1. Introduction
Petroselinum crispum (Mill.) Fuss is a biennial cultivated plant commonly known as parsley that belongs to the family Apiaceae or Umbellliferae and the genus
Petroselinum [
1]. The Mediterranean region, and more precisely Sardinia, has been recognized as its primary center of origin, whereas its cultivation dates back to the 3rd century BC [
2]. Parsley can be divided into three main types, namely the plain-leaf type (
Petroselinum crispmum ssp.
neapolitanum, Danert), the curly-leaf type (
Petroselinum crispum ssp.
crispum) that is mainly cultivated for its aromatic foliage and, lastly, the turnip-rooted or ‘Hamburg’ type (
Petroselinum crispum ssp.
tuberosum) cultivated for its fleshy taproots [
3]. It is widely cultivated as an annual species throughout the world, while the fresh and dried herbs of the plant are widely used for flavoring and garnishing in many food products due to the characteristic aroma [
4]. Similarly, the essential oils and oleoresins from the aerial plant parts are widely exploited in perfume manufacturing as fragrances, as well as in traditional and folk medicine [
4,
5,
6]. In particular, the aerial parts of parsley have been associated with several health effects and have been used since ancient times for the treatment of hemorrhoids, gastrointestinal disorders, blurred vision and urinary tract and skin diseases, whereas extracts of parsley have been associated with antidiabetic, antihypertensive, spasmolytic, antibacterial, antifungal, analgesic and immunosuppressant activities [
6,
7,
8].
The aerial plant parts of parsley, such as the stems, leaves, fruits and seeds, are the most commonly used and considered a rich source of polyphenols, carotenoids, furanocoumarins, essential oils, minerals and fatty acid compounds, as well as vitamins such as tocopherols, B complex, A and C [
9,
10,
11,
12,
13,
14]. On the other hand, turnip-rooted parsley is appreciated for its edible fleshy roots that contain essential oils [
3,
15,
16], as well as iron and polyphenols [
17], vitamin C [
13], carotenoids [
18] and flavonoids [
19]. In contrast, according to the literature, the consumption of parsley roots may be associated with health risks related to nitrate intake depending on the cultivation system (organic vs. conventional cropping), the genotype and the time of harvest, which may also affect nitrate content [
20,
21,
22]. However, Pokluda [
23] suggested that the nitrate content of 15 root parsley cultivars was within tolerance limits. Moreover, in the study by Kolarovic et al. [
24], it was suggested that parsley root juice might show protective effects against doxorubicin-induced cardiotoxicity. Crop diversification in the Mediterranean basin, through the introduction of new species and/or cultivars of conventional crops, is imminent due to the severe effects of climate change and the increasing need for genotypes better adapted to the new conditions [
25]. The Apiaceae family includes several aromatic and medicinal plants and shows great genetic diversity that could be further exploited with the aim of improving the agro-biodiversity of the region [
25,
26]. Our previous experiments, where various parsley cultivars of all three types were evaluated, showed promising results regarding the fresh herb and root yield, the chemical composition and the bioactive compound content of the aerial parts of the plant [
27,
28,
29]. In this follow-up study, several turnip-rooted parsley cultivars were evaluated for their yield potential, as well as for the existence of variability in the chemical composition and bioactive properties of parsley germplasm.
3. Results and Discussion
The results of the root yield analysis of the studied turnip-rooted parsley varieties are presented in
Table 1. A significant variation in fresh yield was recorded among the genotypes, with the best performance being observed for the common “Root parsley” variety (164 g/pot), followed by the varieties “Osborne” (109 g/plot), “Sonata” (104 g/pot), “Kaśka” (104 g/pot) and “Halblange Berlinska” (103 g/pot), whereas the lowest yield was recorded for the “Hanacka” variety (69 g/pot). A similar variability in the yield of root parsley germplasms was recorded by Pokluda et al. [
23], Rahimić et al. [
36] and Petropoulos et al. [
29], who suggested significant differences between root parsley cultivars, as well as by Fernandes et al. [
28], who evaluated the fresh foliage yield of the same cultivars as in the present study and recorded genotypic differences. The observed differences may be attributed to genotypic differences, as well as differences in the length of the growth cycle of each cultivar, which suggests the application of different sowing dates to facilitate the best performance of each genotype [
29]. Moreover, considering that most of these genotypes are cultivated in northern Europe, this probably suggests an adaptation to cooler climates than those of the south Mediterranean that could be overcome through the selection of suitable growing sites and corresponding growing periods.
The nutritional values of the studied parsley germplasms are presented in
Table 1. Significant variations were recorded for all the determined parameters, with the roots of the “Sonata” genotype showing the highest fat content and “Berlinski Halblange Springer” the lowest. On the other hand, the roots of the “Arat” cultivar were the richest in protein, whereas “Cucrowa” and “Alba” contained the lowest amount of protein. “Arat”, “Osborne” and “Olomuńcka” had the highest ash content, with no significant differences between them, whereas “Vistula”, “Konika” and “Cucrowa” had the lowest amounts of ash. The “Alba” cultivar contained significantly higher amounts of carbohydrate than the rest of the tested genotypes, whereas the carbohydrate contents in the “Olomuńcka”, “Osborne” and “Sonata” roots were the lowest overall. Finally, the “Vistula” and “Arat” cultivars showed the highest and lowest energetic value among the tested genotypes, respectively. To the best of our knowledge, this is the first report regarding the nutritional value of turnip-rooted parsley roots. However, similar variations were observed in the nutritional value of the leaves of the same turnip-rooted parsley cultivars by our team [
28], while Pokluda et al. [
23] suggested a varied composition of minerals in various root parsley cultivars. Moreover, Dobričević et al. [
13] and Golubkina et al. [
36] reported a great variation in the total soluble solid content of the roots and seeds, respectively, of various parsley types (e.g., plain- and curly-leaf and turnip-rooted parsley), which could be associated with differences in proximate analysis parameters. Finally, Pokluda [
37] suggested a great variation in the dry matter content of root parsley cultivars, indicating differences in the parameters that constitute the nutritional value.
The composition of tocopherols in the roots of the studied germplasms is presented in
Table 2. The only detected isoforms of vitamin E were α- and δ-tocopherols, which showed variable content levels depending on the cultivar. Therefore, although in most cultivars α-tocopherol was the most abundant compound, there were also genotypes, such as “Pólna”, “Linga”, “Alba”, “Arat” and “Root parsley”, where similar amounts of both tocopherols were detected. Regarding the variation among the tested cultivars, “Arat” roots were the richest in α- and total tocopherols; the same cultivar, as well as “Pólna” and “Root parsley”, had the highest levels of δ-tocopherol. A-tocopherol was previously reported in parsley roots by Horbowicz [
38], while Gómez-Coronado [
39] and Fernandes et al. [
28] suggested α- and γ-tocopherol as the only detected vitamin E vitamers in parsley leaves. In contrast, Saleh et al. [
40] identified all tocopherols in parsley aerial parts, with α-tocopherol being the most abundant isoform. However, apart from genotypic effects, growing conditions, such as red light dose, wavelength and pressure from stress factors, may also affect tocopherol composition [
41,
42].
The composition of free sugars in the roots of the studied parsley germplasms is presented in
Table 3. Four individual free sugars were detected, with sucrose being the most abundant compound (13.53–16.96 g/100 g dw), followed by apiose (2.93–5.55 g/100 g dw), glucose (1.3–3.47 g/100 g dw) and fructose (1.37–3.03 g/100 g dw). The “Sonata” cultivar had the highest sucrose content, while “Olomuńcka”, “Arat” and “Root parsley” were the richest in apiose; “Pólna” and “Kaśka” were the most abundant in glucose, and “Pólna” “Lenka”, “Kaśka” and “Cucrowa” contained the highest amounts of fructose. In contrast, the lowest sucrose content was detected in the “Osborne” and “Vistula” cultivars, while apiose and glucose levels were the lowest in “Berlinski Halblange Springer” and that of fructose was lowest in the “Osborne” cultivar. Finally, the highest and lowest total sugar contents were recorded in the “Kaśka” and “Berlinski Halblange Springer” cultivars, respectively. The same compounds constitute the free sugars identified in parsley leaves following a genotype-dependent composition pattern [
28], while Tkacz et al. [
43] also identified rhamnose but not apiose in a sea-buckthorn-based smoothie that contained the pulp of parsley roots. In contrast, in an early study by Horbowjcz et al. [
44], only sucrose, mannitol, raffinose and traces of fructose and glucose were detected, a difference which may be attributable to different protocols and analytical equipment. Apiose was identified in parsley in very early studies during the first half of the 20th century and is typical of the
Apium genus and species such as celery and parsley [
45].
The composition of organic acids in the studied roots is presented in
Table 4. The identified compounds included oxalic, malic, citric and succinic acid, while traces of ascorbic and fumaric acid were also detected. A variable composition was recorded among the tested parsley germplasms, with malic acid being the most abundant compound in most of the studied cultivars, namely “Olomuńcka”, “Pólna”, “Hanacka”, “Halblange Eagle”, “Alba”, “Arat”, “Root parsley” and “Berlinski Halblange Springer”. Moreover, succinic acid was the richest compound in the cultivars “Linga”, “Lenka”, “Sonata”, “Vistula” and “Konica”. Finally, oxalic acid was detected in the highest amounts in the cultivars “Osborne” and “Kaśka,” while citric acid was the most profound organic acid in the “Cucrowa” cultivar. Regarding the individual organic acids, the highest content of oxalic acid was recorded in the “Osborne” cultivar; malic acid in the “Root parsley” cultivar; citric acid in the “Linga” cultivar, where the highest total organic acid content was also detected; and succinic acid in the “Sonata” cultivar. In contrast, the lowest amounts of oxalic acid were detected in “Root parsley” and “Berlinski Halblange Springer”, and the lowest level of malic acid in “Vistula”; citric acid was the lowest in “Konika”, “Alba” and “Berlinski Halblange Springer”, while succinic acid was detected in the smallest amounts in “Cucrowa” and “Alba”. Finally, the lowest total organic acid content was identified in the “Alba” cultivar. The detected differences indicate a significant genotypic effect on organic acid composition, considering that all the cultivars were grown under the same conditions. Similar compositions of organic acids were reported for parsley shoots and leaves by Saleh et al. [
40] and Fernandes et al. [
28], although the former also identified isobutyric acid and the latter shikimic acid. Moreover, Gîrd al. [
46] suggested that parsley leaves should be considered a source of ascorbic acid, contributing to more than 140% of daily dietary intake. These contradictions are attributable to the different plant parts studied in these reports since, to the best of our knowledge, this is the first report on the organic acid composition of turnip-rooted parsley roots. However, Tkacz et al. [
43] reported a similar composition of organic acids in a sea-buckthorn-based beverage that contained the pulp of parsley roots, while Priecina et al. [
47] reported the presence of the same compounds, as well as salicylic and butyric acid, in celery roots.
The main fatty acid composition (relative %) of the studied parsley roots is presented in
Table 5. Twenty-one individual fatty acids were identified in all the studied samples (
Supplementary Table S1). The most abundant compounds were linoleic (47.9–57.1%, in “Olomuńcka” and “Sonata”, respectively) and palmitic acid (20.66–20.5% in “Halblange Eagle” and “Root parsley”, respectively), followed by oleic (5.57–10.43% in “Halblange Berlinska” and “Pólna”, respectively) and linolenic acid (5.12–9.63% in “Lenka” and “Kaśka”, respectively). Other fatty acids detected in quantities >1% were palmitic (0.928–1.847%, in “Halblange Eagle” and “Olomuńcka”, respectively), behenic (0.669–1.45%, “Konika” and “Olomuńcka”, respectively), eicosapentaenoic (0.87–3.98%, in “Berlinski Halblange Springer” and “Halblange Berlinska”, respectively) and lignoceric acids (0.813–2.19%, “Konika” and “Olomuńcka”, respectively). Moreover, the most abundant class of fatty acids was polyunsaturated fatty acids (PUFA: 56.7–66.5%), followed by saturated fatty acids (SFA: 25.7–35.57%) and monounsaturated fatty acids (MUFA: 6.03–10.9%). Similar results were reported by Makarenko et al. [
48], who also identified linoleic and palmitic acids as the major fatty acids in parsley roots and further suggested the effect of temperature on fatty acid composition. The same authors also highlighted the importance of the high PUFA content in vacuole membranes, since they are responsible for membrane plasticity and integrity [
48]. Moreover, in the early study by Ellenbracht et al. [
49] it was reported that, apart from linoleic and palmitic acids, there were two C18:1 fatty acids identified as (Z)-9 (oleic acid) and (Z)-11 (vaccenic acid) isomers, whereas petroselinic acid, which was detected mostly in seeds and in lesser amounts in leaves, was not detected in parsley roots. Another nutritional aspect related to fatty acid composition is that the ratio of n6/n3 fatty acids was higher than 4.0 for all the studied cultivars (values ranged between 4.45 and 7.49 in “Kaśka” and “Lenka”, respectively), which does not meet the recommended criteria for beneficial health effects (the recommended value of this ratio is below 4.0) [
50]. On the other hand, the ratio of PUFA/SFA was higher than 0.45 (values ranged between 1.59 and 2.56 in “Olomuńcka” and “Halblange Eagle”, respectively) in all the tested cultivars, which is typical of a healthy diet [
51].
The details regarding the identification of the detected phenolic compounds are presented in
Table 6; nineteen individual phenolic compounds were tentatively identified (a detailed profile of phenolic compounds is provided in
Supplementary Table S2). In particular, three phenolic acids were identified as caffeic,
p-coumaric acid and cinnamic acid derivatives (peaks 1 to 4), while the rest of the compounds were classified as hydrolyzable tannins (peaks 7 and 10) and flavonoids (peaks 4 to 6, 8, 9 and 11 to 19). The composition of the main phenolic compounds detected in parsley roots is presented in
Table 7. Variable content levels were recorded among the studied cultivars, and the most abundant compound was apigenin-
O-pentoside-
O-hexoside (peak 12), which was detected at 24 mg/g extract in “Berlinski Halblange Springer”, followed by apigenin-
O-acetyl-hexosyl-pentoside (peak 17: detected at 18.3 mg/g of extract in “Vistula”) and acetylated luteolin hexoxyl-rhamnoside (peak 18: detected at 7.36 mg/g extract in “Berlinski Halblange Springer”). Flavonoids were the most abundant class of polyphenols, with the highest overall content being recorded for “Konica” and “Berlinski Halblange Springer” (54 mg/g extract and 56 mg/g extract, respectively). The total hydrolyzable tannins and total phenolic acids were less abundant, and the highest content levels were observed in the “Pólna” and “Olomuńcka” cultivars (2.89 mg/g extract and 1.9 mg/g extract, respectively). Finally, “Berlinski Halblange Springer” was the cultivar with the highest total content of phenolic compounds (59 mg/g extract). The prevalence of flavonoids compared to phenolic acids was also reported by Emad et al. [
52], who evaluated total phenolic and flavonoid contents in parsley plant parts and also suggested that roots were more abundant in phenolic compounds than shoots. However, this finding was not confirmed by our team; a genotypic effect was recorded, and the phenolic content of the roots was not always higher than leaves in the tested cultivars [
27]. Moreover, in the study by Emad et al. [
52], a significant impact of the solvent was observed on the recovery of phenolic compounds, with methanol and water being more effective in leaf and root extracts, respectively.
In the recent study by Arsenov [
53], the phenolic compound profile of parsley roots was evaluated, and it was suggested that apiin was the most abundant polyphenol, followed by chlorogenic acid, scopoletin and ferulic acid. These differences in phenolic compound composition could be attributed to different protocols implemented since, according to Emad et al. [
52], the extraction method may affect the recovery efficiency of polyphenols. Phenolic acids, such as gallic and ferulic acid, have been previously reported in parsley leaves [
54], while Mazzucotelli et al. [
55] also identified gallic and hydroxybenzoic acids, Grúz et al. [
56] identified dicoumaric acid, Slighoua et al. [
57] identified gallic, ferulic and cinnamic acid and Derouich et al. [
58] identified caffeic, chlorogenic,
p-coumaric, ferulic, gallic, syringic and vannilic acid. Moreover, in the study by Tkacz et al. [
43], it was suggested that the addition of pulp from parsley roots significantly increased by almost seven times the total phenolic acid content in sea-buckthorn-based smoothies, while the increase was less profound for procyanidin polymers (approximately 1.5 times). However, there are several other studies where the presence of flavonoids was reported (e.g., apigenin and kaempferol derivatives [
27]; quercetin, apigenin, luteolin, isorhamnetin, kaempferol and chrysoeriol derivatives [
59,
60]; apigenin, apiin and cosmosiin [
61]; isorhamnetin, apigenin, diosmetin and hesperetin [
62];
p-coumaroyl hexoside, apigenin, luteolin, isorhamnetin, apigenin and diosmetin [
11] and other flavonoids [
63]).
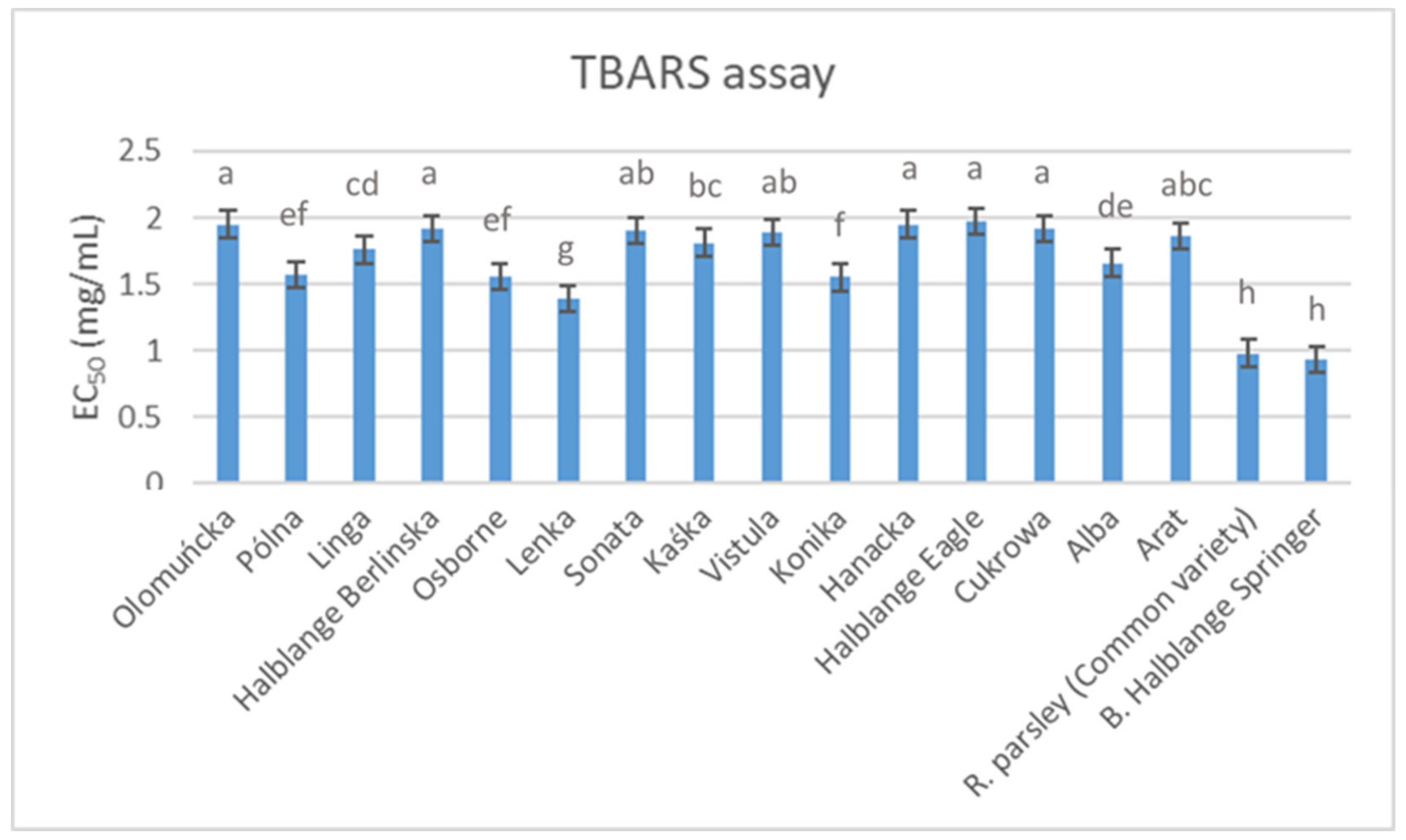
The antioxidant activity levels of the root extracts obtained from the studied parsley germplasms are presented in
Figure 1 and
Figure 2. The results obtained from the TBARS (
Figure 1) and OxHLIA (
Figure 2) assays differed, and the most effective (i.e., lower EC
50 values) cultivars for the TBARS assay (“Root parsley” and “Berlinski Halblange Springer”) were those with the lowest antioxidant activity for the OxHLIA assay after 120 min. In contrast, the “Vistula” cultivar, which was among the cultivars with the lowest effectiveness as determined by the TBARS assay, was the best-performing cultivar in the OXHLIA assay, recording the lowest overall IC
50 values at both 60 and 120 min. Comparing these results with those presented in
Table 7, it seems that there is no correlation between the phenolic compound content and the antioxidant activity in parsley roots. This finding is similar to previous reports regarding the antioxidant activity of parsley leaves [
27], roots [
52] and seeds [
64], whereas Epifanio et al. [
59] attributed the antioxidant activity observed in parsley seeds to apiin and apigenin. Moreover, El-Zaeddi et al. [
11], who evaluated the phenolic compound composition and the antioxidant activity in three Apiaceae species (coriander, dill and parsley), suggested a variable association between phenolic compound content and antioxidant activity, depending on the species and the tested assay. In contrast to these reports, several other studies identified phenolic compounds as significant contributors to the overall antioxidant activity of parsley plant parts [
14,
54,
58,
65], highlighting the important effect of the implemented assay, the extraction protocol, the plant part used for the extraction and the growing conditions.
The antimicrobial properties of the tested parsley root extracts are presented in
Table 8 and
Table 9. The results for the tests of antimicrobial activity of the studied extracts against several bacteria showed variable effects depending on the cultivar and the bacteria tested (
Table 8). In most cases, the root extracts were more efficient or similarly effective compared to the positive controls, and the MBC values of E211 against
Bacilus cereus and those of E224 against
Staphylococcus aureus and
Salmonella typhimurium were the most effective ones. Moreover, the root extracts of the “Olomuńcka” cultivar were effective against all the tested bacteria. Similarly, both the MIC and MBC values of E224 were the lowest against
Trichoderma viride. On the other hand, all the tested root extracts were more effective than positive controls against all the studied fungi (
Table 9), while the extracts of the “Root parsley” cultivar were among the most effective against almost all the tested fungi (except for
Aspergilus niger). Variable responses against the same microbial agents were also observed for the leaf extracts of the same cultivars tested in the current study [
27], while Abdu and Hauwa [
66] suggested a significant antibacterial activity for parsley leaf extracts that varied depending on the extraction protocol. Moreover, Farah et al. [
67] and Bodeta et al. [
68] highlighted the efficiency of parsley ethanolic extracts against
Candida tropical,
Salmonella typhi and
Staphylococcus aureus, while hot water extracts of parsley leaves were effective against
S. aureus,
Pseudomonas aeruginosa and
Streptococcus pyogenes [
7]. Other plant parts, such as seeds and fruit, exhibited strong antibacterial properties [
40,
69]; moreover, apart from solvent extracts, essential oils of parsley have shown significant antimicrobial properties, indicating the presence of potent bioactive compounds produced mostly via the shikimate pathway [
70,
71,
72,
73]. According to Marín et al. [
68], the antimicrobial properties of natural matrices are usually associated with the presence of dietary polyphenols that are metabolized by gut microbiota into simple bioactive compounds with diverse effects. The studies of Wolny-Koładka et al. [
74] and Roy et al. [
75] also suggested using the waste from parsley leaves or stems to produce silver nanoparticles with significant antimicrobial effects against
Klebsiella pneumonia,
S. aureus and
Escherichia coli.