Comprehensive Evaluation of Low Temperature and Salt Tolerance in Grafted and Rootstock Seedlings Combined with Yield and Quality of Grafted Tomato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Low Temperature Stress Treatment
2.3. Salt Stress Treatment
2.4. Effect of Rootstock on the Yield and Quality of Grafted Tomatoes
2.5. Seedling Growth Determinations
2.6. Fruit Quality Determinations
2.7. Statistical Analysis
3. Results
3.1. Effects of Rootstock on the Growth of Grafted Tomato Seedlings
3.2. Effect of Rootstock on the Low Temperature Tolerance of Grafted Tomato Seedlings
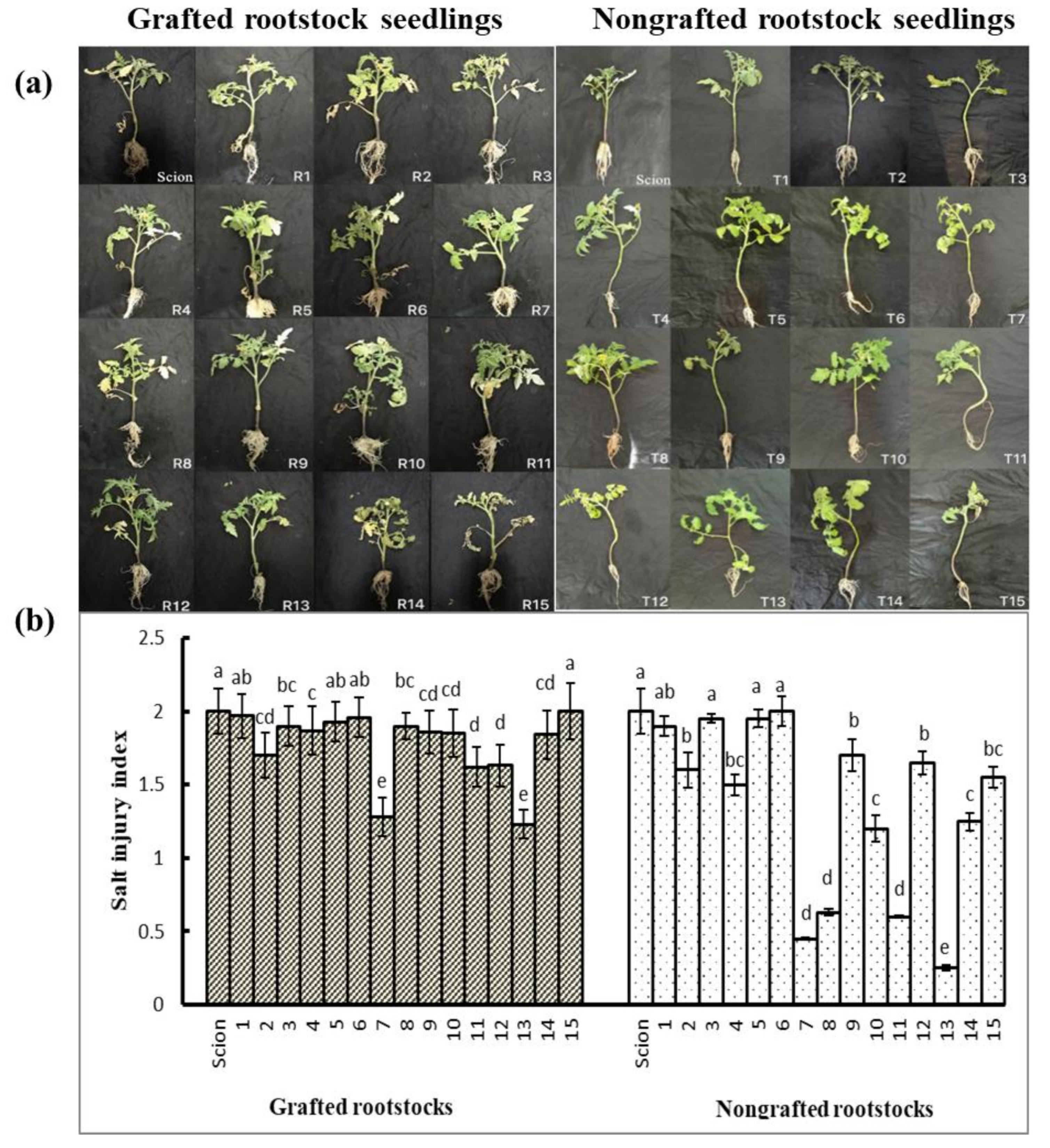
3.3. Effects of Rootstock on the Salt Tolerance of Grafted Tomato Seedlings
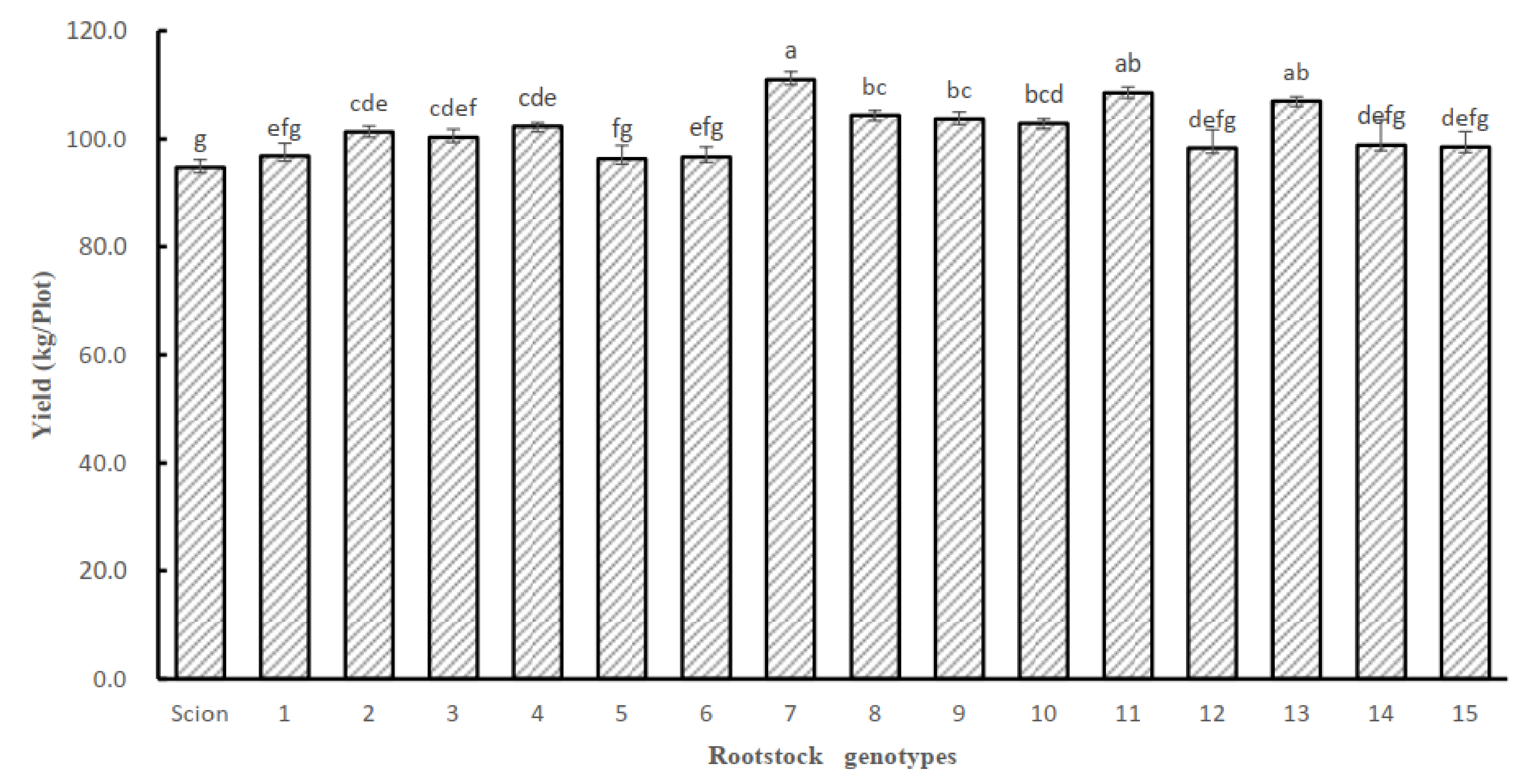
3.4. Effects of Rootstock on the Fruit Yield and Quality of Grafted Tomatoes
4. Discussion
4.1. Effects of Rootstock on the Growth of Grafted Tomato Seedlings
4.2. Effects of Rootstock on Salt Tolerance of Grafted Seedlings
4.3. Effects of Rootstock on the Low Temperatures Tolerance of Grafted Seedlings
4.4. Effect of Rootstock on the Fruit Yield of Tomato Plants
4.5. Effect of Rootstock on the Fruit Quality of Tomato Plants
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bawa, I. Management strategies of Fusarium wilt disease of tomato incited by Fusarium oxysporum f. sp. lycopersici (Sacc.) A Review. Int. J. Adv. Acad. Res. 2016, 2, 32–42. [Google Scholar]
- Tieman, D.; Zhu, G.; Resende, M.F.R.; Lin, T.; Nguyen, C.; Bies, D.; Rambla, J.L.; Beltran, K.S.O.; Taylor, M.; Zhang, B.; et al. A chemical genetic roadmap to improved tomato flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Darwish, T.; Atallah, T.; Moujabber, M.E.; Khatib, N. Salinity evolution and crop response to secondary soil salinity in two agro-climatic zones in Lebanon. Agric. Water Manag. 2005, 78, 152–164. [Google Scholar] [CrossRef]
- Yu, H.Y.; Li, T.X.; Zhou, J.M. Second salinization of greenhouse soil and its effects on soil properties. Soils 2005, 37, 581–586. (In Chinese) [Google Scholar]
- Boari, F.; Donadio, A.; Pace, B.; Schiattone, M.I.; Cantore, V. Kaolin improves salinity tolerance, water use efficiency and quality of tomato. Agric. Water Manag. 2016, 167, 29–37. [Google Scholar] [CrossRef]
- Venema, J.H.; Dijk, B.E.; Bax, J.M.; Hasselt, P.R.V.; Elzenga, J.T.M. Grafting tomato (Solanum lycopersicum) onto the rootstock of a high-altitude accession of Solanum habrochaites improves suboptimal-temperature tolerance. Environ. Exp. Bot. 2008, 63, 359–367. [Google Scholar] [CrossRef]
- Dezhabad, F.; Haghighi, M. Bottom-cold stress was less harmful than cold-air stress on tomato seedling production treated with boric acid. Acta Physiol. Plant. 2020, 42, 44. [Google Scholar] [CrossRef]
- Ranawat, B.; Mishra, S.; Singh, A. Enterobacter hormaechei (MF957335) enhanced yield, disease and salinity tolerance in tomato. Arch. Microbiol. 2021, 203, 2659–2667. [Google Scholar] [CrossRef]
- Schwarz, D.; Rouphael, Y.; Colla, G.; Venema, J.H. Grafting as a tool to improve tolerance of vegetables to abiotic stresses: Thermal stress, water stress and organic pollutants. Sci. Hortic. 2010, 127, 162–171. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; Romero, L. Can grafting in tomato plants strengthen resistance to thermal stress? J. Sci. Food Agric. 2003, 83, 1315–1319. [Google Scholar] [CrossRef]
- Riga, P. Effect of rootstock on growth, fruit production and quality of tomato plants grown under low temperature and light conditions. Hortic. Environ. Biotechnol. 2015, 56, 626–638. [Google Scholar] [CrossRef]
- Gaion, L.A.; Monteiro, C.C.; Cruz, F.J.R.; Rossatto, D.R.; López-Díaz, I.; Carrera, E.; Carvalho, R.F. Constitutive gibberellin response in grafted tomato modulates root-to-shoot signaling under drought stress. J. Plant Physiol. 2018, 221, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estañ, M.T.; Martinez, M.M.; Francisco, P.A.; Flowers, T.J.; Bolarin, M.C. Grafting raises the salt tolerance of tomato through limiting the transport of sodium and chloride to the shoot. J. Exp. Bot. 2005, 56, 703–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koleška, I.; Hasanagić, D.; Todorović, V.; Murtić, S.; Maksimović, I. Grafting influence on the weight and quality of tomato fruit under salt stress. Ann. Appl. Biol. 2018, 172, 187–196. [Google Scholar] [CrossRef]
- Mackey, M.; Kurosky, A.; Robb, E.J.; Nazar, R.N. A graft mimic strategy for verticillium resistance in tomato. Mol. Biotechnol. 2018, 60, 665–669. [Google Scholar] [CrossRef]
- Albacete, A.; Martinez-Andujar, C.; Martinez-Perez, A.; Thompson, A.J.; Dodd, I.C.; Perez-Alfocea, F. Unravelling rootstock×scion interactions to improve food security. J. Exp. Bot. 2015, 66, 2211–2226. [Google Scholar] [CrossRef] [Green Version]
- Kyriacou, M.C.; Rouphael, Y.; Colla, G.; Zrenner, R.; Schwarz, D. Vegetable grafting: The implications of a growing agronomic imperative for vegetable fruit quality and nutritive value. Front. Plant Sci. 2017, 8, 741. [Google Scholar] [CrossRef]
- Thapa, R.; Thapa, P.; Ahamad, K.; Vahdati, K. Effect of grafting methods and dates on the graft take rate of persian walnut in open field condition. Int. J. Hortic. Sci. Technol. 2021, 8, 133–147. [Google Scholar]
- Rezaee, R.; Vahdati, K.; Grigoorian, W.; Valizadeh, M. Walnut grafting success and bleeding rate as affected by different grafting methods and seedling vigor. J. Hortic. Sci. Biotechnol. 2008, 83, 94–99. [Google Scholar] [CrossRef]
- Asins, M.J.; Raga, V.; Roca, D.; Belver, A.; Carbonell, E.A. Genetic dissection of tomato rootstock effects on scion traits under moderate salinity. Theor. Appl. Genet. 2015, 128, 667–679. [Google Scholar] [CrossRef]
- Santa-Cruz, A.; Martínez-Rodríguez, M.M.; Perez-Alfocea, F.; Romero-Aranda, R.; Bolarin, M.C. The rootstock effect on the tomato salinity response depends on the shoot genotype. Plant Sci. 2002, 162, 825–831. [Google Scholar] [CrossRef]
- Albacete, A.; Martinez-Andujar, C.; Dodd, I.; Giuffrida, F.; Hichri, I.; Lutts, S.; Thompson, A.; Asins, M. Rootstock-mediated variation in tomato vegetative growth under drought, salinity and soil impedance stresses. Acta Hortic. 2015, 1086, 141–146. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, M.M.; Estañ, M.T.; Moyano, E.; Garcia-Abellan, J.O.; Flores, F.B.; Campos, J.F.; Al-Azzawi, M.J.; Flowers, T.J.; Bolarín, M.C. The effectiveness of grafting to improve salt tolerance in tomato when an excluder genotype is used as scion. Environ. Exp. Bot. 2008, 63, 392–401. [Google Scholar] [CrossRef]
- Zhang, G.W.; Liu, Z.L.; Zhou, J.C.; Zhu, Y.L. Effects of Ca(NO3)2 stress on oxidative damage, antioxidant enzymes activities and polyamine contents in roots of grafted and non-grafted tomato plants. Plant Growth Regul. 2008, 56, 7–19. [Google Scholar] [CrossRef]
- Ntatsi, G.; Savvas, D.; Druege, U.; Schwarz, D. Contribution of phytohormones in alleviating the impact of sub-optimal temperature stress on grafted tomato. Sci. Hortic. 2013, 149, 28–38. [Google Scholar] [CrossRef]
- Ntatsi, G.; Savvas, D.; Kläring, H.P.; Schwarz, D. Growth, yield, and metabolic responses of temperature-stressed tomato to grafting onto rootstocks differing in cold tolerance. J. Am. Soc. Hortic. Sci. 2014, 139, 230–243. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, R.M.; Upreti, K.K.; Divya, M.H.; Bhat, S.; Pavithra, C.B.; Sadashiva, A.T. Interspecific grafting to enhance physiological resilience to flooding stress in tomato (Solanum lycopersicum L.). Sci. Hortic. 2015, 182, 8–17. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, T.; Ge, H.; Pang, W.; Gao, L.; Re, L.; Chen, H. SSR mapping of QTLs conferring cold tolerance in an interspecific cross of tomato. Int. J. Genomics. 2016, 2016, 3219276. [Google Scholar] [CrossRef] [Green Version]
- Niu, M.L.; Xie, J.J.; Sun, J.Y.; Huang, Y.; Kong, Q.S.; Nawaz, M.A.; Bie, Z.L. A shoot based Na+, tolerance mechanism observed in pumpkin-An important consideration for screening salt tolerant rootstocks. Sci. Hortic. 2017, 218, 38–47. [Google Scholar] [CrossRef]
- Schwarz, D.; Öztekin, G.B.; Tüzel, Y.; Brückner, B.; Krumbein, A. Rootstocks can enhance tomato growth and quality characteristics at low potassium supply. Sci. Hortic. 2013, 149, 70–79. [Google Scholar] [CrossRef]
- Turhan, A.; Ozmen, N.; Serbeci, M.S.; Seniz, V. Effects of grafting on different rootstocks on tomato fruit yield and quality. Hortic. Sci. 2011, 38, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Öztekin, G.B.; Giuffrida, F.; Tuzel, Y.; Leonardi, C. Is the vigour of grafted tomato plants related to root characteristics? J. Food Agric. Environ. 2009, 7, 364–368. [Google Scholar]
- Sánchez-Rodríguez, E.; Leyva, R.; Constán-Aguilar, C.; Romero, L.; Ruiz, J. How does grafting affect the ionome of cherry tomato plants under water stress? Soil Sci. Plant Nutr. 2014, 60, 145–155. [Google Scholar] [CrossRef]
- Fullana-Pericàs, M.; Ponce, J.; Conesa, M.À.; Juan, A.; Ribas-Carbó, M.; Galmés, J. Changes in yield, growth and photosynthesis in a drought-adapted Mediterranean tomato landrace (Solanum lycopersicum “Ramellet”) when grafted onto commercial rootstocks and Solanum pimpinellifolium. Sci. Hortic. 2018, 233, 70–77. [Google Scholar] [CrossRef]
- King, S.R.; Davis, A.R.; Zhang, X.; Crosby, K. Genetics, breeding and selection of rootstocks for Solanaceae and Cucurbitaceae. Sci. Hortic. 2010, 127, 106–111. [Google Scholar] [CrossRef]
- 3Savvas, D.; Savva, A.; Ntatsi, G.; Ropokis, A.; Karapanos, I.; Krumbein, A.; Olympios, C. Effects of three commercial rootstocks on mineral nutrition, fruit yield, and quality of salinized tomato. J. Plant Nutr. Soil Sci. 2011, 174, 154–162. [Google Scholar] [CrossRef]
- 3Djidonou, D.; Simonne, A.H.; Koch, K.E.; Brecht, J.K.; Zhao, X. Nutritional quality of field-grown tomato fruit as affected by grafting with interspecific hybrid rootstocks. HortScience 2016, 51, 1618–1624. [Google Scholar] [CrossRef] [Green Version]
- 3Mohammed, S.T.M.; Humidan, M.; Boras, M.; Abdalla, O.A. Effect of grafting tomato on different rootstocks on growth and productivity under glasshouse conditions. Asian J. Agric. Res. 2009, 3, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Leonardi, C.; Giuffrida, F. Variation of plant growth and macronutrient uptake in graftedtomatoes and eggplants on three different rootstocks. Eur. J. Hortic. Sci. 2006, 71, 97–101. [Google Scholar]
- Khah, E.M.; Kakava, E.; Mavromatis, A.; Chachalis, D.; Goulas, C. Effect of grafting on growth and yield of tomato (Lycopersicon esculentum Mill.) in greenhouse and open-field. J. Appl. Hortic. 2006, 8, 3–7. [Google Scholar] [CrossRef]
- Krumbein, A.; Schwarz, D. Grafting: A possibility to enhance health promoting and flavour compounds in tomato fruits of shaded plants? Sci. Hortic. 2013, 149, 97–107. [Google Scholar] [CrossRef]
- Rouphael, Y.; Schwarz, D.; Krumbein, A.; Colla, G. Impact of grafting on product quality of fruit vegetables. Sci. Hortic. 2010, 127, 172–179. [Google Scholar] [CrossRef]
- Huang, W.; Liao, S.; Lv, H.; Khaldun, A.B.M.; Wang, Y. Characterization of the growth and fruit quality of tomato grafted on a woody medicinal plant, Lycium chinense. Sci. Hortic. 2015, 197, 447–453. [Google Scholar] [CrossRef]
- Gajc-Wolska, J.; Kowalczyk, K.; Marcinkowska, M.; Radzanowska, J.; Bujalski, D. Influence of growth conditions and grafting on the yield, chemical composition and sensory quality of tomato fruit in greenhouse cultivation. J. Elem. 2015, 20, 73–81. [Google Scholar]
- Brajović, B.; Kastelec, D.; Šircelj, H.; Maršić, N.K. The effect of scion/rootstock combination and ripening stage on the composition of carotenoids and some carpometric characteristics of tomato fruit. Eur. J. Hortic. Sci. 2012, 77, 261–271. [Google Scholar]
- Barrett, C.E.; Zhao, X.; Sims, C.A.; Brecht, J.K.; Dreyer, E.Q.; Gao, Z.F. Fruit composition and sensory attributes of organic heirloom tomatoes as affected by grafting. Hortic. Technol. 2012, 22, 804–809. [Google Scholar] [CrossRef] [Green Version]
- Flores, F.B.; Sanchez-Bel, P.; Estañ, M.T.; Martinez-Rodriguez, M.M.; Moyano, E.; Morales, B.; Campos, J.F.; Garcia-Abellán, J.O.; Egea, M.I.; Fernández-Garcia, N.; et al. The effectiveness of grafting to improve tomato fruit quality. Sci. Hortic. 2010, 125, 211–217. [Google Scholar] [CrossRef]
- Martinez-Ballesta, M.C.; Alcaraz-Lopez, C.; Muries, B.; Carvajal, M. Physiological aspects of rootstock-scion interactions. Sci. Hortic. 2010, 127, 112–118. [Google Scholar] [CrossRef]



| Code of Rootstocks | Name of Variety | Seeds Source | Germination Rate (%) |
|---|---|---|---|
| Scion | Mingzhi 88 | Beijing Baimutian Seedling Co., Ltd., Beijing, China | 100 |
| 1 | Guozhen No.1 | Jingyan Yinong(Beijing) Seed Sci-Tech Co.,Ltd., Beijing, China | 95 |
| 2 | Aihao | RIJK ZWAAN(China) Seed Co., Ltd., Qingdao, China | 100 |
| 3 | Rootstock B1 | RIJK ZWAAN(China) Seed Co., Ltd., Qingdao, China | 100 |
| 4 | Jinba | Zhaowei Seed Co., Ltd., Shijiazhuang, China | 100 |
| 5 | Qiangli | Shandong Shouhe Seed, Shouguang, China | 100 |
| 6 | Alamu | Shandong Shouguang Lilin Seed, Shouguang, China | 99 |
| 7 | CHEONG GANG | De Ruiter Seeds Co., Ltd., Beijing, China | 100 |
| 8 | Zhenmu No.2 | Shanghai Wells Seed Co., Ltd., Shanghai, China | 97 |
| 9 | Ouzhen006 | Seminis Seeds (Beijing) Co., Ltd., Beijing, China | 100 |
| 10 | Aoni | Asahi Chemical Co., Ltd., Tokyo, Japan | 89 |
| 11 | TMS150 | Sakata Seed Corporation, Yokohama, Japan | 100 |
| 12 | Tomato rootstock405 | Shanghai Wells Seed Co., Ltd., Shanghai, China | 100 |
| 13 | Tomato Rootstock 1 | Xi’an Jinpeng Seedings Co., Ltd., Xi’an, China | 50 |
| 14 | Tomato Rootstock 3 | Guangzhou Huayan Seed Technology Company, Guangzhou, China | 100 |
| 15 | Saiqingsong | Shandong Huasheng Seed Co., Ltd., Qingzhou, China | 83 |
| Rootstock Genotypes | Plant Height (cm) | Stem Diameter (mm) | Hypocotyl Height (cm) | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Seeding index Value | Root-Shoot Ratio |
|---|---|---|---|---|---|---|---|
| Scion | 14.25 ± 0.74 a | 3.46 ± 0.10 gh | 3.88 ± 0.19 ab | 4.08 ± 0.27 d | 0.73 ± 0.03 k | 1.86 ± 0.124 f | 0.13 ± 0.0094 g |
| 1 | 11.16 ± 0.41 cde | 4.07 ± 0.08 def | 3.19 ± 0.18 cdef | 4.7 ± 0.18 abcd | 0.98 ± 0.11 j | 2.11 ± 0.069 de | 0.21 ± 0.0145 f |
| 2 | 10.47 ± 0.26 de | 4.33 ± 0.10 cd | 3.62 ± 0.13 abc | 4.31 ± 0.21 cd | 1.34 ± 0.10 efghi | 2.33 ± 0.079 cde | 0.32 ± 0.0296 cde |
| 3 | 11.08 ± 0.31 cde | 3.96 ± 0.08 def | 3.43 ± 0.12 bcd | 4.54 ± 0.30 bcd | 1.79 ± 0.08 abc | 2.26 ± 0.11 cde | 0.41 ± 0.0275 a |
| 4 | 11.25 ± 0.20 cde | 3.76 ± 0.06 fg | 3.48 ± 0.13 bcd | 5.03 ± 0.22 abcd | 1.30 ± 0.08 ghi | 2.11 ± 0.09 de | 0.26 ± 0.0151 ef |
| 5 | 10.72 ± 0.26 de | 4.85 ± 0.13 b | 2.90 ± 0.15 defg | 4.89 ± 0.24 abcd | 1.62 ± 0.12 bcde | 2.96 ± 0.19 b | 0.33 ± 0.0164 cde |
| 6 | 11.31 ± 0.56 cde | 5.22 ± 0.10 a | 3.19 ± 0.12 cdef | 5.63 ± 0.37 a | 1.68 ± 0.09 abcd | 3.40 ± 0.18 a | 0.31 ± 0.027 cde |
| 7 | 11.00 ± 0.35 cde | 3.40 ± 0.10 def | 3.27 ± 0.11 cde | 5.33 ± 0.46 abc | 1.91 ± 0.11 a | 2.62 ± 0.16 bc | 0.39 ± 0.0458 ab |
| 8 | 10.09 ± 0.33 e | 3.96 ± 0.14 def | 3.37 ± 0.15 bcd | 4.71 ± 0.23 abcd | 1.23 ± 0.09 hij | 2.35 ± 0.16 cde | 0.26 ± 0.0139 ef |
| 9 | 10.55 ± 0.35 de | 4.18 ± 0.10 de | 3.28 ± 0.10 cde | 4.59 ± 0.16 bcd | 1.59 ± 0.06 bcde | 2.45 ± 0.06 cd | 0.35 ± 0.0181 bcd |
| 10 | 11.50 ± 0.31 cde | 4.60 ± 0.20 bc | 4.03 ± 0.33 a | 4.43 ± 0.30 abc | 1.46 ± 0.07 defgh | 2.36 ± 0.16 cde | 0.34 ± 0.0207 bcd |
| 11 | 11.20 ± 0.45 cde | 4.14 ± 0.14 de | 2.63 ± 0.20 fgh | 5.19 ± 0.5 abc | 1.84 ± 0.11 ab | 2.65 ± 0.21 bc | 0.36 ± 0.0125 bc |
| 12 | 11.57 ± 0.51 cd | 3.86 ± 0.10 ef | 2.31 ± 0.30 hi | 5.48 ± 0.40 ab | 1.51 ± 0.14 cdef | 2.32 ± 0.14 cde | 0.28 ± 0.0231 ef |
| 13 | 13.19 ± 0.48 ab | 3.99 ± 0.17 def | 2.06 ± 0.11 i | 5.24 ± 0.19 abc | 1.21 ± 0.11 ij | 1.97 ± 0.13 e | 0.23 ± 0.0203 f |
| 14 | 12.20 ± 0.48 bc | 3.97 ± 0.07 def | 2.72 ± 0.11 efgh | 5.61 ± 0.38 a | 1.85 ± 0.08 ab | 2.42 ± 0.09 cd | 0.34 ± 0.0197 bcd |
| 15 | 11.37 ± 0.53 cde | 4.28 ± 0.10 cd | 2.39 ± 0.24 ghi | 5.18 ± 027 abc | 1.31 ± 0.08 fghi | 2.46 ± 0.10 cd | 0.26 ± 0.0195 ef |
| Rootstock Genotypes | Plant Height (cm) | Stem Diameter (mm) | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Seeding Index Value |
|---|---|---|---|---|---|
| Scion | 15.9 ± 0.355 d | 3.88 ± 0.04 bc | 4.59 ± 0.26 cdef | 0.60 ± 0.03 def | 0.130 ± 0.005 cde |
| 1 | 17.92 ± 0.56 abcd | 4.01 ± 0.12 abc | 3.90 ± 0.69 efg | 0.87 ± 0.14 bcde | 0.090 ± 0.003 h |
| 2 | 17.38 ± 0.81 abcd | 4.17 ± 0.07 abc | 4.48 ± 0.40 def | 0.79 ± 0.17 bcdef | 0.113 ± 0.009 efgh |
| 3 | 16.8 ± 0.43 cd | 4.22 ± 0.09 abc | 5.92 ± 0.13 abcd | 1.47 ± 0.19 a | 0.143 ± 0.005 bc |
| 4 | 17.08 ± 0.35 bcd | 4.35 ± 0.11 a | 6.54 ± 0.41 ab | 0.83 ± 0.09 bcde | 0.137 ± 0.003 bcd |
| 5 | 17.56 ± 1.16 abcd | 4.03 ± 0.16 abc | 4.01 ± 0.36 efg | 0.66 ± 0.11 def | 0.113 ± 0.006 efgh |
| 6 | 15.93 ± 0.43 d | 4.33 ± 0.22 ab | 5.05 ± 0.35 bcde | 0.76 ± 0.20 cdef | 0.130 ± 0.010 bcde |
| 7 | 18.04 ± 0.46 abcd | 4.03 ± 0.15 abc | 6.88 ± 0.93 a | 1.23 ± 0.15 ab | 0.149 ± 0.010 b |
| 8 | 19.42 ± 0.55 a | 3.83 ± 0.14 c | 6.22 ± 0.38 abc | 0.81 ± 0.09 bcde | 0.112 ± 0.006 efgh |
| 9 | 18.32 ± 1.17 abc | 4.02 ± 0.14 abc | 5.19 ± 0.78 bcde | 0.53 ± 0.06 efg | 0.090 ± 0.003 h |
| 10 | 17.62 ± 0.16 abcd | 4.04 ± 0.14 abc | 4.83 ± 0.64 cde | 1.15 ± 0.22 abc | 0.116 ± 0.011 defg |
| 11 | 18.02 ± 0.89 abcd | 4.18 ± 0.08 abc | 5.23 ± 0.37 cde | 0.64 ± 0.09 def | 0.152 ± 0.005 b |
| 12 | 16.62 ± 0.92 cd | 4.03 ± 0.12 abc | 5.19 ± 0.67 g | 1.01 ± 0.20 bcd | 0.096 ± 0.005 gh |
| 13 | 19.12 ± 0.48 ab | 4.00 ± 0.23 abc | 2.85 ± 0.07 g | 0.57 ± 0.04 efg | 0.100 ± 0.008 fgh |
| 14 | 17.74 ± 0.48 abcd | 3.94 ± 0.10 abc | 6.18 ± 0.32 abc | 0.84 ± 0.14 bcde | 0.121 ± 0.007 cdef |
| 15 | 11.26 ± 0.4946 e | 3.93 ± 0.12 abc | 3.06 ± 0.20 fg | 0.35 ± 0.06 fg | 0.200 ± 0.006 a |
| Rootstock Genotypes | Plant Height (cm) | Stem Diameter (mm) | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Seeding Index Value |
|---|---|---|---|---|---|
| Scion | 14.25 ± 0.33 g | 3.42 ± 0.06 e | 6.02 ± 0.05 gh | 1.10 ± 0.022 d | 0.298 ± 0.036 g |
| 1 | 15.70 ± 0.59 def | 3.98 ± 0.05 de | 6.53 ± 0.26 fgh | 1.17 ± 0.03 d | 0.344 ± 0.026 def |
| 2 | 16.22 ± 0.14 de | 4.39 ± 0.04 cd | 7.75 ± 0.11 cde | 1.97 ± 0.11 cd | 0.381 ± 0.015 cd |
| 3 | 16.03 ± 0.22 de | 4.14 ± 0.07 cde | 6.70 ± 0.25 efg | 1.96 ± 0.215 cd | 0.355 ± 0.034 de |
| 4 | 16.11 ± 0.29 de | 4.17 ± 0.03 cde | 6.73 ± 0.15 efg | 1.36 ± 0.67 cd | 0.371 ± 0.011 cd |
| 5 | 15.17 ± 0.18 efg | 4.11 ± 0.04 cde | 6.65 ± 0.24 efg | 1.80 ± 0.10 cd | 0.331 ± 0.045 efg |
| 6 | 14.69 ± 0.20 fg | 4.33 ± 0.11 cd | 6.49 ± 0.33 fgh | 1.82 ± 0.10 cd | 0.325 ± 0.106 fg |
| 7 | 21.69 ± 0.36 a | 5.47 ± 0.04 a | 10.23 ± 0.21 a | 2.49 ± 0.21 a | 0.593 ± 0.114 a |
| 8 | 15.21 ± 0.28 efg | 4.33 ± 0.08 cd | 6.49 ± 0.22 fgh | 1.59 ± 0.05 cd | 0.338 ± 0.024 efg |
| 9 | 15.72 ± 0.49 def | 4.06 ± 0.03 cde | 7.07 ± 0.26 defg | 1.87 ± 0.04 cd | 0.347 ± 0.081 def |
| 10 | 15.78 ± 0.20 def | 4.01 ± 0.15 de | 7.28 ± 0.51 def | 1.56 ± 0.05 cd | 0.352 ± 0.014 de |
| 11 | 17.53 ± 0.23 c | 4.73 ± 0.05 abcd | 8.16 ± 0.08 bcd | 2.08 ± 0.07 c | 0.382 ± 0.091 c |
| 12 | 16.57 ± 0.27 cd | 4.62 ± 0.05 bcd | 7.88 ± 0.14 cd | 1.99 ± 0.04 cd | 0.381 ± 0.085 cd |
| 13 | 19.03 ± 0.22 b | 4.88 ± 0.05 abc | 8.54 ± 0.10 bc | 2.28 ± 0.06 b | 0.403 ± 0.079 bc |
| 14 | 16.20 ± 0.09 de | 4.06 ± 0.05 cde | 7.63 ± 0.49 cdef | 1.93 ± 0.07 cd | 0.379 ± 0.105 cd |
| 15 | 14.47 ± 0.15 g | 4.04 ± 0.04 cde | 6.09 ± 0.26 gh | 1.15 ± 0.03 d | 0.305 ± 0.034 g |
| Rootstock Genotypes | Lycopene mg/100 g FW | Soluble Proteins mg/g | Soluble Sugar % | Ascorbic Acid mg/100 g FW | Titratable Acid mg/100 g FW | Soluble Solids % |
|---|---|---|---|---|---|---|
| Scion | 5.72 ± 0.073 e | 2.13 ± 0.027 d | 4.09 ± 0.32 ab | 6.59 ± 0.58 e | 435.2 ± 47.3 abc | 5.78 ± 0.21 ab |
| 1 | 9.89 ± 0.064 bc | 2.29 ± 0.017 cd | 3.49 ± 0.11 cd | 7.25 ± 0.70 de | 438.1 ± 12.16 abc | 5.57 ± 0.34 ab |
| 2 | 10.34 ± 0.110 bc | 1.97 ± 0.019 d | 2.92 ± 0.27 ef | 8.72 ± 0.46 cde | 395.2 ± 36.52 bc | 5.64 ± 0.17 ab |
| 3 | 7.85 ± 0.117 cd | 1.89 ± 0.004 d | 3.68 ± 0.11 bc | 8.45 ± 0.74 cde | 457.1 ± 35.82 abc | 5.67 ± 0.26 ab |
| 4 | 9.76 ± 0.152 bc | 1.58 ± 0.008 d | 2.52 ± 0.08 fg | 9.52 ± 0.80 bcd | 423.8 ± 27.43 bc | 5.13 ± 0.24 b |
| 5 | 8.86 ± 0.065 bc | 2.13 ± 0.007 d | 2.41 ± 0.12 fg | 8.05 ± 0.58 de | 423.8 ± 35.13 abc | 5.67 ± 0.43 ab |
| 6 | 11.10 ± 0.064 ab | 2.28 ± 0.002 cd | 4.24 ± 0.01 ab | 6.45 ± 0.12 e | 490.5 ± 11.67 a | 5.53 ± 0.12 ab |
| 7 | 13.17 ± 0.147 a | 2.04 ± 0.015 d | 4.34 ± 0.13 a | 7.12 ± 0.68 de | 455.7 ± 46.62 abc | 5.92 ± 0.36 ab |
| 8 | 9.01 ± 0.089 bc | 2.17 ± 0.026 d | 2.86 ± 0.13 ef | 8.98 ± 0.92 cde | 485.7 ± 30.59 a | 5.56 ± 0.15 ab |
| 9 | 5.63 ± 0.120 de | 2.19 ± 0.009 d | 3.75 ± 0.17 bc | 6.85 ± 0.26 e | 471.4 ± 36.17 ab | 6.03 ± 0.37 a |
| 10 | 4.77 ± 0.035 e | 1.79 ± 0.009 d | 3.48 ± 0.27 cd | 11.38 ± 0.92 ab | 485.7 ± 27.30 a | 5.93 ± 0.32 ab |
| 11 | 9.53 ± 0.061 bc | 3.10 ± 0.022 bc | 3.77 ± 0.02 bc | 10.85 ± 1.26 abc | 452.4 ± 46.35 abc | 5.53 ± 0.09 ab |
| 12 | 9.49 ± 0.067 bc | 4.22 ± 0.039 a | 3.29 ± 0.05 cde | 8.72 ± 0.80 cde | 466.7 ± 18.46 abc | 5.63 ± 0.07 ab |
| 13 | 9.43 ± 0.076 bc | 3.29 ± 0.029 b | 4.07 ± 0.07 ab | 12.18 ± 0.74 a | 466.6 ± 15.83 abc | 5.46 ± 0.21 ab |
| 14 | 9.25 ± 0.040 bc | 3.60 ± 0.048 ab | 3.09 ± 0.30 de | 7.25 ± 1.04 de | 471.4 ± 14.29 abc | 5.72 ± 0.25 ab |
| 15 | 8.24 ± 0.044 bcd | 3.83 ± 0.063 ab | 2.05 ± 0.04 g | 7.65 ± 0.48 de | 481.0 ± 29.26 ab | 5.65 ± 0.15 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, S.; Chen, J.; Wu, X.; Gao, H.; Lü, G. Comprehensive Evaluation of Low Temperature and Salt Tolerance in Grafted and Rootstock Seedlings Combined with Yield and Quality of Grafted Tomato. Horticulturae 2022, 8, 595. https://doi.org/10.3390/horticulturae8070595
Fu S, Chen J, Wu X, Gao H, Lü G. Comprehensive Evaluation of Low Temperature and Salt Tolerance in Grafted and Rootstock Seedlings Combined with Yield and Quality of Grafted Tomato. Horticulturae. 2022; 8(7):595. https://doi.org/10.3390/horticulturae8070595
Chicago/Turabian StyleFu, Shijie, Jiaqian Chen, Xiaolei Wu, Hongbo Gao, and Guiyun Lü. 2022. "Comprehensive Evaluation of Low Temperature and Salt Tolerance in Grafted and Rootstock Seedlings Combined with Yield and Quality of Grafted Tomato" Horticulturae 8, no. 7: 595. https://doi.org/10.3390/horticulturae8070595
APA StyleFu, S., Chen, J., Wu, X., Gao, H., & Lü, G. (2022). Comprehensive Evaluation of Low Temperature and Salt Tolerance in Grafted and Rootstock Seedlings Combined with Yield and Quality of Grafted Tomato. Horticulturae, 8(7), 595. https://doi.org/10.3390/horticulturae8070595






