Production of Bacillus velezensis Strain GB1 as a Biocontrol Agent and Its Impact on Bemisia tabaci by Inducing Systemic Resistance in a Squash Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Laboratory Culture of B. tabaci
2.2. Isolation and Molecular Identification of the Bacterial Isolate
2.3. Production of the Bacterial Isolate
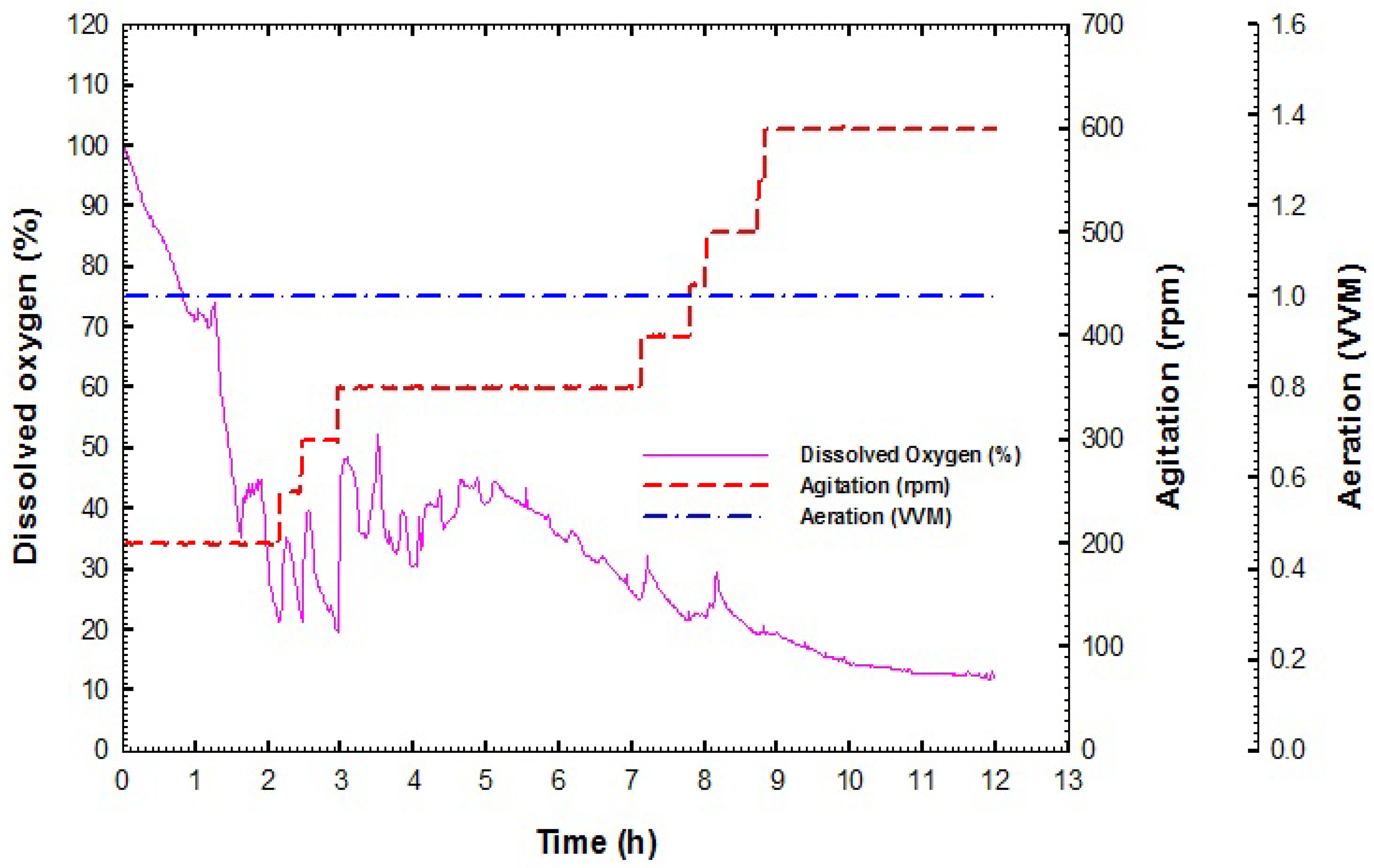
2.3.1. Preparation of Stirred Tank Bioreactor
2.3.2. Batch Fermentation Process
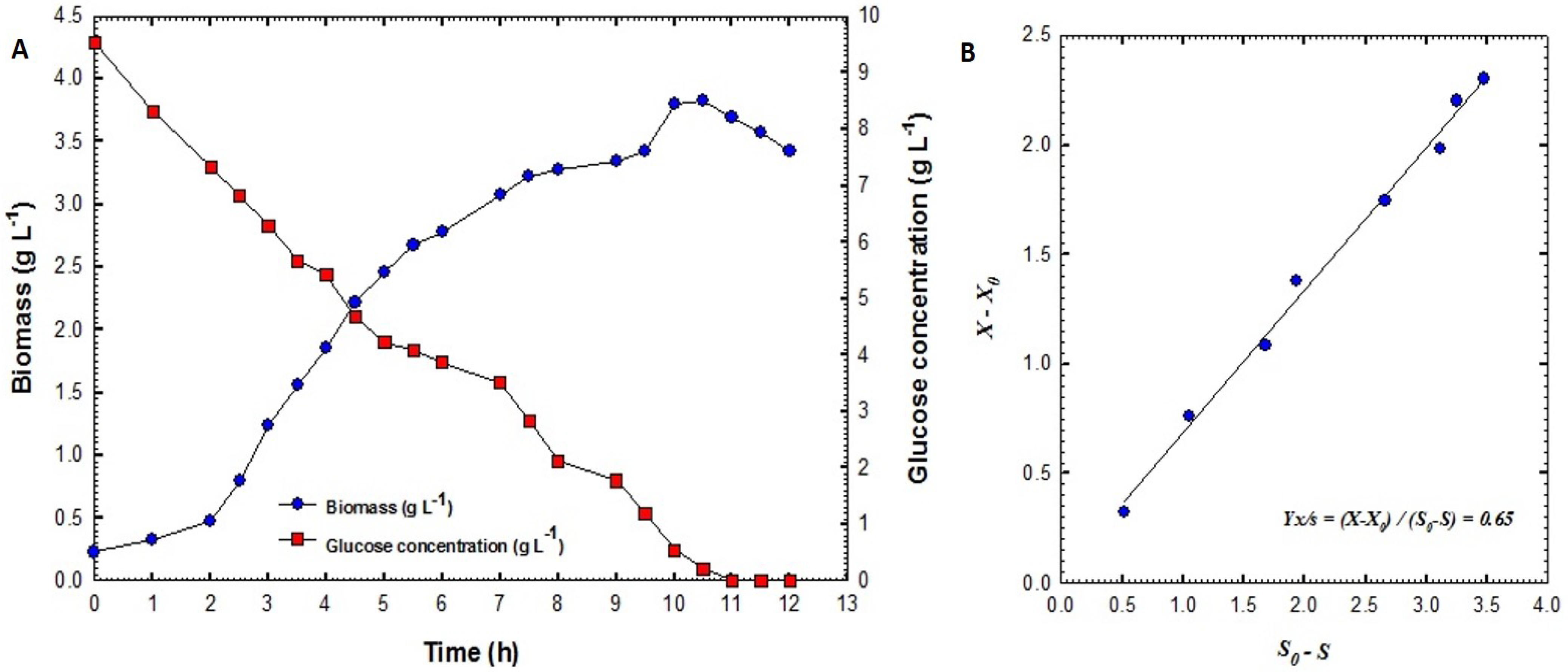
2.3.3. Biomass Estimation
2.3.4. Glucose Estimation
2.4. Induction of Pathogenesis-Related (PR) Proteins in Squash Plants
2.4.1. Experimental Design
2.4.2. Sample Extraction for Determination of Enzymes Activity
2.4.3. Assay of β-1,3-Glucanase Activity
2.4.4. Assay of Chitinase Activity
2.4.5. Assay of Polyphenol Oxidase Activity
2.4.6. Assay of Peroxidase Activity
2.5. The Effect of the Bacterial Isolate on B. tabaci Population Density
2.6. The Effect of Inoculation of Squash Plants by the Bacterial Isolate on Egg-Laying and Hatchability of B. tabaci
2.7. Statistical Analysis
3. Results
3.1. Molecular Identification of the Bacterial Isolate
3.2. Production of B. velezensis Strain GB1
3.3. Induction of Pathogenesis-Related (PR) Proteins in Squash Plants
3.3.1. β-1,3-Glucanase Activity
3.3.2. Chitinase Activity
3.3.3. Polyphenol Oxidase Activity
3.3.4. Peroxidase Activity
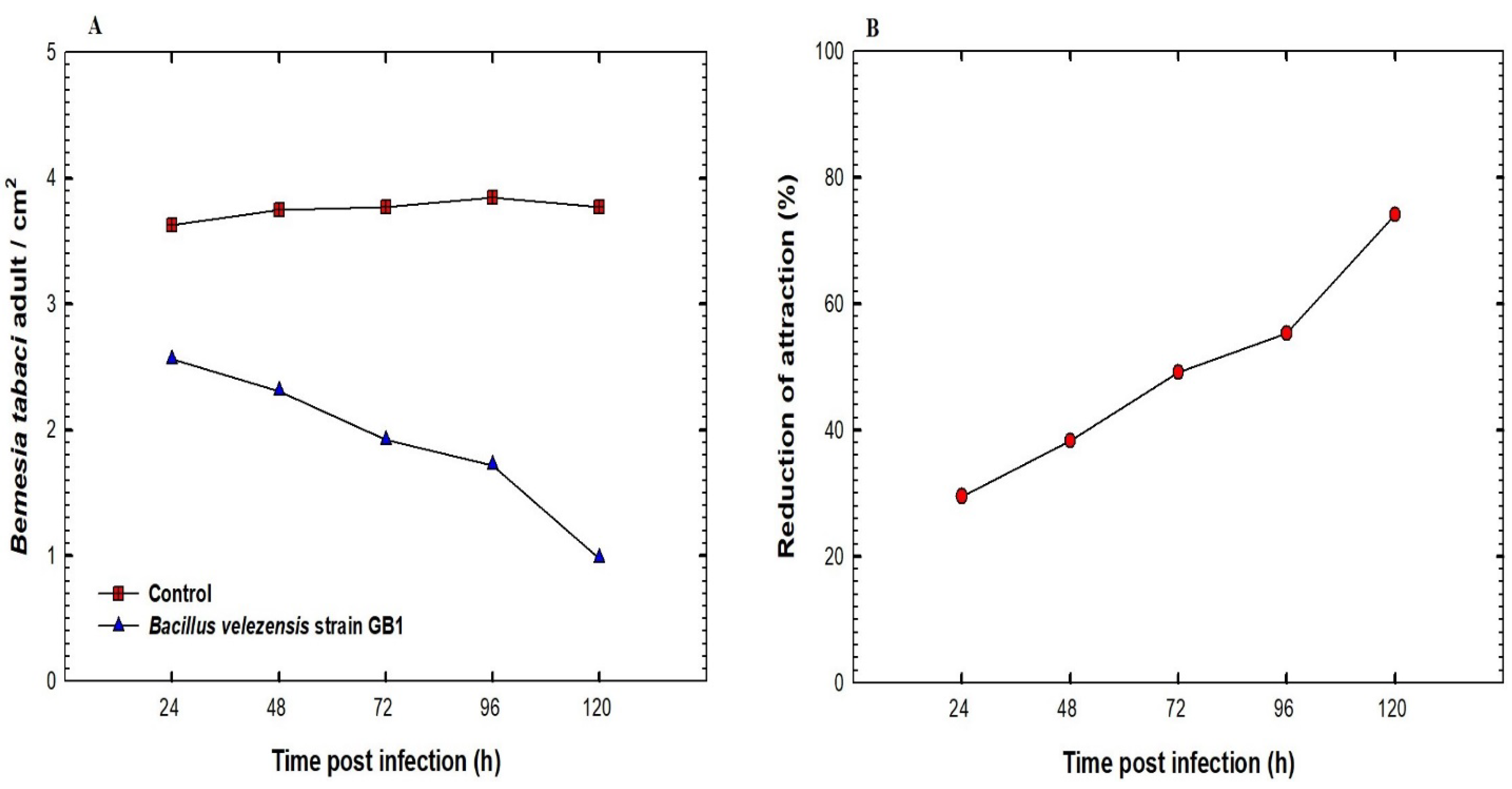
3.4. The Effect of the Inoculation of Squash Plants with B. velezensis Strain GB1 on B. tabaci Population Density
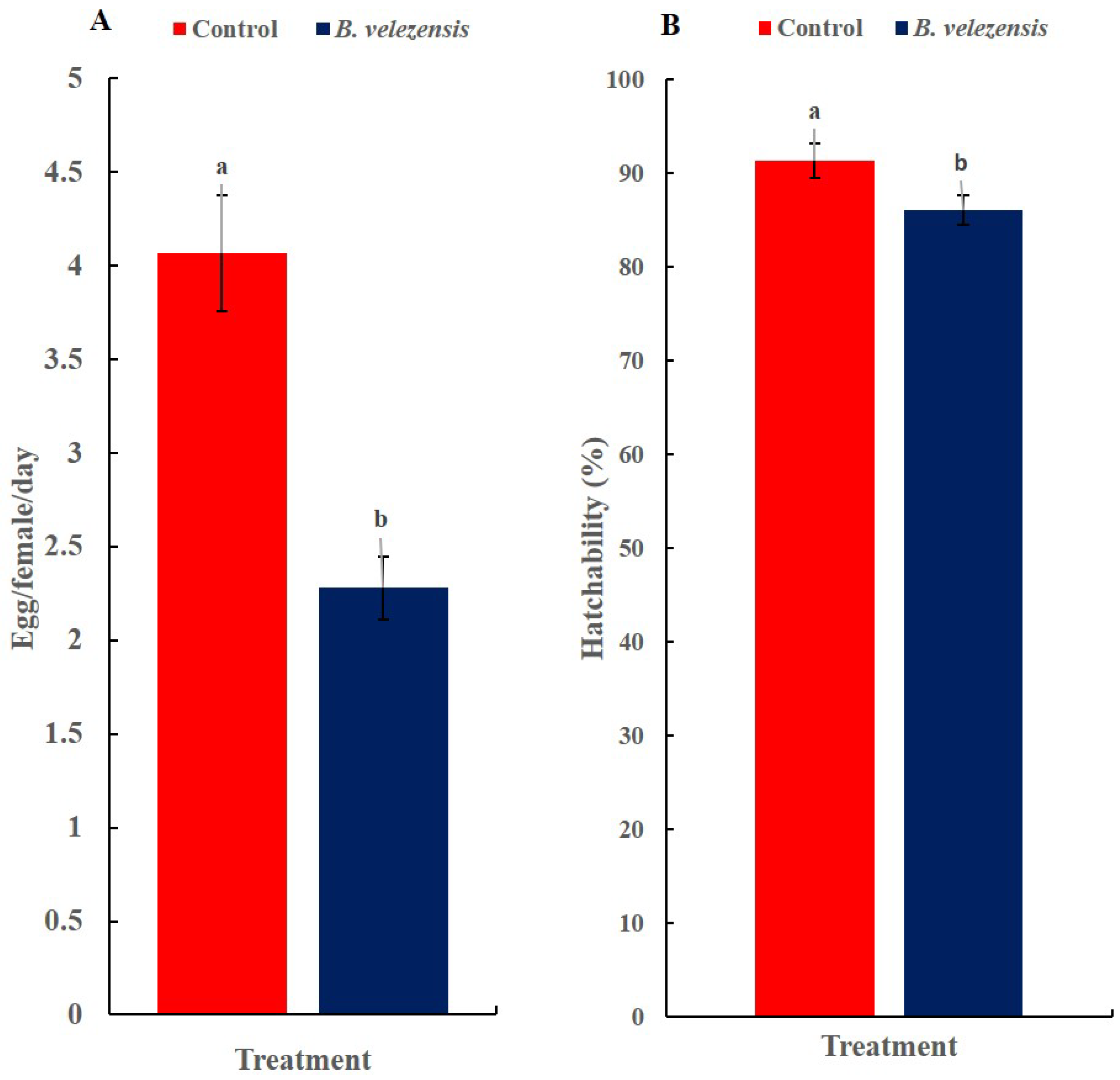
3.5. The Effect of the Inoculation of Squash Plants with B. velezensis Strain GB1 on Egg-Laying and Hatchability of B. tabaci
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Oliveira, M.R.V.; Henneberry, T.J.; Anderson, P. History, current status and collaborative research projects for Bemisia tabaci. Crop Prot. 2001, 20, 709–723. [Google Scholar] [CrossRef] [Green Version]
- Hoelmer, K.A.; Osborne, L.S.; Yokomi, R.K. Foliage disorders in Florida associated with feeding by sweet potato whitefly, Bemisia tabaci. Fla. Entomol. 1991, 74, 162–166. [Google Scholar] [CrossRef]
- Summers, C.G.; Estrada, D. Chlorotic streak of bell pepper: A new toxicogenic disorder induced by feeding of the Silverleaf whitefly, Bemisia argentifolii. Plant Dis. 1996, 80, 822. [Google Scholar] [CrossRef]
- McAuslane, H.J.; Cheng, J.; Carle, R.B.; Schmalstig, J. Influence of Bemisia argentifolii (Homoptera: Aleyrodidae) infestation and squash silver leaf disorder on zucchini seedling growth. J. Econ. Entomol. 2004, 97, 1096–1105. [Google Scholar] [CrossRef]
- Brown, J.K.; Czosnek, H. Whitefly transmission of plant viruses. Adv. Bot. Res. 2002, 36, 65–92. [Google Scholar]
- Jones, D.R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Ng, J.C.; Tian, T.; Falk, B.W. Quantitative parameters determining whitefly (Bemisia tabaci) transmission of lettuce infectious yellows virus and an engineered defective RNA. J. Gen. Virol. 2004, 86, 2697–2707. [Google Scholar] [CrossRef]
- Al-Momany, A.; Al-Antary, T.M. Pests of Garden and Home, 2nd ed.; Publications of University of Jordan: Amman, Jordan, 2008; p. 518. [Google Scholar]
- Al Arabiat, O.W.; Araj, S.A.; Alananbeh, K.M.; Al-Antary, T.M. Effect of three Bacillus spp. on tobacco whitefly Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae). Fresen. Environ. Bull. 2018, 27, 3706–3712. [Google Scholar]
- Sani, I.; Ismail, S.I.; Abdullah, S.; Jalinas, J.; Jamian, S.; Saad, N. A review of the biology and control of whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), with special reference to biological control using entomopathogenic fungi. Insects 2020, 11, 619. [Google Scholar] [CrossRef]
- Bellotti, A.C.; Arias, B. Host plant resistance to whiteflies with emphasis on cassava as a case study. Crop Prot. 2001, 20, 813–823. [Google Scholar] [CrossRef] [Green Version]
- Kant, M.R.; Jonckheere, W.; Knegt, B.; Lemos, F.; Liu, J.; Schimmel, B.C.J.; Villarroel, C.A.; Ataide, L.M.S.; Dermauw, W.; Glas, J.J.; et al. Mechanisms and ecological consequences of plant defense induction and suppression in herbivore communities. Ann. Bot. 2015, 115, 1015–1051. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microb. Res. 2018, 212–213, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; De Bashan, L. Plant growth-promoting. Encycl. Soils Environ. 2005, 1, 103–115. [Google Scholar]
- Al Arabiat, O.W.; Araj, S.E.A.; Alananbeh, K.M.; Al-Antary, T.M. Efficacy of three Bacillus spp. on development of tobacco whitefly Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae). Fresen. Environ. Bull. 2018, 27, 4965–4972. [Google Scholar]
- Van Loon, L.C. Plant responses to plant growth-promoting rhizobacteria. Eur. J. Plant Pathol. 2007, 119, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, M.; Chun, S.C. Expression of PR-protein genes and induction of defense-related enzymes by Bacillus subtilis CBR05 in tomato (Solanum lycopersicum) plants challenged with Erwinia carotovora subsp. carotovora. Biosci. Biotechnol. Biochem. 2016, 80, 2277–2283. [Google Scholar] [CrossRef] [Green Version]
- Van Hulten, M.; Pelser, M.; Van Loon, L.C.; Pieterse, C.M.; Ton, J. Costs and benefits of priming for defense in Arabidopsis. Proc. Nat. Acad. Sci. USA 2006, 103, 5602–5607. [Google Scholar] [CrossRef] [Green Version]
- Chung, E.J.; Hossain, M.T.; Khan, A.; Kim, K.H.; Jeon, C.O.; Chung, Y.R. Bacillus oryzicola sp. nov., an endophytic bacterium isolated from the roots of rice with antimicrobial, plant growth promoting, and systemic resistance inducing activities in rice. Plant Pathol. J. 2015, 31, 152. [Google Scholar] [CrossRef]
- Hossain, M.T.; Khan, A.; Chung, E.J.; Rashid, H.; Chung, Y.R. Biological control of rice bakanae by an endophytic Bacillus oryzicola YC7007. Plant Pathol. J. 2016, 32, 228–241. [Google Scholar] [CrossRef] [Green Version]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeOliveira Araujo, E. Rhizobacteria in the control of pest insects in agriculture. Afr. J. Plant Sci. 2015, 9, 368–373. [Google Scholar]
- Zebelo, S.; Song, Y.; Kloepper, J.W.; Fadamiro, H. Rhizobacteria activates (C)-d-cadinene synthase genes and induces systemic resistance in cotton against beet armyworm (Spodoptera exigua). Plant Cell Environ. 2016, 9, 935–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zehnder, G.; Kloepper, J.; Tuzun, S.; Yao, C.; Wei, G.; Chambliss, O.; Shelby, R. Insect feeding on cucumber mediated by rhizobacteria- induced plant resistance. Entomol. Exp. Appl. 1997, 83, 81–85. [Google Scholar] [CrossRef]
- Ruiz-García, C.; Bejar, V.; Martinez-Checa, F.; Llamas, I.; Quesada, E. Bacillus velezensis sp. nov., a surfactant-producing bacterium isolated from the river Velez in Malaga, southern Spain. Int. J. Syst. Evol. Microbiol. 2005, 55, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of two plant-growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018, 8, 4360. [Google Scholar] [CrossRef]
- Chen, L.; Shi, H.; Heng, J.; Wang, D.; Bian, K. Antimicrobial, plant growth-promoting and genomic properties of the peanut endophyte Bacillus velezensis LDO2. Microbiol. Res. 2019, 218, 41–48. [Google Scholar] [CrossRef]
- Meng, Q.; Jiang, H.; Hao, J.J. Effects of Bacillus velezensis strain BAC03 in promoting plant growth. Biol. Control 2016, 98, 18–26. [Google Scholar] [CrossRef]
- Kim, S.Y.; Song, H.; Sang, M.K.; Weon, H.Y.; Song, J. The complete genome sequence of Bacillus velezensis strain GH1-13 reveals agriculturally beneficial properties and a unique plasmid. J. Biotechnol. 2017, 259, 221–227. [Google Scholar] [CrossRef]
- Fan, B.; Wang, C.; Song, X.; Ding, X.; Wu, L.; Wu, H.; Gao, X.; Borriss, R. Bacillus velezensis FZB42 in 2018: The gram-positive model strain for plant growth promotion and biocontrol. Front. Microbiol. 2018, 9, 2491. [Google Scholar] [CrossRef] [Green Version]
- Rabbee, M.F.; Ali, M.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K.H. Bacillus velezensis: A valuable member of bioactive molecules within plant microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, M.H.; Kim, H.J.; Yeom, S.I.; Yu, H.A.; Manir, M.M.; Moon, S.S.; Kang, Y.J.; Chung, Y.R. Bacillus velezensis YC7010 enhances plant defenses against brown planthopper through transcriptomic and metabolic changes in rice. Front. Plant Sci. 2018, 9, 1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Istock, C.A.; Ferguson, N.; Istock, N.L.; Duncan, K.E. Geographical diversity of genomic lineages in Bacillus subtilis (Ehrenberg) Cohn sensulato. Org. Divers. Evol. 2001, 1, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Matar, S.M.; El-Kazzaz, S.A.; Wagih, E.E.; El-Diwany, A.I.; Hafez, E.E.; Moustafa, H.E.; Abo-Zaid, G.A.; Serour, E.A. Molecular characterization and batch fermentation of Bacillus subtilis as biocontrol agent II. Biotechnology 2009, 8, 35–43. [Google Scholar] [CrossRef]
- Asaka, O.; Shoda, M. Biocontrol of Rhizoctonia solani damping-off of tomato with Bacillus subtilis RB14. Appl. Environ. Microb. 1996, 62, 4081–4408. [Google Scholar] [CrossRef] [Green Version]
- Van Dam-Mieras, M.C.E.; Jeu, W.H.; Vries, J.; Currell, B.R.; James, J.W.; Leach, C.K.; Patmore, R.A. Techniques Used in Bioproduct Analysis; Butterworth-Heinemann Ltd.: Oxford, UK, 1992. [Google Scholar]
- Saikia, R.; Singh, B.P.; Kumar, R.; Arora, D.K. Detection of pathogenesis-related proteins-chitinase and β-1,3-glucanase in induced chickpea. Curr. Sci. 2005, 89, 659–663. [Google Scholar]
- Monreal, J.; Reese, E.T. The chitinase of Serratia marcescens. Can. J. Microb. 1969, 15, 689–696. [Google Scholar] [CrossRef]
- Mayer, A.M.; Harel, E.; Shaul, R.B. Assay of catechol oxidase a critical comparison of methods. Phytochemistry 1965, 5, 783–789. [Google Scholar] [CrossRef]
- Hammerschmidt, R.; Nuckles, E.M.; Kuc, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–76. [Google Scholar] [CrossRef]
- SAS (Statistical Analysis System). SAS/STAT User’s Guide, 8.6th ed.; SAS institute Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Xu, H.-X.; Yang, Y.-J.; Lu, Y.-H.; Zheng, X.-S.; Tian, J.-C.; Lai, F.-X.; Fu, Q.; Lu, Z.-X. Sustainable management of rice insect pests by non-chemical-insecticide technologies in China. Rice Sci. 2017, 24, 61–72. [Google Scholar]
- Li, M.; Li, S.; Xu, A.; Lin, H.; Chen, D.; Wang, H. Selection of Beauveria isolates pathogenic to adults of Nilaparvata lugens. J. Insect Sci. 2014, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, L.M. Plant growth promoting rhizobacteria (PGPR): Prospects for new inoculants. Crop Manag. 2004, 10, 301–305. [Google Scholar] [CrossRef]
- Abdel-Gayed, M.A.; Abo-Zaid, G.A.; Matar, S.M.; Hafez, E.E. Fermentation, formulation and evaluation of PGPR Bacillus subtilis isolate as a bioagent for reducing occurrence of peanut soil-borne diseases. J. Integr. Agric. 2019, 18, 2080–2092. [Google Scholar]
- Rashid, M.H.; Khan, A.; Hossain, M.T.; Chung, Y.R. Induction of systemic resistance against aphids by endophytic Bacillus velezensis YC7010 via expressing PHYTOALEXIN DEFICIENT4 in Arabidopsis. Front. Plant Sci. 2017, 8, 211. [Google Scholar] [CrossRef] [Green Version]
- Van Oosten, V.R.; Bodenhausen, N.; Reymond, P.; Van Pelt, J.A.; Van Loon, L.C.; Dicke, M.; Pieterse, C.M. Differential effectiveness of microbially induced resistance against herbivorous insects in Arabidopsis. Mol. Plant Microbe Interact. 2008, 21, 919–930. [Google Scholar] [CrossRef] [Green Version]
- De Vleesschauwer, D.; Höfte, M. Rhizobacteria-induced systemic resistance. Adv. Bot. Res. 2009, 51, 223–281. [Google Scholar]
- Pineda, A.; Zheng, S.J.; Van Loon, J.J.A.; Pieterse, C.M.J.; Dicke, M. Helping plants to deal with insects: The role of beneficial soil-borne microbes. Trends Plant Sci. 2010, 15, 507–514. [Google Scholar] [CrossRef]
- Jiméncz, D.R.; Yokomi, R.K.; Mayer, R.T.; Shapiro, J.P. Cytology and physiology of silverleaf whitefly-induced squash silverleaf. Physiol. Mol. Plant Pathol. 1995, 46, 227–242. [Google Scholar] [CrossRef]
- Mayer, R.T.; Inbar, M.; Doostdar, H. Are Pathogenesis-related proteins involved in plant resistance to insects? Am. Soc. Plant Biol. Oster Plant Interact. Organ. 1997, 114, 1120. [Google Scholar]
- Inbar, M.; Doostdar, H.; Mayer, R.T. Effects of sessile whitefly nymphs (Homoptera: Aleyrodidae) on leaf-chewing larvae (Lepidoptera: Noctuidae). Physiol. Chem. Ecol. 1999, 28, 353–357. [Google Scholar] [CrossRef]
- Van de Ven, W.T.G.; LeVesque, C.S.; Perring, T.M.; Walling, L.L. Local and systemic changes in squash gene expression in response to silverleaf whitefly feeding. Plant Cell 2000, 12, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Antony, B.; Palaniswami, M.S. Bemisia tabaci feeding induces pathogenesis-related proteins in cassava (Manihotesculenta Crantz). Ind. J. Biochem. Biophys. 2006, 43, 182–185. [Google Scholar]
- Gorovits, R.; Akad, F.; Beery, H.; Vidavsky, F.; Mahadav, A.; Czosnek, H. Expression of stress-response proteins upon whitefly-mediated inoculation of Tomato yellow leaf curl virus in susceptible and resistant tomato plants. Mol. Plant Microbe Interact. 2007, 20, 1376–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taggar, G.K.; Gill, R.S.; Gupta, A.K.; Sandhu, J.S. Fluctuations in peroxidase and catalase activities of resistant and susceptible black gram (Vignamungo (L.) Hepper) genotypes elicited by Bemisia tabaci (Gennadius) feeding. Plant Sing. Behav. 2012, 7, 1321–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Sun, X.; Xue, M.; Zhang, X.; Li, Q. Antioxidant enzyme responses induced by whiteflies in tobacco plants in defense against aphids: Catalase may play a dominant role. PLoS ONE 2016, 27, e0165454. [Google Scholar] [CrossRef] [Green Version]
- Soliman, A.M.; Idriss, M.H.; El-Meniawi, F.A.; Rawash, I.A. Induction of pathogenesis-related (PR) proteins as a plant defense mechanism for controlling the cotton whitefly Bemisia tabaci. Alex. J. Agric. Sci. 2019, 64, 107–122. [Google Scholar] [CrossRef] [Green Version]
- Graham, L.S.; Sticklen, M.B. Plant chitinases. Can. J. Bot. 1994, 72, 1057–1083. [Google Scholar] [CrossRef]
- Singh, R.; Upadhyay, S.K.; Singh, M.; Sharma, I.; Sharma, P.; Kamboj, P.; Saini, A.; Voraha, R.; Sharma, A.K.; Upadhyay, T.K.; et al. Chitin, chitinases and chitin derivatives in biopharmaceutical, agricultural and environmental perspective. Biointerface Res. Appl. Chem. 2021, 11, 9985–10005. [Google Scholar]
- Lawrence, S.D.; Novak, N.G. Expression of poplar chitinase in tomato leads to inhibition of development in Colorado potato beetle. Biotechnol. Lett. 2006, 28, 593–599. [Google Scholar] [CrossRef]
- Castro, D.; Torres, M.; Sampedro, I.; Martínez-Checa, F.; Torres, B.; Béjar, V. Biological Control of verticillium wilt on olive trees by the salt-tolerant strain Bacillus velezensis XT1. Microorganisms 2020, 8, 1080. [Google Scholar] [CrossRef]
- Sherzad, Z.; Canming, T. A new strain of Bacillus velezensis as a bioagent against Verticillium dahliae in cotton: Isolation and molecular identification. Egypt. J. Biol. Pest Control 2020, 30, 118. [Google Scholar] [CrossRef]
- Ramiro, D.A.; Guerreiro-Filho, O.; Mazzafera, P. Phenol contents, oxidase activities, and the resistance of coffee to the leaf miner Leucoptera coffeella. J. Chem. Ecol. 2006, 32, 1977–1988. [Google Scholar] [CrossRef] [PubMed]
- Marusek, C.M.; Trobaugh, N.M.; Flurkey, W.H.; Inlow, J.K. Comparative analysis of polyphenol oxidase from plant and fungal species. J. Inorg. Biochem. 2006, 100, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Constabel, C.P.; Barbehenn, R. Defensive roles of polyphenol oxidase in plants. In Induced Plant Resistance to Herbivory; Springer: Dordrecht, The Netherlands, 2008; pp. 253–270. [Google Scholar]
- Schuman, M.C.; Baldwin, I.T. The layers of plant responses to insect herbivores. Annu. Rev. Entomol. 2016, 61, 373–394. [Google Scholar] [CrossRef]
- Summers, C.B.; Felton, G.W. Prooxidant effects of phenolic acids on the generalist herbivore Helicoverpazea (Lepidoptera: Noctuidae): Potential mode of action for phenolic compounds in plant anti-herbivore chemistry. Insect Biochem. Mol. Biol. 1994, 24, 943–953. [Google Scholar] [CrossRef]
- Mishra, B.B.; Gautam, S. Polyphenol oxidases: Biochemical and molecular characterization, distribution, role and its control. Enzym. Eng. 2016, 5, 2329–6674. [Google Scholar]
- Erb, M.; Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef] [Green Version]
- Castañera, P.; Steffens, J.C.; Tingey, W.M. Biological performance of Colorado potato beetle larvae on potato genotypes with differing levels of polyphenol oxidase. J. Chem. Ecol. 1996, 22, 91–101. [Google Scholar]
- Bhonwong, A.; Stout, M.J.; Attajarusit, J.; Tantasawat, P. Defensive role of tomato polyphenol oxidases against cotton bollworm (Helicoverpa armigera) and beet armyworm (Spodoptera exigua). J. Chem. Ecol. 2009, 35, 28–38. [Google Scholar] [CrossRef]
- Mahanil, S.; Attajarusit, J.; Stout, M.J.; Thipyapong, P. Overexpression of tomato polyphenol oxidase increases resistance to common cutworm. Plant Sci. 2008, 174, 456–466. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Luthe, D.S.; Felton, G.W. Arthropod-inducible proteins: Broad spectrum defenses against multiple herbivores. Plant Physiol. 2008, 146, 852–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffey, S.S.; Stout, M.J. Anti-ntritive and toxic components of plant defense against insects. Arch. Insect Biochem. Physiol. 1996, 32, 3–37. [Google Scholar] [CrossRef]
- Prabhukarthikeyan, S.R.; Keerthana, U.; Archana, S.; Raguchander, T. Induced resistance in tomato plants to Helicoverpa armigera by mixed formulation of Bacillus subtilis and Beauveria bassiana. Res. J. Biotechnol. 2017, 12, 53–59. [Google Scholar]
- Senthilraja, G.; Anand, T.; Kennedy, J.S.; Raguchander, T.; Samiyappan, R. Plant growth promoting rhizobacteria (PGPR) and entomopathogenic fungus bioformulation enhance the expression of defense enzymes and pathogenesis-related proteins in groundnut plants against leaf miner insect and collar rot pathogen. Physiol. Mol. Plant Pathol. 2013, 82, 10–19. [Google Scholar] [CrossRef]
- Barbehenn, R.; Dukatz, C.; Holt, C.; Reese, A.; Martiskainen, O.; Salminen, J.P.; Yip, L.; Tran, L.; Constabel, C.P. Feeding on poplar leaves by caterpillars potentiates foliar peroxidase action in their guts and increases plant resistance. Oecologia 2010, 164, 993–1004. [Google Scholar] [CrossRef]
- Dowd, P.F.; Lagrimini, L.M. Examination of the biological effects of high anionic peroxidase production in tobacco plants grown under field conditions. I. Insect pest damage. Transgenic Res. 2006, 15, 197–204. [Google Scholar]
- Qiu, Y.; Yan, H.; Sun, S.; Wang, Y.; Zhao, X.; Wang, H. Use of Bacillus velezensis SDTB022 against tobacco black shank (TBS) and the biochemical mechanism involved. Biol. Control 2022, 165, 104785. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ngumbi, E.N.; Nangle, K.W.; Fadamiro, H.Y. Inoculants Including Bacillus Bacteria for Inducing Production of Volatile Organic Compounds in Plants. U.S. Patent EA201390851A1, 30 January 2014. pp. 2025–2037. [Google Scholar]
- Disi, J.O.; Zebelo, S.; Kloepper, J.W.; Fadamiro, H. Seed inoculation with beneficial rhizobacteria affects European corn borer (Lepidoptera: Pyralidae) oviposition on maize plants. Entomol. Sci. 2018, 21, 48–58. [Google Scholar] [CrossRef]
- Falqueto, S.A.; Pitaluga, B.F.; de Sousa, J.R.; Targanski, S.K.; Campos, M.G.; Mendes, T.A.O.; Silva, F.G.; Silva, D.H.S.; Soares, M.A. Bacillus spp. metabolites are effective in eradicating Aedes aegypti (Diptera: Culicidae) larvae with low toxicity to non-target species. J. Invertebr. Pathol. 2021, 179, 107525. [Google Scholar] [CrossRef]
- Rajendran, L.; Ramanathan, A.; Durairaj, C.; Samiyappan, R. Endophytic Bacillus subtilis enriched with chitin offer induced systemic resistance in cotton against aphid infestation. Arch. Phytopathol Plant Prot. 2011, 44, 1375–1389. [Google Scholar] [CrossRef]
- Hanafi, A.; Traore, M.; Schnitzler, W.H.; Woitke, M. Induced resistance of tomato to whiteflies and Phytium with the PGPR Bacillus subtilis in a soilless crop grown under greenhouse conditions. Acta Hortic. 2007, 747, 315–322. [Google Scholar] [CrossRef]
- Valenzuela-Soto, J.H.; Estrada-Hernández, M.G.; Ibarra-Laclette, E.; Délano-Frier, J.P. Inoculation of tomato plants (Solanumlycopersicum) with growth-promoting Bacillus subtilis retards whitefly Bemisia tabaci development. Planta 2010, 231, 397–410. [Google Scholar] [CrossRef] [PubMed]

| Treatments | β-1,3-Glucanase Activity (U g−1 Fresh Weight) | ||||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | 120 h | |
| Control | * 15.11 ± 0.99 jk,** | 14.83 ± 0.76 k | 16.34 ± 1.68 j | 13.52 ± 1.42 k | 14.70 ± 0.82 k |
| B. tabaci | 15.31 ± 0.81 jk | 23.69 ± 0.51g | 20.29 ± 0.43 h | 18.81 ± 0.75 i | 25.22 ± 0.51 f |
| B. velezensis | 24.73 ± 0.67 f | 33.66 ± 0.63 d | 35.79 ± 0.71 c | 40.34 ± 0.55 b | 40.20 ± 0.51 b |
| B. velezensis + B. tabaci | 30.76 ± 0.87 e | 29.71 ± 1.4 e | 35.86 ± 0.31 c | 40.17 ± 0.19 b | 42.21 ± 0.47 a |
| Treatments | Chitinase Activity (U g−1 Fresh Weight) | ||||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | 120 h | |
| Control | * 0.0054 ± 0.00015 j,** | 0.0055 ± 0.00025 j | 0.0053 ± 0.0002 j | 0.0057 ± 0.00011 j | 0.0055 ± 0.0001 j |
| B. tabaci | 0.0057 ± 0.00005 j | 0.0060 ± 0.00011 j | 0.0066 ± 0.0001 ij | 0.0070 ± 0.0001 i | 0.0078 ± 0.00025 h |
| B. velezensis | 0.0142 ± 0.0003 g | 0.0379 ± 0.00052 e | 0.0426 ± 0.0010 d | 0.0474 ± 0.0007 c | 0.0497 ± 0.0006 a |
| B. velezensis + B. tabaci | 0.01843 ± 0.0006 f | 0.0378 ± 0.0004 e | 0.0383 ± 0.0005 e | 0.0486 ± 0.00005 b | 0.0382 ± 0.0005 e |
| Treatments | Polyphenol Oxidase Activity (U g−1 Fresh Weight) | ||||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | 120 h | |
| Control | * 4.33 ± 0.02 n,** | 6.25 ± 0.09 l | 3.80 ± 0.23 o | 4.71 ± 0.16 n | 5.43 ± 0.4 m |
| B. tabaci | 9.73 ± 0.55 k | 14.17 ± 0.045 j | 19.20 ± 0.20 h | 18.62 ± 0.36 i | 14.15 ± 0.09 j |
| B. velezensis | 25.48 ± 0.22 c | 28.82 ± 0.3 a | 27.82 ± 0.43 b | 25.87 ± 0.08 c | 23.82 ± 0.29 d |
| B. velezensis + B. tabaci | 22.16 ± 0.50 f | 23.25 ± 0.8 e | 24.15 ± 0.19 d | 19.12 ± 0.14 h | 20.34 ± 0.17 g |
| Treatments | Peroxidase Activity (U g−1 Fresh Weight) | ||||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | 120 h | |
| Control | * 932.67 ± 7.63 m,** | 1004.66 ± 7.37 k | 987.00 ± 14.52 l | 985.66 ± 10.07 l | 1005.33 ± 9.07 k |
| B. tabaci | 1187.33 ± 9.71 j | 1235.00 ± 4.00 i | 1351.33 ± 22.01 h | 1375.67 ± 5.03 gh | 1363.67 ± 5.13 h |
| B. velezensis | 1535.33 ± 15.63 f | 1542.67 ± 11.59 f | 1785.00 ± 15.1 cd | 1838.66 ± 6.51 b | 1883.67 ± 12.58 a |
| B. velezensis + B. tabaci | 1387.67 ± 7.02 g | 1390.33 ± 8.02 g | 1782.33 ± 13.01 d | 1801.33 ± 9.50 c | 1764.00 ± 14.11 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, A.; Matar, S.; Abo-Zaid, G. Production of Bacillus velezensis Strain GB1 as a Biocontrol Agent and Its Impact on Bemisia tabaci by Inducing Systemic Resistance in a Squash Plant. Horticulturae 2022, 8, 511. https://doi.org/10.3390/horticulturae8060511
Soliman A, Matar S, Abo-Zaid G. Production of Bacillus velezensis Strain GB1 as a Biocontrol Agent and Its Impact on Bemisia tabaci by Inducing Systemic Resistance in a Squash Plant. Horticulturae. 2022; 8(6):511. https://doi.org/10.3390/horticulturae8060511
Chicago/Turabian StyleSoliman, Ahmed, Saleh Matar, and Gaber Abo-Zaid. 2022. "Production of Bacillus velezensis Strain GB1 as a Biocontrol Agent and Its Impact on Bemisia tabaci by Inducing Systemic Resistance in a Squash Plant" Horticulturae 8, no. 6: 511. https://doi.org/10.3390/horticulturae8060511
APA StyleSoliman, A., Matar, S., & Abo-Zaid, G. (2022). Production of Bacillus velezensis Strain GB1 as a Biocontrol Agent and Its Impact on Bemisia tabaci by Inducing Systemic Resistance in a Squash Plant. Horticulturae, 8(6), 511. https://doi.org/10.3390/horticulturae8060511







