Hormone–Flavonoid Patterns in Two Genotypes of Campanula portenschlagiana with Distinct Adventitious Rooting Competence
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Morphological and Physiological Analyses
2.3. Plant Hormone Analyses
2.4. Flavonoid Analyses
2.5. Statistical Analyses
3. Results
3.1. Deep Blue Ocean and White Ocean Genotypes Present Differences in the Flavonoid Composition of Petals
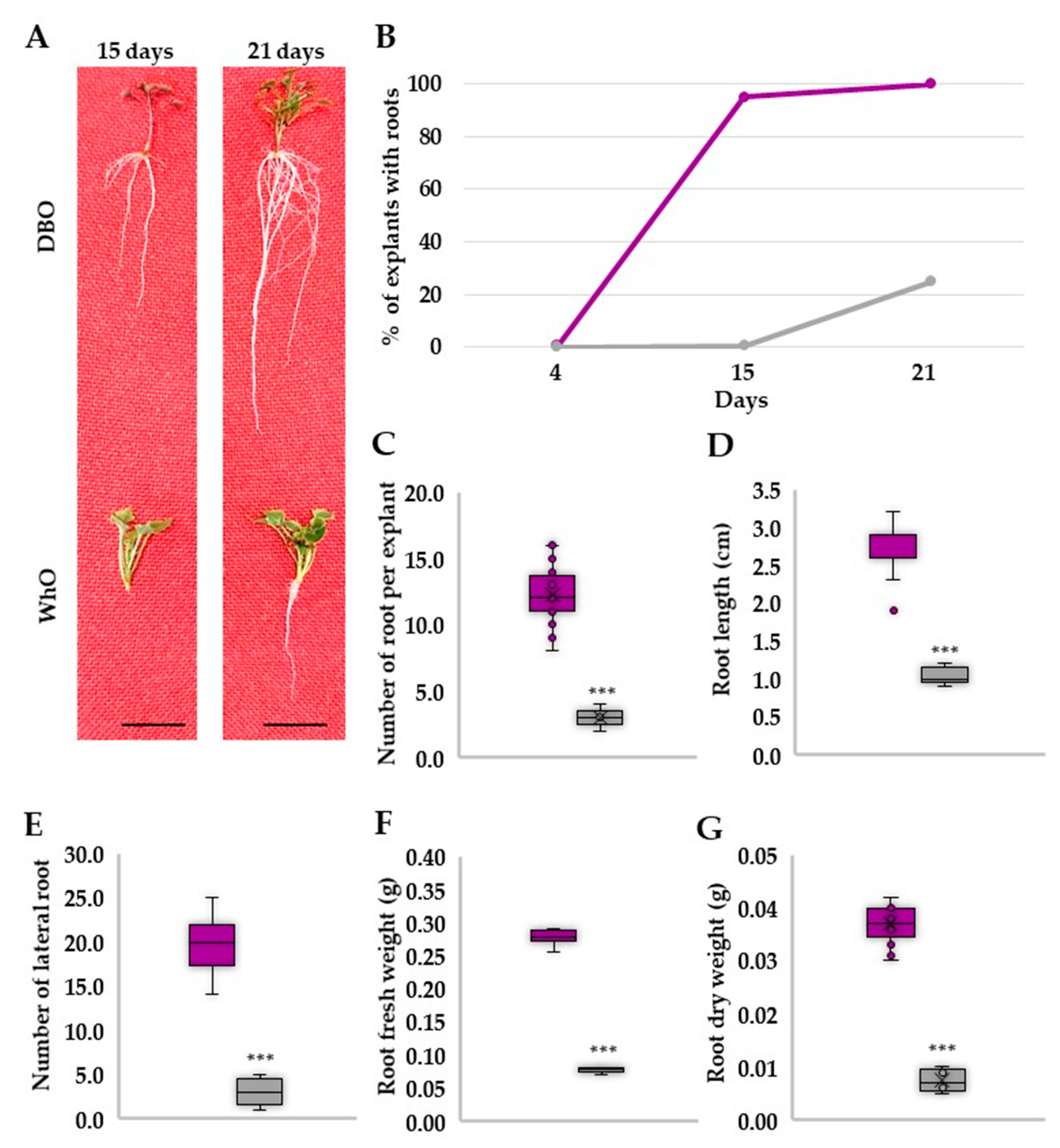
3.2. Deep Blue Ocean and White Ocean Genotypes Have Contrasting Efficiency to Adventitious Root Formation
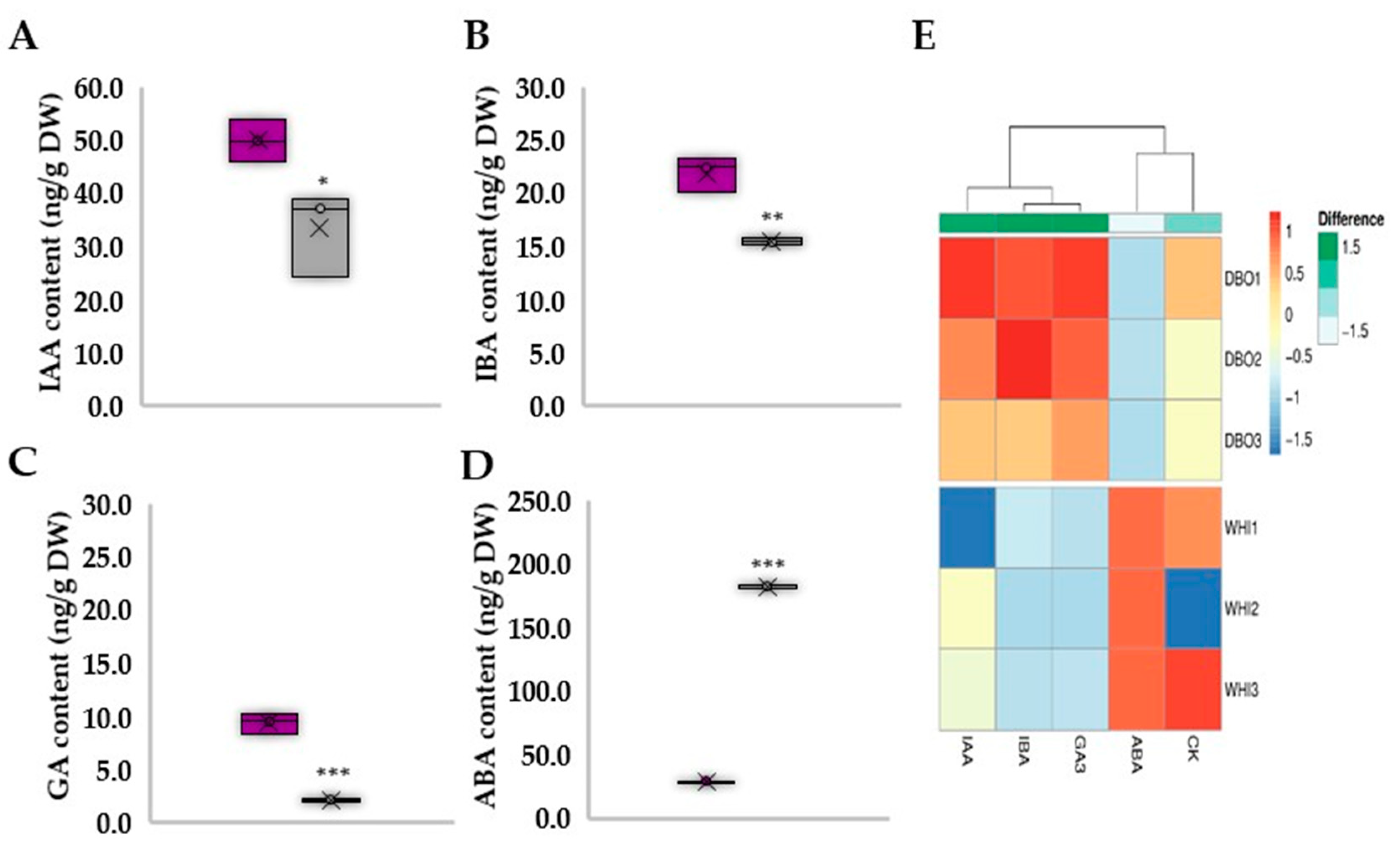
3.3. Deep Blue Ocean and White Ocean Genotypes Show Discrepant Concentrations of Endogenous Plant Hormones
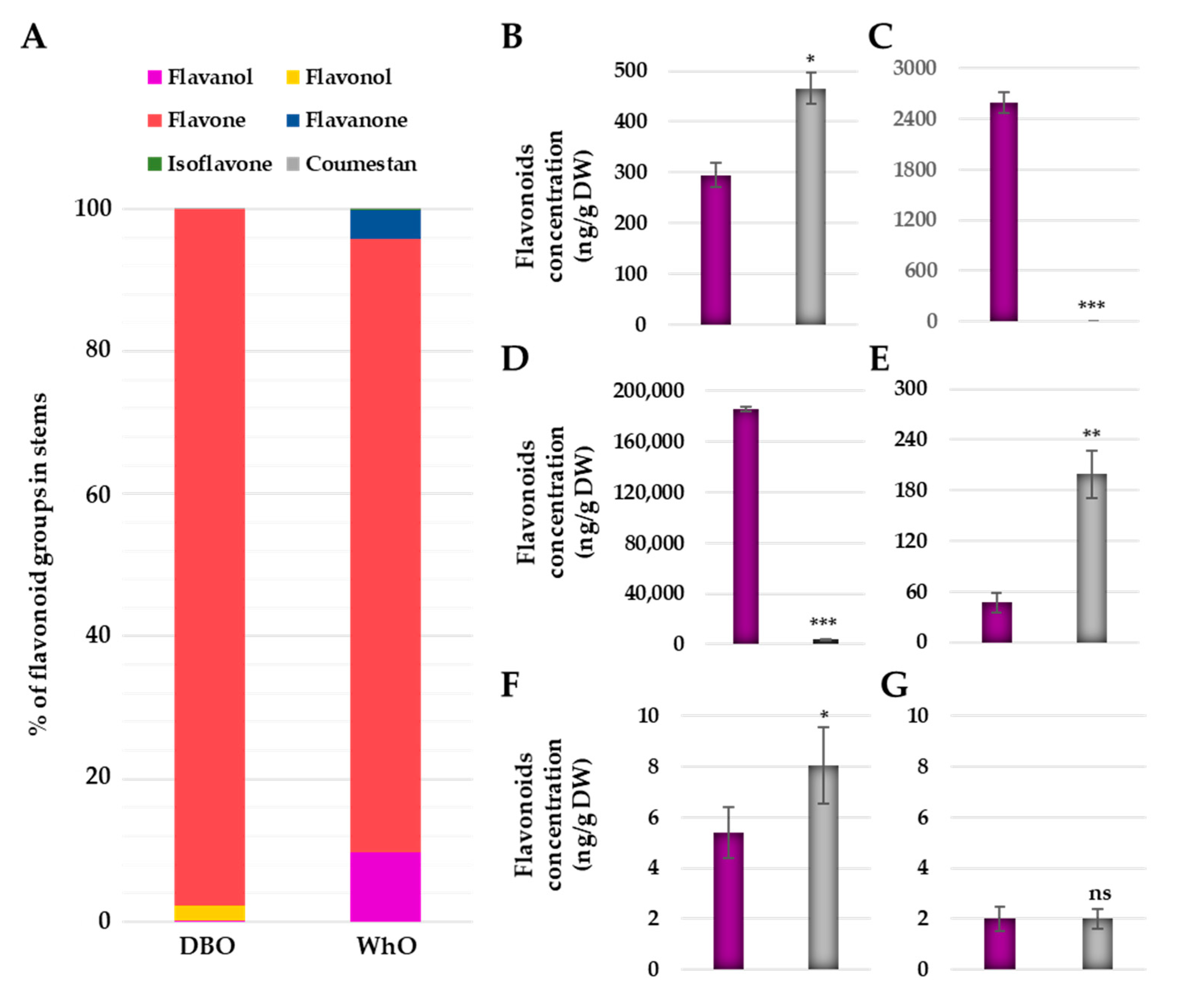
3.4. Deep Blue Ocean and White Ocean Genotypes Present Differences in the Flavonoid Composition in the Basal Part of the Stems
4. Discussion
4.1. Crosstalk of Flavonoids, Auxin, and ROS
4.2. ABA Is a Potential Additional Regulator of Ar Development
5. Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steffens, B.; Rasmussen, A. The physiology of adventitious roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Buer, C.S.; Imin, N.; Djordjevic, M.A. Flavonoids: New roles for old molecules. J. Integr. Plant Biol. 2010, 52, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Teale, W.D.; Ditengou, F.A.; Dovzhenko, A.D.; Li, X.; Molendijk, A.M.; Ruperti, B.; Paponov, I.; Palme, K. Auxin as a model for the integration of hormonal signal processing and transduction. Mol. Plant 2008, 1, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Auxin biosynthesis: A simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Mol. Plant 2012, 5, 334–338. [Google Scholar] [CrossRef]
- Paponov, I.A.; Teale, W.D.; Trebar, M.; Blilou, K.; Palme, K. The PIN auxin efflux facilitators: Evolutionary and functional perspectives. Trends Plant Sci. 2005, 10, 170–177. [Google Scholar] [CrossRef]
- Cho, M.; Cho, H.T. The function of ABCB transporters in auxin transport. Plant Signal. Behav. 2013, 8, e22990. [Google Scholar] [CrossRef]
- Brown, D.E.; Rashotte, A.M.; Murphy, A.S.; Normanly, J.; Tague, B.W.; Peer, W.A.; Taiz, L.; Muday, G.K. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant. Physiol. 2001, 126, 524–535. [Google Scholar] [CrossRef]
- Peer, W.A.; Murphy, A.S. Flavonoids and auxin transport: Modulators or regulators? Trends Plant Sci. 2007, 12, 556–563. [Google Scholar] [CrossRef]
- Peer, W.A.; Bandyopadhyay, A.; Blakeslee, J.J.; Makam, S.I.; Chen, R.J.; Masson, P.H.; Murphy, A.S. Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 2004, 16, 1898–1911. [Google Scholar] [CrossRef]
- Paponov, I.A.; Budnyk, V.; Paponov, M.; Teale, W.; Palme, K. Butylated Hydroxytoluene (BHT) Inhibits PIN1 Exocytosis From BFA Compartments in Arabidopsis Roots. Front. Plant Sci. 2020, 11, 393. [Google Scholar] [CrossRef]
- Teale, W.D.; Pasternak, T.; Dal Bosco, C.; Dovzhenko, A.; Kratzat, K.; Bildl, W.; Schwörer, M.; Falk, T.; Ruperti, B.; Schaefer, J.V.; et al. Flavonol-mediated stabilization of PIN efflux complexes regulates polar auxin transport. EMBO J. 2021, 40, e104416. [Google Scholar] [CrossRef] [PubMed]
- De Klerk, G.-J.; Guan, H.; Huisman, P.; Marinova, S. Effects of phenolic compounds on adventitious root formation and oxidative decarboxylation of applied indoleacetic acid in Malus ‘Jork 9’. Plant Growth Regul. 2011, 63, 175–185. [Google Scholar] [CrossRef]
- Denaxa, N.-K.; Roussos, P.A.; Vemmos, S.N. Assigning a role to the endogenous phenolic compounds on adventitious root formation of olive stem cuttings. J. Plant Growth Regul. 2020, 39, 411–421. [Google Scholar] [CrossRef]
- Gayomba, S.R.; Muday, G.K. Flavonols regulate root hair development by modulating accumulation of reactive oxygen species in the root epidermis. Development 2020, 147, 185819. [Google Scholar] [CrossRef]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.F.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.G.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Tsukagoshi, H.; Busch, W.; Benfey, P.N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 2010, 143, 606–616. [Google Scholar] [CrossRef]
- Orman-Ligeza, B.; Parizot, B.; de Rycke, R.; Fernandez, A.; Himschoot, E.; Van Breusegem, F.; Bennett, M.J.; Périlleux, C.; Beeckman, T.; Draye, X. RBOH-mediated ROS production facilitates lateral root emergence in Arabidopsis. Development 2016, 143, 3328–3339. [Google Scholar] [CrossRef]
- Manzano, C.; Pallero-Baena, M.; Casimiro, I.; De Rybel, B.; Orman-Ligeza, B.; Van Isterdael, G.; Beeckman, T.; Draye, X.; Casero, P.; Del Pozo, J.C. The emerging role of reactive oxygen species signaling during lateral root development. Plant Physiol. 2014, 165, 1105–1119. [Google Scholar] [CrossRef]
- Huang, A.; Wang, Y.; Liu, Y.; Wang, G.; She, X. Reactive oxygen species regulate auxin levels to mediate adventitious root induction in Arabidopsis hypocotyl cuttings. J. Integr. Plant Biol. 2020, 62, 912–926. [Google Scholar] [CrossRef]
- Osterc, G.; Štefančič, M.; Solar, A.; Stampar, F. Potential involvement of flavonoids in the rooting response of chestnut hybrid (Castanea crenata × Castanea sativa) clones. Aust. J. Exp. Agr. 2007, 47, EA05149. [Google Scholar] [CrossRef]
- Vilasboa, J.; da Costa, C.T.; Ransan, L.G.; Mariath, J.E.D.A.; Fett-Neto, A.G. Microcutting redox profile and anatomy in Eucalyptus spp. with distinct adventitious rooting competence. Front Plant Sci 2021, 11, 620832. [Google Scholar] [CrossRef] [PubMed]
- Curir, P.; Sulis, S.; Mariani, F.; van Sumere, C.F.; Marchesini, A.; Dolci, M. Influence of endogenous phenols on rootability of Chamaelaucium uncinatum Schauer stem cuttings. Sci. Hortic. 1993, 55, 303–314. [Google Scholar] [CrossRef]
- Van Overbeek, J.; Gordon, S.; Gregory, L. An analysis of the function of the leaf in the process of root formation in cuttings. Am. J. Bot. 1946, 33, 100–107. [Google Scholar] [CrossRef]
- Barba de la Rosa, A.; León-Rodríguez, A.; Laursen, B.; Fomsgaard, I. Influence of the growing conditions on the flavonoids and phenolic acids accumulation in amaranth (Amaranthus hypochondriacus L.) leaves. Rev. Terra Latinoam. 2019, 37, 449. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Bergonci, T.; Paponov, I.A. Amino acid treatments induce adventitious root formation in two different genotypes of Campanula portenschlagiana. Biol. Life Sci. Forum 2022, 16, 8. [Google Scholar]
- Fattorini, L.; Falasca, G.; Kevers, C.; Rocca, L.M.; Zadra, C.; Altamura, M.M. Adventitious rooting is enhanced by methyl jasmonate in tobacco thin cell layers. Planta 2009, 231, 155–168. [Google Scholar] [CrossRef]
- Chen, L.; Tong, J.; Xiao, L.; Ruan, Y.; Liu, J.; Zeng, M.; Huang, H.; Wang, J.W.; Xu, L. YUCCA-mediated auxin biogenesis is required for cell fate transition occurring during de novo root organogenesis in Arabidopsis. J. Exp. Bot. 2016, 67, 4273–4284. [Google Scholar] [CrossRef]
- An, H.; Zhang, J.; Xu, F.; Jiang, S.; Zhang, X. Transcriptomic profiling and discovery of key genes involved in adventitious root formation from green cuttings of highbush blueberry (Vaccinium corymbosum L.). BMC Plant Biol. 2020, 20, 182. [Google Scholar] [CrossRef]
- Gonin, M.; Bergougnoux, V.; Nguyen, T.D.; Gantet, P.; Champion, A. What Makes Adventitious Roots? Plants 2019, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.M.; Silva, E.M.; Saldanha, L.L.; Adachi, S.A.; Schley, T.R.; Rodrigues, T.M.; Dokkedal, A.L.; Nogueira, F.T.; Rolim de Almeida, L.F. Flavonoids modify root growth and modulate expression of SHORT-ROOT and HD-ZIP III. J. Plant Physiol. 2015, 188, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Lakehal, A.; Bellini, C. Control of adventitious root formation: Insights into synergistic and antagonistic hormonal interactions. Physiol. Plantarum 2019, 165, 90–100. [Google Scholar] [CrossRef]
- Buer, C.S.; Muday, G.K.; Djordjevic, M.A. Implications of long-distance flavonoid movement in Arabidopsis thaliana. Plant Signal. Behav. 2008, 3, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.T. Role of phenolic inhibitors in peroxidase-mediated degradation of indole-3-acetic acid. Plant Physiol. 1977, 59, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, V.B.; Van Aken, O.; Morreel, K.; Vandenbroucke, K.; van de Cotte, B.; De Clercq, I.; Chiwocha, S.; Fenske, R.; Prinsen, E.; Boerjan, W.; et al. Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell 2010, 22, 2660–2679. [Google Scholar] [CrossRef]
- Frick, E.M.; Strader, L.C. Roles for IBA-derived auxin in plant development. J. Exp. Bot. 2017, 69, 169–177. [Google Scholar] [CrossRef]
- Korasick, D.A.; Enders, T.A.; Strader, L.C. Auxin biosynthesis and storage forms. J. Exp. Bot. 2013, 64, 2541–2555. [Google Scholar] [CrossRef]
- Strader, L.C.; Bartel, B. Transport and metabolism of the endogenous auxin precursor indole-3-butyric acid. Mol. Plant 2011, 4, 477–486. [Google Scholar] [CrossRef]
- Strader, L.C.; Culler, A.H.; Cohen, J.D.; Bartel, B. Conversion of endogenous indole-3-butyric acid to indole-3-acetic acid drives cell expansion in Arabidopsis seedlings. Plant Physiol. 2010, 153, 1577–1586. [Google Scholar] [CrossRef]
- Ravelo-Ortega, G.; López-Bucio, J.S.; Ruiz-Herrera, L.F.; Pelagio-Flores, R.; Ayala-Rodríguez, J.Á.; de la Cruz, H.R.; Guevara-García, Á.A.; López-Bucio, J. The growth of Arabidopsis primary root is repressed by several and diverse amino acids through auxin-dependent and independent mechanisms and MPK6 kinase activity. Plant Sci. 2021, 302, 110717. [Google Scholar] [CrossRef] [PubMed]
- Ahkami, A.H.; Melzer, M.; Ghaffari, M.R.; Pollmann, S.; Ghorbani Javid, M.; Shahinnia, F.; Hajirezaei, M.R.; Druege, U. Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 2013, 238, 499–517. [Google Scholar] [CrossRef]
- Yin, R.; Han, K.; Heller, W.; Albert, A.; Dobrev, P.I.; Zažímalová, E.; Schäffner, A.R. Kaempferol 3-O-rhamnoside-7-O-rhamnoside is an endogenous flavonol inhibitor of polar auxin transport in Arabidopsis shoots. New Phytol. 2014, 201, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.; Blakeslee, J.J.; Bouchard, R.; Lee, O.R.; Vincenzetti, V.; Bandyopadhyay, A.; Titapiwatanakun, B.; Peer, W.A.; Bailly, A.; Richards, E.L.; et al. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J. 2005, 44, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Noh, B.; Murphy, A.S.; Spalding, E.P. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 2001, 13, 2441–2454. [Google Scholar]
- Murphy, A.S.; Hoogner, K.R.; Peer, W.A.; Taiz, L. Identification, purification, and molecular cloning of N-1- naphthylphthalmic acid-binding plasma membrane-associated aminopeptidases from Arabidopsis. Plant Physiol. 2002, 128, 935–950. [Google Scholar] [CrossRef]
- Bashandy, T.; Meyer, Y.; Reichheld, J.-P. Redox regulation of auxin signaling and plant development in Arabidopsis. Plant Signal. Behav. 2011, 6, 117–119. [Google Scholar] [CrossRef]
- Csepregi, K.; Hideg, É. Phenolic compound diversity explored in the context of photo-oxidative stress protection. Phytochem. Anal. 2018, 29, 129–136. [Google Scholar] [CrossRef]
- Li, S.-W.; Xue, L.; Xu, S.; Feng, H.; An, L. Hydrogen peroxide acts as a signal molecule in the adventitious root formation of mung bean seedlings. Environ. Exp. Bot. 2009, 65, 63–71. [Google Scholar] [CrossRef]
- Huang, A.-X.; She, X.-P.; Cao, B.-H.; Ren, Y. Distribution of hydrogen peroxide during adventitious roots initiation and development in mung bean hypocotyls cuttings. Plant Growth Regul. 2011, 64, 109–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Z.A.; Zhan, C.; Liu, M.; Xia, W.; Wang, N. Comprehensive analysis of dynamic gene expression and investigation of the roles of hydrogen peroxide during adventitious rooting in poplar. BMC Plant Biology. 2019, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Liu, G.; Wei, Y.; Chan, Z. The zinc-finger transcription factor ZAT6 is essential for hydrogen peroxide induction of anthocyanin synthesis in Arabidopsis. Plant Mol. Biol. 2018, 97, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Verstraeten, I.; Trinh, H.K.; Heugebaert, T.; Stevens, C.V.; Garcia-Maquilon, I.; Rodriguez, P.L.; Vanneste, S.; Geelen, D. Arabidopsis hypocotyl adventitious root formation is suppressed by ABA signaling. Genes 2021, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- De Smet, I.; Signora, L.; Beeckman, T.; Inzé, D.; Foyer, C.H.; Zhang, H. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J. 2003, 33, 543–555. [Google Scholar] [CrossRef]
- Brunetti, C.; Sebastiani, F.; Tattini, M. Review: ABA, flavonols, and the evolvability of land plants. Plant Sci. 2019, 280, 448–454. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef]
- Tossi, V.; Lamattina, L.; Cassia, R. An increase in the concentration of abscisic acid is critical for nitric oxide-mediated plant adaptive responses to UV-B irradiation. New Phytol. 2009, 181, 871–879. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Jia, K.P.; Dickinson, A.J.; Mi, J.; Cui, G.; Xiao, T.T.; Kharbatia, N.M.; Guo, X.; Sugiono, E.; Aranda, M.; Blilou, I.; et al. Anchorene is a carotenoid-derived regulatory metabolite required for anchor root formation in Arabidopsis. Sci. Adv. 2019, 5, eaaw6787. [Google Scholar] [CrossRef]
- Mauriat, M.; Petterle, A.; Bellini, C.; Moritz, T. Gibberellins inhibit adventitious rooting in hybrid aspen and Arabidopsis by affecting auxin transport. Plant J. 2014, 78, 372–384. [Google Scholar] [CrossRef]
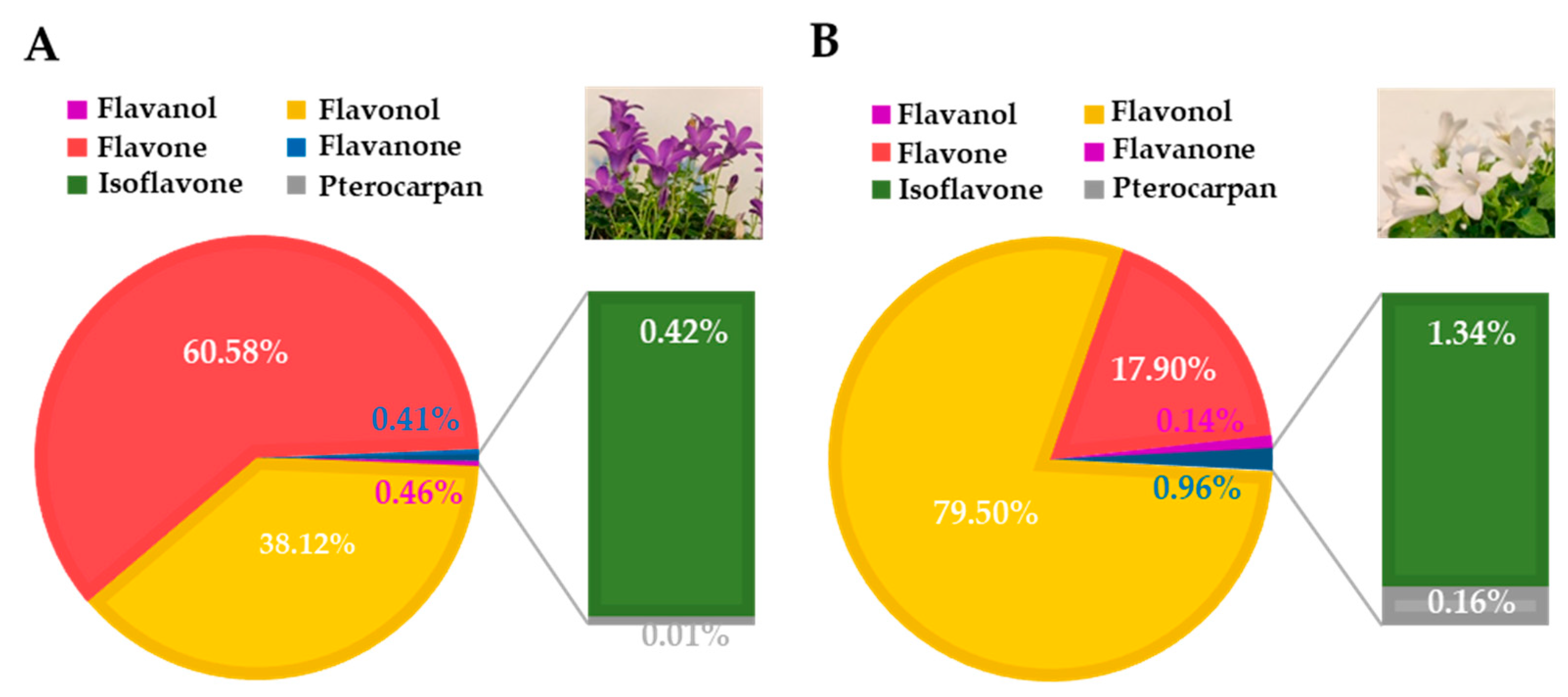
| Common Name | Systematic Name | Chemical Group |
|---|---|---|
| Epicatechin | 2,3-trans-catechin | Flavanol |
| Kaempferol | 3,4‘,5,7-tetrahydroxyflavone | Flavonol |
| Nicotiflorin | Kaempferol-3-O-rutinoside | |
| Quercetin-rha-xyl-gal | Quercetin-3-O-D-rhamnosyl-(1-6)D-[xylosyl-(1-2])—D-galactoside | |
| Quercetin-xyl-gal | Quercetin-3-O-[xyl(1-2)-gal] | |
| Rutin | Quercetin-3-O-rutinoside | |
| Apigenin | 4‘,5,7-trihydroxyflavone | Flavone |
| Orientin | Luteolin-8-C-glucoside | |
| Isovitexin | 6-(β-D-glucopyranosyl)-4’,5,7-trihydroxyflavone | |
| Luteolin | 3‘,4‘,5,7-tetrahydroxyflavone | |
| Luteolin-4-O-glc | Luteolin-4-O-glucoside | |
| Luteolin-di-glc | Luteolin-3‘,7-di-O-glucoside | |
| Myricetin | 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one | |
| Naringenin | 4‘,5,7-trihydroxyflavanone | Flavanone |
| Naringin | Naringenin-7-O-neohesperidoside | |
| Biochanin A | 5,7-dihydroxy-4‘-methoxyisoflavone | Isoflavone |
| Daidzein | 4‘,7-dihydroxyisoflavone | |
| Daidzin | Daidzein-7-O-glucoside | |
| Formononetin | 7-hydroxy-4‘-methoxyisoflavone | |
| Genistein | 4‘,5,7-trihydroxyisoflavone | |
| Genistin | Genistein-7-O-D-glucoside | |
| Sissotrin | Biochanin A 7-O-β-D-glucopyranoside | |
| Coumestrol | 3,9-dihydroxy-[1]benzofuro[3,2-c]chromen—6-one | Coumestan |
| Medicarpin | 3-hydroxy-9-methoxypterocarpan | Pterocarpan |
| Flavonoid Compound | Chemical Group | Deep Blue Ocean | SD | White Ocean | SD | t-Test |
|---|---|---|---|---|---|---|
| Epicatechin | Flavanol | 132 | 12 | 9 | 2 | *** |
| Kaempferol | Flavonol | 0 | --- | 228 | --- | --- |
| Nicotiflorin | 7160 | 125 | 4400 | 110 | ** | |
| Quercetin-rha-xyl-gal | 158 | 14 | 8 | 1 | *** | |
| Quercetin-xyl-gal | 2004 | 210 | 25 | 4 | *** | |
| Rutin | 1726 | 123 | 240 | 21 | *** | |
| Apigenin | Flavone | 0 | --- | 228 | --- | --- |
| Luteolin | 580 | 36 | 213 | 15 | ** | |
| Luteolin-4-O-glc | 184 | 21 | 158 | 16 | ns | |
| Luteolin-di-glc | 16,500 | 255 | 7 | 1 | *** | |
| Myricetin | 270 | 25 | 602 | 30 | ** | |
| Naringenin | Flavanone | 38 | 10 | 7 | 2 | ** |
| Naringin | 80 | 12 | 53 | 8 | * | |
| Genistein | Isoflavone | 0 | --- | 74 | --- | --- |
| Sissotrin | 106 | 18 | 1 | 0.6 | *** |
| Flavonoid Compound | Chemical Group | Deep Blue Ocean | SD | White Ocean | SD | t-Test |
|---|---|---|---|---|---|---|
| Epicatechin | Flavanol | 295 | 58 | 466 | 56 | * |
| Nicotiflorin | Flavonol | 1476 | 145 | 0 | --- | --- |
| Rutin | 2586 | 236 | 0 | --- | --- | |
| Orientin | Flavone | 26 | 12 | 18 | 3 | ns |
| Isovitexin | 184 | 56 | 280 | 25 | ** | |
| Luteolin | 130 | 26 | 190 | 24 | * | |
| Luteolin-4-O-glc | 22 | 3 | 0 | --- | --- | |
| Luteolin-di-glc | 185,200 | 2541 | 3670 | 265 | *** | |
| Naringenin | Flavanone | 21 | 3 | 146 | 25 | ** |
| Naringin | 26 | 5 | 53 | 12 | * | |
| Daidzein | Isoflavone | 0.6 | 0.1 | 0 | --- | --- |
| Formononetin | 0 | --- | 0.04 | 0.01 | --- | |
| Genistin | 4 | 2 | 8 | 2 | * | |
| Sissotrin | 0.8 | 0.1 | 0 | --- | --- | |
| Coumestrol | Coumestan | 2 | 0.8 | 2 | 1 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergonci, T.; Fomsgaard, I.S.; Kjaer, K.H.; Paponov, I.A. Hormone–Flavonoid Patterns in Two Genotypes of Campanula portenschlagiana with Distinct Adventitious Rooting Competence. Horticulturae 2023, 9, 121. https://doi.org/10.3390/horticulturae9010121
Bergonci T, Fomsgaard IS, Kjaer KH, Paponov IA. Hormone–Flavonoid Patterns in Two Genotypes of Campanula portenschlagiana with Distinct Adventitious Rooting Competence. Horticulturae. 2023; 9(1):121. https://doi.org/10.3390/horticulturae9010121
Chicago/Turabian StyleBergonci, Tábata, Inge S. Fomsgaard, Katrine H. Kjaer, and Ivan A. Paponov. 2023. "Hormone–Flavonoid Patterns in Two Genotypes of Campanula portenschlagiana with Distinct Adventitious Rooting Competence" Horticulturae 9, no. 1: 121. https://doi.org/10.3390/horticulturae9010121
APA StyleBergonci, T., Fomsgaard, I. S., Kjaer, K. H., & Paponov, I. A. (2023). Hormone–Flavonoid Patterns in Two Genotypes of Campanula portenschlagiana with Distinct Adventitious Rooting Competence. Horticulturae, 9(1), 121. https://doi.org/10.3390/horticulturae9010121









