Variation in Fruit Morphology and Seed Oil Fatty Acid Composition of Camellia oleifera Collected from Diverse Regions in Southern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Environmental Factors
2.4. Determination of Fatty Acid Content in Oil Tea
2.5. Determination of Phenotypic Characteristic
2.6. Statistical Analysis
3. Result
3.1. Fruit Phenotypic Characters and Variation Characteristics
3.2. The Relationship between Fruit Characters and Environmental Factors
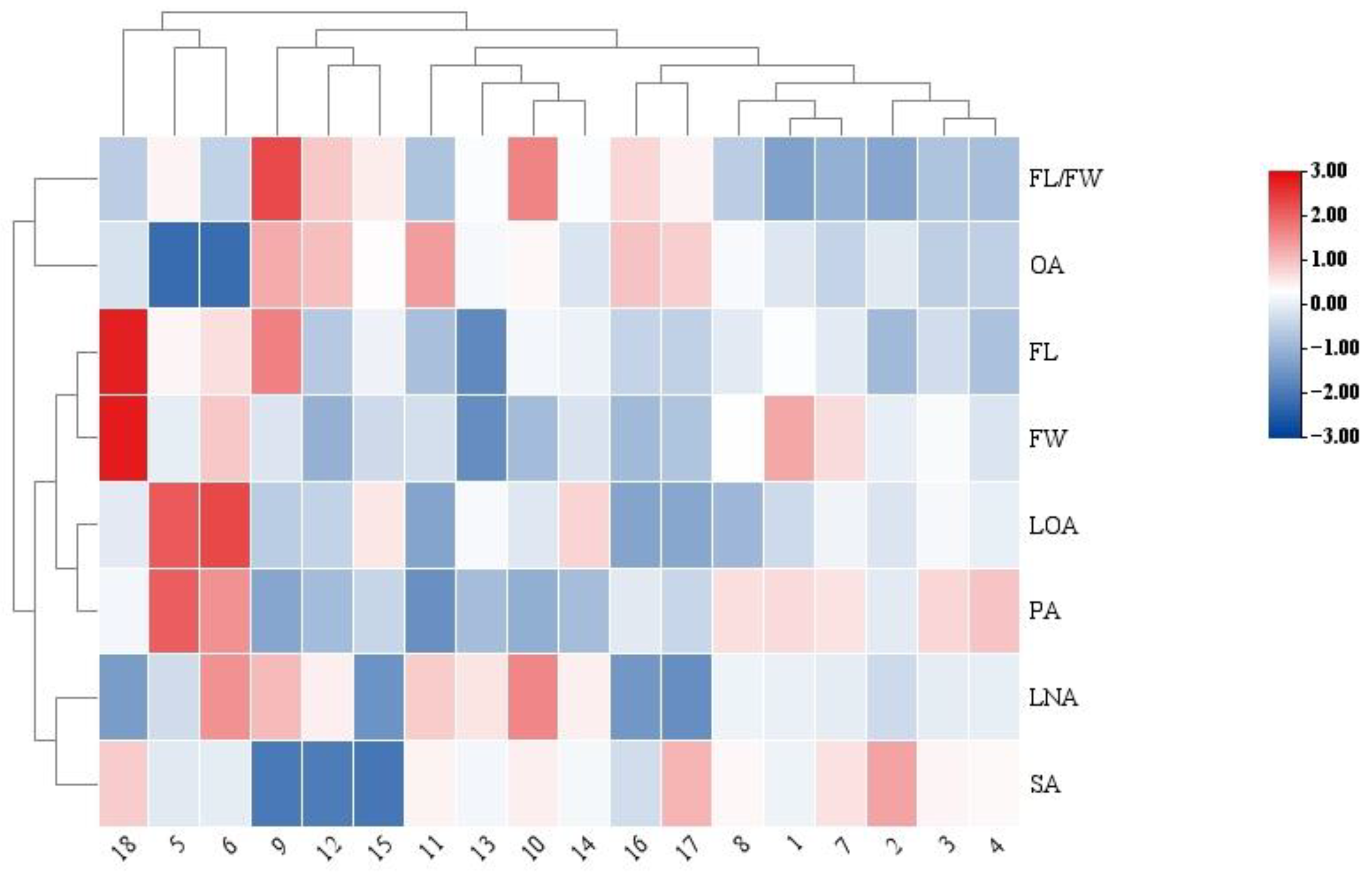
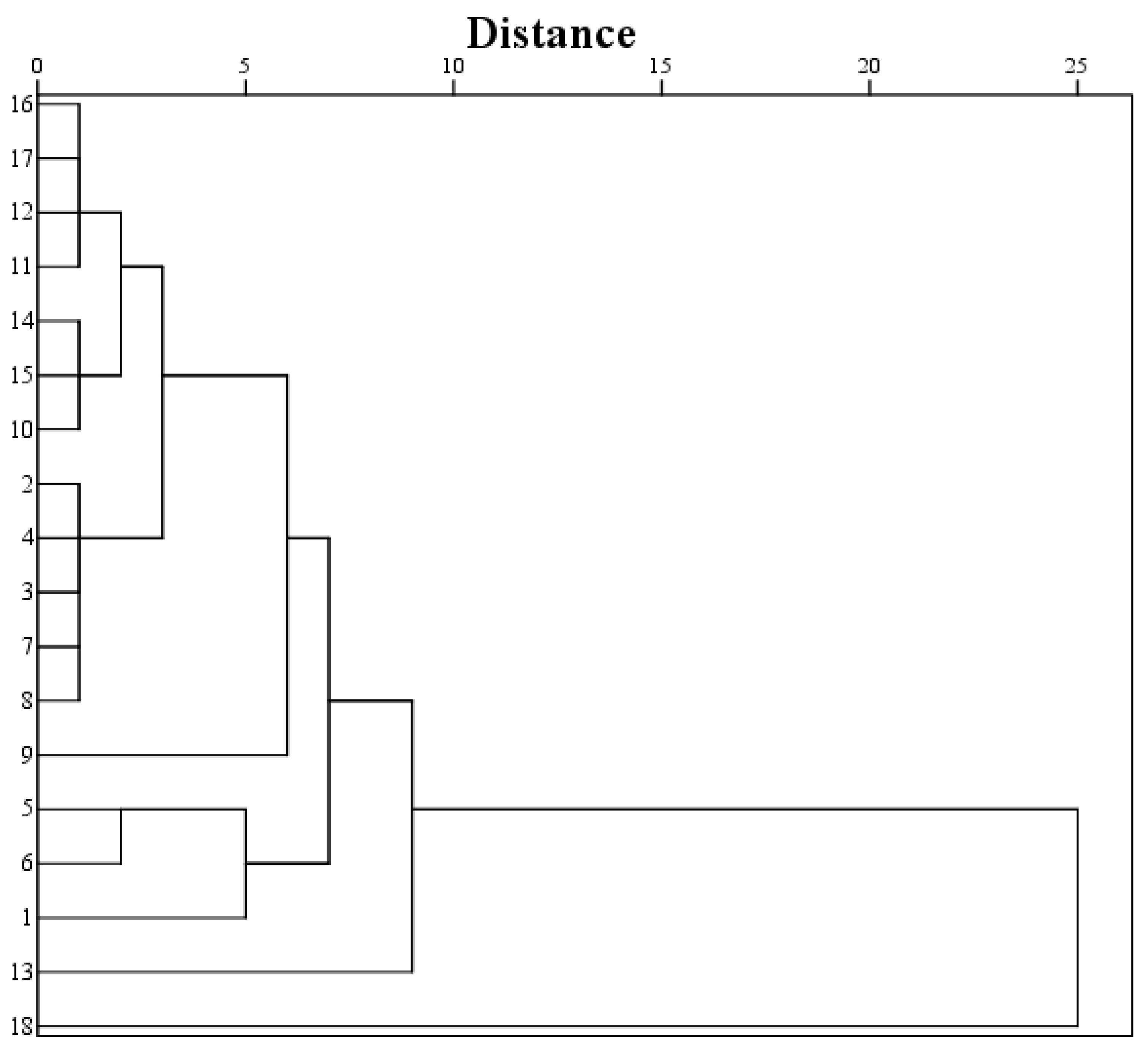
3.3. Principal Component Analysis, and Cluster Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Zhao, Y.L.; Yang, G.Y.; Peng, J.; Chen, S.W.; Xu, Z.G. Determination of the evolutionary pressure on Camellia oleifera on Hainan Island using the complete chloroplast genome sequence. PeerJ 2019, 7, e7210. [Google Scholar] [CrossRef]
- Yuan, J.; Han, Z.Q.; He, S.Y.; Huang, L.Y.; Zhou, N.F. Investigation and cluster analysis of main morphological and economical characters for oil tea resource in Hainan province. J. Plant Genet. Resour. 2014, 15, 1380–1384. [Google Scholar]
- Lin, P.; Wang, K.L.; Zhou, C.F.; Xie, Y.H.; Yao, X.H.; Yin, H.F. Seed transcriptomics analysis in Camellia oleifera uncovers genes associated with oil content and fatty acid composition. Int. J. Mol. Sci. 2018, 19, 118. [Google Scholar] [CrossRef]
- Fang, X.Z.; Du, M.H.; Luo, F.; Jin, Y.F. Physicochemical properties and lipid composition of Camellia fruit oil (Camellia oleifera Abel.) extracted using different methods. Food Sci. Technol. Res. 2015, 21, 779–785. [Google Scholar] [CrossRef]
- Luan, F.; Zeng, J.S.; Yang, Y.; He, X.R.; Wang, B.J.; Gao, Y.B.; Zeng, N. Recent advances in Camellia oleifera Abel: A review of nutritional constituents, biofunctional properties, and potential industrial applications. J. Funct. Foods 2020, 75, 104242. [Google Scholar] [CrossRef]
- McKevith, B. Nutritional aspects of oilseeds. Nutr. Bull. 2005, 30, 13–26. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Souza, P.R.; Peghini, B.C.; da Silva, J.S.; Cardoso, C.R. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [PubMed]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary linoleic acid and risk of coronary heart disease: A systematic review and meta-analysis of prospective cohort studies. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhang, S.B.; Zhang, Y.P.; Kitajima, K. Effects of phylogeny and climate on seed oil fatty acid composition across 747 plant species in China. Ind. Crops Prod. 2015, 63, 1–8. [Google Scholar] [CrossRef]
- Yang, C.Y.; Liu, X.M.; Chen, Z.Y.; Lin, Y.S.; Wang, S.Y. Comparison of oil content and fatty acid profile of ten new Camellia oleifera cultivars. J. Lipids 2016, 2016, 3982486. [Google Scholar] [CrossRef]
- Munasinghe, M.; Wansapala, J. Study on variation in seed morphology, oil content and fatty acid profile of Madhuca longifolia grown in different agro-climatic zones in Sri Lanka. Sci. Res. 2015, 3, 105–109. [Google Scholar] [CrossRef]
- Ruraż, K.; Piwowarczyk, R.; Gajdoš, P.; Krasylenko, Y.; Certík, M. Fatty acid composition in seeds of holoparasitic Orobanchaceae from the Caucasus region: Relation to species, climatic conditions and nutritional value. Phytochemistry 2020, 179, 112510. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Lu, X.H.; Wang, Q.Q.; Han, H.Y.; Zhang, X.M.; Ma, Y.H.; Gan, X.H. Leaf phenotypic variation of endangered plant Tetracentron sinense Oliv. and influence of geographical and climatic factors. J. For. Res. 2021, 32, 623–636. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; Zhang, S.; Azam, M.; Shaibu, A.S.; Feng, Y.; Li, Y.; Tian, Y.; Hong, H.; Li, B.; Sun, J. Profiling of fruit fatty acid composition in 1025 Chinese soybean accessions from diverse ecoregions. Crop J. 2022, 8, 635–644. [Google Scholar] [CrossRef]
- Kumar, V.; Rani, A.; Solanki, S.; Hussain, S.M. Influence of growing environment on the biochemical composition and physical characteristics of soybean fruit. J. Food Compos. Anal. 2006, 19, 188–195. [Google Scholar] [CrossRef]
- Song, W.W.; Yang, R.P.; Wu, T.T.; Wu, C.X.; Sun, S.; Zhang, S.W.; Jiang, B.J.; Tian, S.Y.; Liu, X.B.; Han, T.F. Analyzing the effects of climate factors on soybean protein, oil contents, and composition by extensive and high-density sampling in China. J. Agric. Food Chem. 2016, 64, 4121–4130. [Google Scholar] [CrossRef]
- Aslam, M.N.; Nelson, M.N.; Kailis, S.G.; Bayliss, K.L.; Speijers, J.; Cowling, W.A. Canola oil increases in polyunsaturated fatty acids and decreases in oleic acid in drought-stressed Mediterranean-type environments. Plant Breed. 2009, 128, 348–355. [Google Scholar] [CrossRef]
- Singer, S.D.; Zou, J.; Weselake, R.J. Abiotic factors influence plant storage lipid accumulation and composition. Plant Sci. 2016, 243, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, S.; Asadi-Gharneh, H.A.; Miransari, M. The phytochemical variability of fatty acids in basil seeds (Ocimum basilicum L.) affected by genotype and geographical differences. Food Chem. 2019, 276, 700–706. [Google Scholar] [CrossRef]
- Yaklich, R.W.; Vinyard, B.; Camp, M.; Douglass, S. Analysis of fruit protein and oil from soybean northern and southern region uniform tests. Crop Sci. 2002, 42, 1504–1515. [Google Scholar] [CrossRef]
- Wu, T.T.; Yang, X.S.; Sun, S.; Wang, C.J.; Wang, Y.; Jia, H.C.; Man, W.Q.; Fu, L.S.; Song, W.W.; Wu, C.X.; et al. Temporal–spatial characterization of fruit proteins and oil in widely grown soybean cultivars across a century of breeding in China. Crop Sci. 2017, 57, 748–759. [Google Scholar] [CrossRef]
- Alcántara-Ayala, O.; Oyama, K.; Ríos-Muñoz, C.A.; Rivas, G.; Ramirez-Barahona, S.; Luna-Vega, I. Morphological variation of leaf traits in the Ternstroemia lineata species complex (Ericales: Penthaphylacaceae) in response to geographic and climatic variation. PeerJ 2020, 8, e8307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, X.Q.; Yu, M.K.; Han, Y.Z.; Wu, T.G. Variations in seed size and seed mass related to tree growth over 5 years for 23 provenances of Quercus acutissima from across China. J. For. Res. 2017, 28, 917–923. [Google Scholar] [CrossRef]
- Wirth, L.R.; Graf, R.; Gugerli, F.; Landergott, U.; Holderegger, R. Between-year variation in fruit weights across altitudes in the high-alpine plant Eritrichium nanum. Plant Ecol. 2010, 207, 227–231. [Google Scholar] [CrossRef]
- Yi, F.Y.; Wang, Z.R.; Baskin, C.C.; Baskin, J.M.; Ye, R.H.; Sun, H.L.; Zhang, Y.Y.; Ye, X.H.; Liu, G.F.; Yang, X.J.; et al. Seed germination responses to seasonal temperature and drought stress are species-specific but not related to fruit size in a desert steppe: Implications for effect of climate change on community structure. Ecol. Evol. 2019, 9, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.F.; Chen, Y.Z.; Zhang, R.Q.; Yang, X.H.; Wang, X.N.; Lu, J. Classification of Fruit Shape and Color and Analysis of the Economic Traits of Oil-tea Camellia. J. Cent. South Univ. For. Technol. 2018, 27, 33–39. [Google Scholar]
- Qi, J.M.; Zhang, P.; Xi, R.C.; Gao, L.; Lu, C. Genetic variation and correlation analysis of fruit characters of Camellia gauchowensis Chang. J. Cent. South Univ. For. Technol. 2018, 38, 108–113. [Google Scholar]
- Zhong, F.X.; Wang, R.H.; Liao, W.T.; Li, T.; Zhou, Y.J. Effects of environmental factors on fruit diameter growth in Camellia oleifera at high temperature and less rain period. Nonwood For. Res. 2015, 33, 50–55. [Google Scholar]
- Su, S.; Wu, J.; Peng, X.Y.; Li, B.; Li, Z.J.; Wang, W.; Ni, J.W.; Xu, X.Q. Genetic and agro-climatic variability in seed fatty acid profiles of Akebia trifoliata (Lardizabalaceae) in China. J. Food Compos. Anal. 2021, 102, 104064. [Google Scholar] [CrossRef]
- Fang, H.; Bai, H. Cultural distribution and site classification for Camellia oleifera. Sci. Silvae Sin. 2002, 38, 64–72. [Google Scholar]
- Ma, Y.X.; Wang, S.X.; Liu, X.J.; Yu, H.Y.; Dan, Y.; Li, G.T.; Wang, L.B. Oil content, fatty acid composition and biodiesel properties among natural provenances of Siberian apricot (Prunus sibirica L.) from China. GCB Bioenergy 2021, 13, 112–132. [Google Scholar] [CrossRef]
- Wang, B.F.; Zou, F.; Yuan, D.Y.; Yuan, J.; Xiao, S.X.; Gao, C.; Li, Z.; Peng, X.B. Comprehensive evaluation of fruit economic character and premium species selection of Camellia oleifera in Hainan. J. Fujian Agric. For. Univ. 2016, 45, 156–161. [Google Scholar]
- Liu, J.F.; Deng, Y.P.; Wang, X.F.; Ni, Y.Y.; Wang, Q.; Xiao, W.F.; Lei, J.P.; Jiang, Z.P.; Li, M.H. The concentration of non-structural carbohydrates, N, and P in Quercus variabilis does not decline toward its northernmost distribution range along a 1500 km transect in China. Front. Plant Sci. 2018, 9, 1444. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Slave, C. Using arcmap to create a cadastral database. Case study. J. Inf. Syst. Oper. Manag. 2018, 12, 180–190. [Google Scholar]
- Alena, J.; Gösta, E.; Ingegerd, D.; Jan, I. Studies on frost hardiness of Pinus contorta Dougl. seedlings grown in climate chambers. Studia For. Suec. 1981, 157, 4–47. [Google Scholar]
- Zhang, F.H.; Li, Z.; Zhou, J.Q.; Gu, Y.Y.; Tan, X.F. Comparative study on fruit development and oil synthesis in two cultivars of Camellia oleifera. BMC Plant Biol. 2021, 21, 348. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.F.; Zang, R.G.; Liu, H.; Bai, Z.Q.; Guo, Z.J.; Ding, Y. Geographic variation of seed morphological traits of Picea schrenkiana var. tianschanica in Tianshan Mountains, Xinjiang of Northwest China. Chin. J. Appl. Ecol 2012, 23, 1455–1461. [Google Scholar]
- Smith, I.M.; Forbes, A.B. Algorithms and software for fitting polynomial functions constrained to pass through the origin. J. Phys. Conf. Ser. 2018, 1065, 212022. [Google Scholar] [CrossRef]
- Sun, J.C.; Shi, W.S.; Wu, Y.Y.; Ji, J.; Feng, J.; Zhao, J.B.; Shi, X.R.; Du, C.J.; Chen, W.; Liu, J.F.; et al. Variations in Acorn Traits in Two Oak Species: Quercus mongolica Fisch. ex Ledeb. and Quercus variabilis Blume. Forests 2021, 12, 1755. [Google Scholar] [CrossRef]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using Canoco; Cambridge University Press: Cambridge, UK, 2003; p. 282. [Google Scholar]
- Li, H.C.; Gan, X.H.; Zhang, Z.P.; Zhang, C.; Song, L. Effects of altitudes and the DBH of seed trees on biological characteristics of Tetracentron sinense (Tetracentraceae) seeds. Plant Divers. Resour. 2015, 37, 177–183. [Google Scholar]
- Poljak, I.; Idžojti’c, M.; Šapi’c, I.; Korijan, P.; Vukeli’c, J. Diversity and structure of Croatian continental and Alpine-Dinaric populations of grey alder (Alnus incana L./Moench subsp. incana): Isolation by distance and environment explains phenotypic divergence. Sumar. List 2018, 142, 19–32. [Google Scholar]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Yang, L.R.; Zhang, Z.L.; Chen, J.L.; Cheng, X.; Ji, Q.M.; Zhang, D.J. The quantitative characters and diversity of oil tea fruit in Hainan province. Nonwood For. Res. 2018, 36, 69–76. [Google Scholar]
- Zhang, Y.Z.; Li, Y.Q.; Lin, M.; Liu, Y.J.; Ding, X.G.; Cai, J. Variation analysis of fruit characteristics in Camellia gauchowensis. Nonwood For. Res. 2014, 32, 1–8. [Google Scholar]
- Zhou, W.C.; Wang, Z.W.; Dong, L.; Wen, Q.; Huang, W.Y.; Li, T.; Ye, J.S.; Xu, L.A. Analysis on the character diversity of fruit and seed of Camellia chekiangoleosa. J. Nanjing For. Univ. 2021, 45, 51–59. [Google Scholar]
- Yang, L.; Gao, C.; Xie, J.J.; Qiu, J.; Deng, Q.E.; Zhou, Y.C.; Liao, D.S.; Deng, C.Y. Fruit economic characteristics and yields of 40 superior Camellia oleifera Abel plants in the low-hot valley area of Guizhou Province, China. Sci. Rep. 2022, 12, 7068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Lu, X.J.; Zhou, Q.; Deng, J.F. Morphological Variability between Geographical Provenances of Walnut Fruit (Juglans mandshurica) in the Eastern Liaoning Province, PR China. Pol. J. Environ. Stud. 2021, 30, 4353–4364. [Google Scholar] [CrossRef]
- Liang, S.; Rong, X.; Sai, L.; Chen, J.; Xu, C.Q.; Xie, C.X.; Liu, T.N. Phenotypic variation of seed traits of Haloxylon ammodendron and its affecting factors. Biochem. Syst. Ecol. 2015, 60, 81–87. [Google Scholar] [CrossRef]
- Sun, C.W.; Wang, J.W.; Duan, J.; Zhao, G.C.; Weng, X.H.; Jia, L.M. Association of fruit and seed traits of Sapindus mukorossi germplasm with environmental factors in Southern China. Forests 2017, 8, 491. [Google Scholar] [CrossRef]
- Sarikhani, S.; Arzani, K.; Roozban, M.R. Correlations of certain high heritability horticultural traits in Persian walnut (Juglans regia L.). Acta Hortic. 2014, 1050, 61–68. [Google Scholar] [CrossRef]
- Li, Z.X.; Wang, W.H.; Zhang, H.X.; Liu, J.H.; Shi, B.Y.; Dai, W.Z.; Liu, K.W.; Zhang, H.G. Diversity in Fruit Morphology and Nutritional Composition of Juglans mandshurica Maxim in Northeast China. Front. Plant Sci. 2022, 13, 820457. [Google Scholar] [CrossRef]
- She, C.Q.; Fang, S.Z.; Yang, W.X. Geographical variation of morphologic characteristics of Cyclocarya paliurus seeds. J. Nanjing For. Univ. 2008, 32, 63–66. [Google Scholar]
- Wu, Y.H.; Fan, Z.L.; Li, J.; Guo, J.H.; Guo, Y.K.; Wang, Y.L. Phenotypic diversity of seeds and fruits in natural populations of Acer ginnala in China. Guihaia 2018, 38, 795–803. [Google Scholar]
- Mustiga, G.M.; Morrissey, J.; Stack, J.C.; DuVal, A.; Royaert, S.; Jansen, J.; Bizzotto, C.; Villela-Dias, C.; Mei, L.; Cahoon, E.B.; et al. Identification of climate and genetic factors that control fat content and fatty acid composition of Theobroma cacao L. beans. Front. Plant Sci. 2019, 10, 1159. [Google Scholar] [CrossRef] [PubMed]
- Rouina, Y.B.; Zouari, M.; Zouari, N.; Rouina, B.B.; Bouaziz, M. Olive tree (Olea europaea L. cv. Zelmati) grown in hot desert climate: Physio-biochemical responses and olive oil quality. Sci. Hortic. 2020, 261, 108915. [Google Scholar] [CrossRef]
- Martínez-Díaz, Y.; González-Rodríguez, A.; Rico-Ponce, H.R.; Rocha-Ramírez, V.; Ovando-Medina, I.; Espinosa-García, F.J. Fatty acid diversity is not associated with neutral genetic diversity in native populations of the biodiesel plant Jatropha curcas L. Chem. Biodivers 2017, 14, e1600188. [Google Scholar] [CrossRef] [PubMed]
- Ghebretinsae, A.G.; Graham, S.A.; Camilo, G.R.; Barber, J.C. Natural infraspecific variation in fatty acid composition of Cuphea (Lythraceae) seed oils. Ind. Crops Prod. 2008, 27, 279–287. [Google Scholar] [CrossRef]
- Sun, C.W.; Jia, L.M.; Xi, B.Y.; Wang, L.C.; Weng, X.H. Natural variation in fatty acid composition of Sapindus spp. seed oils. Ind. Crops Prod. 2017, 102, 97–104. [Google Scholar] [CrossRef]
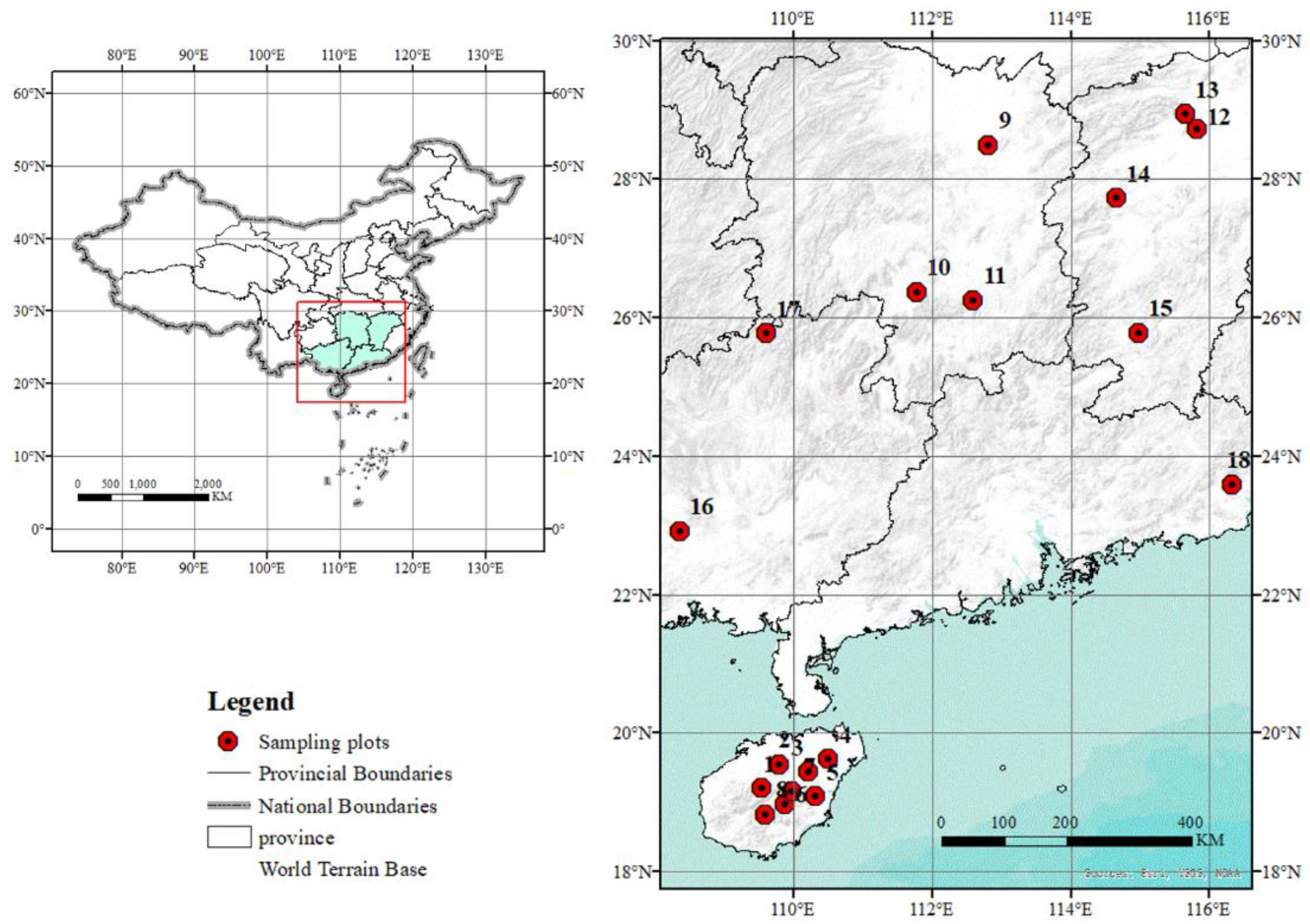


| Site | Site Location, Province | Code | LON (° E) | LAT (° N) | ALT (m) | MAT (°C) | MTW (°C) | AP (mm) | PWQ (mm) | ELAT (°) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Baisha City, Hainan | BSH | 109.54 | 19.21 | 210 | 23.72 | 31.44 | 1597 | 773 | 18.76 |
| 2 | Chengmai City, Hainan | CMH | 109.78 | 19.56 | 157 | 23.93 | 32.11 | 1739 | 830 | 18.84 |
| 3 | Dingan City, Hainan | DAH | 110.21 | 19.45 | 110 | 24.13 | 32.16 | 1713 | 784 | 18.50 |
| 4 | Haikou City, Hainan | HKH | 110.50 | 19.63 | 41 | 24.14 | 32.28 | 1743 | 784 | 18.34 |
| 5 | Qionghai City, Hainan | QHH | 110.31 | 19.09 | 60 | 24.72 | 32.44 | 1669 | 780 | 17.89 |
| 6 | Qiongzhong City, Hainan | QZH | 109.86 | 18.98 | 352 | 23.31 | 30.59 | 1654 | 800 | 19.35 |
| 7 | Tunchang City, Hainan | TCH | 109.98 | 19.16 | 217 | 23.67 | 31.21 | 1676 | 800 | 18.75 |
| 8 | Wuzhishan City, Hainan | WZH | 109.58 | 18.83 | 748 | 22.05 | 28.90 | 1581 | 788 | 22.03 |
| 9 | Changsha City, Hunan | CSH | 112.81 | 28.51 | 34.5 | 17.45 | 32.79 | 1347 | 575 | 27.18 |
| 10 | Yongzhou City, Hunan | YZH | 111.77 | 26.38 | 163.1 | 18.20 | 33.17 | 1368 | 593 | 25.70 |
| 11 | Hengyang City, Hunan | HYH | 112.59 | 26.27 | 115.3 | 18.43 | 33.68 | 1422 | 618 | 25.35 |
| 12 | Nanchang City, Jiangxi | NCJ | 115.82 | 28.74 | 29.3 | 17.76 | 33.49 | 1572 | 734 | 27.39 |
| 13 | Jiujiang City, Jiangxi | JJJ | 115.65 | 28.95 | 25.9 | 17.40 | 33.29 | 1498 | 696 | 27.58 |
| 14 | Xinyu City, Jiangxi | XYJ | 114.65 | 27.74 | 129.5 | 16.97 | 32.08 | 1540 | 680 | 26.89 |
| 15 | Ganzhou City, Jiangxi | GZJ | 114.98 | 25.79 | 249.6 | 19.08 | 33.32 | 1481 | 623 | 25.54 |
| 16 | Nanning City, Guangxi | NNG | 108.35 | 22.92 | 117.2 | 21.16 | 31.61 | 1480 | 705 | 22.01 |
| 17 | Liuzhou City, Guangxi | LZG | 109.60 | 25.79 | 182.6 | 18.18 | 31.98 | 1512 | 709 | 25.20 |
| 18 | Jieyang City, Guangdong | JYG | 116.33 | 23.60 | 12 | 21.75 | 32.02 | 1546 | 661 | 22.16 |
| Site | FL (mm) | FW (mm) | FL/FW | OA (%) | LOA (%) | LNA (%) | PA (%) | SA (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 43.80 cd | 55.04 b | 0.80 d | 78.94 efg | 7.43 de | 0.60 efg | 11.28 bcd | 1.75 efgh |
| 2 | 36.00 h | 44.34 ef | 0.81 d | 79.09 efg | 7.86 cd | 0.50 g | 9.85 cdef | 2.70 a |
| 3 | 40.05 efg | 46.21 de | 0.87 cd | 77.31 g | 8.75 bcd | 0.58 fg | 11.37 bcd | 1.99 de |
| 4 | 36.81 h | 43.04 fg | 0.86 cd | 77.36 g | 8.33 bcd | 0.59 fg | 11.76 bc | 1.96 def |
| 5 | 44.67 cd | 44.23 ef | 1.01 abcd | 70.21 h | 13.78 a | 0.51 g | 13.89 a | 1.62 gh |
| 6 | 46.44 c | 51.88 c | 0.90 cd | 70.30 h | 14.26 a | 0.98 ab | 12.80 ab | 1.66 fgh |
| 7 | 41.58 def | 50.11 c | 0.83 cd | 77.62 fg | 8.56 bcd | 0.58 fg | 11.09 bcd | 2.16 cd |
| 8 | 41.62 def | 46.82 d | 0.89 cd | 80.37 defg | 5.87 ef | 0.61 efg | 11.18 bcd | 1.97 def |
| 9 | 53.47 b | 43.00 fg | 1.24 a | 84.57 a | 6.83 def | 0.86 bc | 7.63 fg | 0.12 i |
| 10 | 42.95 de | 37.11 hi | 1.16 ab | 81.05 cde | 8.00 cd | 1.01 a | 7.89 fg | 2.04 de |
| 11 | 36.53 h | 42.16 fg | 0.87 cd | 85.23 ab | 5.03 f | 0.81 cd | 6.93 g | 2.01 de |
| 12 | 37.76 gh | 35.42 i | 1.07 abc | 83.7 bc | 7.09 de | 0.71 def | 8.34 efg | 0.16 i |
| 13 | 30.16 i | 30.33 j | 0.99 bcd | 80.28 defg | 8.81 bcd | 0.74 cde | 8.36 efg | 1.8 efg |
| 14 | 42.47 de | 42.74 g | 0.99 bcd | 78.87 efg | 10.24 b | 0.71 def | 8.35 efg | 1.82 efg |
| 15 | 42.55 de | 41.54 g | 1.02 abcd | 80.83 cdef | 9.75 bc | 0.18 h | 9.16 def | 0.08 i |
| 16 | 38.92 fgh | 36.9 hi | 1.05 abcd | 83.5 bc | 5.02 f | 0.20 h | 9.82 cdef | 1.46 h |
| 17 | 38.54 fgh | 38.22h | 1.01 abcd | 82.95 bcd | 5.13 f | 0.16 h | 9.21 def | 2.55 ab |
| 18 | 60.77 a | 68.26 a | 0.89 cd | 78.66 efg | 8.14 cd | 0.22 h | 10.24 cde | 2.35 bc |
| Max | 60.77 | 68.26 | 1.24 | 85.23 | 14.26 | 1.01 | 13.89 | 2.70 |
| Min | 30.16 | 30.33 | 0.80 | 70.21 | 5.02 | 0.16 | 6.93 | 0.08 |
| Mean | 41.95 | 44.30 | 0.96 | 79.49 | 8.27 | 0.59 | 9.95 | 1.68 |
| SD | 6.63 | 8.25 | 0.12 | 4.05 | 2.52 | 0.25 | 1.84 | 0.76 |
| CV/% | 15.81 | 18.63 | 12.51 | 5.09 | 30.42 | 43.27 | 18.53 | 45.24 |
| LON (° E) | ELAT (°) | ALT (m) | MAT (°C) | MTW (°C) | AP (mm) | PWQ (mm) | |
|---|---|---|---|---|---|---|---|
| FL | 0.191 | −0.070 | −0.029 | 0.092 | −0.185 | −0.193 | −0.289 |
| FW | −0.039 | −0.516 * | 0.144 | 0.531 * | −0.438 | 0.312 | 0.216 |
| FL/FW | 0.309 | 0.680 ** | −0.261 | −0.675 ** | 0.418 | −0.728 ** | −0.697 ** |
| OA | 0.261 | 0.681 ** | −0.121 | −0.682 ** | 0.361 | −0.661 ** | −0.598 ** |
| LOA | 0.070 | −0.340 | −0.040 | 0.349 | −0.055 | 0.405 | 0.294 |
| LNA | 0.057 | 0.154 | 0.051 | −0.166 | 0.082 | −0.149 | −0.093 |
| PA | −0.529 * | −0.867 ** | 0.269 | 0.880 ** | −0.582 * | 0.763 ** | 0.765 ** |
| SA | −0.411 | −0.449 | 0.131 | 0.392 | −0.353 | 0.388 | 0.411 |
| Comparison | r | p-Value | Significance |
|---|---|---|---|
| Morphological, Geographic | 0.304 | 0.0014 | ** |
| Morphological, Climate | 0.0826 | 0.192 | ns |
| Fatty acid composition, Geographic | 0.243 | 0.0081 | ** |
| Fatty acid composition, Climate | 0.272 | 0.0029 | ** |
| Principal Component | PC1 | PC2 | PC3 |
|---|---|---|---|
| FL | 0.359 | 0.365 | 0.831 |
| FW | 0.715 | −0.131 | 0.621 |
| FL/FW | −0.607 | 0.659 | 0.159 |
| OA | −0.905 | −0.277 | 0.295 |
| LOA | 0.687 | 0.596 | −0.293 |
| LNA | −0.067 | 0.480 | −0.416 |
| PA | 0.876 | 0.021 | −0.145 |
| SA | 0.409 | −0.692 | −0.192 |
| Eigenvalue | 3.24 | 1.73 | 1.50 |
| Variance (%) | 40.50 | 21.57 | 18.80 |
| % Total Variance | 40.50 | 62.07 | 80.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Wang, B.; Liu, F.; Zhao, J.; Yuan, J.; Xiao, S.; Masabni, J.; Zou, F.; Yuan, D. Variation in Fruit Morphology and Seed Oil Fatty Acid Composition of Camellia oleifera Collected from Diverse Regions in Southern China. Horticulturae 2022, 8, 818. https://doi.org/10.3390/horticulturae8090818
Gao S, Wang B, Liu F, Zhao J, Yuan J, Xiao S, Masabni J, Zou F, Yuan D. Variation in Fruit Morphology and Seed Oil Fatty Acid Composition of Camellia oleifera Collected from Diverse Regions in Southern China. Horticulturae. 2022; 8(9):818. https://doi.org/10.3390/horticulturae8090818
Chicago/Turabian StyleGao, Shuang, Bifang Wang, Fandeng Liu, Junru Zhao, Jun Yuan, Shixin Xiao, Joseph Masabni, Feng Zou, and Deyi Yuan. 2022. "Variation in Fruit Morphology and Seed Oil Fatty Acid Composition of Camellia oleifera Collected from Diverse Regions in Southern China" Horticulturae 8, no. 9: 818. https://doi.org/10.3390/horticulturae8090818
APA StyleGao, S., Wang, B., Liu, F., Zhao, J., Yuan, J., Xiao, S., Masabni, J., Zou, F., & Yuan, D. (2022). Variation in Fruit Morphology and Seed Oil Fatty Acid Composition of Camellia oleifera Collected from Diverse Regions in Southern China. Horticulturae, 8(9), 818. https://doi.org/10.3390/horticulturae8090818







