The Main Morphological Characteristics and Chemical Components of Fruits and the Possibilities of Their Improvement in Raspberry Breeding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location of Experiment and Study Conditions
2.2. Biological Material and Experimental Procedures
2.3. Chemical Analysis
2.4. Statistical Analyses
3. Results
3.1. The Morphological Traits of the Fruits
3.2. The Traits That Contribute to the Production and Marketing Potential of Fruits
3.3. Chemical Compounds of Interest in the Fruits
3.4. Correlations between Morphological Traits and Fruit Content in Useful Substances
3.5. Multivariate Analysis
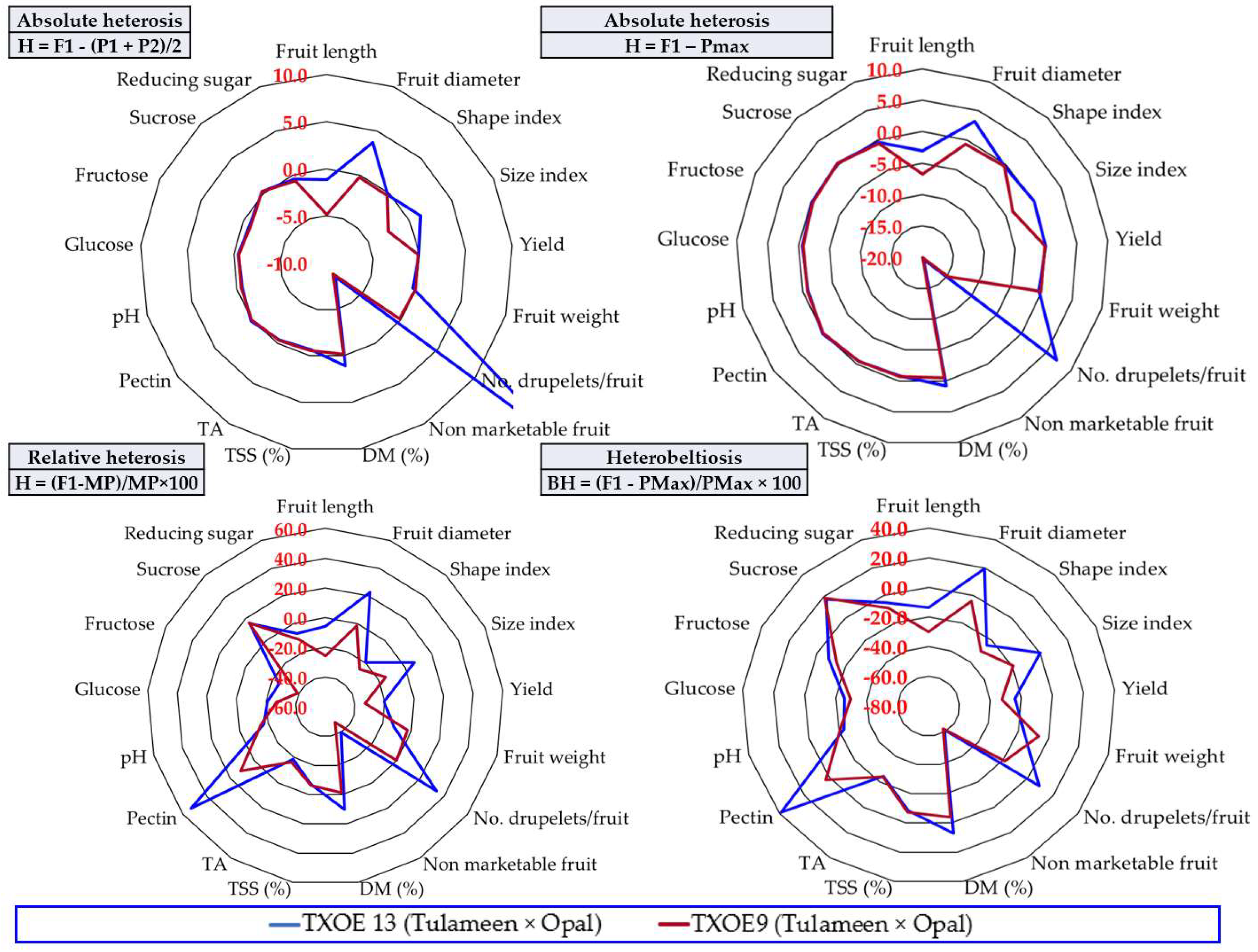
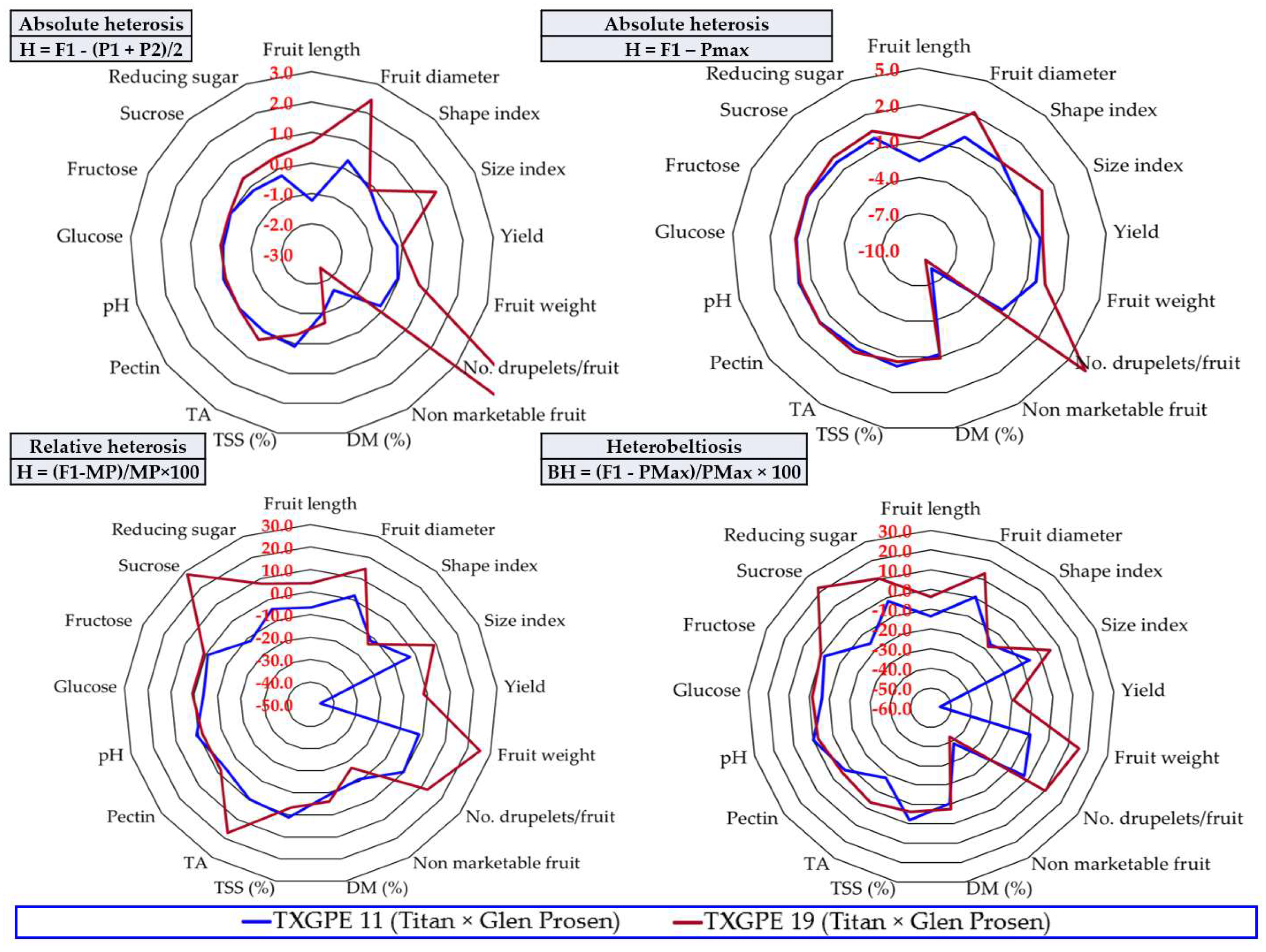
3.6. The Heterosis Effect
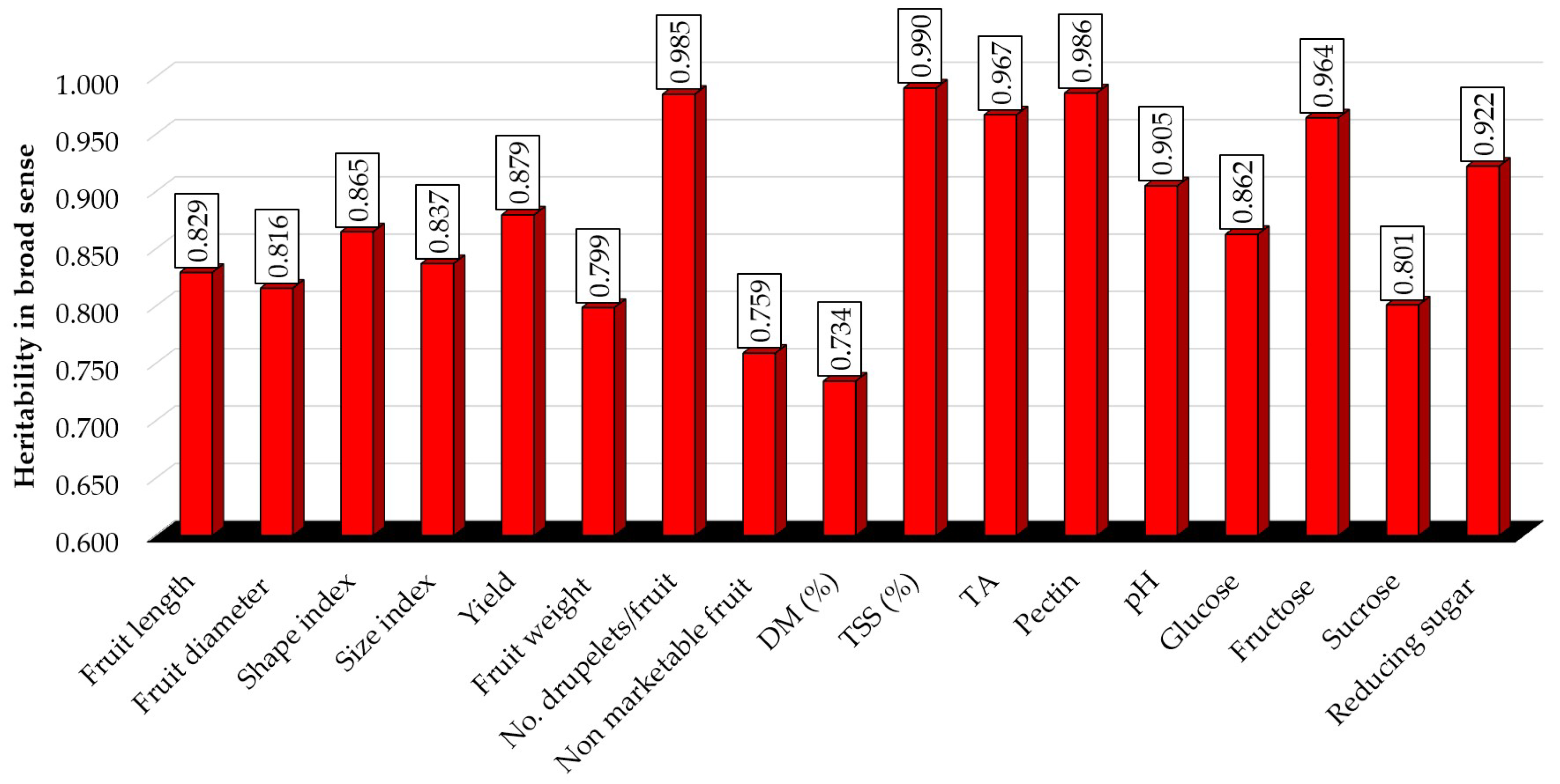
3.7. Heritability
4. Discussion
4.1. Raspberry Breeding Objectives: General and Topical Considerations Related to the Current Study
4.2. The Usefulness of Information Provided by Calculating Heterosis to Raspberry Breeding
4.3. The Usefulness of Information Provided by Heritability Coefficients to Raspberry Breeding
4.4. The Usefulness of Information Provided by the Calculation of Correlation Coefficients and Multivariate Analyses to Raspberry Breeding
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hummer, K.E. Rubus diversity. HortScience 1996, 31, 182–183. [Google Scholar] [CrossRef] [Green Version]
- Foster, T.M.; Bassil, N.V.; Dossett, M.; Worthington, M.L.; Graham, J. Genetic and genomic resources for Rubus breeding: A roadmap for the future. Hortic. Res. 2019, 6, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dale, A.; Moore, P.P.; McNicol, R.J.; Sjulin, T.M.; Burmistrov, L.A. Genetic diversity of red raspberry varieties throughout the-world. J. Am. Soc. Hortic. Sci. 1993, 118, 119–129. [Google Scholar] [CrossRef]
- Veljković, B.; Šoštarić, I.; Dajić-Stevanović, Z.; Liber, Z.; Šatović, Z. Genetic structure of wild raspberry populations in the central Balkans depends on their location and on their relationship to commercial cultivars. Sci. Hortic. 2019, 256, 108606. [Google Scholar] [CrossRef]
- Dulf, F.V.; Andrei, S.; Bunea, A.; Socaciu, C. Fatty acid and phytosterol contents of some Romanian wild and cultivated berry pomaces. Chem. Pap. 2012, 66, 925–934. [Google Scholar] [CrossRef]
- Lācis, G.; Kota-Dombrovska, I.; Strautiņa, S. Evaluation of red raspberry cultivars used for breeding and commercial growing in the Baltic region. Proc. Latv. Acad. Sci. Sect. B 2017, 71, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Milivojevic, J.M.; Nikolic, M.D.; Maksimovic, J.J.D.; Radivojevic, D.D. Generative and fruit quality characteristics of primocane fruiting red raspberry cultivars. Turk. J. Agric. For. 2011, 35, 289–296. [Google Scholar] [CrossRef]
- Kula, P.J. Raspberry Pi 2 Server Essentials; PACKT Publishing: Birmingham, UK, 2016. [Google Scholar]
- Qiu, C.; Xu, Q.; Gaudreau, L.; Gosselin, A.; Gauthier, L.; Van Sterthem, A.; Desjardins, Y. Yield improvement of red raspberry by soilless cultivation with two propagation methods under northern Canadian climate. Acta Hortic. 2016, 1133, 195–200. [Google Scholar] [CrossRef]
- Palonen, P.; Pinomaa, A.; Tommila, T. The influence of high tunnel on yield and berry quality in three floricane raspberry cultivars. Sci. Hortic. 2017, 214, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Stavang, J.A.; Freitag, S.; Foito, A.; Verrall, S.; Heide, O.M.; Stewart, D.; Sønsteby, A. Raspberry fruit quality changes during ripening and storage as assessed by colour, sensory evaluation and chemical analyses. Sci. Hortic. 2015, 195, 216–225. [Google Scholar] [CrossRef]
- Anttonen, M.J.; Karjalainen, R.O. Environmental and genetic variation of phenolic compounds in red raspberry. J. Food Compos. Anal. 2005, 18, 759–769. [Google Scholar] [CrossRef]
- Remberg, S.F.; Sønsteby, A.; Aaby, K.; Heide, O.M. Influence of postflowering temperature on fruit size and chemical composition of glen ample raspberry (Rubus idaeus L.). J. Agric. Food Chem. 2010, 58, 9120–9128. [Google Scholar] [CrossRef] [PubMed]
- Mazur, S.P.; Nes, A.; Wold, A.-B.; Remberg, S.F.; Aaby, K. Quality and chemical composition of ten red raspberry (Rubus idaeus L.) genotypes during three harvest seasons. Food Chem. 2014, 160, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Miletić, N.; Leposavić, A.; Popović, B.; Mitrović, O.; Kandić, M. Chemical and antioxidant properties of fully matured raspberry fruits (Rubus idaeus L.) picked in different moments of harvesting season. Acta Hortic. 2015, 1099, 211–218. [Google Scholar] [CrossRef]
- Leposavić, A.; Đurović, D.; Keserović, Z.; Jevremović, D. Vegetative and yield potential of cultivars and selection of raspberry cultivated in conditions of West Serbia. Bulg. J. Agric. Sci. 2015, 21, 153–159. [Google Scholar]
- Stanisavljevic, M.; Mitrovic, O.; Gavriloviç-Damjanoviç, J. Comparative studies on raspberry cultivars. Acta Hortic. 2002, 585, 241–245. [Google Scholar] [CrossRef]
- Sønsteby, A.; Myrheim, U.; Heiberg, N.; Heide, O.M. Production of high yielding red raspberry long canes in a northern climate. Sci. Hortic. 2009, 121, 289–297. [Google Scholar] [CrossRef]
- Woznicki, T.L.; Heide, O.M.; Remberg, S.F.; Sønsteby, A. Effects of controlled nutrient feeding and different temperatures during floral initiation on yield, berry size and drupelet numbers in red raspberry (Rubus idaeus L.). Sci. Hortic. 2016, 212, 148–154. [Google Scholar] [CrossRef]
- Cañas-Gutiérrez, G.P.; Alcaraz, L.; Hormaza, J.I.; Arango-Isaza, R.E.; Saldamando-Benjumea, C.I. Diversity of avocado (Persea americana Mill.) cultivars from Antioquia (Northeast Colombia) and comparison with a worldwide germplasm collection. Turk. J. Agric. For. 2019, 43, 437–449. [Google Scholar] [CrossRef]
- Zivotic, A.; Micic, N.; Zabic, M.; Bosancic, B.; Cvetkovic, M. Precision cane meristem management can influence productivity and fruit quality of floricane red raspberry cultivars. Turk. J. Agric. For. 2019, 43, 405–413. [Google Scholar] [CrossRef]
- Durán-Soria, S.; Pott, D.M.; Will, F.; Mesa-Marín, J.; Lewandowski, M.; Celejewska, K.; Masny, A.; Żurawicz, E.; Jennings, N.; Sønsteby, A.; et al. Exploring genotype-by-environment interactions of chemical composition of raspberry by using a metabolomics approach. Metabolites 2021, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Peinado, I.; Rosa, E.; Heredia, A.; Andrés, A. Rheological characteristics of healthy sugar substituted spreadable strawberry product. J. Food Eng. 2012, 113, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Koraqi, H.; Durmishi, N.; Rizani, K.L.; Rizani, S. Chemical composition and nutritional value of raspberry fruit (Rubus idaeus L.). In Proceedings of the UBT International Conference, Pristina, Kosovo, 26–28 October 2019; p. 397. [Google Scholar]
- Ponder, A.; Hallmann, E. The nutritional value and vitamin C content of different raspberry cultivars from organic and conventional production. J. Food Compos. Anal. 2020, 87, 103429. [Google Scholar] [CrossRef]
- Tosun, M.; Ercisli, S.; Karlidag, H.; Sengul, M. Characterization of red raspberry (Rubus idaeus L.) genotypes for their physicochemical properties. J. Food Sci. 2009, 74, C575–C579. [Google Scholar] [CrossRef] [PubMed]
- Kirina, I.B.; Belosokhov, F.G.; Titova, L.V.; Suraykina, I.A.; Pulpitow, V.F. Biochemical assessment of berry crops as a source of production of functional food products. IOP Conf. Series Earth Environ. Sci. 2020, 548, 82068. [Google Scholar] [CrossRef]
- Bošnjak, D.; Marković, M.; Agić, D.; Vinković, T.; Tkalec Kojić, M.; Ravnjak, B.; Stanisavljević, A. The influence of nutrient media modification on the morphological parameters in raspberry (Rubus idaeus L.) micropropagation in the liquid and semi-solid media. Poljoprivreda 2021, 27, 22–29. [Google Scholar] [CrossRef]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Red raspberries and their bioactive polyphenols: Cardiometabolic and neuronal health links. Adv. Nutr. Int. Rev. J. 2016, 7, 44–65. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, anti-inflammatory and cytotoxic activity of phenolic compound family extracted from rasp-berries (Rubus idaeus): A general review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef]
- Shoukat, S.; Mahmudiono, T.; Al-Shawi, S.G.; Abdelbasset, W.K.; Yasin, G.; Shichiyakh, R.A.; Iswanto, A.H.; Kadhim, A.J.; Kadhim, M.M.; Al-Rekaby, H.Q. Determination of the antioxidant and mineral contents of raspberry varieties. Food Sci. Technol. 2022, 42, e118521. [Google Scholar] [CrossRef]
- De Souza, V.R.; Pereira, P.A.P.; Da Silva, T.L.T.; De Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Zhou, X.; Chen, S.; Sun, Y.; Shen, Y.; Ye, X. Chemical composition and antioxidant activity of Chinese wild raspberry (Rubus hirsutus Thunb.). LWT Food Sci. Technol. 2014, 60, 1262–1268. [Google Scholar] [CrossRef]
- Duarte, W.F.; Dias, D.R.; Oliveira, J.M.; Vilanova, M.; Teixeira, J.A.; e Silva, J.B.A.; Schwan, R.F. Raspberry (Rubus idaeus L.) wine: Yeast selection, sensory evaluation and instrumental analysis of volatile and other compounds. Food Res. Int. 2010, 43, 2303–2314. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.-T.; Wang, C.Y. The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chem. 2009, 112, 676–684. [Google Scholar] [CrossRef]
- Maro, L.A.C.; Pio, R.; Guedes, M.N.S.; De Abreu, C.M.P.; Moura, P.H.A. Environmental and genetic variation in the post-harvest quality of raspberries in subtropical areas in Brazil. Acta Sci. Agron. 2014, 36, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Gecer, M.K.; Kan, T.; Gundogdu, M.; Ercisli, S.; Ilhan, G.; Sagbas, H.I. Physicochemical characteristics of wild and cultivated apricots (Prunus armeniaca L.) from Aras valley in Turkey. Genet. Resour. Crop Evol. 2020, 67, 935–945. [Google Scholar] [CrossRef]
- Maeda, H.; Akagi, T.; Tao, R. Quantitative characterization of fruit shape and its differentiation pattern in diverse persimmon (Diospyros kaki) cultivars. Sci. Hortic. 2018, 228, 41–48. [Google Scholar] [CrossRef]
- Ozkan, G.; Ercisli, S.; Zeb, A.; Agar, G.; Sagbas, H.I.; Ilhan, G.; Gundogdu, M. Some morphological and biochemical characteristics of wild grown Caucasian whortleberry (Vaccinium arctostaphylos L.) genotypes from northeastern Turkey. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 378–383. [Google Scholar] [CrossRef] [Green Version]
- Sestras, R.E.; Pamfil, D.; Ardelean, M.; Botez, C.; Sestras, A.F.; Mitre, I.; Dan, C.; Mihalte, L. Use of phenotypic and MAS selection based on bulk segregant analysis to reveal the genetic variability induced by artificial hybridization in apple. Not. Bot. Horti Agrobo. Cluj-Napoca 2009, 37, 273–277. [Google Scholar] [CrossRef]
- Evdokimenko, S.N.; Podgaetskiy, M.A. Current status and prospects of raspberry breeding. Hortic. Vitic. 2022, 4, 5–15. [Google Scholar] [CrossRef]
- Puskás, M.; Höfer, M.; Sestras, R.E.; Peil, A.; Sestras, A.F.; Hanke, M.-V.; Flachowsky, H. Molecular and flow cytometric evaluation of pear (Pyrus L.) genetic resources of the German and Romanian national fruit collections. Genet. Resour. Crop. Evol. 2016, 63, 1023–1033. [Google Scholar] [CrossRef]
- Dan, C.; Sestras, A.F.; Bozdog, C.; Sestras, R.E. Investigation of wild species potential to increase genetic diversity useful for apple breeding. Genetika 2015, 47, 993–1011. [Google Scholar] [CrossRef]
- Robbins, J.; Moore, P.P. Fruit morphology and fruit strength in a seedling population of red raspberry. HortScience 1991, 26, 294–295. [Google Scholar] [CrossRef] [Green Version]
- Sagbas, H.I.; Ilhan, G.; Zitouni, H.; Anjum, M.A.; Hanine, H.; Necas, T.; Ondrasek, I.; Ercisli, S. Morphological and biochemical characterization of diverse strawberry tree (Arbutus unedo L.) genotypes from northern Turkey. Agronomy 2020, 10, 1581. [Google Scholar] [CrossRef]
- Haskell, G. Biometrical characters and selection in cultivated raspberry. Euphytica 1960, 9, 17–34. [Google Scholar] [CrossRef]
- Hernández-Bautista, A.; Lobato-Ortiz, R.; García-Zavala, J.J.; Rocandio-Rodríguez, M.; Mejía-Contreras, J.A.; Chávez-Servia, J.L.; García-Velazquez, J.A. Relationship of parental genetic distance with agronomic performance, specific combining ability, and predicted breeding values of raspberry families. Euphytica 2018, 214, 37. [Google Scholar] [CrossRef]
- Evdokimenko, S.; Kulikov, I.; Podgaetsky, M. Some aspects of raspberry breeding with predominant fruiting on annual shoots. E3S Web Conf. 2021, 254, 1019. [Google Scholar] [CrossRef]
- Bautista, A.H.; Ortiz, R.L.; Mejía-Contreras, J.A.; García-Zavala, J.J.; Chávez-Servia, J.L.; Rocandio-Rodríguez, M.; Bautista, A.L.H.; Castañeda-Villa, A.M.; Rodríguez-Bautista, G. Heterosis and agronomic performance of raspberry families: Breeding raspberry. Agro Product. 2022, 15, 3–11. [Google Scholar]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 2002. [Google Scholar]
- Shelukhina, N.P.; Fedichkina, L.G. A rapid method for quantitative determination of pectic substances. Acta Bot. Neerlandica 1994, 43, 205–207. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4–9. [Google Scholar]
- Felföldi, Z.; Ranga, F.; Socaci, S.A.; Farcas, A.; Plazas, M.; Sestras, A.F.; Vodnar, D.C.; Prohens, J.; Sestras, R.E. Physico-chemical, nutritional, and sensory evaluation of two new commercial tomato hybrids and their parental lines. Plants 2021, 10, 2480. [Google Scholar] [CrossRef]
- Sestras, A.F.; Pamfil, D.; Dan, C.; Bolboaca, S.D.; Jäntschi, L.; Sestras, R.E. Possibilities to improve apple scab (Venturia inaequalis (Cke.) Wint.) and powdery mildew [Podosphaera leucotricha (Ell. et Everh.) Salm.] resistance on apple by increasing genetic diversity using potentials of wild species. Aust. J. Crop Sci. 2011, 5, 748–755. [Google Scholar]
- Falconer, D.S.; Mackay, T.F. Introduction to Quantitative Genetics; Longman Group Ltd.: London, UK, 1996. [Google Scholar]
- Mărcășan, L.I.S.; Hulujan, I.B.; Florian, T.; Alpar, S.P.; Militaru, M.; Sestras, A.F.; Oltean, I.; Sestras, R.E. The importance of assessing the population structure and biology of psylla species for pest monitoring and management in pear orchards. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 13022. [Google Scholar] [CrossRef]
- Hall, H.; Hummer, K.E.; Jamieson, A.; Jennings, S.N.; Weber, C. Raspberry breeding and genetics. In Plant Breeding Reviews; Janick, J., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; Volume 32. [Google Scholar]
- Graham, J.; Jennings, S.N. Raspberry breeding. In Breeding Tree Crops; Jain, S.M., Priyadarshan, M., Eds.; IBH & Science Publication: Oxford, UK, 2009; pp. 233–248. [Google Scholar]
- Hernández-Bautista, A.; Lobato-Ortiz, R.; García-Zavala, J.J.; Contreras, J.A.M.; Chávez-Servia, J.L.; Rodríguez, M.R.; Cruz-Izquierdo, S.; Velázquez, J.A.G. Adaptability of raspberry primocane genotypes in a tropical environment and its implication in the raspberry production in Mexico. J. Berry Res. 2019, 9, 155–163. [Google Scholar] [CrossRef]
- Ozherelieva, Z.E.; Lupin, M.V.; Bogomolova, N.I. Study of raspberry genotypes by biologically valuable traits under conditions of central Russia. Agronomy 2022, 12, 630. [Google Scholar] [CrossRef]
- Black, B.L.; Lindstrom, T.; Heflebower, R.; Hunter, B.; Olsen, S.; Alston, D.G. Adaptability of primocane raspberry cultivars to a high-elevation arid climate. J. Am. Pomol. Soc. 2013, 67, 47–56. [Google Scholar]
- Hansen, S.; Black, B.; Alston, D.; Lindstrom, T.; Olsen, S. A comparison of nine primocane fruiting raspberry cultivars for suitability to a high-elevation, arid climate. Int. J. Fruit Sci. 2021, 21, 500–508. [Google Scholar] [CrossRef]
- Hall, H.K.; Kempler, C. Raspberry breeding. Fruit Veg. Cereal Sci. Biotech. 2011, 5, 44–62. [Google Scholar]
- Zejak, D.; Glisic, I.; Spalevic, V.; Maskovic, P.; Dudic, B. Sustainable management of fruit growing in rural areas of Montenegro: The impact of location on the phenological and nutritional properties on raspberry (Rubus idaeus L.). Agronomy 2021, 11, 1663. [Google Scholar] [CrossRef]
- Zejak, D.; Radović, A.; Spalevic, V.; Glišić, I. Production of planting material of raspberry variety Glen Ample in the North Montenegro. Agric. For. Poljopr. I Sumar. 2021, 67, 245–259. [Google Scholar] [CrossRef]
- Leposavić, A.; Janković, M.; Đurović, D.; Veljković, B.; Keserović, Z.; Popović, B. Fruit quality of red raspberry cultivars and selections grown in western Serbia. Hortic. Sci. 2013, 40, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Akšić, M.F.; Nešović, M.; Ćirić, I.; Tešić, Ž.; Pezo, L.; Tosti, T.; Gašić, U.; Dojčinović, B.; Lončar, B.; Meland, M. Chemical fruit profiles of different raspberry cultivars grown in specific Norwegian agroclimatic conditions. Horticulturae 2022, 8, 765. [Google Scholar] [CrossRef]
- Dan, C.; Șerban, C.; Sestraş, A.F.; Militaru, M.; Morariu, P.; Sestraş, R.E. Consumer perception concerning apple fruit quality, depending on cultivars and hedonic scale of evaluation—A case study. Not. Sci. Biol. 2015, 7, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Lebedev, V.G.; Subbotina, N.M.; Maluchenko, O.P.; Krutovsky, K.V.; Shestibratov, K.A. Assessment of genetic diversity in differently colored raspberry cultivars using SSR markers located in flavonoid biosynthesis genes. Agronomy 2019, 9, 518. [Google Scholar] [CrossRef] [Green Version]
- Acquaah, G. Principles of Plant Genetics and Breeding; Blackwell Publishers: Oxford, UK, 2007; p. 1166. [Google Scholar]
- Yu, S.B.; Li, J.X.; Xu, C.G.; Tan, Y.F.; Gao, Y.J.; Li, X.H.; Zhang, Q.; Maroof, M.A.S. Importance of epistasis as the genetic basis of heterosis in an elite rice hybrid. Proc. Natl. Acad. Sci. USA 1997, 94, 9226–9231. [Google Scholar] [CrossRef] [Green Version]
- Kempler, C.; Hall, H.; Finn, C.E. Raspberry. In Fruit Breeding; Badenes, M.L., Byrne, D.H., Eds.; Springer: Boston, MA, USA, 2012; pp. 263–304. [Google Scholar]
- Hernández-Bautista, A.; Lobato-Ortíz, R.; García-Zavala, J.J.; Servia, J.L.C.; Mejía-Contreras, J.A.; García-Velazquez, J.A. Breeding potential of raspberry primocane selections based on their combining abilities. Can. J. Plant Sci. 2018, 98, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Marchi, P.; Carvalho, I.; Höhn, D.; Carra, B.; Szareski, V.; Dini, M.; Vizzotto, M.; Antunes, L. Research article genetic parameters prediction (REML/BLUP) for morphological, physical-chemical, phytochemical, and mineral components of primocane-fruiting raspberries. J. Genet. Mol. Res. 2019, 18, 18254. [Google Scholar] [CrossRef]
- Fotirić-Akšić, M.; Radović, A.; Milivojević, J.; Nikolić, M.; Nikolić, D. Genetic parameters of yield components and pomo-logic properties in raspberry seedlings. Genetika 2011, 43, 667–674. [Google Scholar] [CrossRef]
- Cash, C.B. Studies of Linkage Mapping, Trait Heritability, and Pedigrees for Breeding Improvement of Southeastern Black and Red Raspberry. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2016. [Google Scholar]
- Nestby, R. Heritability estimates in raspberry breeding. In Progress in Temperate Fruit Breeding. Developments in Plant Breeding; Schmidt, H., Kellerhals, M., Eds.; Springer: Dordrecht, The Netherlands, 1994; Volume 1. [Google Scholar]
- Fehr, W.R. Heterosis. In Principles of Cultivar Development: Theory and Techniques; Macmillan Publishing Company: New York, NY, USA, 1987; Volume 1. [Google Scholar]
- Dossett, M.; Lee, J.; Finn, C.E. Inheritance of phenological, vegetative, and fruit chemistry traits in black raspberry. J. Am. Soc. Hortic. Sci. 2008, 133, 408–417. [Google Scholar] [CrossRef] [Green Version]
- Connor, A.M.; Stephens, M.J.; Hall, H.K.; Alspach, P.A. Variation and heritabilities of antioxidant activity and total phenolic content estimated from a red raspberry factorial experiment. J. Am. Soc. Hortic. Sci. 2005, 130, 403–411. [Google Scholar] [CrossRef] [Green Version]
- González, M. Heritability of primocane-yield components in red raspberry. Acta Hortic. 2016, 1133, 75–80. [Google Scholar] [CrossRef]
- Stephens, M.J.; Alspach, P.A.; Beatson, R.A.; Winefield, C.; Buck, E.J. Genetic parameters and development of a selection index for breeding red raspberries for processing. J. Am. Soc. Hortic. Sci. 2012, 137, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Stephens, M.J.; Alspach, P.A.; Beatson, R.A.; Winefield, C.; Buck, E.J. Genetic parameters and breeding for yield in red raspberry. J. Am. Soc. Hortic. Sci. 2012, 137, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Fejer, S.O.; Spangelo, L.P.S. Three generations of inbreeding and S2 factorial test crosses in red raspberry cultivars. Can. J. Genet. Cytol. 1974, 16, 419–432. [Google Scholar] [CrossRef]
- Fejer, S.O. Inheritance of yield, yield components and fall fruiting habit in red raspberry diallel crosses. Can. J. Genet. Cytol. 1977, 19. [Google Scholar] [CrossRef]
- Radovich, A.; Fotirich Aksich, M.; Rakonjac, V.; Milivojevich, J.; Nikolich, D.; Nikolich, M. Diversity and relationship of yield components and fruit quality in promising floricane raspberry hybrids. Bulg. J. Agric. Sci. 2013, 19, 750–755. [Google Scholar]
- Cormack, M.R.; Woodward, P.J. Raspberry cultivar assessments at the national fruit trials and the scottish horticultural research institute. Expt. Hort. 1977, 29, 1–14. [Google Scholar]
- Clark, A. Senescence and the genetic-correlation hang-up. Am. Nat. 1987, 129, 932–940. [Google Scholar] [CrossRef]
- Sgrò, C.M.; Hoffmann, A. Genetic correlations, tradeoffs and environmental variation. Heredity 2004, 93, 241–248. [Google Scholar] [CrossRef]
- Haffner, K.; Rosenfeld, H.J.; Skrede, G.; Wang, L. Quality of red raspberry Rubus idaeus L. cultivars after storage in controlled and normal atmospheres. Postharvest Biol. Technol. 2002, 24, 279–289. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, G.; Sun, L.; Song, X.; Bao, Y.; Luo, T.; Wang, J. Comprehensive evaluation of 24 red raspberry varieties in northeast China based on nutrition and taste. Foods 2022, 11, 3232. [Google Scholar] [CrossRef]
- Bradish, C.M.; Perkins-Veazie, P.; Fernandez, G.E.; Xie, G.; Jia, W. Comparison of flavonoid composition of red raspberries (Rubus idaeus L.) grown in the southern United States. J. Agric. Food Chem. 2012, 60, 5779–5786. [Google Scholar] [CrossRef]
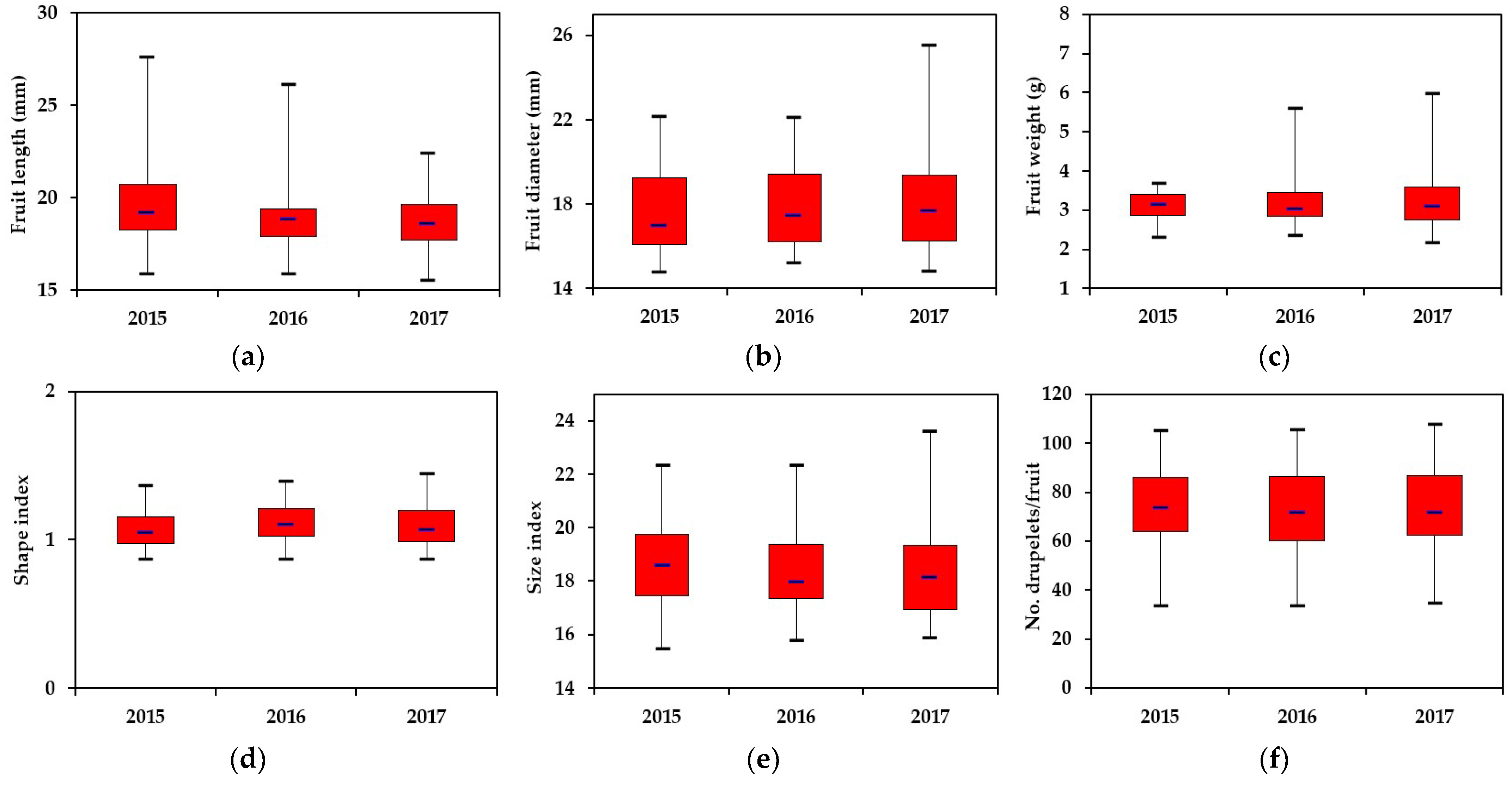
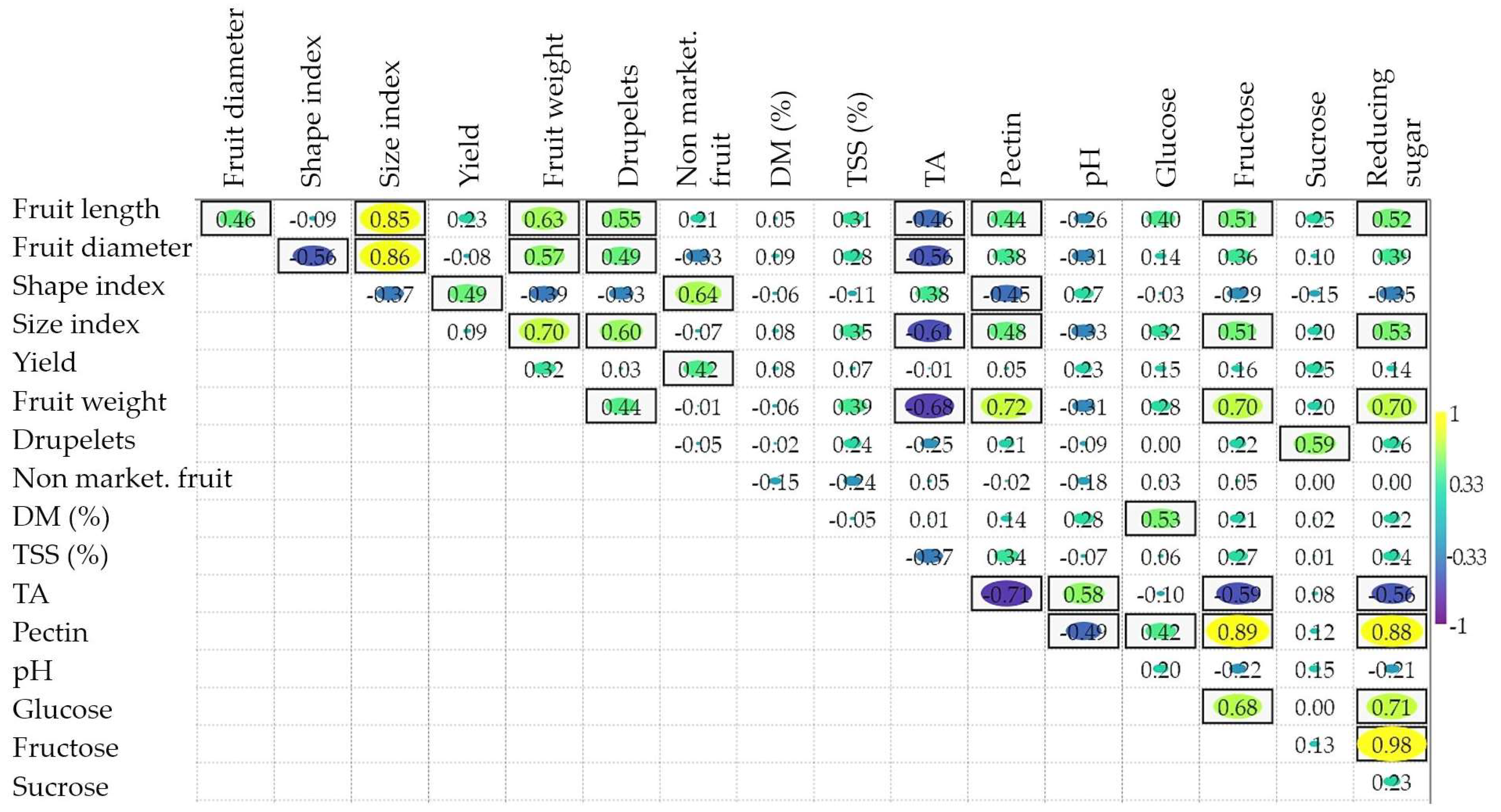
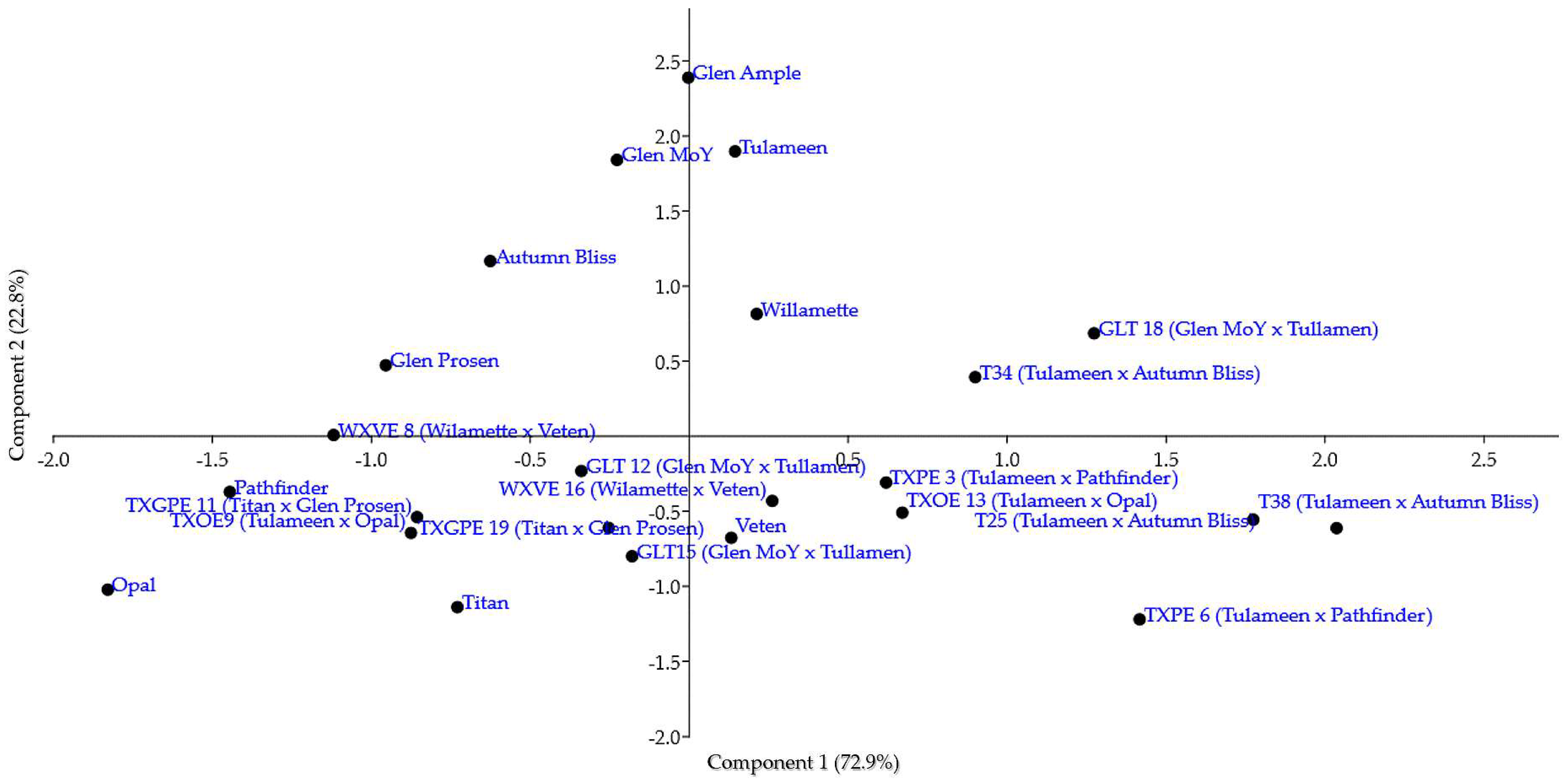
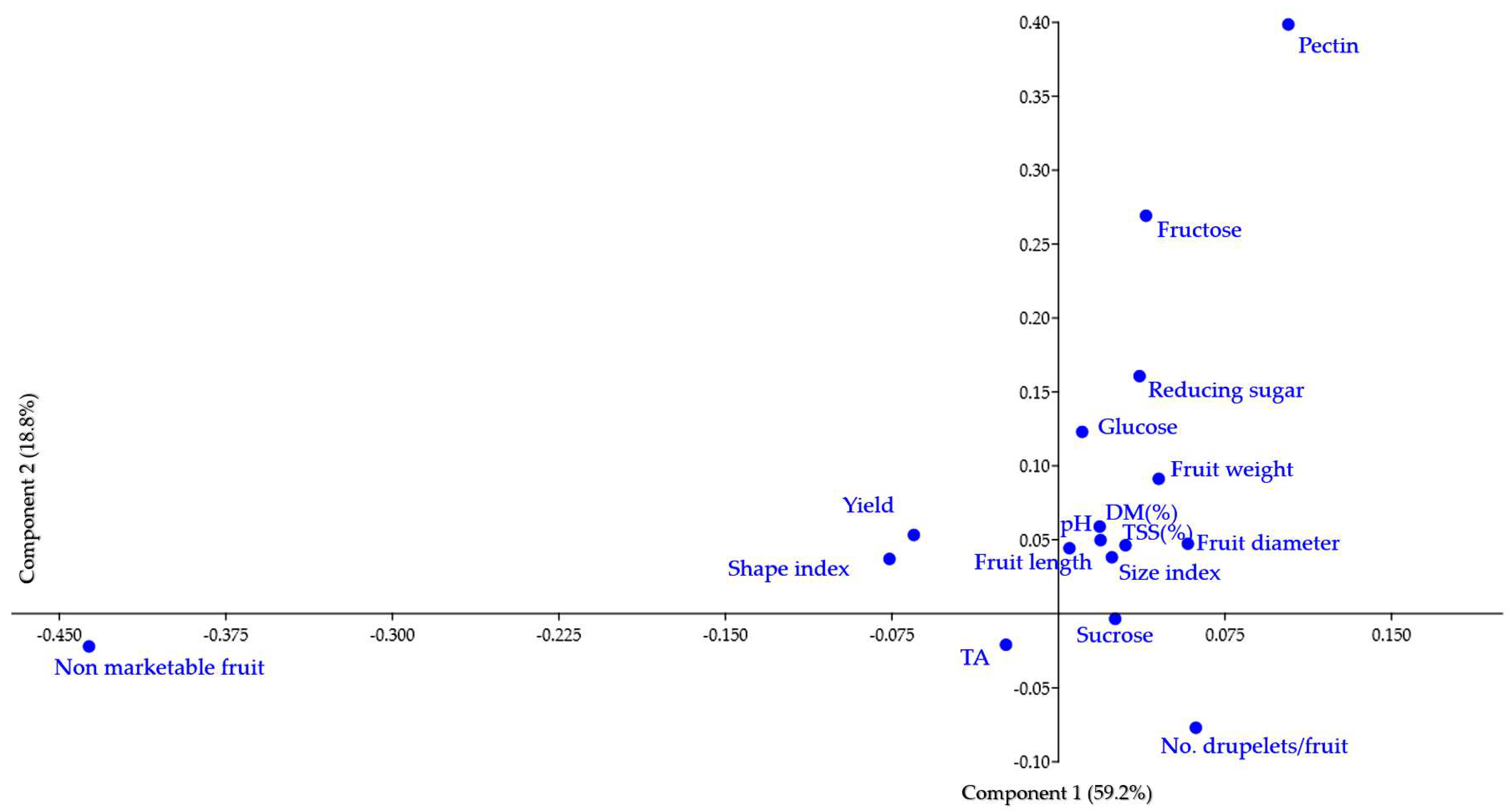
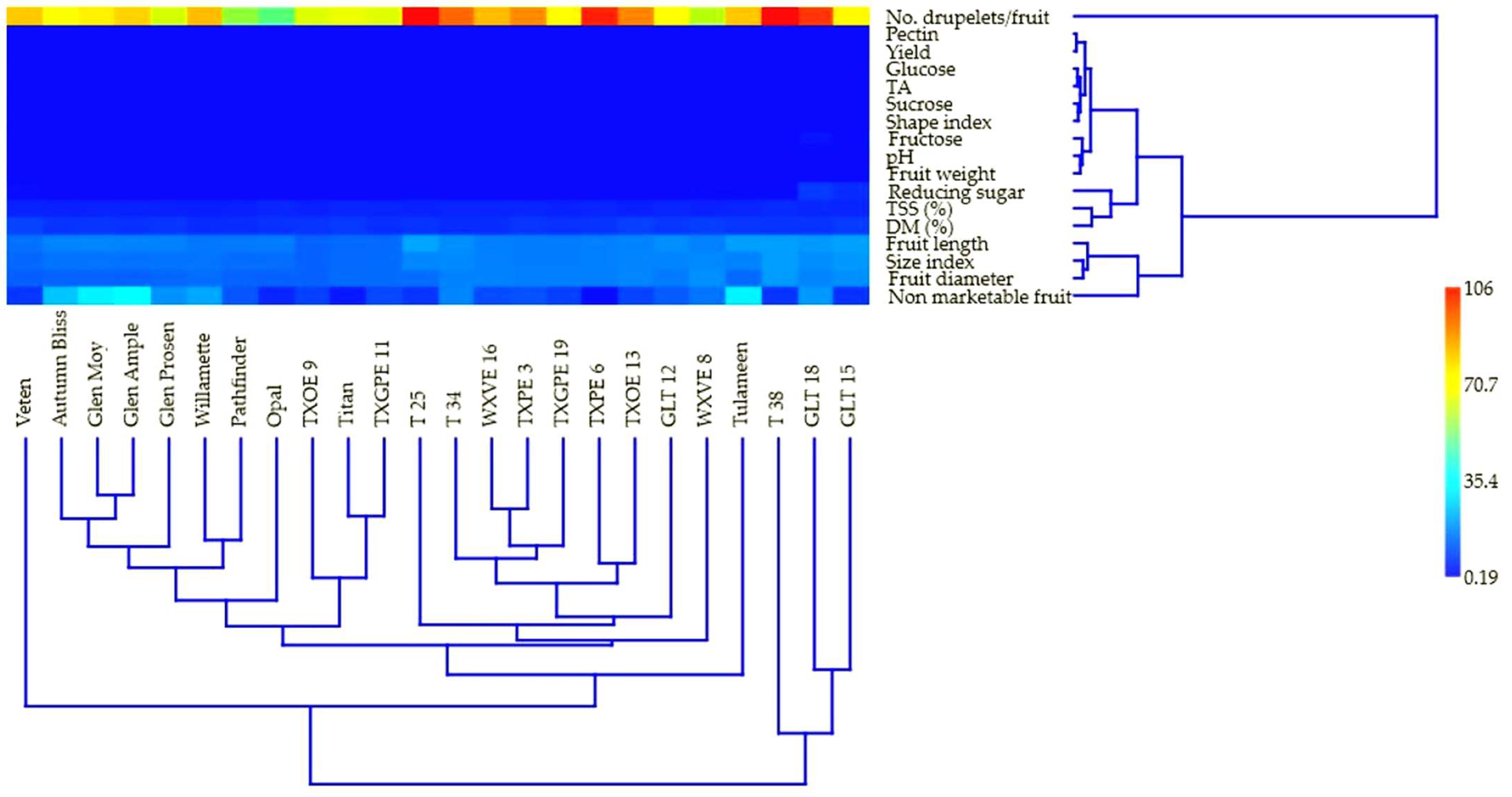
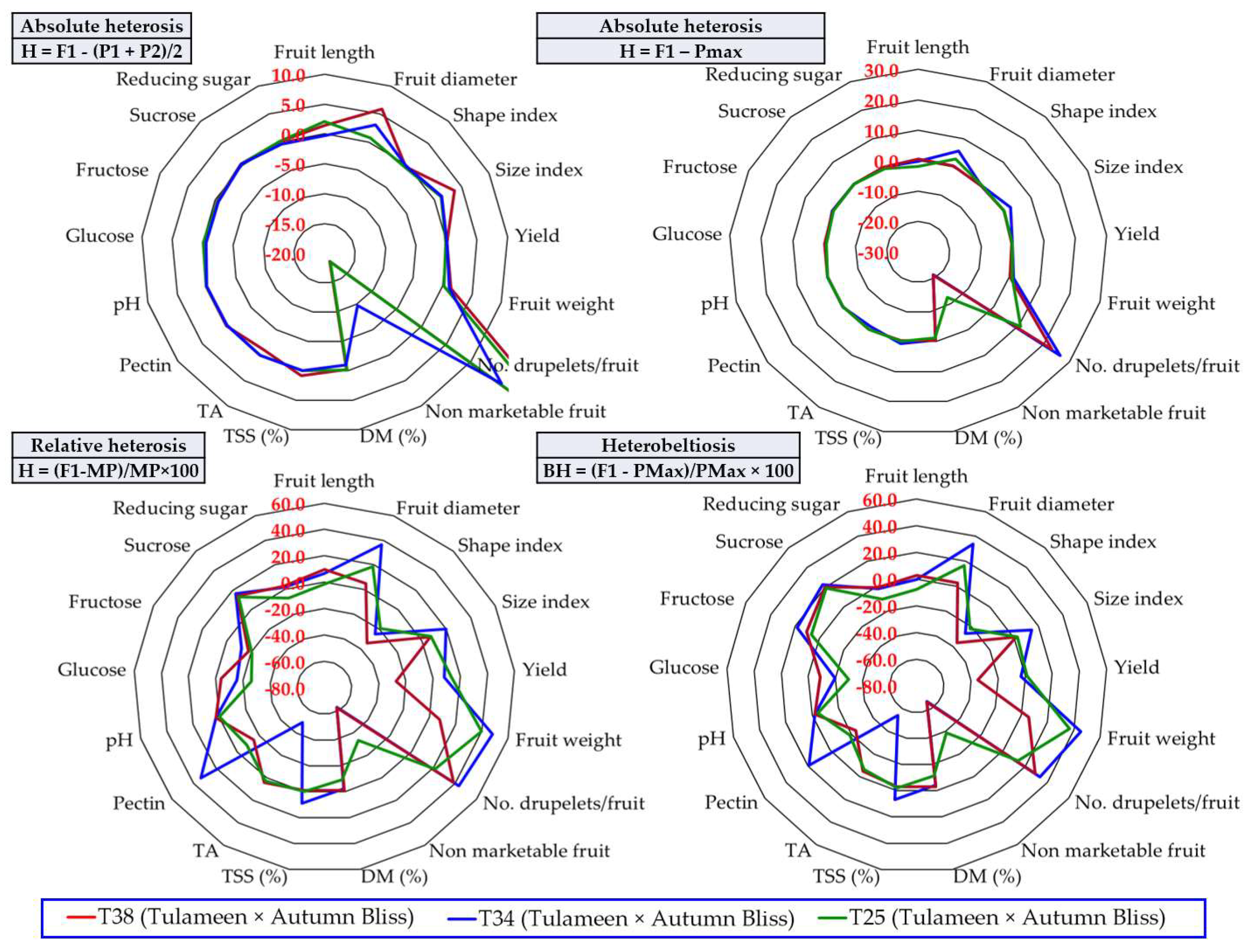
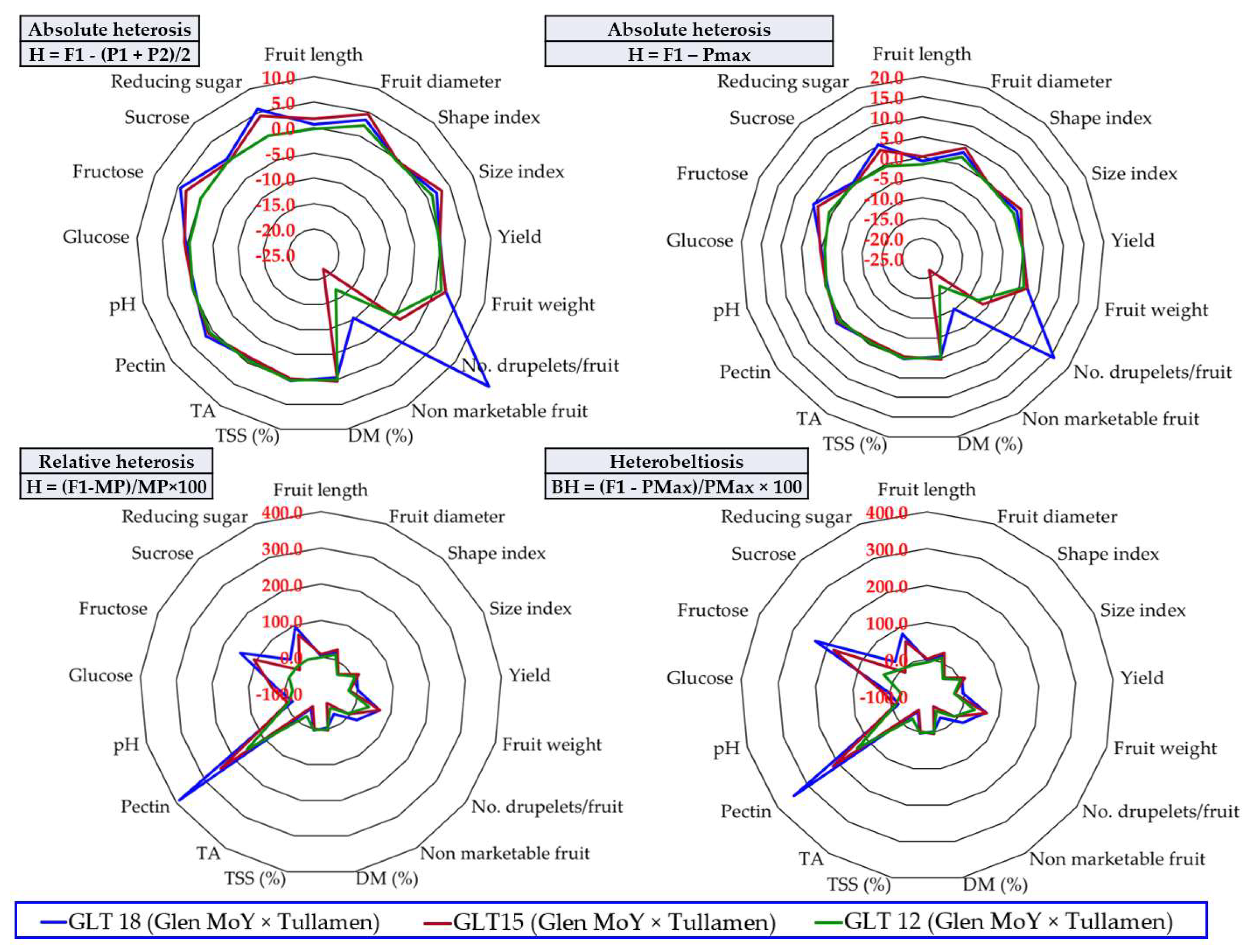
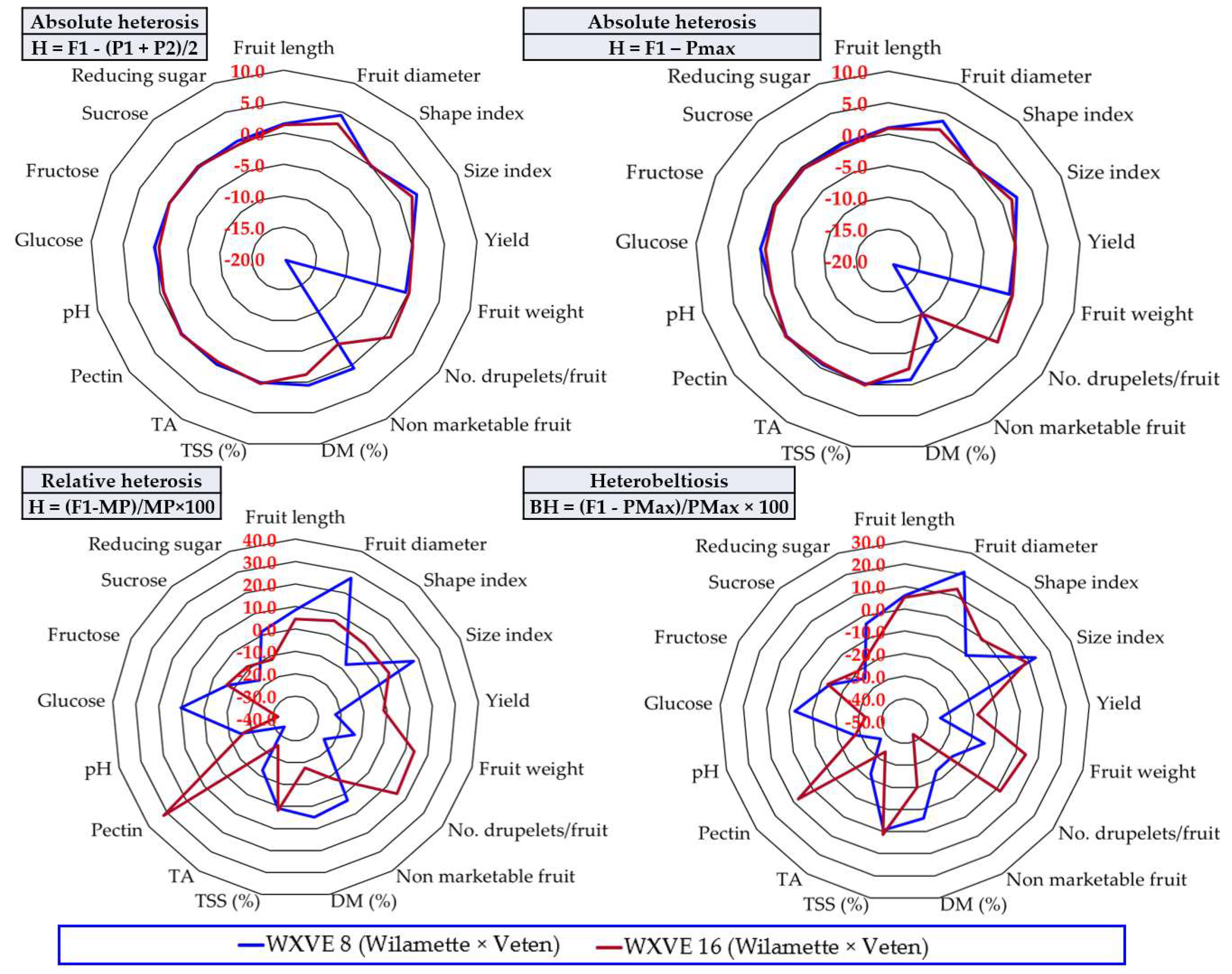


| No. | Genotype | Fruit Length (mm) | Fruit Diameter (mm) | Shape Index | Size Index |
|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | ||
| 1 | Tulameen | 22.54 a ± 0.12 | 16.58 d ± 0.33 | 1.36 a ± 0.03 | 19.56 b ± 0.18 |
| 2 | Autumn Bliss | 19.51 a,b ± 0.19 | 15.99 d ± 0.29 | 1.22 b ± 0.02 | 17.75 c ± 0.17 |
| 3 | T 38 (Tulameen × Autumn Bliss) | 22.43 a ± 0.39 | 22.21 a ± 0.52 | 1.01 d ± 0.04 | 22.32 a ± 0.29 |
| 4 | T 25 (Tulameen × Autumn Bliss) | 23.10 a ± 0.48 | 17.04 d ± 0.41 | 1.36 a ± 0.06 | 20.07 b ± 0.25 |
| 5 | T 34 (Tulameen × Autumn Bliss) | 20.75 a,b ± 0.62 | 19.32 c ± 0.42 | 1.07 d± 0.02 | 20.03 b ± 0.47 |
| 6 | Glen Moy | 19.61 a,b ± 0.20 | 15.89 d ± 0.29 | 1.23 b ± 0.02 | 17.75 c ± 0.18 |
| 7 | GLT 18 (Glen Moy × Tulameen) | 21.69 a ± 0.36 | 19.71 c ± 0.41 | 1.10 c ± 0.03 | 20.70 b ± 0.34 |
| 8 | GLT 15 (Glen Moy × Tulameen) | 22.78 a ± 0.42 | 20.85 a ± 0.35 | 1.09 c ± 0.02 | 21.82 a ± 0.35 |
| 9 | GLT 12 (Glen Moy × Tulameen) | 20.81 a,b ± 0.67 | 18.50 c ± 0.86 | 1.12 b,c ± 0.03 | 19.66 b ± 0.54 |
| 10 | Willamette | 18.18 c ± 0.26 | 17.24 d ± 0.20 | 1.05 c ± 0.03 | 17.71 c ± 0.15 |
| 11 | Veten | 17.30 c,d ± 0.64 | 15.60 d ± 0.26 | 1.11 b,c ± 0.04 | 16.45 c,d ± 0.12 |
| 12 | WXVE 8 (Willamette × Veten) | 19.25 b ± 0.74 | 20.88 a ± 0.39 | 0.92 e ± 0.06 | 20.06 b ± 0.36 |
| 13 | WXVE 16 (Willamette × Veten) | 19.07 b ± 0.72 | 19.48 b,c ± 0.41 | 0.98 d ± 0.04 | 19.27 b ± 0.42 |
| 14 | Pathfinder | 18.34 b ± 0.44 | 15.95 d ± 0.31 | 1.15 b ± 0.04 | 17.14 c ± 0.30 |
| 15 | TXPE 3 (Tulameen × Pathfinder) | 18.78 b ± 0.27 | 19.00 b,c ± 0.15 | 0.99 d ± 0.01 | 18.89 b ± 0.15 |
| 16 | TXPE 6 (Tulameen × Pathfinder) | 19.03 b ± 0.26 | 18.93 c ± 0.16 | 1.01 d ± 0.01 | 18.98 b ± 0.15 |
| 17 | Opal | 18.75 b ± 0.46 | 15.64 d ± 0.26 | 1.19 b ± 0.03 | 17.20 c ± 0.24 |
| 18 | TXOE 13 (Tulameen × Opal) | 19.45 b ± 0.43 | 19.81 b,c ± 0.34 | 0.98 d ± 0.01 | 19.63 b ± 0.14 |
| 19 | TXOE 9 (Tulameen × Opal) | 15.77 b ± 0.31 | 15.87 d ± 0.41 | 0.99 d ± 0.02 | 15.82 d ± 0.18 |
| 20 | Titan | 16.45 d ± 0.17 | 16.89 d ± 0.25 | 0.97 d ± 0.02 | 16.67 c,d ± 0.12 |
| 21 | Glen Prosen | 19.28 b ± 0.25 | 16.36 d ± 0.27 | 1.18 b ± 0.02 | 17.82 c ± 0.22 |
| 22 | TXGPE 11 (Titan × Glen Prosen) | 16.63 d ± 0.20 | 16.92 d ± 0.27 | 0.97 d ± 0.02 | 16.78 c ± 0.22 |
| 23 | TXGPE 19 (Titan × Glen Prosen) | 18.54 b ± 0.22 | 19.07 b,c ± 0.17 | 0.98 d ± 0.01 | 18.80 b,c ± 0.14 |
| 24 | Glen Ample | 19.40 a,b ± 0.33 | 15.83 d ± 0.17 | 1.23 b ± 0.03 | 17.61 c ± 0.18 |
| No. | Genotype | Yield (kg/cane) | Fruit Weight (g) | No. Drupelets/Fruit | Non-Marketable Fruit (g/cane) |
|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | ||
| 1 | Tulameen | 0.42 a ± 0.05 | 2.97 c ± 0.02 | 79.44 c ± 1.27 | 32.07 a ± 4.03 |
| 2 | Autumn Bliss | 0.33 c ± 0.01 | 2.84 c ± 0.02 | 68.35 d± 5.02 | 26.63 b ± 1.63 |
| 3 | T 38 (Tulameen × Autumn Bliss) | 0.41 a ± 0.01 | 4.31 a ± 0.10 | 105.91 a ± 0.92 | 10.89 d ± 0.68 |
| 4 | T 25 (Tulameen × Autumn Bliss) | 0.28 c,d ± 0.04 | 3.13 c ± 0.06 | 102.58 a ± 1.28 | 11.04 d ± 4.70 |
| 5 | T 34 (Tulameen × Autumn Bliss) | 0.43 a ± 0.03 | 4.07 a,b ± 0.08 | 90.03 b ± 1.08 | 19.62 b,c ± 7.16 |
| 6 | Glen Moy | 0.38 b ± 0.01 | 2.79 c ± 0.08 | 74.43 c ± 4.14 | 31.93 a ± 4.01 |
| 7 | GLT 18 (Glen Moy × Tulameen) | 0.41 a ± 0.00 | 4.86 a± 0.17 | 95.16 a ± 0.22 | 21.73 b ± 9.71 |
| 8 | GLT 15 (Glen Moy × Tulameen) | 0.32 c ± 0.03 | 4.90 a ± 0.14 | 73.16 c ± 0.88 | 10.42 d ± 5.12 |
| 9 | GLT 12 (Glen Moy × Tulameen) | 0.31 c ± 0.02 | 3.95 a,b ± 0.28 | 71.67 c ± 0.77 | 15.13 c ± 5.97 |
| 10 | Willamette | 0.32 c ± 0.03 | 2.97 c ± 0.03 | 80.58 b,c ± 1.81 | 23.43 b ± 3.93 |
| 11 | Veten | 0.46 a ± 0.02 | 2.90 a ± 0.19 | 79.19 c ± 0.67 | 17.82 c ± 9.72 |
| 12 | WXVE 8 (Willamette × Veten) | 0.30 c ± 0.04 | 2.55 d ± 0.09 | 60.26 b,c ± 0.22 | 11.19 d ± 4.30 |
| 13 | WXVE 16 (Willamette × Veten) | 0.38 b ± 0.01 | 3.11 c ± 0.06 | 80.58 b,c ± 0.70 | 13.32 c,d ± 3.72 |
| 14 | Pathfinder | 0.35 b ± 0.01 | 2.85 c ± 0.05 | 55.98 e ± 4.04 | 14.53 c ± 9.27 |
| 15 | TXPE 3 (Tulameen × Pathfinder) | 0.39 b ± 0.01 | 3.96 b ± 0.04 | 85.97 b ± 0.29 | 14.12 c ± 5.36 |
| 16 | TXPE 6 (Tulameen × Pathfinder) | 0.19 d ± 0.02 | 2.52 d ± 0.08 | 97.41 a ± 0.42 | 6.17 d ± 1.20 |
| 17 | Opal | 0.39 b ± 0.01 | 2.72 c ± 0.06 | 50.09 f ± 0.37 | 9.36 d ± 2.70 |
| 18 | TXOE 13 (Tulameen × Opal) | 0.32 c ± 0.02 | 2.49 d ± 0.03 | 86.51 b ± 0.23 | 12.48 c,d ± 5.24 |
| 19 | TXOE 9 (Tulameen × Opal) | 0.29 c,d ± 0.02 | 2.77 c ± 0.07 | 64.57 d ± 0.39 | 12.11 c,d ± 3.82 |
| 20 | Titan | 0.30 c ± 0.05 | 2.40 d ± 0.03 | 66.41 d ± 2.95 | 8.03 d ± 2.59 |
| 21 | Glen Prosen | 0.47 a ± 0.04 | 2.85 c ± 0.05 | 63.30 d ± 4.34 | 21.17 b ± 4.55 |
| 22 | TXGPE 11 (Titan × Glen Prosen) | 0.21 d ± 0.01 | 2.58 c ± 0.10 | 64.70 d ± 0.16 | 13.00 c,d ± 4.03 |
| 23 | TXGPE 19 (Titan × Glen Prosen) | 0.38 b ± 0.01 | 3.29 c ± 0.08 | 73.07 c ± 0.31 | 12.14 c,d ± 3.41 |
| 24 | Glen Ample | 0.42 a ± 0.03 | 3.05 c ± 0.05 | 77.9 c ± 5.31 | 36.30 a ± 2.09 |
| No. | Genotype | Dry Matter (%) | Total Soluble Solids (%) | Titratable Acidity (g/L Tartaric Acid) | Pectin (%) | pH |
|---|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | ||
| 1 | Tulameen | 11.27 a ± 0.27 | 9.24 b ± 0.29 | 1.89 b ± 0.01 | 0.37 d ± 0.11 | 3.16 a ± 0.04 |
| 2 | Autumn Bliss | 10.83 a ± 0.33 | 8.87 c ± 0.27 | 2.28 a ± 0.15 | 0.47 d ± 0.04 | 2.87 b ± 0.04 |
| 3 | T 38 (Tulameen × Autumn Bliss) | 10.77 a ± 0.15 | 9.85 a ± 0.14 | 1.05 d ± 0.02 | 0.56 d ± 0.02 | 3.10 a ± 0.06 |
| 4 | T 25 (Tulameen × Autumn Bliss) | 10.92 a ± 0.06 | 8.98 c ± 0.03 | 2.17 a ± 0.04 | 0.36 d ± 0.03 | 3.10 a ± 0.01 |
| 5 | T 34 (Tulameen × Autumn Bliss) | 10.00 b ± 0.46 | 9.03 c ± 0.02 | 2.14 a ± 0.03 | 0.38 d ± 0.02 | 3.02 a ± 0.04 |
| 6 | Glen Moy | 11.17 a ± 0.12 | 8.94 c ± 0.20 | 1.81 b ± 0.09 | 0.45 d ± 0.04 | 2.97 a,b ± 0.02 |
| 7 | GLT 18 (Glen Moy × Tulameen) | 10.90 b ± 0.06 | 9.47 b ± 0.15 | 0.93 d ± 0.03 | 2.00 a ± 0.06 | 2.51 c ± 0.02 |
| 8 | GLT 15 (Glen Moy × Tulameen) | 11.70 a ± 0.12 | 9.01 c ± 0.01 | 0.80 d ± 0.09 | 1.41 b ± 0.12 | 2.88 b ± 0.31 |
| 9 | GLT 12 (Glen Moy × Tulameen) | 11.30 a ± 0.21 | 9.32 b ± 0.01 | 1.37 c ± 0.02 | 1.05 c ± 0.01 | 2.81 b ± 0.13 |
| 10 | Willamette | 10.03 b ± 0.18 | 8.62 d ± 0.29 | 2.07 a ± 0.07 | 0.33 d ± 0.06 | 3.20 a ± 0.00 |
| 11 | Veten | 12.70 a ± 0.47 | 8.90 c ± 0.20 | 2.65 a ± 0.14 | 0.36 d ± 0.03 | 4.47 a ± 0.01 |
| 12 | WXVE 8 (Willamette × Veten) | 11.93 a ± 0.04 | 8.83 c ± 0.10 | 2.06 a ± 0.02 | 0.23 d ± 0.01 | 3.26 a ± 0.04 |
| 13 | WXVE 16 (Willamette × Veten) | 10.17 b ± 0.07 | 9.02 c ± 0.01 | 1.75 b ± 0.05 | 0.39 d ± 0.01 | 3.22 a ± 0.02 |
| 14 | Pathfinder | 10.20 b ± 0.64 | 8.83 c ± 0.14 | 1.88 b ± 0.02 | 0.30 d ± 0.06 | 3.25 a ± 0.03 |
| 15 | TXPE 3 (Tulameen × Pathfinder) | 11.11 a ± 0.05 | 9.10 c ± 0.06 | 1.63 b ± 0.02 | 0.24 d ± 0.01 | 3.22 a ± 0.01 |
| 16 | TXPE 6 (Tulameen × Pathfinder) | 10.80 a ± 0.12 | 9.13 c ± 0.08 | 1.84 b ± 0.09 | 0.37 d ± 0.03 | 3.33 a ± 0.01 |
| 17 | Opal | 10.63 a ± 0.14 | 9.51 b ± 0.10 | 2.22 a ± 0.01 | 0.30 d ± 0.05 | 3.74 a ± 0.03 |
| 18 | TXOE 13 (Tulameen × Opal) | 12.07 a ± 0.46 | 8.72 c,d ± 0.02 | 1.67 b ± 0.03 | 0.51 d ± 0.02 | 2.87 b ± 0.04 |
| 19 | TXOE 9 (Tulameen × Opal) | 10.80 a ± 0.15 | 8.80 c ± 0.09 | 1.69 b ± 0.02 | 0.38 d ± 0.01 | 2.97 a,b ± 0.02 |
| 20 | Titan | 11.50 a ± 0.26 | 9.41 b ± 0.11 | 2.17a ± 0.01 | 0.46 d ± 0.02 | 2.90 a,b ± 0.01 |
| 21 | Glen Prosen | 11.13 a ± 0.35 | 8.85 c ± 0.01 | 1.41 c ± 0.01 | 0.50 d ± 0.00 | 2.86 b ± 0.02 |
| 22 | TXGPE 11 (Titan × Glen Prosen) | 10.30 a,b ± 0.06 | 9.23 b,c ± 0.12 | 1.78 b ± 0.01 | 0.46 d ± 0.02 | 2.90 a,b ± 0.01 |
| 23 | TXGPE 19 (Titan × Glen Prosen) | 10.62 a,b ± 0.06 | 8.83 c ± 0.01 | 2.10 a ± 0.01 | 0.47 d ± 0.03 | 2.83 b ± 0.02 |
| 24 | Glen Ample | 10.53 a,b ± 0.47 | 8.90 c ±0.20 | 1.94 a,b ± 0.04 | 0.28 d ± 0.02 | 3.25 a ± 0.05 |
| No. | Genotype | Glucose (g/100 g) | Fructose (g/100 g) | Sucrose (g/100 g) | Reducing Sugar (g/100 g) |
|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | ||
| 1 | Tulameen | 2.33 b ± 0.16 | 3.73 c ± 1.14 | 1.45 b ± 0.17 | 6.37 c ± 0.06 |
| 2 | Autumn Bliss | 2.10 c ± 0.36 | 2.21 d ± 0.05 | 1.59 a,b ± 0.19 | 5.90 c ± 0.17 |
| 3 | T 38 (Tulameen × Autumn Bliss) | 1.87 d ± 0.03 | 2.60 d ± 0.01 | 1.77 a ± 0.01 | 6.24 c ± 0.03 |
| 4 | T 25 (Tulameen × Autumn Bliss) | 2.12 c ± 0.05 | 2.44 d ± 0.13 | 1.74 a ± 0.02 | 6.30 c ± 0.11 |
| 5 | T 34 (Tulameen × Autumn Bliss) | 1.63 d ± 0.12 | 2.35 d ± 0.08 | 1.72 a ± 0.01 | 5.70 c,d ± 0.10 |
| 6 | Glen Moy | 1.77 d ± 0.02 | 2.20 d ± 0.05 | 1.48 b ± 0.28 | 5.45 d ± 0.30 |
| 7 | GLT 18 (Glen Moy × Tulameen) | 2.40 b ± 0.06 | 7.34 a ± 0.07 | 1.83 a ± 0.10 | 11.57 a ± 0.11 |
| 8 | GLT 15 (Glen Moy × Tulameen) | 2.72 a ± 0.12 | 6.13 b ± 0.08 | 1.27 c ± 0.04 | 10.12 b ± 0.18 |
| 9 | GLT 12 (Glen Moy × Tulameen) | 1.61 d ± 0.03 | 2.86 d ± 0.03 | 1.44 b ± 0.03 | 5.91 c,d ± 0.11 |
| 10 | Willamette | 1.91 c ± 0.27 | 2.61 d ± 0.05 | 1.46 b ± 0.29 | 5.97 c,d ± 0.17 |
| 11 | Veten | 2.46 b ± 0.01 | 3.07 c ± 0.05 | 1.78 a ± 0.01 | 6.64 c ± 0.33 |
| 12 | WXVE 8 (Willamette × Veten) | 2.40 b ± 0.02 | 2.64 d ± 0.06 | 1.35 b,c ± 0.02 | 6.39 c ± 0.06 |
| 13 | WXVE 16 (Willamette × Veten) | 1.64 d ± 0.02 | 2.67 d ± 0.03 | 1.43 b ± 0.02 | 5.74 c,d ± 0.07 |
| 14 | Pathfinder | 1.68 d ± 0.05 | 2.22 d ± 0.06 | 1.45 b ± 0.11 | 5.35 d ± 0.15 |
| 15 | TXPE 3 (Tulameen × Pathfinder) | 1.74 d ± 0.03 | 2.23 d ± 0.07 | 1.35 b,c ± 0.03 | 5.32 d ± 0.12 |
| 16 | TXPE 6 (Tulameen × Pathfinder) | 1.78 d ± 0.02 | 2.31 d ± 0.01 | 1.68 a ± 0.02 | 5.77 c,d ± 0.02 |
| 17 | Opal | 2.06 c ± 0.04 | 2.56 d ± 0.05 | 1.49 b ± 0.02 | 6.11 c ± 0.47 |
| 18 | TXOE 13 (Tulameen × Opal) | 1.73 d ± 0.01 | 2.36 d ± 0.03 | 1.70 a ± 0.01 | 5.79 c,d ± 0.09 |
| 19 | TXOE 9 (Tulameen × Opal) | 1.63 d ± 0.06 | 2.21 d ± 0.01 | 1.72 a ± 0.06 | 5.56 c,d ± 0.09 |
| 20 | Titan | 1.83 c,d ± 0.03 | 2.07 d ± 0.04 | 1.22 c ± 0.05 | 5.12 d ± 0.05 |
| 21 | Glen Prosen | 1.92 c ± 0.10 | 2.10 d ± 0.01 | 1.35 b,c ± 0.17 | 5.38 c,d ± 0.28 |
| 22 | TXGPE 11 (Titan × Glen Prosen) | 1.80 c,d ± 0.02 | 2.07 d ± 0.04 | 1.13 c ± 0.02 | 5.01 d ± 0.04 |
| 23 | TXGPE 19 (Titan × Glen Prosen) | 1.89 c,d ± 0.02 | 2.11 d ± 0.01 | 1.65 a,b ± 0.02 | 5.65 c,d ± 0.09 |
| 24 | Glen Ample | 1.74 d ± 0.06 | 2.25 d ± 0.07 | 1.60 a,b ± 0.09 | 5.60 c,d ± 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titirică, I.; Roman, I.A.; Nicola, C.; Sturzeanu, M.; Iurea, E.; Botu, M.; Sestras, R.E.; Pop, R.; Militaru, M.; Ercisli, S.; et al. The Main Morphological Characteristics and Chemical Components of Fruits and the Possibilities of Their Improvement in Raspberry Breeding. Horticulturae 2023, 9, 50. https://doi.org/10.3390/horticulturae9010050
Titirică I, Roman IA, Nicola C, Sturzeanu M, Iurea E, Botu M, Sestras RE, Pop R, Militaru M, Ercisli S, et al. The Main Morphological Characteristics and Chemical Components of Fruits and the Possibilities of Their Improvement in Raspberry Breeding. Horticulturae. 2023; 9(1):50. https://doi.org/10.3390/horticulturae9010050
Chicago/Turabian StyleTitirică, Irina, Ioana A. Roman, Claudia Nicola, Monica Sturzeanu, Elena Iurea, Mihai Botu, Radu E. Sestras, Rodica Pop, Mădălina Militaru, Sezai Ercisli, and et al. 2023. "The Main Morphological Characteristics and Chemical Components of Fruits and the Possibilities of Their Improvement in Raspberry Breeding" Horticulturae 9, no. 1: 50. https://doi.org/10.3390/horticulturae9010050
APA StyleTitirică, I., Roman, I. A., Nicola, C., Sturzeanu, M., Iurea, E., Botu, M., Sestras, R. E., Pop, R., Militaru, M., Ercisli, S., & Sestras, A. F. (2023). The Main Morphological Characteristics and Chemical Components of Fruits and the Possibilities of Their Improvement in Raspberry Breeding. Horticulturae, 9(1), 50. https://doi.org/10.3390/horticulturae9010050











