jiang, G.; Yu, D.; Zhong, Z.; et al. Ohr and OhrR are critical for organic peroxide resistance and symbiosis in Azorhizobium caulinodans ORS571. Genes 2020, 11, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Yang, J.; Sun, Y.; Liu, X.; Li, Y.; Zhang, Z.; Xie, Z. Azorhizobium caulinodans Transmembrane chemoreceptor TlpA1 involved in host colonization and nodulation on roots and stems. Front. Microbiol. 2017, 8, 1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Liu, W.; Sun, Y.; Xia, C.; Elmerich, C.; Xie, Z. A cheZ-like gene in Azorhizobium caulinodans is a key gene in the control of chemotaxis and colonization of the host plant. Appl. Environ. Microbiol. 2018, 84, e01827-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Wang, X.; Qi, H.; Wang, Q.; Chen, Y.; Li, Q.; Zhang, Y.; Qiu, L.; Fontana, J.E.; Zhang, B.; et al. The infection and impact of Azorhizobium caulinodans ORS571 on wheat (Triticum aestivum, L.). PLoS ONE 2017, 12, e0187947. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.Y.; Yuan, J.G.; Xin, G.R.; Chang, H.T.; Wong, M.H. Germination, growth, and nodulation of Sesbania rostrata grown in Pb/Zn mine tailings. Environ. Manag. 1997, 21, 617–622. [Google Scholar] [CrossRef]
- Jian, S.; Shen, W.; Yang, Z. Enhanced adaptability of Sesbania rostrata to Pb/Zn tailings via stem nodulation. J. Environ. Sci. 2009, 21, 1135–1141. [Google Scholar] [CrossRef]
- Abdelaziz, M.E.; Kim, D.; Ali, S.; Fedoroff, N.V.; Al-Babili, S. The endophytic fungus Piriformospora indica enhances Arabidopsis thaliana growth and modulates Na+/K+ homeostasis under salt stress conditions. Plant Sci. 2017, 263, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, A.; Omran, V.O.G.; Razavi, S.M.; Pirdashti, H.; Ranjbar, M. Piriformospora indica confers salinity tolerance on tomato (Lycopersicon esculentum Mill.) through amelioration of nutrient accumulation, K+/Na+ homeostasis and water status. Plant Cell Rep. 2019, 38, 1151–1163. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/country-showcase/selected-product-detail/en/c/1287945 (accessed on 12 June 2020).
- Žižková, E.; Dobrev, P.I.; Muhovski, Y.; Hošek, P.; Hoyerová, K.; Haisel, D.; Procházková, D.; Lutts, S.; Motyka, V.; Hichri, I. Tomato (Solanum lycopersicum L.) SlIPT3 and SlIPT4 isopentenyltransferases mediate salt stress response in tomato. BMC Plant Biol. 2015, 15, 85. [Google Scholar] [CrossRef]
- Johnson, J.M.; Sherameti, I.; Ludwig, A.; Nongbri, P.L.; Sun, C.; Lou, B.; Varma, A.; Oelmuller, R. Protocols for Arabidopsis thaliana and Piriformospora indica co-cultivation—A model system to study plant beneficial traits. J. Endocytobiol. Cell Res. 2011, 21, 1001–1013. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Lee, S.-C.; Kim, J.-Y.; Kim, S.-J.; Aye, S.S.; Kim, S.-R. Over-expression of dehydrin gene, OsDhn1, improves drought and salt stress tolerance through scavenging of reactive oxygen species in rice (Oryza sativa L.). J. Plant Biol. 2014, 57, 383–393. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Becana, M.; Aparicio-Tejo, P.; Irigoyen, J.J.; Sanchez-Diaz, M. Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of Medicago sativa. Plant Physiol. 1986, 82, 1169–1171. [Google Scholar] [CrossRef] [Green Version]
- Aebi, H.E. Catalase. In Methods of Enzymatic Analyses; Berameyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1983; Volume 3, pp. 273–282. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidase. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA, 1955; Volume 2, pp. 764–775. [Google Scholar]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985, 113, 548–555. [Google Scholar] [CrossRef]
- Law, M.Y.; Charles, S.A.; Halliwell, B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplast. The effect of hydrogen peroxide and paraquat. Biochem. J. 1983, 210, 899–903. [Google Scholar] [CrossRef] [Green Version]
- Fazeli, F.; Ghorbanli, M.; Niknam, V. Effect of drought on biomass, protein content, lipid peroxidation and antioxidant enzymes in two sesame cultivars. Biol. Plant 2007, 51, 98–103. [Google Scholar] [CrossRef]
- Queiroz, C.R.A.D.A.; Morais, S.A.L.D.; Nascimento, E.A.D. Characterization of aroeira-preta (Myracrodruon urundeuva) wood tannins. Rev. Árvore 2002, 26, 493–497. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, E.L.; da Silva, F.A.; da Silva, F.S.B. Arbuscular mycorrhizal fungi increase the phenolic compounds concentration in the bark of the stem of Libidibia ferrea in field conditions. Open Microbiol. J. 2017, 11, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Faraloni, C.; Cutino, I.; Petruccelli, R.; Leva, A.R.; Lazzeri, S.; Torzillo, G. Chlorophyll fluorescence technique as a rapid tool for in vitro screening of olive cultivars (Olea europaea L.) tolerant to drought stress. Environ. Exp. Bot. 2011, 73, 49–56. [Google Scholar] [CrossRef]
- Wang, N.Y.; Ho, J.; Yi, L.S.; Gil, C.H.; Hyeon, K.S.; Rae, R.I. Chlorophyll fluorescence as a diagnostic tool for abiotic stress tolerance in wild and cultivated strawberry species. Hortic. Environ. Biotechnol. 2014, 54, 280–286. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Baczewska, A.H.; Pawluśkiewicz, B.; Paunov, M.; Alexantrov, V.; Goltsev, V.; Kalaji, M.H. Prompt chlorophyll a fluorescence as a rapid tool for diagnostic changes in PSII structure inhibited by salt stress in perennial ryegrass. J. Photochem. Chem. Photobiol. B-Biol. 2016, 157, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.; Haro, R.; Conchillo, L.B.; Benito, B. The endophyte Serendipita indica reduces the sodium content of Arabidopsis plants exposed to salt stress: Fungal ENA ATPases are expressed and regulated at high pH and during plant co-cultivation in salinity. Environ. Microbiol. 2019, 21, 3364–3378. [Google Scholar] [CrossRef] [PubMed]
- Heidarianpour, M.B.; Aliasgharzad, N.; Olsson, P.A. Positive effects of co-inoculation with Rhizophagus irregularis and Serendipita indica on tomato growth under saline conditions, and their individual colonization estimated by signature lipids. Mycorrhiza 2020, 30, 455–466. [Google Scholar] [CrossRef]
- Heydari, S.; Pirzad, A. Mycorrhizal fungi and Thiobacillus co-inoculation improve the physiological indices of Lallemantia iberica under salinity stress. Curr. Microbiol. 2020, 77, 2523–2534. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Abd Elgawad, H.; Xiong, Y.C.; Macovei, A.; Brestic, M.; Skalicky, M.; Shaghaleh, H.; Alhaj Hamoud, Y.; El-Sawah, A.M. Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress and improve seed yield and quality of soybean plant. Physiol. Plant 2021, 172, 2153–2169. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Wang, X.; Zhu, P.; Wu, H.; Qi, S. Plant growth-promoting endophyte Piriformospora indica alleviates salinity stress in Medicago truncatula. Plant Physiol. Biochem. 2017, 119, 211–223. [Google Scholar] [CrossRef]
- Nivedita; Gazara, R.K.; Khan, S.; Iqrar, S.; Ashrafi, K.; Abdin, M.Z. Comparative transcriptome profiling of rice colonized with beneficial endophyte, Piriformospora indica, under high salinity environment. Mol. Biol. Rep. 2020, 47, 7655–7673. [Google Scholar] [CrossRef]
- Sepehri, M.; Ghaffari, M.R.; Khayam Nekoui, M.; Sarhadi, E.; Moghadam, A.; Khatabi, B.; Hosseini Salekdeh, G. Root endophytic fungus Serendipita indica modulates barley leaf blade proteome by increasing the abundance of photosynthetic proteins in response to salinity. J. Appl. Microbiol. 2021, 131, 1870–1889. [Google Scholar] [CrossRef]
- Baltruschat, H.; Fodor, J.; Harrach, B.D.; Niemczyk, E.; Barna, B.; Gullner, G.; Janeczko, A.; Kogel, K.H.; Schäfer, P.; Schwarczinger, I.; et al. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 2008, 180, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Couto, E.; Wichmann, J.; Ferrari, P.; Trichopoulos, D.; Bueno-de-Mesquita, H.B.; Van Duijnhoven, F.J.; Büchner, F.L.; Key, T.; Boeing, H.; et al. Fruit and vegetable intake and overall cancer risk in the European prospective investigation into cancer and nutrition (EPIC). J. Natl. Cancer Inst. 2010, 102, 529–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Safe 2013, 12, 483–508. [Google Scholar] [CrossRef] [PubMed]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling anthocyanin bioavailability for human health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Redman, R.S. Balancing the generation and elimination of reactive oxygen species. Proc. Natl. Acad. Sci. USA 2005, 102, 3175–3176. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Yadav, V.; Tuteja, N.; Johri, A.K. Antioxidant enzyme activities in maize plants colonized with Piriformospora indica. Microbiology 2009, 155, 780–790. [Google Scholar] [CrossRef] [Green Version]






| Chl a (mg/g FW) | Chl b (mg/g FW) | Car (mg/g FW) | Total Chlorophyll | |
|---|---|---|---|---|
| CK | 17.65 ± 0.63 abc | 6.75 ± 0.34 a | 3.45 ± 0.30 ab | 27.84 ± 0.56 abc |
| Azac | 18.61 ± 0.73 ab | 7.95 ± 0.39 a | 4.16 ± 0.10 a | 30.72 ± 1.22 a |
| Piin | 19.07 ± 1.13 a | 7.67 ± 1.14 a | 3.60 ± 0.60 ab | 30.34 ± 0.79 ab |
| NaCl | 15.11 ± 0.41 c | 6.50 ± 0.25 a | 2.52 ± 0.20 b | 24.13 ± 0.80 d |
| NaCl + Azca | 16.90 ± 0.96 abc | 7.29 ± 0.54 a | 3.25 ± 0.19 ab | 27.44 ± 0.58 bc |
| NaCl + Piin | 15.80 ± 0.90 bc | 7.21 ± 1.22 a | 3.20 ± 0.51 ab | 26.22 ± 1.61 cd |
| NaCl + Azca + Piin | 17.56 ± 0.92 abc | 7.50 ± 0.45 a | 3.63 ± 0.25 ab | 28.68 ± 0.78 abc |
| Y(II) | ETR | qP | Fv/Fm | |
|---|---|---|---|---|
| CK | 0.55 ± 0.00 f | 29.00 ± 0.08 f | 0.88 ± 0.00 d | 0.5591 ± 0.00 e |
| Azca | 0.62 ± 0.00 b | 32.55 ± 0.10 b | 0.94 ± 0.00 b | 0.6171 ± 0.00 d |
| Piin | 0.60 ± 0.00 d | 31.36 ± 0.17 d | 0.90 ± 0.00 d | 0.6397 ± 0.00 c |
| NaCl | 0.56 ± 0.00 e | 29.55 ± 0.38 e | 1.00 ± 0.05 a | 0.4140 ± 0.01 f |
| NaCl + Azca | 0.61 ± 0.00 c | 32.01 ± 0.11 c | 0.90 ± 0.00 cd | 0.6768 ± 0.00 b |
| NaCl + Piin | 0.64 ± 0.00 a | 33.82 ± 0.13 a | 0.93 ± 0.00 bc | 0.6848 ± 0.00 a |
| NaCl + Azca + Piin | 0.55 ± 0.00 f | 28.61 ± 0.11 f | 0.85 ± 0.040 e | 0.6436 ± 0.00 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Pehlivan, N.; Ghorbani, A.; Wu, C. Effects of Azorhizobium caulinodans and Piriformospora indica Co-Inoculation on Growth and Fruit Quality of Tomato (Solanum lycopersicum L.) under Salt Stress. Horticulturae 2022, 8, 302. https://doi.org/10.3390/horticulturae8040302
Xu Z, Pehlivan N, Ghorbani A, Wu C. Effects of Azorhizobium caulinodans and Piriformospora indica Co-Inoculation on Growth and Fruit Quality of Tomato (Solanum lycopersicum L.) under Salt Stress. Horticulturae. 2022; 8(4):302. https://doi.org/10.3390/horticulturae8040302
Chicago/Turabian StyleXu, Zhiwen, Necla Pehlivan, Abazar Ghorbani, and Chu Wu. 2022. "Effects of Azorhizobium caulinodans and Piriformospora indica Co-Inoculation on Growth and Fruit Quality of Tomato (Solanum lycopersicum L.) under Salt Stress" Horticulturae 8, no. 4: 302. https://doi.org/10.3390/horticulturae8040302







