Sustainable Management of Diseases in Horticulture: Conventional and New Options
Abstract
:1. Introduction
2. The Basis for a Sustainable Disease Control: The Preventive Measures
2.1. Suitability and Selection of the Site and Cultivars
2.2. Healthy Seeds and Plant Material
2.3. Optimal Soil Fertility and Agronomical Techniques
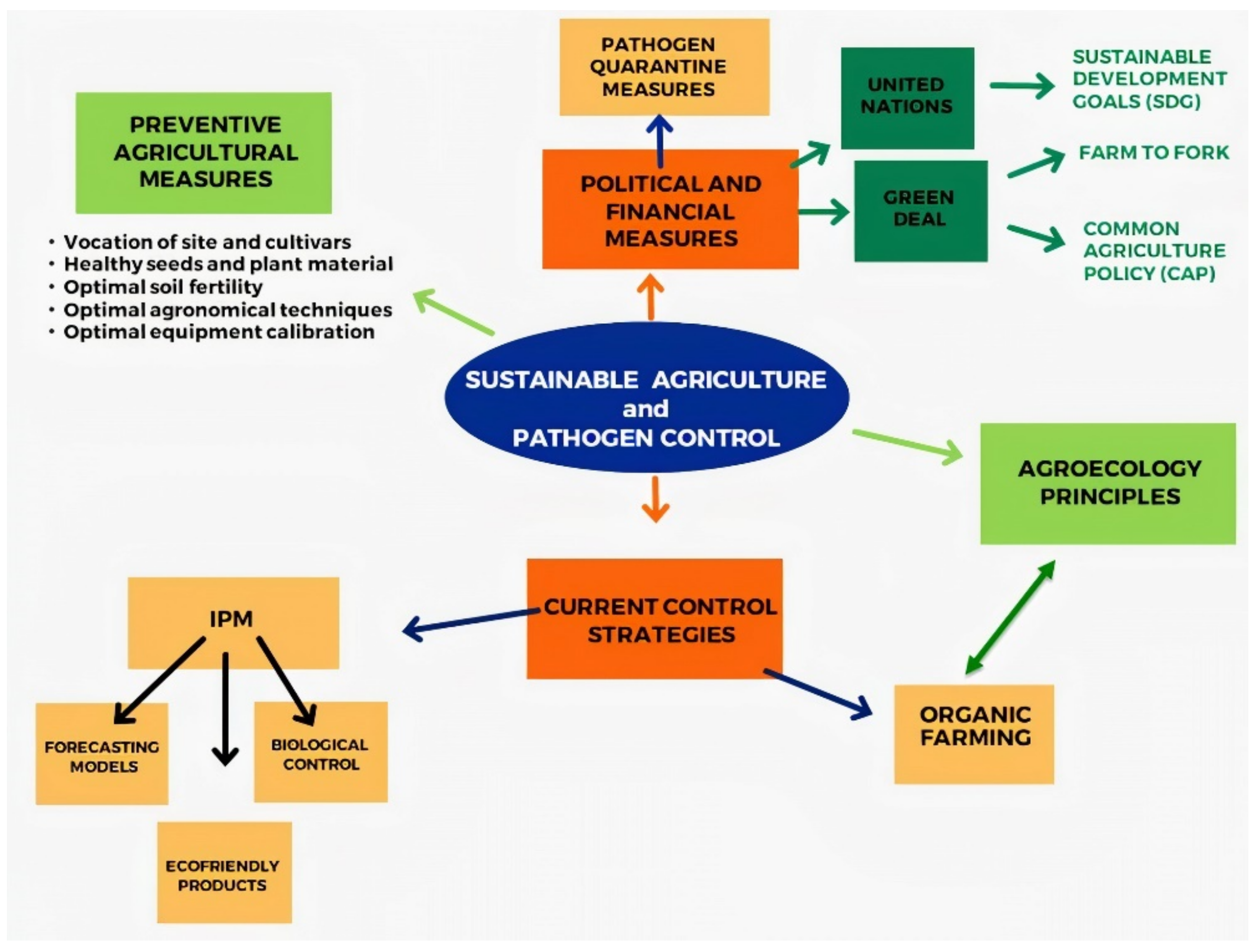
3. Sustainable Agriculture and Pathogen Control
3.1. The Basis for an Effective Sustainable Pathogen Control
3.2. Current Control Strategies
3.2.1. Disease-Forecasting Models
3.2.2. Biological Control
3.2.3. Natural Products and Compounds
4. Developing Control Strategies
4.1. Technical Support
4.1.1. Precise Timing Decision for Pathogen Control
4.1.2. Precision Farming and Pathogen Control
4.1.3. Nanotechnology
4.1.4. Endotherapy
4.2. New Bioproducts and Sustainability
4.2.1. Biocontrol Agents
4.2.2. Natural Products
4.2.3. Nutrition Management
4.2.4. Systemic Resistance Inducers
4.2.5. Gene Silencing
5. Concluding Remarks and Perspectives
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siebrecht, N. Sustainable agriculture and its implementation gap-overcoming obstacles to implementation. Sustainability 2021, 12, 3853. [Google Scholar] [CrossRef]
- Carlisle, L.; Montenegro de Wit, M.; Delonge, M.S.; Iles, A.; Calo, A.; Getz, C.; Ory, J.; Munden-Dixon, K.; Galt, R.; Melone, B.; et al. Transitioning to sustainable agriculture requires growing and sustaining an ecologically skilled workforce. Front. Sustain. Food Syst. 2019, 3, 96. [Google Scholar] [CrossRef]
- McNeill, D. The contested discourse of sustainable agriculture. Glob. Policy 2019, 10 (Suppl. 1), 16–27. [Google Scholar] [CrossRef] [Green Version]
- Hertoge, K. Mals/Malles Venosta Referendum. 2014. Available online: http://www.marcozullo.it/wp-content/uploads/Malles-Venosta-Referendum.pdf (accessed on 22 May 2022).
- Damalas, C.A.; Koutroubas, S.D. Current status and recent development in biopesticides use. Agriculture 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Fenibo, E.O.; Ijoma, G.N.; Matambo, T. Biopesticides in sustainable agriculture: A critical sustainable development driver governed by green chemistry principles. Front. Sust. Food Syst. 2021, 5, 619058. [Google Scholar] [CrossRef]
- Garrett, K.A. Climate change and plant disease risk. In Global Climate Change and Extreme Weather Events: Understanding the Contributions to Infectious Disease Emergence; Relman, D.A., Hamburg, M.A., Choffnes, E.R., Mack, A., Eds.; National Academies Press: Washington, DC, USA, 2008; pp. 143–155. [Google Scholar]
- Juroszek, P.; Von Tiedemann, A. Potential strategies and future requirements for plant disease management under a changing climate. Plant Pathol. 2011, 60, 100–112. [Google Scholar] [CrossRef]
- Soussana, J.-F.; Graux, A.-I.; Tubiello, F.N. Improving the use of modelling for projections of climate change impacts on crops and pastures. J. Exp. Bot. 2010, 61, 2217–2228. [Google Scholar] [CrossRef] [Green Version]
- Zhan, J.; Thrall, P.H.; Burdon, J.J. Achieving sustainable plant disease management through evolutionary principles. Trends Plant Sci. 2014, 19, 570–575. [Google Scholar] [CrossRef]
- Leung, K.; Ras, E.; Ferguson, K.B.; Ariens, S.; Babendreier, D.; Bijma, P.; Bourtzis, K.; Brodeur, J.; Bruins, M.A.; Centurion, A.; et al. Next-generation biological control: The need for integrating genetics and genomics. Biol. Rev. 2020, 95, 1838–1854. [Google Scholar] [CrossRef]
- Fenu, G.; Malloci, F.M. Forecasting plant and crop disease: An explorative study on current algorithms. Big Data Cogn. Comput. 2021, 5, 2. [Google Scholar] [CrossRef]
- Fontana, D.C.; De Paula, S.; Torres, A.G.; Moura de Souza, V.H.; Pascholati, S.F.; Schmidt, D.; Dourado Neto, D. Endophytic fungi: Biological control and induced resistance to phytopathogens and abiotic stresses. Pathogens 2021, 10, 570. [Google Scholar] [CrossRef]
- He, D.-C.; He, M.-H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological control of plant diseases: An evolutionary and eco-economics consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef]
- Ferrante, P.; Scortichini, M. Frost promotes the pathogenicity of Pseudomonas syringae pv. actinidiae in Actinidia chinensis and A. deliciosa plants. Plant Pathol. 2014, 63, 12–19. [Google Scholar]
- Nuttall, J.G.; Perry, E.M.; Delahunty, A.J.; O’Leary, G.J.; Barlow, K.M.; Wallace, A.J. Frost response in wheat and early detection using proximal sensors. J. Agron. Crop Sci. 2019, 205, 220–234. [Google Scholar] [CrossRef] [Green Version]
- Barlow, K.M.; Christy, B.P.; O’Leary, G.J.; Riffkin, P.A.; Nuttall, J.G. Simulating the impact of heat and frost events on wheat crop production: A review. Field Crop Res. 2015, 171, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Haworth, M.; Marino, G.; Brunetti, C.; Killi, D.; De Carlo, A.; Centritto, M. The impact of the heat stress and water deficit on the photosynthetic and stomatal physiology of olive (Olea europea L.)—A case study of the 2017 heat wave. Plants 2018, 7, 76. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, C.; Bacchetta, L.; Bellincontro, A.; Cristofori, V. Advances in cultivar choice, hazelnut orchard management, and nut storage to enhance product quality and safety: An overview. Sci. Food Agric. 2021, 101, 27–43. [Google Scholar] [CrossRef]
- Macholdt, J.; Honermeier, B. Importance of variety choice: Adapting to climate change in organic and conventional farming system in Germany. Outlook Agric. 2017, 46, 178–184. [Google Scholar] [CrossRef]
- Hulme, P.E. Unwelcome exchange: International trade as direct and indirect driver of biological invasions worldwide. One Earth 2021, 4, 666–679. [Google Scholar] [CrossRef]
- Garbelotto, M.; Pautasso, M. Impacts of exotic forest pathogens on Mediterranean ecosystems: Four case studies. Eur. J. Plant Pathol. 2012, 133, 101–116. [Google Scholar] [CrossRef]
- European Commission. Proposal for a regulation of the european parliament and of the council establishing rules on support for strategic plans to be drawn up by Member States under the Common agricultural policy (CAP Strategic Plans) and financed by the European Agricultural Guarantee Fund (EAGF) and by the European Agricultural Fund for Rural Development (EAFRD) and repealing Regulation (EU); No 1305/2013 of the European Parliament and of the Council and Regulation (EU) No 1307/2013 of the European Parliament and of the Council; COM/2018/392 final—2018/0216 (COD). 2018. [Google Scholar]
- Wezel, A.; Casagrande, M.; Celette, F.; Vian, J.-F.; Ferrer, A.; Peigné, J. Agroecological practices for sustainable agriculture. A review. Agron. Sustain. Dev. 2014, 34, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Peeters, A.; Ambhul, E.; Barberi, P.; Migliorini, P.; Ostermann, O.; Goris, M.; Donham, J.; Wezel, A.; Batello, C. Integrating Agroecology into European Agricultural Policies. Position Paper and Recommendations to the European Commission on Eco-Schemes. 2021. Available online: https://www.agroecology-europe.org/wp-content/uploads/2021/07/AEEU_Positionpaper_Ecoschemes_FINAL_english.pdf (accessed on 22 May 2022).
- Larkin, R.P.; Lynch, R.P. Use and effects of different Brassica and other rotation crops on soilborne diseases and yield of potato. Horticulturae 2018, 4, 37. [Google Scholar] [CrossRef] [Green Version]
- Muscolo, A.; Sidari, M.; Nardi, S. Humic substance: Relationships between structure and activity. Deeper information suggests univocal findings. J. Geochem. Explor. 2013, 129, 57–63. [Google Scholar] [CrossRef]
- Rillig, M.C.; Sosa-Hernandez, M.A.; Roy, J.; Aguilar-Trigueros, C.A.; Valyi, K.; Lehmann, A. Towards an integrated mycorrhizal technology: Harnessing mycorrhiza for sustainable intensification in agriculture. Front. Plant Sci. 2016, 7, 1625. [Google Scholar] [CrossRef] [Green Version]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslikova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanism of action, and roadmap to commercialization of biostimulant for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [Green Version]
- Semida, W.M.; Beheiry, H.R.; Sétamou, M.; Simpson, C.R.; Abd El-Mageed, T.A.; Rady, M.M.; Nelson, S.D. Biochar implications for sustainable agriculture and environment: A review. S. Afr. J. Bot. 2019, 127, 333–347. [Google Scholar] [CrossRef]
- Seenivasagan, R.; Babalola, O.O. Utilization of microbial consortia as biofertilizers and biopesticides for the production of feasible agricultural product. Biology 2021, 10, 1111. [Google Scholar] [CrossRef]
- Elnahal, A.S.M.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
- Fu, L.; Penton, C.R.; Ruan, Y.; Shen, Z.; Xue, C.; Li, R.; Shen, Q. Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol. Biochem. 2017, 104, 39–48. [Google Scholar] [CrossRef]
- Suarez-Estrella, F.; Arcos-Nievas, M.A.; Lopez, M.J.; Vargas-Garcia, M.C.; Moreno, J. Biological control of plant pathogens by microorganisms isolated from agro-industrial composts. Biol. Control 2013, 67, 509–515. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Lu, C.; Liao, Q.; Gudda, F.O.; Ling, W. Antibiotics in animal manure and manure-based fertilizers: Occurrence and ecological risk assessment. Chemosphere 2020, 255, 127006. [Google Scholar] [CrossRef] [PubMed]
- Dordas, C. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron. Sustain. Dev. 2008, 28, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Michael, C.; Gil, E.; Gallart, M.; Stavrinides, M.C. Influence of spray technology and application rate on leaf deposit and ground losses in mountain viticulture. Agriculture 2020, 10, 615. [Google Scholar] [CrossRef]
- Gamliel, A. Application of soil solarization in the open field. In Soil Solarization THEORY and Practice; Gamliel, A., Katan, J., Eds.; American Phytopathological Society: St. Paul, MN, USA, 2017; pp. 175–180. [Google Scholar]
- Van Bruggen, A.H.C.; Gamliel, A.; FinckH, M.R. Plant disease management in organic farming systems. Pest Sci. Manag. 2016, 72, 30–44. [Google Scholar] [CrossRef]
- Hansen, Z.R.; Everts, K.L.; Fry, W.E.; Gevens, A.J.; Grunwald, N.J.; Gugino, B.K.; Johnson, D.A.; Johnson, S.B.; Knaus, B.J.; McGrath, M.T.; et al. Genetic variation within clonal lineages of Phytophthora infestans revealed through genotyping-by-sequencing, and implications for late blight epidemiology. PLoS ONE 2016, 11, e065690. [Google Scholar] [CrossRef] [Green Version]
- Cohen, Y.; Ben Naim, Y.; Falach, L.; Rubin, A.E. Epidemiology of basil downy mildew. Phytopathology 2017, 107, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Newberry, A.A.; Babu, B.; Roberts, P.D.; Dufault, N.S.; Goss, E.M.; Jones, J.B.; Paret, M.L. Molecular epidemiology of Pseudomonas syringae pv. syringae causing bacterial leaf spot of watermelon and squash in Florida. Plant Dis. 2018, 102, 511–518. [Google Scholar]
- Pegg, K.G.; Coates, L.M.; O’Neill, W.T.; Turner, D.W. The epidemiology of Fusarium wilt of banana. Front. Plant Sci. 2019, 10, 1395. [Google Scholar] [CrossRef] [Green Version]
- Pruvost, O.; Boyer, K.; Ravigné, V.; Richard, D.; Vernière, C. Deciphering how plant pathogenic bacteria disperse and meet: Molecular epidemiology of Xanthomonas citri pv. citri at a microgeographic scales in a tropical area of Asiatic citrus canker endemicity. Evol. Appl. 2019, 12, 1523–1538. [Google Scholar]
- Donati, I.; Cellini, A.; Sangiorgio, D.; Vanneste, J.L.; Scortichini, M.; Balestra, G.M.; Spinelli, F. Pseudomonas syringae pv. actinidiae: Ecology, infection dynamics and disease epidemiology. Environ. Microbiol. 2020, 80, 81–102. [Google Scholar]
- Ostos, E.; Garcia-Lopez, M.T.; Porras, R.; Lopez-Escudero, F.J.; Trapero-Casas, A.; Michaelides, T.J.; Moral, J. Effect of cultivar resistance and soil management on spatial-temporal development of Verticillium wilt of olive: A long-term study. Front. Plant Sci. 2020, 11, 584496. [Google Scholar] [CrossRef]
- Caffi, T.; Rossi, V. Fungicide models are components of multiple modeling approaches for decision-making in crop protection. Phytopathol. Medit. 2018, 57, 153–169. [Google Scholar]
- Kim, K.-H.; Jung, I. Development of a daily epidemiological model for rice blast tailored for seasonal disease early warning in South Korea. Plant Pathol. J. 2020, 36, 406–417. [Google Scholar] [CrossRef]
- He, X.; Fu, P.; Aker, W.G.; Hwang, H.-M. Toxicity of engineered nanomaterials mediated by nano–bio–eco interactions. J. Environ. Sci. Health Part C 2018, 36, 21–42. [Google Scholar] [CrossRef]
- Gusberti, M.; Klemm, U.; Meier, M.S.; Maurhofer, M.; Hunger-Glaser, I. Fire blight control: The struggle goes on. A comparison of different fire blight control methods in Switzerland with respect to biosafety, efficacy and durability. Int. J. Environ. Res. Public Health 2015, 12, 11422–11447. [Google Scholar] [CrossRef] [Green Version]
- Moser, R.; Pertot, I.; Elad, Y.; Raffaelli, R. Farmers’ attitudes toward the use of biocontrol agents in IPM strawberry production in three countries. Biol. Control 2008, 47, 125–132. [Google Scholar] [CrossRef]
- Barzmann, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P.; et al. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Jorgensen, L.N.; Hovmoller, M.S.; Hansen, J.G.; Lassen, P.; Clark, B.; Bayles, R.; Rodemann, B.; Flath, K.; Jahn, M.; Goral, T.; et al. IPM strategies and their dilemmas including an introduction to www.eurowheat.org. J. Integr. Agric. 2014, 13, 265–281. [Google Scholar] [CrossRef]
- Berlin, A.; Nordström Kälström, H.; Lindgren, A.; Olson, A. Scientific evidence for sustainable plant disease protection strategies for the main arable crops in Sweden. A systematic map protocol. Environ. Evid. 2018, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Holb, I.J.; Abpnyi, F.; Bourma, J.; Heijne, B. On-farm and on-station evaluations of three orchard management approaches against apple scab and apple powdery mildew. Crop Prot. 2017, 97, 109–118. [Google Scholar] [CrossRef]
- Shipp, L.; Elliott, D.; Gillespie, D.; Brodeur, J. From chemical to biological control in Canadian greenhouse crops. In Biological Control: A Global Perspective; Vincent, C., Goettel, M.S., Lazarovitis, C., Eds.; CABI: Wallingford, UK, 2007; pp. 118–127. [Google Scholar]
- Jacobsen, B.J.; Zidack, N.K.; Larson, B.J. The role of Bacillus-based biological control agents in integrated pest management systems: Plant diseases. Phytopathology 2004, 94, 1272–1275. [Google Scholar] [CrossRef] [Green Version]
- Galletti, S.; Paris, R.; Cianchetta, S. Selected isolates of Trichoderma gamsii induce different pathways of systemic resistance in maize upon Fusarium verticillioides challenge. Microbiol. Res. 2020, 233, 126406. [Google Scholar] [CrossRef]
- Muneret, L.; Mitchell, M.; Seufert, V.; Aviron, S.; Djoud, E.A.; Pétillon, J.; Plantegenest, M.; Thiéry, D.; Rusch, A. Evidence that organic farming promotes pest control. Nat. Sustain. 2018, 1, 361–368. [Google Scholar] [CrossRef]
- Baker, B.P.; Green, T.A.; Cooley, D.; Futrell, S.; Garling, L.; Gershuny, G.; Moyer, J.; Rajotte, E.G.; Seaman, A.J.; Young, S.L. Organic Agriculture and Integrated Pest Management: A Synergistic Partnership to Improve Sustainable Agriculture and Food Systems. 2015. Available online: https://organicipmwg.files.wordpress.com/2015/07/white-paper.pdf (accessed on 22 May 2022).
- Rossi, V.; Salinari, F.; Poni, S.; Caffi, T.; Bettati, T. Addressing the implementation problem in decision support systems: The example of vite.net®. Comput. Electron. Agric. 2014, 100, 88–99. [Google Scholar] [CrossRef]
- Minchinton, E.J.; Auer, D.P.F.; Thomson, F.M.; Trapnell, L.; Petkowski, J.E.; Galea, V.J.; Faggian, R.; Kita, N.; Murdoch, C.; Kennedy, R. Evaluation of the efficacy and economics of irrigation management, plant resistance and BrassicaspotTM models for management of white blister on Brassica crops. Austalasian Plant Pathol. 2013, 42, 169–178. [Google Scholar] [CrossRef]
- Pavan, W.; Fraisse, C.W.; Peres, N.A. Development of a web-based disease forecasting system for strawberry. Comput. Electron. Agric. 2011, 75, 169–175. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sund, I.; Eilenberg, J. Why has the authorization of microbial biocontrol agents been slower in the EU than in comparable jurisdictions? Pest Manag. Sci. 2021, 77, 2170–2178. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Hinz, H.; Mulema, J.; Weyl, P.; Ryan, M.J. Biological control and the Nagoya Protocol on access and benefit sharing-a case of effective due diligence. Biocontrol Sci. Technol. 2018, 28, 914–926. [Google Scholar] [CrossRef]
- Pliego, C.; Ramos, C.; De Vicente, A.; Cazorla, F.M. Screening for candidate bacterial biocontrol agents against soilborne fungal pathogens. Plant Soil 2011, 340, 505–520. [Google Scholar] [CrossRef] [Green Version]
- Ajouz, S.; Nicot, P.C.; Bardin, M. Adaptation to pyrrolnitrin of Botrytis cinerea and cost of resistance. Plant Pathol. 2010, 59, 556–566. [Google Scholar] [CrossRef]
- Wei, W.; Xu, Y.; Li, S.; Zhu, L.; Song, J. Developing suppressive soil for root disease of soybean with continuous long-term cropping of soybean in black soils of Northeast China. Acta Agric. Scand. B Soil Plant Sci. 2015, 65, 279–285. [Google Scholar] [CrossRef]
- Milgroom, M.G.; Cortesi, P. Biological control of chestnut blight with hypovirulence: A critical analysis. Annu. Rev. Phytopathol. 2004, 42, 311–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicot, P.C.; Bardin, M.; Alabouvette, C.; Köhl, J.; Ruocco, M. Potential of biological control based on published research. 1. Protection against plant pathogens of selected crops. In Classical and Augmentative Biological Control against Diseases and Pests: Critical Status Analysis and Review of Factors Influencing Their Success; Nicot, P.C., Ed.; IOBC/WPRS, 2011; pp. 1–11. [Google Scholar]
- Moore, L.W.; Warren, G. Agrobacterium radiobacter strain K84 and biological control of crown gall. Annu. Rev. Phytopathol. 1979, 17, 163–179. [Google Scholar] [CrossRef]
- Köhl, J.; Scheer, C.; Holb, I.J.; Masny, S.; Molhoek, W.M.L. Toward an integrated use of biological control of Cladosporium cladosporioides H39 in apple scab (Venturia inaequalis) management. Plant Dis. 2015, 99, 535–543. [Google Scholar] [CrossRef] [Green Version]
- Haddoudi, I.; Cabrefiga, J.; Mora, I.; Mhadhbi, H.; Montesinos, E.; Mrabet, M. Biological control of fusarium wilt caused by Fusarium equiseti in Vicia faba with broad spectrum antifungal plant-associated Bacillus spp. Biol. Control 2021, 160, 104671. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef] [Green Version]
- Sood, M.; Kapoor, D.; Sheteiwy, M.; Ramakhrisnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell Infect. Microbiol. 2020, 10, 604293. [Google Scholar] [CrossRef]
- Wisniewski, M.; Droby, S.; Norelli, J.; Liu, J.; Schena, L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 2016, 122, 3–10. [Google Scholar] [CrossRef]
- Höfte, M. The use of Pseudomonas spp. as bacterial biocontrol agents to control plant diseases. In Microbial Bioprotectants for Plant Disease Management; Köhl, J., Ravensberg, V., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2021; p. 74. [Google Scholar]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapon for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 3. [Google Scholar] [CrossRef] [Green Version]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019, 35, 154. [Google Scholar] [CrossRef] [Green Version]
- Kunz, S.; Schmitt, A.; Haug, P. Field testing of strategies for fire blight control in organic fruit growing. Acta Hortic. 2011, 896, 431–436. [Google Scholar] [CrossRef]
- Iqbal, M.; Jamshaid, M.; Zahid, M.A.; Andreasson, E.; Vetukuri, R.R.; Stenberg, J. Biological control of strawberry crown rot, root rot and grey mould by the beneficial fungus Aureubasidium Pullulans. BioControl 2021, 66, 535–545. [Google Scholar] [CrossRef]
- Guetsky, R.; Shtienberg, D.; Elad, Y.; Dinoor, A. Combining biocontrol agents to reduce the variability of biological control. Phytopathology 2001, 91, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Raupach, G.S.; Kloepper, J.W. Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology 1998, 88, 1158–1164. [Google Scholar] [CrossRef] [Green Version]
- Leibinger, W.; Breuker, B.; Hahn, M.; Mendgen, K. Control of postharvest pathogens and colonization of the apple surface by antagonistic microorganisms in the field. Phytopathology 1997, 87, 1103–1110. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.-M.; Jeffries, P.; Pautasso, M.; Jeger, M.J. Combined use of biocontrol agents to manage plant disease in theory and practice. Phytopathology 2011, 101, 1024–1031. [Google Scholar] [CrossRef] [Green Version]
- Sarrocco, S.; Valenti, F.; Manfredini, S.; Esteban, P.; Bernardi, R.; Puntoni, G.; Baroncelli, R.; Haidukowski, M.; Moretti, A.; Vannacci, G. Is exploitation competition involved in a multitrophic strategy for the biocontrol of Fusarium head blight? Phytopathology 2019, 109, 560–570. [Google Scholar] [CrossRef] [Green Version]
- Roselló, G.; Francés, J.; Daranas, N.; Montesinos, E.; Bonaterra, A. Control of fire blight of pear trees with mixed inocula of two Lactobacillus plantarum strains and lactic acid. J. Plant Pathol. 2017, 99, 111–120. [Google Scholar]
- Slusarenko, A.J.; Patel, A.; Portz, D. Control of plant diseases by natural products: Allicin from garlic as a case study. Eur. J. Plant Pathol. 2008, 121, 313–322. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Datta, S.; Dhangial, D.S.; Singh, J. Plant disease management by bioactive natural products. In Natural Bioactive Products in Sustainable Agriculture; Singh, J.l., Yadav, A.l., Eds.; Springer: Singapore, 2022; pp. 15–19. [Google Scholar]
- Yan, Y.; Liu, Q.; Jacobsen, S.E.; Tang, Y. The impact and prospect of natural product discovery in agriculture. EMBO Rep. 2018, 19, e46824. [Google Scholar] [CrossRef]
- Sundin, G.W.; Wang, N. Antibiotic resistance in plant pathogenic bacteria. Annu. Rev. Phytopathol. 2018, 56, 161–180. [Google Scholar] [CrossRef]
- Taylor, P.; Reeder, R. Antibiotics use on crops in low and middle-income countries based on recommendations made by agricultural advisors. CABI Agric. Biosci. 2020, 1, 1. [Google Scholar] [CrossRef]
- Wei, Z.-M.; Beer, S. Harpin from Erwinia amylovora induces plant resistance. Acta Hortic. 1996, 411, 223–226. [Google Scholar] [CrossRef]
- De Capdeville, G.; Beer, S.V.; Wilson, C.L.; Aist, J.R. Alternative disease control agents induce resistance to blue mold in harvested “Red Delicious” apple fruit. Phytopathology 2002, 92, 900–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daayf, F.; Schmitt, A.; Bèlanger, R.R. The effects of plant extracts of Reynouria sachalinensis on powdery mildew development and leaf physiology of long English cucumber. Plant Dis. 1995, 79, 577–580. [Google Scholar] [CrossRef]
- Trottin-Caudal, Y.; Fournier, C.; Leyre, J.-M.; Decognet, V.; Nicot, P.C.; Bardin, M. Efficiency of Plant Extract from Reynoutria Sachalinensis (Milsana) to Control Powdery Mildew on Tomato (Oidium Neolycopersici); ffhal-02764311f; Colloque International: Avignon, France, 2003. [Google Scholar]
- Margaritopoulos, T.; Toufexi, E.; Kizis, D.; Balayiannis, G.; Anagnostopoulos, C.; Theocharis, A.; Rempelos, L.; Troyanos, Y.; Leifert, C.; Markellou, E. Reynoutria sachalinensis elicits SA-dependent defense responses in courgetti genotypes against powdery mildew caused by Podosphaera xanthii. Sci. Rep. 2020, 10, 3354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Ghaouth, A.; Arul, J.; Asselin, A.; Benhamou, N. Antifungal activity of chitosan on post-harvest pathogens: Induction of morphological and cytological alterations in Rhizopus stolonifer. Mycol Res. 1992, 96, 769–779. [Google Scholar] [CrossRef]
- Gould, F.; Brown, Z.S.; Kuzma, J. Wicked evolution: Can we address the sociobiological dilemma of pesticide resistance? Science 2018, 360, 728–732. [Google Scholar] [CrossRef] [Green Version]
- Newlands, N.K. Model-based forecasting of agricultural crop disease risk at the regional scale, integrating airborne inoculum, environmental, and satellite-based monitoring data. Front. Environ. Sci. 2018, 6, 63. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Pu, R.; Yuan, L.; Huang, W.; Nie, C.; Yang, G. Integrating remotely sensed and meteorological observations to forecast wheat powdery mildew at a regional scale. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 4328–4339. [Google Scholar] [CrossRef]
- Gold, K.M.; Townsend, P.A.; Chlus, A.; Hermann, I.; Couture, J.J.; Larson, E.R.; Gevens, A.J. Hyperspectral measurements enable pre-symptomatic detection and differentiation of contrasting physiological effects of late blight and early blight in potato. Remote Sens. 2020, 12, 286. [Google Scholar] [CrossRef] [Green Version]
- Lindblom, J.; Lundstrom, C.; Ljung, J.; Jonsson, A. Promoting sustainable intensification in precision agriculture: Review of decision support systems development and strategies. Precis. Agric. 2017, 18, 309–331. [Google Scholar] [CrossRef] [Green Version]
- Newbery, F.; Qi, A.; Fitt, B.D.L. Modelling impacts of climate change on arable crop diseases: Progress, challenge and applications. Curr. Opin. Plant Biol. 2016, 32, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.P.; Short, N.M., Jr.; Sill, J.; Lakshman, D.K.; Hu, X.; Buser, M. Precision agriculture and geospatial techniques for sustainable disease control. Ind. Phytopathol. 2021, 74, 287–305. [Google Scholar] [CrossRef]
- Simko, I.; Jimenez-Berni, J.A.; Sirault, X.R.R. Phenomic approach and tool for phytopathologists. Phytopathology 2017, 107, 6–17. [Google Scholar] [CrossRef] [Green Version]
- Khattab, A.; Habib, S.E.D.; Ismail, H.; Zayan, S.; Fahmy, Y.; Khairy, M.M. An IoT-based cognitive monitoring system for early plant disease forecast. Comput. Electron. Agric. 2019, 166, 105028. [Google Scholar] [CrossRef]
- Lu, X.; Lee, W.; Minzan, L.; Ehsani, R.; Mishra, A.; Yang, C.; Mangan, R. Feasibility study on huanglongbing (citrus greening) detection based on worldview-2 satellite imagery. Biosyst. Eng. 2015, 132, 28–38. [Google Scholar]
- Yang, C. Remote sensing and precision agriculture technologies for crop disease detection and management with a practical application example. Engineering 2020, 6, 528–532. [Google Scholar] [CrossRef]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future overlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Kah, M.; Hofmann, T. Nanopesticide research: Current trend and future priority. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, L.M.; Pourzahedi, L.; Laughton, S.; Gao, X.; Zimmermann, J.B.; Theis, T.L.; Westerhoff, P.; Lowry, G.V. Guiding the design space for nanotechnology to advance sustainable crop production. Nature Nanotechnol. 2020, 15, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Mazzaglia, A.; Balestra, G.M. Sustainable control strategies for plant protection and food packaging sectors by natural substances and novel nanotechnologies approaches. J. Sci. Food Agric. 2019, 99, 986–1000. [Google Scholar] [CrossRef]
- De Oliveira, J.L. Nano-biopesticides: Present concepts and future perspectives in integrated pest management. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture. A Smart Delivery System for Crop Improvement; Jogaiah, S., Singh, H.B., Fraceto, L.F., De Lima, R., Eds.; Elsevier Science Publishing: Amsterdam, The Netherlands, 2021; pp. 1–27. [Google Scholar]
- Sanchez-Hernandez, E.; Langa-Lomba, N.; Gonzalez-Garcia, V.; Casanova-Gascon, J.; Martin-Gil, J.; Santiago-Aliste, A.; Torres-Sanchez, S.; Martin-Ramos, P. Lignin-chitosan nanocarriers for the delivery of bioactive natural products against wood-decay phytopathogens. Agronomy 2022, 12, 461. [Google Scholar] [CrossRef]
- Kalia, A.; Kaur, H. Nano-biofertilizers: Harnessing dual benefts of nano-nutrient and bio-fertilizers for enhanced nutrient use efficiency and sustainable productivity. In Nanoscience for Sustainable Agriculture; Pudake, R., Chauhan, N., Kole, C., Eds.; Springer: New York, NY, USA, 2019; pp. 51–73. [Google Scholar]
- Borgatta, J.; Ma, C.; Hudson-Smith, N.; Elmer, W.; Plaza Peréz, C.D.; De La Torre-Roche, R.; Zuverza-Mena, N.; Haynes, C.L.; White, J.C.; Hamers, R.J. Copper based nanomaterials suppress root fungal disease in watermelon (Citrullus lanatus): Role of particle morphology, composition and dissolution behaviour. ACS Sustain. Chem. Eng. 2018, 6, 11. [Google Scholar] [CrossRef]
- Elmer, W.H.; White, J.C. The use of metallic oxide nanoparticles to enhance growth of tomatoes and eggplants in diseased infected soil or soilless medium. Environ. Sci. Nano 2016, 3, 1072–1079. [Google Scholar] [CrossRef]
- Song, J.J.; Soytong, K.; Kanokmedhakul, S.; Poeaim, S. Natural products of nanoparticles constructed from Chaetomium spp. to control rice blast disease caused by Magnaporthe oryzae. Int. J. Agron. Biol. 2020, 23, 1013–1020. [Google Scholar]
- Sharma, J.; Singh, V.K.; Kumar, A.; Shankarayan, R.; Mallubhotla, S. Role of silver nanoparticles in treatment of plant diseases. In Microbial Biotechnology; Patra, J., Das, G., Shin, H.S., Eds.; Springer: Singapore, 2018; pp. 435–454. [Google Scholar]
- Kookana, R.S.; Boxall, A.B.A.; Reeves, P.T.; Ashauer, R.; Beulke, S.; Chaudry, K.; Cornelis, G.; Fernandes, T.F.; Gan, J.; Kah, M.; et al. Nanopesticides: Guiding principles for regulatory evaluation of environmental risk. J. Agric. Food Chem. 2014, 62, 4227–4240. [Google Scholar] [CrossRef] [Green Version]
- Freepons, D. Chitosan, does it have a place in agriculture? Proc. Plant Growth Regul. Soc. Am. 1991, 10, 11–19. [Google Scholar]
- Montesinos, E.; Badosa, E.; Cabrefiga, J.; Planas, M.; Feliu, L.; Bardají, E. Antimicrobial peptides for plant disease control. From discovery to application. In Small Wonders: Peptides for Disease Control; Rajasekaran, K., Cary, J., Jaynes, J., Montesinos, E., Eds.; Blackwell: Oxford, UK, 2012; pp. 235–261. [Google Scholar]
- Montesinos, L.; Gascon, B.; Ruz, L.; Badosa, E.; Planas, M.; Feliu, L.; Montesinos, E. A bifunctional synthetic peptide with antimicrobial and plant elicitation properties that protect tomato plants from bacterial and fungal infections. Front. Plant Sci. 2021, 12, 756357. [Google Scholar] [CrossRef]
- Berger, C.; Laurent, F. Trunk injection of plant protection products to protect trees from pests and pathogens. Crop Prot. 2019, 124, 104831. [Google Scholar] [CrossRef]
- Akinsamni, O.A.; Drenth, A. Phosphite and metalaxyl rejuvenate macadamia trees in decline caused by Phytophtora cinnamomi. Crop Prot. 2013, 53, 29–36. [Google Scholar] [CrossRef]
- Archer, L.; Albrecht, U.; Crane, J. Trunk injection to deliver crop protection materials: An overview of basic principles and practical considerations. University of Florida, Horticultural Science Department, IFAS Extension. EDIS 2021, 221, HFS1426. [Google Scholar]
- Montecchio, L. A Venturi effect can help cure our trees. J. Vis. Exp. 2013, 80, e51199. [Google Scholar] [CrossRef] [Green Version]
- Acimovic, S.G.; Cregg, B.M.; Sundin, G.; Wise, J.C. Comparison of drill- and needle-based tree injection technologies in healing of trunk injection ports on apple trees. Urban For. Urban Green 2016, 19, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Scortichini, M.; Loreti, S.; Pucci, N.; Scala, V.; Tatulli, G.; Verweire, D.; Oehl, M.; Widmer, U.; Massana Codina, J.; Hertl, P.; et al. Progress towards a sustainable control of Xylella fastidiosa subsp. pauca in olive groves of Salento (Apulia, Italy). Pathogens 2021, 10, 668. [Google Scholar] [CrossRef]
- Girelli, C.R.; Hussain, M.; Verweire, D.; Oehl, M.; Massana-Codina, J.; Avendano, M.S.; Migoni, D.; Scortichini, M.; Fanizzi, F.P. Agro-active endo-therapy treated Xylella fastidiosa subsp. pauca-infected olive trees assessed by the first 1H-NMR-based metabolomic study. Sci. Rep. 2022, 12, 5973. [Google Scholar]
- Del Frari, G.; Costa, J.; Oliveira, H.; Boavida Ferreira, R. Endotherapy of infected grapevine cuttings for the control of Phaeomoniella chlamydospora and Phaeoacremonium minimum. Phytopathol. Medit. 2018, 57, 439–445. [Google Scholar]
- Barratt, B.I.P.; Moran, V.C.; Bigler, F.; Van Lenteren, J.C. The status of biological control and recommendations for improving uptake for the future. BioControl 2017, 63, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Lamichhane, J.R.; Bischoff-Schaefer, M.; Bluemel, S.; Dachbrodt-Saaydeh, S.; Dreux, L.; Jansen, J.-P.; Kiss, J.; Kohl, J.; Kudsk, P.; Malausa, T.; et al. Identifying obstacles and ranking common biological research priorities for Europe to manage most economically important pests in arable, vegetable and perennial crops. Pest Manag. Sci. 2017, 73, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Bonaterra, A.; Badosa, E.; Cabriefiga, J.; Francés, J.; Montesinos, E. Prospects and limitations of microbial pesticides for control of bacterial and fungal pomefruit tree diseases. Trees 2012, 26, 215–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velivelli, S.L.S.; De Vos, P.; Kromann, P.; Declerck, S.; Prestwich, B.D. Biological control agents: From field to market, problems, and challenges. Trends Biotechnol. 2014, 32, 493–496. [Google Scholar] [CrossRef]
- Raymaekers, K.; Ponet, L.; Holtappels, D.; Berckmans, B.; Cammue, B.P.A. Screening for new biocontrol agents applicable in plant disease management—A review. Biol. Cont. 2020, 144, 104240. [Google Scholar] [CrossRef]
- Stefani, E.; Obradovic, A.; Gasic, K.; Altin, I.; Nagy, I.K.; Kovacs, T. Bacteriophage-mediated control of phytopathogenic xanthomonads: A promising green solution for the future. Microorganisms 2021, 9, 1056. [Google Scholar] [CrossRef]
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and bacterial plant diseases. Front. Microbiol. 2017, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Holtappels, D.; Fortuna, K.; Lavigne, R.; Wagemans, J. The future of phage biocontrol in integrated plant protection for sustainable crop production. Curr. Opin. Biotechnol. 2021, 68, 60–71. [Google Scholar] [CrossRef]
- Balogh, B.; Nga, N.T.T.; Jones, J.B. Relative level of bacteriophage multiplication in vitro or phyllosphere may not predict in planta efficacy for controlling bacterial leaf spot on tomato caused by Xanthomonas perforans. Front. Microbiol. 2018, 9, 2176. [Google Scholar] [CrossRef] [Green Version]
- Vu, N.T.; Oh, C.-S. Bacteriophage usage for bacterial disease management and diagnosis in plant. Plant Pathol. J. 2020, 36, 204–217. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Li, Q.; Zhang, J.; Ji, C.; Sui, J.; Liu, Z.; Song, X.; Liu, X. Isolation and characterization of antagonistic bacteria with the potential for biocontrol of soil-borne wheat disease. Plant Pathol. 2018, 125, 1868–1880. [Google Scholar]
- Legein, M.; Smets, W.; Vandenheuvel, D.; Eilers, T.; Muyshondt, B.; Prinsen, E.; Samson, R.; Leeber, S. Modes of action of microbial biocontrol in the phyllosphere. Front. Microbiol. 2020, 11, 1619. [Google Scholar] [CrossRef]
- Mota, M.S.; Gomes, C.B.; Souza, I.T., Jr.; Bittencourt Moura, A. Bacterial selection for biological control of plant disease: Criterion determination and validation. Braz. J. Microbiol. 2017, 48, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Lama Cabanas, C.; Legarda, G.; Ruano-Rosa, D.; Pizarro-Tobias, P.; Valverde-Corredor, A.; Niqui, J.L.; Trivino, J.C.; Roca, A.; Mercado-Blanco, J. Indigenous Pseudomonas spp. strains from the olive (Olea europea L.) rhizosphere as effective biocontrol agents against Verticillium dahliae: From the host roots to the bacterial genome. Front. Microbiol. 2018, 9, 277. [Google Scholar] [CrossRef] [Green Version]
- Salvatierra-Martinez, R.; Arancibia, W.; Araya, M.; Aguilera, S.; Olalde, V.; Bravo, J.; Stoll, A. Colonization ability as an indicator of enhanced biocontrol capacity—An example using two Bacillus amyloliquefaciens strains and Botrytis cinerea infection of tomatoes. J. Phytopathol. 2018, 166, 601–612. [Google Scholar] [CrossRef]
- Stockwell, V.O.; Johnson, K.B.; Sugar, D.; Loper, J.E. Control of fire blight by Pseudomonas fluorescens A506 and Pantoea vagans C9-1 applied as single strains and mixed inocula. Phytopathology 2010, 100, 1330–1339. [Google Scholar] [CrossRef] [Green Version]
- Couillerot, O.; Prigent-Combaret, C.; Caballero-Mellado, J.; Moenne-Loccoz, Y. Pseudomonas fluorescens and closely related fluorescent pseudomonads as biocontrol agents of soil-borne phytopathogens. Lett. Appl. Microbiol. 2009, 48, 505–512. [Google Scholar] [CrossRef]
- Nam, H.S.; Anderson, A.J.; Kim, Y.C. Biocontrol efficacy of formulated Pseudomonas chlororaphis O6 against plant diseases and root-knot nematodes. Plant Pathol. J. 2018, 34, 241–249. [Google Scholar] [CrossRef]
- Chen, K.; Tian, Z.; He, H.; Long, C.; Jiang, F. Bacillus species as potential biocontrol agents against citrus diseases. Biol. Cont. 2020, 151, 104419. [Google Scholar] [CrossRef]
- Aiello, D.; Leonardi, G.R.; Di Petro, C.; Vitale, A.; Polizzi, G. A new strategy to improve management of Citrus mal secco disease using bioformulates based on Bacillus amyloliquefaciens strains. Plants 2022, 11, 446. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.; Torres, M.; Sampedro, I.; Martinez-Checa, F.; Torres, B.; Béjar, V. Biological control of Verticillium wilt on olive trees by the salt-tolerant strain Bacillus velezensis XT1. Microorganisms 2020, 8, 1080. [Google Scholar] [CrossRef] [PubMed]
- Fira, D.; Dimkic, I.; Beric, T.; Lozo, J.; Stankovic, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Osei, R.; Yang, C.; Cui, L.; Wei, L.; Jin, M.; Wei, X. Antagonistic bioagent mechanisms of controlling potato soft rot. Plant Prot. Sci. 2022, 58, 18–30. [Google Scholar] [CrossRef]
- Law, J.W.-F.; Ser, H.-L.; Khan, T.M.; Chuah, L.-H.; Pusparajah, P.; Chan, K.-G.; Goh, B.-H.; Lee, L.-H. The potential of Streptomyces as biocontrol agents against the rice blast fungus, Magnaporthe oryzae (Pyricularia oryzae). Front. Microbiol. 2017, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Chaiharn, M.; Theantana, T.; Pathom-aree, W. Evaluation of biocontrol activities of Streptomyces spp. against rice blast disease fungi. Pathogens 2020, 9, 126. [Google Scholar] [CrossRef] [Green Version]
- Daranas, N.; Rossello, G.; Cabrefiga, J.; Donati, I.; Francés, J.; Badosa, E.; Spinelli, F.; Montesinos, E.; Bonaterra, A. Biological control of bacterial plant diseases with Lactobacillus plantarum strains selected for their broad spectrum activity. Ann. Appl. Biol. 2020, 174, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Mohd Taha, M.D.; Mohd Jain, M.F.; Saidi, N.B.; Abdul Rahim, R.; Shah, U.K.; Mohd Hashim, A. Biological control of Erwinia mallotivora, the causal agent of papaya dieback disease by indigenous seed-borne endophytic lactic acid bacteria consortium. PLoS ONE 2019, 14, e0224431. [Google Scholar] [CrossRef]
- Sarrocco, S.; Esteban, P.; Vicente, I.; Bernardi, R.; Plainchamp, T.; Domenichini, S.; Puntoni, G.; Baroncelli, R.; Vannacci, G.; Dufresne, M. Straw competition and wheat root endophytism of Trichoderma gamsii T6085 as useful traits in the biological control of Fusarium head blight. Phytopathology 2021, 111, 1129–1136. [Google Scholar] [CrossRef]
- Tuão, C.A.; Pinto, J.M. Biocontrol of melon wilt caused by Fusarium oxysporum Schlect f. sp. melonis using seed treatment with Trichoderma spp. and liquid compost. Biol. Control 2016, 97, 13–20. [Google Scholar]
- Lahuf, A.A.; Kareem, A.A.; Al-Sweedi, T.M.; Alfarttoosi, H.A. Evaluation the potential of indigenous biocontrol agent Trichoderma harzianum and its interactive effect with nanosized ZnO particles against the sunflower damping-off pathogen, Rhizoctonia Solani. IOP Conf. Ser. Earth Environ. Sci. 2019, 365, 012033. [Google Scholar] [CrossRef]
- Fanelli, F.; Liuzzi, V.C.; Logrieco, A.F.; Altomare, C. Genomic characterization of Trichoderma atrobrunneum (T. harzianum species complex) ITEM 908: Insight into genetic endowment of a multi-target biocontrol strain. BMC Genom. 2018, 19, 662. [Google Scholar] [CrossRef]
- Rush, T.A.; Sherstha, H.K.; Meena, M.G.; Spangler, M.K.; Ellis, J.C.; Labbè, J.L.; Abraham, P.E. Bioprospecting Trichoderma: A systematic roadmap to screen genomes and natural products for biocontrol applications. Front. Fung. Biol. 2021, 2, 716511. [Google Scholar] [CrossRef]
- Niu, B.; Wang, W.; Yuan, Z.; Sederoff, R.R.; Chaing, V.L.; Borriss, R. Microbial interactions within multiple-strain biological control agents impact soil-borne plant disease. Front. Microbiol. 2020, 11, 585404. [Google Scholar] [CrossRef]
- Pertot, I.; Giovannini, O.; Benanchi, M.; Caffi, T.; Rossi, V.; Mugnai, L. Combining biocontrol agents with different mechanism of action in a strategy to control Botrytis cinerea on grapevine. Crop Prot. 2017, 97, 85–93. [Google Scholar] [CrossRef]
- Bubici, G. Streptomyces spp. as biocontrol agents against Fusarium species. CAB Rev. 2018, 13, 050. [Google Scholar] [CrossRef]
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K.D. Use of endophytes as biocontrol agents. Fungal Biol. Rev. 2019, 33, 133–148. [Google Scholar] [CrossRef]
- Sdiri, Y.; Lopes, T.; Rodrigues, N.; Silva, K.; Rodrigues, I.; Pereira, J.A.; Baptista, P. Biocontrol ability and production of volatile organic compounds as a potential mechanism of action of olive endophytes against Colletotrichum Acutatum. Microorganisms 2022, 10, 571. [Google Scholar] [CrossRef]
- Del Carmen Rodríguez, H.; Evans, H.C.; De Abreu, L.M.; De Macedo, D.M.; Ndacnou, M.K.; Bekele, K.B.; Barreto, R.W. New species and records of Trichoderma isolated as mycoparasites and endophytes from cultivated and wild coffee in Africa. Sci. Rep. 2021, 11, 5671. [Google Scholar]
- Vurucunda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, C.E.; Park, J.M. Endophytic bacteria as biocontrol agents against plant pathogens: Current state-of-the-art. Plant Biotechnol. Rep. 2016, 10, 353–357. [Google Scholar] [CrossRef]
- Andrivon, D.; Bardin, M.; Bertrand, C.; Brun, L.; Daire, X.; Fabre, F.; Gary, C.; Montarry, J.; Nicot, P.; Reignault, P.; et al. Can organic agriculture cope without copper for disease control? In Synthesis of the Collective Scientific Assessment Report; INRA: Paris, France, 2018; p. 64. [Google Scholar]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop protection. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Chinnadurai, C.; Ramkissoon, A.; Rajendran, R.; DeAspa, S.; Ramsubhag, A.; Jayaraj, J. Integrated disease management in pumpkin in the southern Caribbean. Trop. Agric. Univ. West Indies 2018, 95, 132–140. [Google Scholar]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahrauoi, A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-Gato, M.; Astray, G.; Mejuto, J.C.; Simal-Gandara, J. Essential oils as antimicrobials in crop protection. Antibiotics 2021, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Amini, L.; Soudi, M.R.; Saboora, A.; Mobasheri, H. Effect of essential oil from Zataria multiflora on local strains of Xanthomonas campestris: An efficient antimicrobial agent for decontamination of seeds of Brassica oleracea var. capitata. Sci. Hort. 2018, 236, 256–264. [Google Scholar] [CrossRef]
- Kumar, P.; Lokesh, V.; Doddaraju, P.; Kumari, A.; Singh, P.; Meti, B.S.; Sharma, J.; Gupta, K.J.; Manjunatha, G. Greenhouse and field experiments revealed that clove oil can effectively reduce bacterial blight and increase yield in pomegranate. Food Energy Secur. 2021, 10, e305. [Google Scholar] [CrossRef]
- Hashem, M.; Moharam, A.M.; Zaied, A.A.; Saleh, F.E.M. Efficacy of essential oils in the control of cumin root rot disease caused by Fusarium spp. Crop Protect. 2010, 29, 1111–1117. [Google Scholar] [CrossRef]
- Katijar, D.; Hemantaranjan, A.; Singh, B.; Nishant Bhanu, A. A future perspective in crop protection: Chitosan and its oligosaccharides. Adv. Plants Agric. Res. 2014, 1, 23–30. [Google Scholar]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef] [Green Version]
- Romanazzi, G.; Feliziani, E.; Sivakumar, D. Chitosan, a biopolymer with triple action on postharvest decay of fruit and vegetables: Eliciting, antimicrobial and film-forming properties. Front. Microbiol. 2018, 9, 2745. [Google Scholar] [CrossRef]
- Scortichini, M. Field efficacy of chitosan to control Pseudomonas syringae pv. actinidiae, the causal agent of kiwifruit bacterial canker. Eur. J. Plant Pathol. 2014, 140, 887–892. [Google Scholar]
- Stanley-Raja, V.; Senthil-Nathan, S.; Chantini, K.M.P.; Sivanesh, H.; Ramasubramanian, R.; Karthi, S.; Shyam-Sundar, N.; Vasantha-Srinivasan, P.; Kalaivani, K. Biological activity of chitosan inducing resistance efficiency of rice (Oryza sativa L.) after treatment with fungal based chitosan. Sci. Rep. 2021, 11, 20488. [Google Scholar] [CrossRef]
- Nandeeshkumar, P.; Sudisha, J.; Ramachandra, K.K.; Prakash, H.S.; Niranjana, S.R. Shekar, S.H. Chitosan induced resistance to downy mildew in sunflower caused by Plasmopara halstedii. Physiological. Mol. Plant Pathol. 2008, 72, 188–194. [Google Scholar] [CrossRef]
- Chandrika, K.S.V.P.; Prasad, R.D.; Godbole, V. Development of chitosan-PEG blended films using Thrichoderma: Enhancement of antimicrobial activity and seed quality. Int. J. Biol. Macromol. 2019, 126, 282–290. [Google Scholar] [CrossRef]
- Kappel, L.; Kosa, N.; Gruber, S. The multilateral efficacy of chitosan and Trichoderma on sugar beet. J. Fungi 2022, 8, 137. [Google Scholar] [CrossRef]
- Lafontaine, J.P.; Benhamou, N. Chitosan treatment: An emerging strategy for enhancing resistance of greenhouse tomato plants to infection by Fusarium oxysporum f. sp. radicis lycopersici. Biocontrol Sci. Technol. 1996, 6, 111–124. [Google Scholar] [CrossRef]
- Aloui, H.; Khwaldia, K.; Licciardello, F.; Mazzaglia, A.; Muratore, G.; Hamdi, M.; Restuccia, C. Efficacy of the combined application of chitosan and locust bean gum with different citrus essential oils to control postharvest spoilage caused by Aspergillus flavus in dates. Int. J. Food Microbiol. 2014, 170, 21–28. [Google Scholar] [CrossRef]
- Wullf, E.G.; Zida, E.; Torp, J.; Lund, O.S. Yucca schidigera extract: A potential biofungicide against seedborne pathogens of sorghum. Plant Pathol. 2012, 61, 331–338. [Google Scholar] [CrossRef]
- Trebbi, G.; Negri, L.; Bosi, S.; Dinelli, G.; Cozzo, R.; Marotti, I. Evaluation of Equisetum arvense (horsetail macerate) as a copper substitute for pathogen management in field-grown organic tomato and durum wheat cultivation. Agriculture 2021, 11, 5. [Google Scholar] [CrossRef]
- Quattrucci, A.; Ovidi, E.; Tiezzi, A.; Vinciguerra, V.; Balestra, G.M. Biological control of tomato bacterial speck using Punica granatum fruit peel extract. Crop Prot. 2013, 46, 18–22. [Google Scholar] [CrossRef]
- Mohamad, T.G.; Khalil, A.A. Effect of agriculture waste: Pomegranate (Punica granatum L.) fruits peel on some important phytopathogenic fungi and control of tomato damping-off. J. Appl. Life Sci. Int. 2015, 3, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Li Destri Nicosia, M.G.; Pangallo, S.; Raphael, G.; Romeo, F.V.; Strano, M.C.; Rapisarda, P.; Droby, S.; Schena, L. Control of postharvest fungal rots on citrus fruit and sweet cherries using a pomegranate peel extract. Postharvest Biol. Technol. 2016, 114, 54–61. [Google Scholar] [CrossRef]
- Lovato, A.; Pignatti, A.; Vitulo, N.; Vandelle, E.; Polverari, A. Inhibition of virulence-related traits in Pseudomonas syringae pv. actinidiae by gunpowder green tea extracts. Front. Microbiol. 2019, 10, 2362. [Google Scholar] [PubMed]
- Liu, H.-W.; Ji, Q.-T.; Ren, G.-G.; Wang, F.; Su, F.; Wang, P.-Y.; Zhou, X.; Wu, Z.-B.; Li, Z.; Yang, S. Antibacterial functions and proposed modes of action of novel 1,2,3,4-tetrahydro-β-carboline derivatives that possess an attractive 1,3-diaminopropan-2-ol pattern against rice bacterial blight, kiwifruit bacterial canker, and citrus bacterial canker. J. Agric. Food. Chem. 2020, 68, 12558–12568. [Google Scholar] [CrossRef] [PubMed]
- Agrios, N.G. Plant Pathology, 5th ed.; Elsevier-Academic Press: Amsterdam, The Netherlands, 2005; p. 635. [Google Scholar]
- Huber, D.M.; Haneklaus, S. Managing nutrition to control plant diseases. Landbauforsch. Völkenrode 2007, 57, 313–322. [Google Scholar]
- Gupta, N.; Debhnat, S.; Sharma, S.; Sharma, P.; Purohit, J. Role of nutrients in controlling the plant diseases in sustainable agriculture. In Agriculturally Important Microbes for Sustainable Agriculture; Meena, V.S., Mishra, P.K., Bisht, J.K., Pattanayak, A., Eds.; Springer Nature: Singapore, 2017; pp. 217–261. [Google Scholar]
- Mitchell, A.F.; Walters, D.R. Potassium phosphate induces systemic protection in barley to powdery mildew infection. Pest Manag. Sci. 2004, 60, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Yogev, E.; Sadowsky, A.; Solei, Z.; Oren, Y.; Orbach, Y. The performance of potassium phosphite for controlling Alternaria brown spot of citrus fruit. J. Plant Dis. Prot. 2006, 113, 207–213. [Google Scholar] [CrossRef]
- Liljeroth, E.; Lankinen, A.; Andreasson, E.; Alexandersson, E. Phosphite integrated the late blight treatment strategies in starch potato does not cause residue in the starch product. Plant Dis. 2020, 104, 3026–3032. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Vasilakakis, M.; Diamantidis, G.; Migani, I. The effect of postharvest calcium application on tissue calcium concentration, quality attributes, incidence of flesh browning and cell wall physicochemical aspects of peach fruits. Food Chem. 2007, 100, 1385–1392. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, C.; Tang, Y.; Ren, D.; Cai, Y.; Zhou, G.; Wang, Y.; Xu, L.; Zhu, P. Inhibition of Botrytis cinerea and control of gray mold of table grapes by calcium propionate. Food Qual. Saf. 2021, 5, fyab016. [Google Scholar] [CrossRef]
- Huber, D.M.; Jones, J.B. The role of magnesium in plant disease. Plant Soil 2013, 368, 73–85. [Google Scholar] [CrossRef]
- Moreira, W.R.; Bispo, W.M.S.; Rios, J.A.; Debona, D.; Nascimento, C.W.A.; Rodrigues, F.A. Magnesium induced alterations in the photosynthetic performance and resistance of the plants infected with Bipolaris oryzae. Sci. Agric. 2015, 72, 328–333. [Google Scholar] [CrossRef] [Green Version]
- Fagnano, M.; Agrelli, D.; Pascale, A.; Adamo, P.; Fiorentino, N.; Rocco, C.; Pepe, O.; Ventorino, V. Copper accumulation in agricultural soils: Risks for the food chain and the soil microbial populations. Sci. Total Environ. 2020, 734, 139434. [Google Scholar] [CrossRef]
- Pearson, C.J.; Jacobs, B.C. Elongation and retarded growth of rice during short-term submergence in three stages of development. Field Crops Res. 1986, 13, 331–344. [Google Scholar] [CrossRef]
- Navarrete, F.; De La Fuente, L. Zinc detoxification is required for full virulence and modification of the host leaf ionome by Xylella fastidiosa. Mol. Plant Microbe Interact. 2015, 28, 497–507. [Google Scholar] [CrossRef] [Green Version]
- Scortichini, M.; Chen, J.; De Caroli, M.; Dalessandro, G.; Pucci, N.; Modesti, V.; L’Aurora, A.; Petriccione, M.; Zampella, L.; Mastrobuoni, F.; et al. A zinc, copper and citric acid biocomplex shows promise for control of Xylella fastidiosa subsp. pauca in olive trees of Apulia region (southern Italy). Phytopathol. Medit. 2018, 57, 48–72. [Google Scholar]
- Tatulli, G.; Modesti, V.; Pucci, N.; Scala, V.; L’Aurora, A.; Lucchesi, S.; Salustri, M.; Scortichini, M.; Loreti, S. Further in vitro assesment and mid-term evaluation of control strategy of Xylella fastidiosa subsp. pauca in olive groves of Salento (Apulia, Italy). Pathogens 2021, 10, 85. [Google Scholar] [CrossRef]
- Del Coco, L.; Migoni, D.; Girelli, C.R.; Angilè, F.; Scortichini, M.; Fanizzi, F.P. Soil and leaf ionome heterogeneity in Xylella fastidiosa subsp. pauca-infected, non infected and treated olive groves in Italy. Plants 2020, 9, 760. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, R.R.; Tyagi, S.K. Pre-harvest foliar application of calcium and boron influences physiological disorders, fruit yield and quality of strawberry (Fragaria × ananassa Duch.). Sci. Hort. 2007, 112, 215–220. [Google Scholar] [CrossRef]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Gong, D.; Zhu, S.; Zhang, L.; Zhang, L. Expression of PPO and POD genes and contents of polyphenolic compounds in harvested mango fruits in relation to benzathiadiazole-induced defense against anthracnose. Sci. Hort. 2011, 130, 85–89. [Google Scholar] [CrossRef]
- Sillero, J.C.; Rojas-Molina, M.M.; Avila, C.M.; Rubiales, D. Induction of systemic acquired resistance against rust, ascochyta blight and broomrape in faba bean by exogenous application of salicylic acid and benzothiadiazole. Crop Prot. 2012, 34, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Monchiero, M.; Gullino, M.L.; Pugliese, M.; Spadaro, D.; Garibaldi, A. Efficacy of different chemical and biological products in the control of Pseudomonas syringae pv. actinidiae on kiwifruit. Eur. J. Plant Pathol. 2015, 44, 13–23. [Google Scholar]
- Hahm, M.-S.; Sumayo, M.; Hwang, Y.-J.; Jeon, S.-A.; Park, S.-J.; Lee, J.Y.; Ahn, J.-H.; Kim, B.-S.; Ryu, C.-M.; Ghim, S.-Y. Biological control and plant growth promoting capacity of rhizobacteria on pepper under greenhouse and field conditions. J. Microbiol. 2012, 50, 380–385. [Google Scholar] [CrossRef]
- Tortora, M.L.; Díaz-Ricci, J.C.; Pedraza, R.O. Protection of strawberry plants (Fragaria ananassa Duch.) against anthracnose disease induced by Azospirillum brasilense. Plant Soil 2012, 356, 279–290. [Google Scholar] [CrossRef]
- Cohen, Y.; Rubin, A.E.; Kilfin, G. Mechanisms of induced resistance in lettuce against Bremia lactucae by DL-β-amino-butyric acid (BABA). Eur. J. Plant Pathol. 2010, 126, 553–573. [Google Scholar] [CrossRef]
- Tamm, L.; Thürig, B.; Fliessbach, A.; Goltlieb, A.E.; Karavani, S.; Cohen, Y. Elicitors and soil management to induce resistance against fungal plant diseases. NJAS Wagening. J. Life Sci. 2011, 58, 131–137. [Google Scholar] [CrossRef]
- Harel, Y.M.; Elad, Y.; Rav-David, D.; Borenstein, M.; Shulchani, R.; Lew, B.; Graber, E.R. Biochar mediates systemic response of strawberry to foliar fungal pathogens. Plant Soil 2012, 357, 245–257. [Google Scholar] [CrossRef]
- Faggioli, F.; Luigi, M.; Mangili, A.; Dragone, I.; Antonelli, M.G.; Contarini, M.; Speranza, S.; Bertin, S.; Tiberini, A.; Gentili, A. Effects of biochar on the growth and development of tomato seedlings, and on the response of tomato plants to the infection of systemic viral agents. Front. Microbiol. 2022, 13, 862075. [Google Scholar]
- Paulert, R.; Talamini, V.; Cassolato, J.E.F.; Duarte, M.E.R.; Noseda, M.D.; Smania, A.; Stadnik, M.J., Jr. Effects of sulphated polysaccharide and alcoholic extracts from green seaweed Ulva fasciata on anthracnose severity and growth of common bean (Phaseolus vulgaris L.). J. Plant Dis. Protect. 2009, 116, 263–270. [Google Scholar] [CrossRef]
- Jaulneau, V.; Lafitte, C.; Corio-Costet, M.-F.; Stadnik, M.J.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.-T.; Dumas, B. An Ulva armoricana extract protects plants against three powdery mildew pathogens. Eur. J. Plant Pathol. 2011, 131, 393–401. [Google Scholar] [CrossRef]
- Koch, A.; Kogel, K.-H. New wind in the sails: Improving the agronomic value of crop plants through RNAi-mediated gene silencing. Plant Biotechnol. J. 2014, 12, 821–831. [Google Scholar] [CrossRef]
- Muhammad, T.; Zhang, F.; Zhang, Y.; Liang, Y. RNA interference: A natural immune system of plants to counteract biotic stressors. Cells 2019, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Gebremichael, D.E.; Mehari Haile, Z.; Negrini, F.; Sabbadini, S.; Capriotti, L.; Mezzetti, B.; Baraldi, E. RNA interference strategies for future management of plant pathogenic fungi: Prospects and challenges. Plants 2021, 10, 650. [Google Scholar] [CrossRef]
- Qiao, L.; Lan, C.; Capriotti, L.; Ah-Fong, A.; Sanchez, J.N.; Hamby, R.; Heller, J.; Zhao, H.; Glass, N.L.; Judelson, H.S.; et al. Spray-induced gene silencing for disease control is dependent on the efficiency of the pathogen RNA uptake. Plant Biotechnol. J. 2021, 19, 1756–1768. [Google Scholar] [CrossRef]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abtellatef, E.; Linicus, L.; Johannsmeier, J.; Jelonek, L.; Goesmann, A.; et al. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog. 2016, 12, e1005901. [Google Scholar] [CrossRef]
- Goodfellow, S.; Zhang, D.; Wang, M.-B.; Zhang, R. Bacterium-mediated RNA interference: Potential application in plant protection. Plants 2019, 8, 572. [Google Scholar] [CrossRef] [Green Version]
- Werner, B.T.; Gaffar, F.Y.; Schuemann, J.; Biedenkopf, D.; Koch, A.M. RNA-spray-mediated silencing of Fusarium graminearum AGO and DCL genes improve barley disease resistance. Front. Plant Sci. 2020, 11, 476. [Google Scholar] [CrossRef]
- Jahan, S.N.; Asman, A.K.M.; Corcoran, P.; Fogelqvist, J.; Vetukuri, R.R.; Dixelius, C. Plant-mediated gene silencing restricts growth of the potato late blight pathogen Phytophthora infestans. J. Exp. Bot. 2015, 66, 2785–2794. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Weiberg, A.; Lin, F.-M.; Thomma, B.; Huang, H.-D.; Jin, H. Bidirection cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants 2016, 2, 16151. [Google Scholar] [CrossRef]
- Nerva, L.; Sandrini, M.; Gambino, G.; Chitarra, W. Double-stranded RNAs (dsRNAs) as a sustainable tool against gray mold (Botrytis cinerea) in grapevine: Effectiveness of different application methods in an open-air environment. Biomolecules 2020, 10, 200. [Google Scholar] [CrossRef] [Green Version]
- Legreve, A.; Duveiller, E. Preventing potential diseases and pest epidemics under a changing climate. In Climate Change and Crop Production; Reynolds, M.P., Ed.; CABI Publishing: Wallingford, UK, 2010; pp. 50–70. [Google Scholar]
- Rippa, M.; Battaglia, V.; Cermola, M.; Sicignano, M.; Lahoz, E.; Mormile, P. Monitoring the copper persistence on plant leaves using pulsed thermography. Environ. Minit. Assess. 2022, 194, 160. [Google Scholar] [CrossRef] [PubMed]
- Wisniewsky, M.; Wilson, C.; Droby, S.; Chalutz, E.; El-Ghaouth, A.; Stevens, C. Postharvest biocontrol: New concepts and applications. In Biological Control: A Global Perspective; Vincent, C., Goettel, M.S., Lazarovitis, C., Eds.; CABI: Wallingford, UK, 2007; pp. 262–273. [Google Scholar]
- Brouwer, H.; Woodhill, J.; Hemmati, M.; Verhoosel, K.; Van Vugt, S. The MSP Guides: How to Design and Facilitate Multistakeholder Partnerships; Centre for Development and Innovation: Wageningen, The Netherlands, 2015. [Google Scholar]
- Pancino, B.; Blasi, E.; Rappoldt, A.; Pascucci, S.; Ruini, L.; Ronchi, C. Partnering for sustainability in agri-food supply chains: The case of Barilla sustainable farming in the Po valley. Agric. Food. Econ. 2019, 7, 13. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scortichini, M. Sustainable Management of Diseases in Horticulture: Conventional and New Options. Horticulturae 2022, 8, 517. https://doi.org/10.3390/horticulturae8060517
Scortichini M. Sustainable Management of Diseases in Horticulture: Conventional and New Options. Horticulturae. 2022; 8(6):517. https://doi.org/10.3390/horticulturae8060517
Chicago/Turabian StyleScortichini, Marco. 2022. "Sustainable Management of Diseases in Horticulture: Conventional and New Options" Horticulturae 8, no. 6: 517. https://doi.org/10.3390/horticulturae8060517
APA StyleScortichini, M. (2022). Sustainable Management of Diseases in Horticulture: Conventional and New Options. Horticulturae, 8(6), 517. https://doi.org/10.3390/horticulturae8060517




