Foliar Application of Salicylic Acid Enhances the Endogenous Antioxidant and Hormone Systems and Attenuates the Adverse Effects of Salt Stress on Growth and Yield of French Bean Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Experimental Design and Growth Conditions
2.2. Data Recorded
2.2.1. Vegetative Growth
2.2.2. Relative Water Content of Leaves
2.2.3. Leaf Membrane Stability Index (LMSI)
2.2.4. Determination of Total Soluble Phenol, Flavonoid, Flavanol, and Hydroxycinnamic Acid Contents
2.2.5. Total Antioxidant Capacity
2.2.6. Measurement of Chlorophylls
2.2.7. Determination of Proline and Lipid Peroxidation
2.2.8. Extraction and Assay of Peroxidase Activity
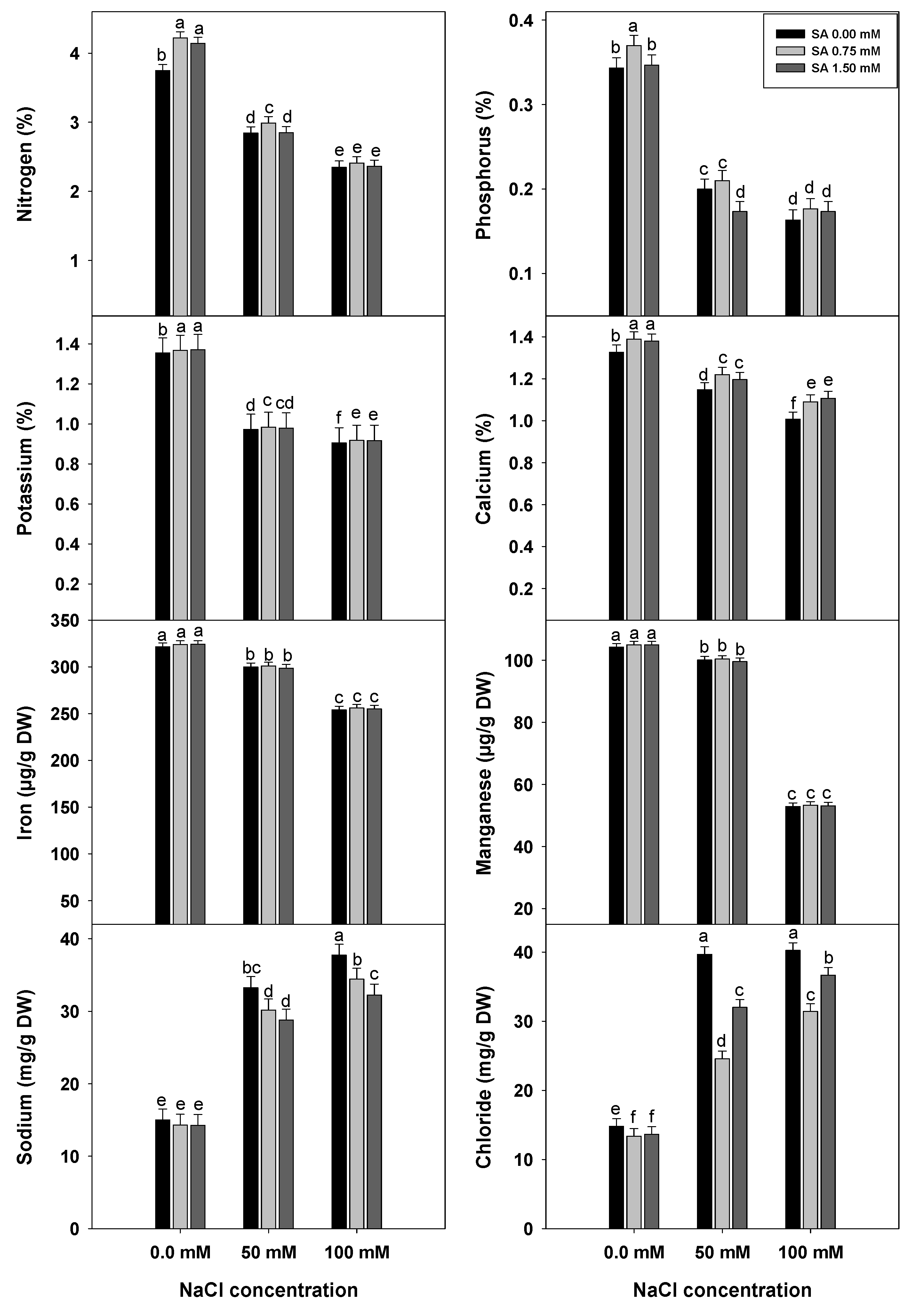
2.2.9. Analysis of Leaf Macro- and Micronutrients
2.2.10. Determination of Endogenous Hormones
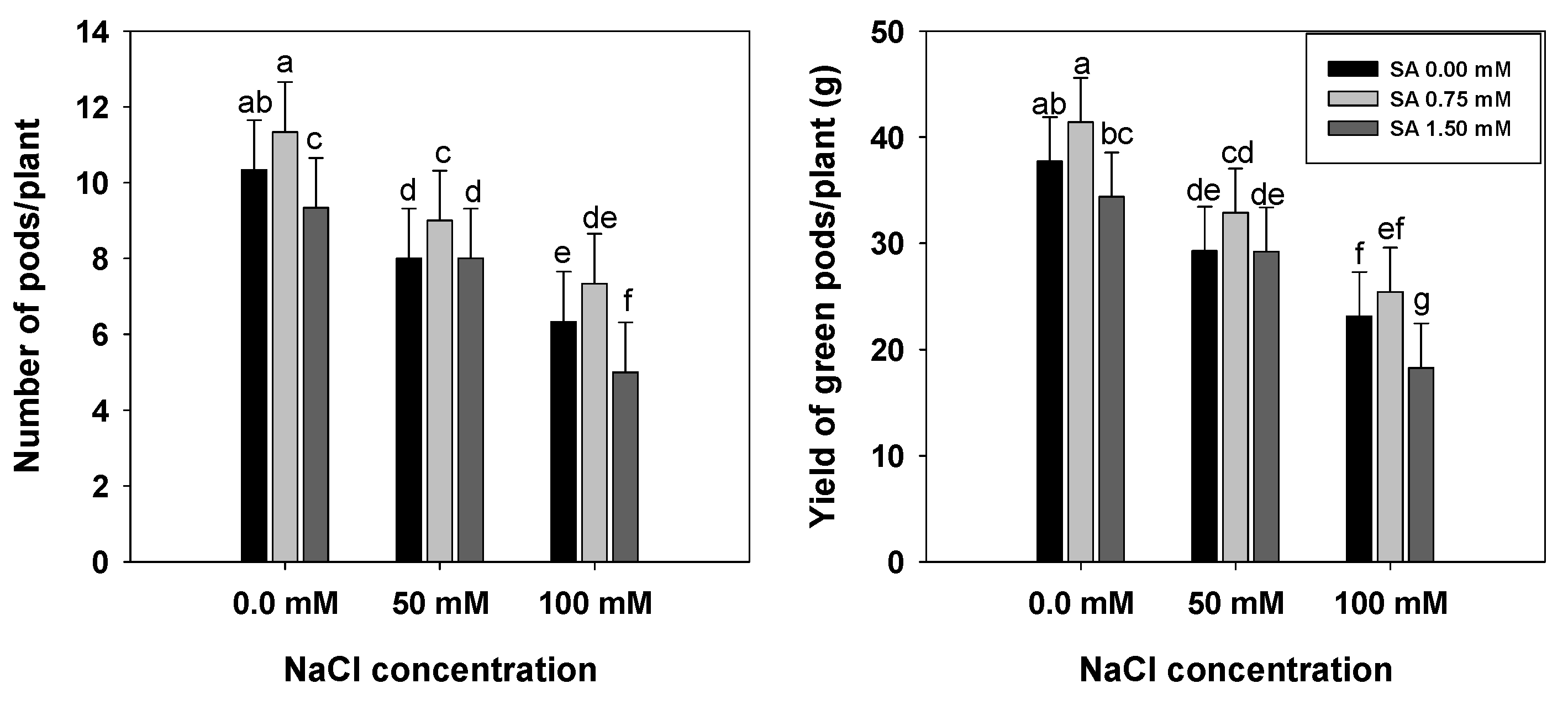
2.2.11. Yield of Green Pods
2.3. Statistical Analysis
3. Results
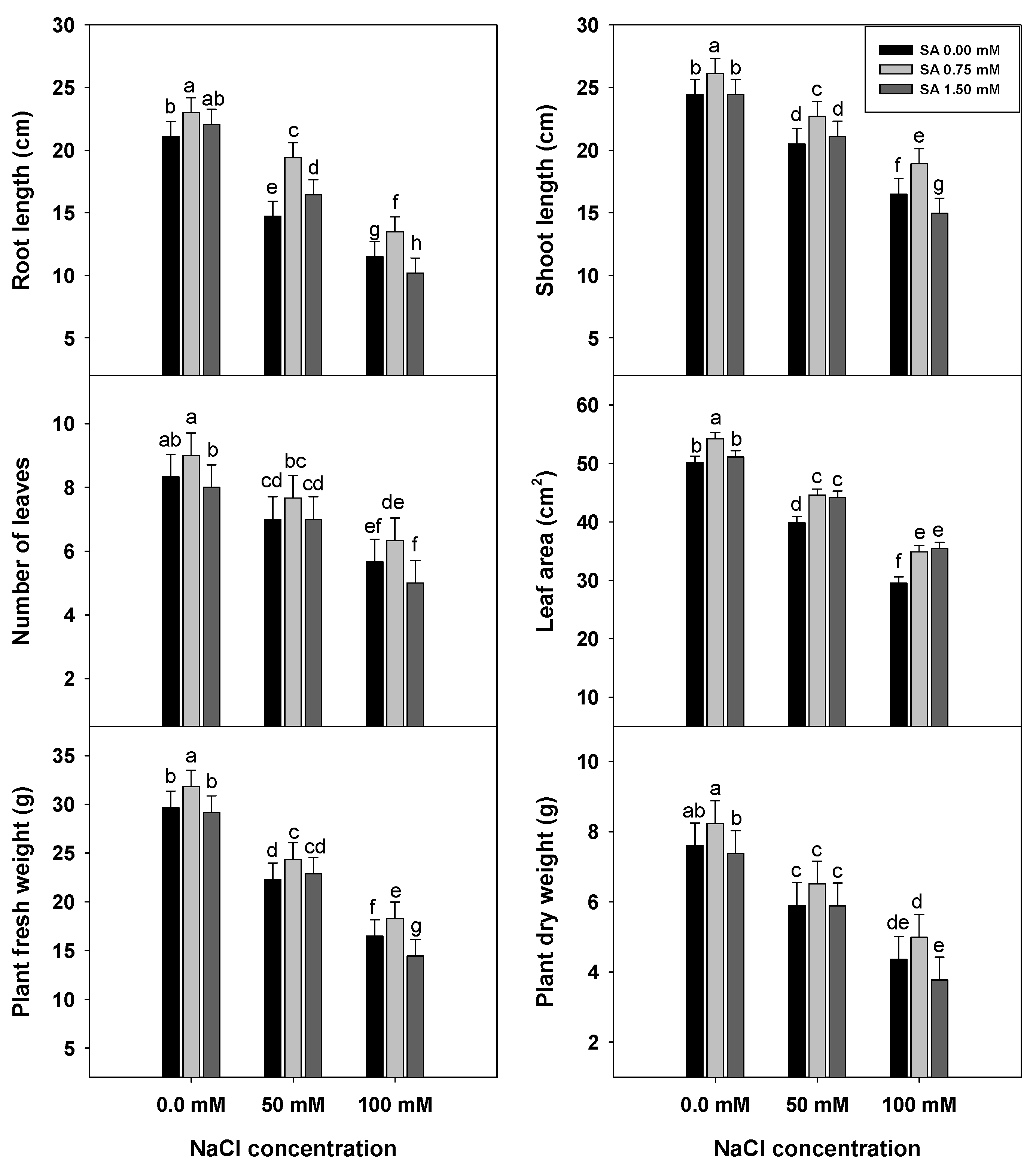
3.1. Plant Growth Traits
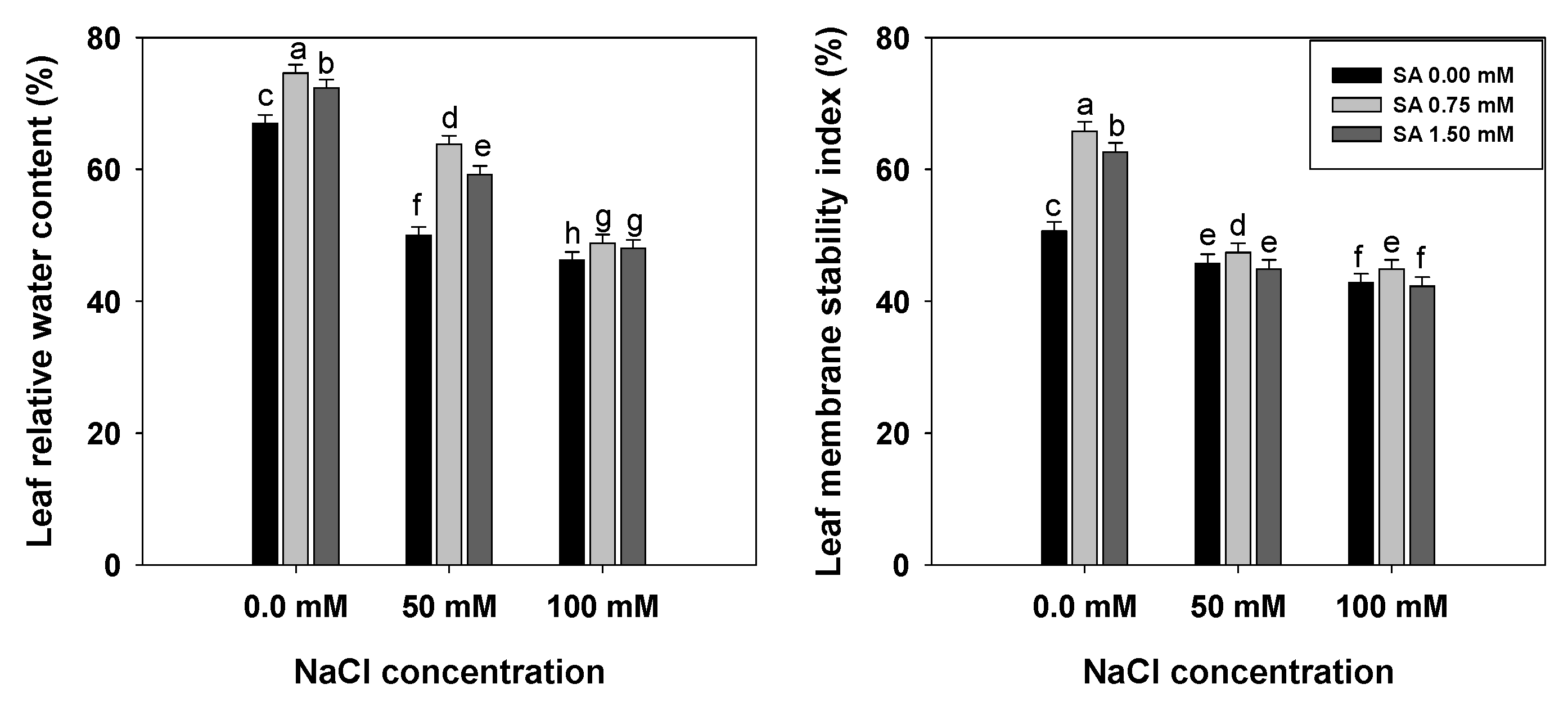
3.2. Leaf Relative Water Content and Leaf Membrane Stability Index
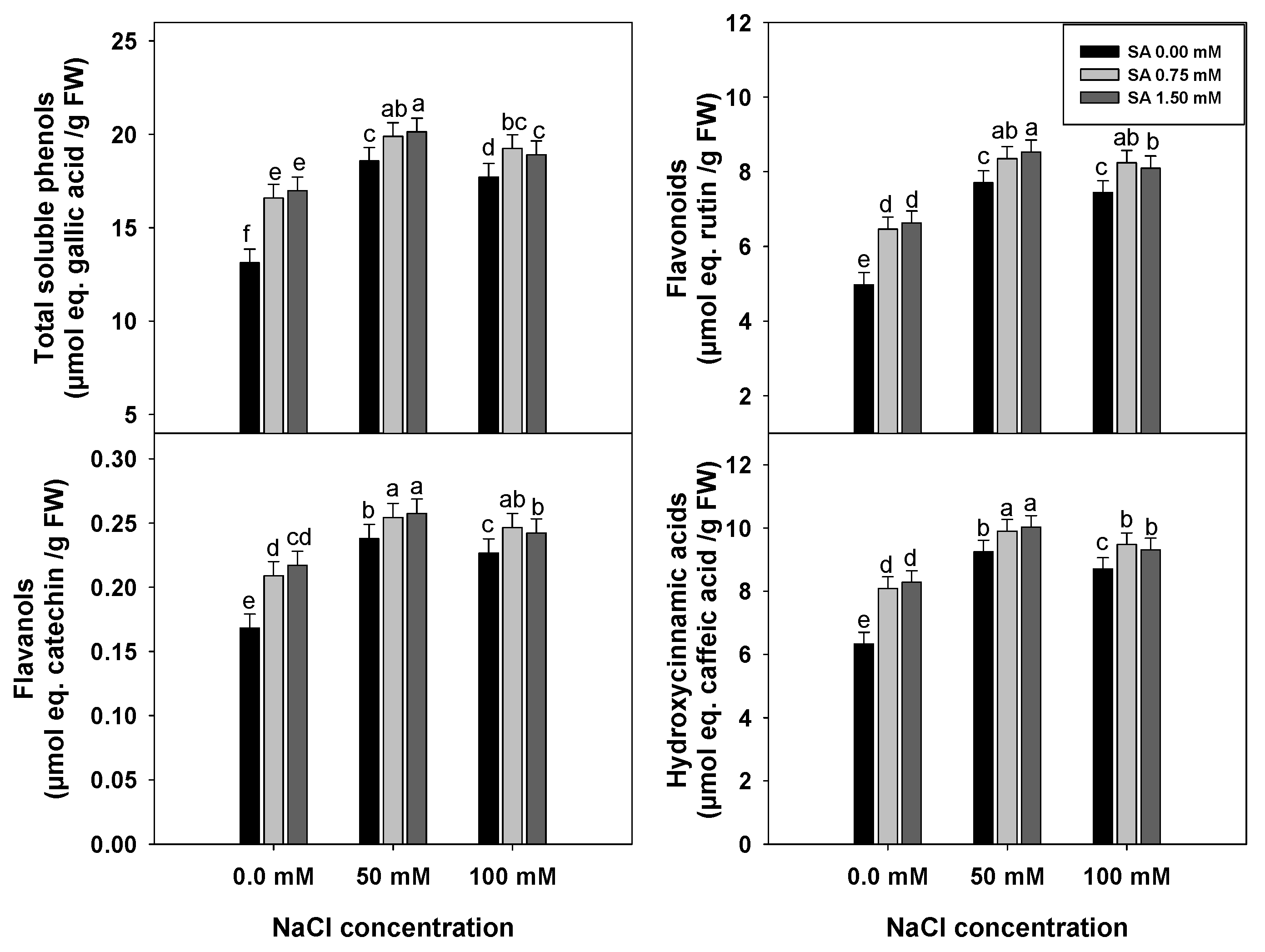
3.3. Levels of Soluble Phenolic Compounds
3.4. Total Antioxidant Capacity
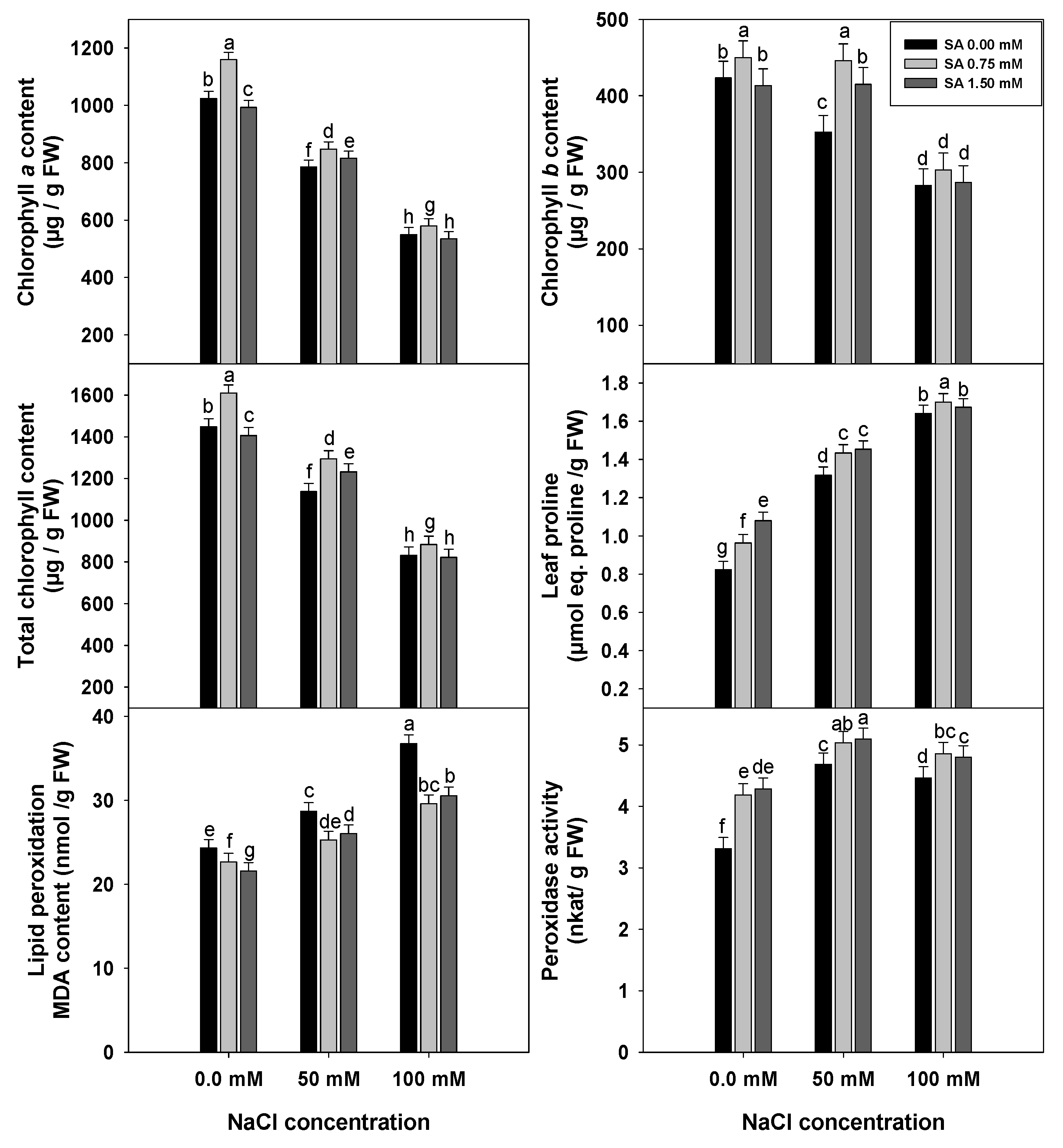
3.5. Chlorophyll and Proline Contents, Levels of Lipid Peroxidation and Peroxidase Activity
3.6. Leaf Macro- and Micronutrient Contents
3.7. Endogenous Hormones
3.8. Number of Pods and Yield of Green Pods
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahid, S.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Zaman, M., Shahid, S.A., Heng, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar]
- Omuto, C.T.; Vargas, R.R.; el Mobarak, A.M.; Mohamed, N.; Viatkin, K.; Yigini, Y. Mapping of Salt-Affected Soils: Technical Manual; FAO: Rome, Italy, 2021; Available online: https://www.fao.org/documents/card/en/c/cb7247en (accessed on 28 November 2022).
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity Tolerance of Crops—What Is the Cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant Cellular and Molecular Responses to High Salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Hernández, J.; Ferrer, M.; Jiménez, A.; Ros-Barceló, A.; Sevilla, F. Antioxidant Systems and O2−/Hydrogen Peroxide Production in the Apoplast of Pea Leaves. Its Relation with Salt-Induced Necrotic Lesions in Minor Veins. Plant Physiol. 2001, 127, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, L.; Rupasinghe, T.W.T.; Roessner, U.; Barkla, B.J. Salt Stress Alters Membrane Lipid Content and Lipid Biosynthesis Pathways in the Plasma Membrane and Tonoplast. Plant Physiol. 2022, 189, 805–826. [Google Scholar] [CrossRef]
- Hameed, A.; Ahmed, M.Z.; Hussain, T.; Aziz, I.; Ahmad, N.; Gul, B.; Nielsen, B.L. Effects of Salinity Stress on Chloroplast Structure and Function. Cells 2021, 10, 2023. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Acosta-Motos, J.; Ortuño, M.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.; Hernandez, J. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic Acid-Induced Abiotic Stress Tolerance and Underlying Mechanisms in Plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Horváth, E.; Szalai, G.; Janda, T. Induction of Abiotic Stress Tolerance by Salicylic Acid Signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Rasheed, F.; Anjum, N.A.; Masood, A.; Sofo, A.; Khan, N.A. The Key Roles of Salicylic Acid and Sulfur in Plant Salinity Stress Tolerance. J. Plant Growth Regul. 2022, 41, 1891–1904. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A. Exogenous Salicylic Acid Improves Photosynthesis and Growth through Increase in Ascorbate-Glutathione Metabolism and S Assimilation in Mustard under Salt Stress. Plant Signal Behav. 2015, 10, e1003751. [Google Scholar] [CrossRef]
- Youssef, S.M.; Elhady, S.A.A.; Aref, R.M.; Riad, G.S. Salicylic Acid Attenuates the Adverse Effects of Salinity on Growth and Yield and Enhances Peroxidase Isozymes Expression More Competently than Proline and Glycine Betaine in Cucumber Plants. Gesunde Pflanz. 2018, 70, 75–90. [Google Scholar] [CrossRef]
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic Acid Improves Salinity Tolerance in Arabidopsis by Restoring Membrane Potential and Preventing Salt-Induced K+ Loss via a GORK Channel. J. Exp. Bot. 2013, 64, 2255–2268. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Du, C.; Li, A.; Xia, X.; Yin, W.; Chen, J. Salicylic Acid Alleviated Salt Damage of Populus euphratica: A Physiological and Transcriptomic Analysis. Forests 2019, 10, 423. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity Induced Physiological and Biochemical Changes in Plants: An Omic Approach towards Salt Stress Tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic Acid in Relation to Other Phytohormones in Plant: A Study towards Physiology and Signal Transduction under Challenging Environment. Environ. Exp. Bot. 2020, 175, 104040. [Google Scholar] [CrossRef]
- Kang, G.; Li, G.; Zheng, B.; Han, Q.; Wang, C.; Zhu, Y.; Guo, T. Proteomic Analysis on Salicylic Acid-Induced Salt Tolerance in Common Wheat Seedlings (Triticum aestivum L.). Biochim. Et Biophys. Acta BBA Proteins Proteom. 2012, 1824, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Celmeli, T.; Sari, H.; Canci, H.; Sari, D.; Adak, A.; Eker, T.; Toker, C. The Nutritional Content of Common Bean (Phaseolus vulgaris L.) Landraces in Comparison to Modern Varieties. Agronomy 2018, 8, 166. [Google Scholar] [CrossRef]
- Gama, P.B.S.; Inanaga, S.; Tanaka, K. Nakazawa Physiological Response of Common Bean ( Phaseolus vulgaris L.) Seedlings to Salinity Stress. Afr. J. Biotechnol. 2007, 6, 79–088. [Google Scholar]
- Mohi-Ud-Din, M.; Talukder, D.; Rohman, M.; Ahmed, J.U.; Jagadish, S.V.K.; Islam, T.; Hasanuzzaman, M. Exogenous Application of Methyl Jasmonate and Salicylic Acid Mitigates Drought-Induced Oxidative Damages in French Bean (Phaseolus vulgaris L.). Plants 2021, 10, 2066. [Google Scholar] [CrossRef] [PubMed]
- Wekesa, C.; Asudi, G.O.; Okoth, P.; Reichelt, M.; Muoma, J.O.; Furch, A.C.U.; Oelmüller, R. Rhizobia Contribute to Salinity Tolerance in Common Beans (Phaseolus vulgaris L.). Cells 2022, 11, 3628. [Google Scholar] [CrossRef]
- Azizi, F.; Amiri, H.; Ismaili, A. Melatonin Improves Salinity Stress Tolerance of Phaseolus vulgaris L. Cv. Pak by Changing Antioxidant Enzymes and Photosynthetic Parameters. Acta Physiol. Plant 2022, 44, 40. [Google Scholar] [CrossRef]
- Ahmad, A.; Blasco, B.; Martos, V. Combating Salinity through Natural Plant Extracts Based Biostimulants: A Review. Front. Plant Sci. 2022, 13, 862034. [Google Scholar] [CrossRef]
- Koller, H.R. Leaf Area-Leaf Weight Relationships in the Soybean Canopy. Crop. Sci. 1972, 12, 180–183. [Google Scholar] [CrossRef]
- Youssef, S.M.; López-Orenes, A.; Ferrer, M.A.; Calderón, A.A. Salicylic-Acid-Regulated Antioxidant Capacity Contributes to Growth Improvement of Okra (Abelmoschus esculentus Cv. Red Balady). Agronomy 2022, 12, 168. [Google Scholar] [CrossRef]
- López-Orenes, A.; Bueso, M.C.; Conesa, H.; Calderón, A.A.; Ferrer, M.A. Seasonal Ionomic and Metabolic Changes in Aleppo Pines Growing on Mine Tailings under Mediterranean Semi-Arid Climate. Sci. Total Environ. 2018, 637–638, 625–635. [Google Scholar] [CrossRef]
- Pérez-Tortosa, V.; López-Orenes, A.; Martínez-Pérez, A.; Ferrer, M.A.; Calderón, A.A. Antioxidant Activity and Rosmarinic Acid Changes in Salicylic Acid-Treated Thymus membranaceus Shoots. Food Chem. 2012, 130, 362–369. [Google Scholar] [CrossRef]
- López-Orenes, A.; Martínez-Moreno, J.M.; Calderón, A.A.; Ferrer, M.A. Changes in Phenolic Metabolism in Salicylic Acid-Treated Shoots of Cistus heterophyllus. Plant Cell Tissue Organ Cult. PCTOC 2013, 113, 417–427. [Google Scholar] [CrossRef]
- Ferrer, M.A.; Calderón, A.A.; Muñoz, R.; Ros Barceló, A. 4-Methoxy-α-Naphthol as a Specific Substrate for Kinetic, Zymographic and Cytochemical Studies on Plant Peroxidase Activities. Phytochem. Anal. 1990, 1, 63–69. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soils, Plants and Water; University of California: Berkeley, CA, USA, 1961. [Google Scholar]
- Shindy, W.W.; Smith, O.E. Identification of Plant Hormones from Cotton Ovules. Plant Physiol. 1975, 55, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.; Lluch, C.; Iribarne, C.; García-Garrido, J.M.; Tejera García, N.A. Combined Effect of Salicylic Acid and Salinity on Some Antioxidant Activities, Oxidative Stress and Metabolite Accumulation in Phaseolus vulgaris. Plant Growth Regul. 2009, 58, 307–316. [Google Scholar] [CrossRef]
- Al Hassan, M.; Morosan, M.; López-Gresa, M.; Prohens, J.; Vicente, O.; Boscaiu, M. Salinity-Induced Variation in Biochemical Markers Provides Insight into the Mechanisms of Salt Tolerance in Common (Phaseolus vulgaris) and Runner (P. Coccineus) Beans. Int. J. Mol. Sci. 2016, 17, 1582. [Google Scholar] [CrossRef]
- Souri, M.K.; Tohidloo, G. Effectiveness of Different Methods of Salicylic Acid Application on Growth Characteristics of Tomato Seedlings under Salinity. Chem. Biol. Technol. Agric. 2019, 6, 26. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ and K+ Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Gunes, A.; Inal, A.; Alpaslan, M.; Eraslan, F.; Bagci, E.G.; Cicek, N. Salicylic Acid Induced Changes on Some Physiological Parameters Symptomatic for Oxidative Stress and Mineral Nutrition in Maize (Zea mays L.) Grown under Salinity. J. Plant Physiol. 2007, 164, 728–736. [Google Scholar] [CrossRef]
- Khan, N.; Syeed, S.; Masood, A.; Nazar, R.; Iqbal, N. Application of Salicylic Acid Increases Contents of Nutrients and Antioxidative Metabolism in Mungbean and Alleviates Adverse Effects of Salinity Stress. Int. J. Plant Biol. 2010, 1, e1. [Google Scholar] [CrossRef]
- Mugwanya, M.; Kimera, F.; Dawood, M.; Sewilam, H. Elucidating the Effects of Combined Treatments of Salicylic Acid and L-Proline on Greenhouse-Grown Cucumber under Saline Drip Irrigation. J. Plant Growth Regul. 2022, 1–17. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the Roles of Osmolytes for Acclimatizing Plants to Changing Environment: A Review of Potential Mechanism. Plant Signal Behav. 2021, 16, 1913306. [Google Scholar] [CrossRef] [PubMed]
- Bayuelo-Jiménez, J.S.; Jasso-Plata, N.; Ochoa, I. Growth and Physiological Responses of Phaseolus Species to Salinity Stress. Int. J. Agron. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Zheng, Y.; Cabassa-Hourton, C.; Planchais, S.; Lebreton, S.; Savouré, A. The Proline Cycle as an Eukaryotic Redox Valve. J. Exp. Bot. 2021, 72, 6856–6866. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.-L.; Liu, L.-N.; Xie, Q.; Sui, N. Photosynthetic Regulation under Salt Stress and Salt-Tolerance Mechanism of Sweet Sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar] [CrossRef]
- Es-sbihi, F.Z.; Hazzoumi, Z.; Aasfar, A.; Amrani Joutei, K. Improving Salinity Tolerance in Salvia officinalis L. by Foliar Application of Salicylic Acid. Chem. Biol. Technol. Agric. 2021, 8, 25. [Google Scholar] [CrossRef]
- Dawood, M.F.A.; Zaid, A.; Latef, A.A.H.A. Salicylic Acid Spraying-Induced Resilience Strategies Against the Damaging Impacts of Drought and/or Salinity Stress in Two Varieties of Vicia faba L. Seedlings. J. Plant Growth Regul. 2022, 41, 1919–1942. [Google Scholar] [CrossRef]
- Husen, A.; Iqbal, M.; Sohrab, S.S.; Ansari, M.K.A. Salicylic Acid Alleviates Salinity-Caused Damage to Foliar Functions, Plant Growth and Antioxidant System in Ethiopian Mustard (Brassica carinata A. Br.). Agric. Food Secur. 2018, 7, 44. [Google Scholar] [CrossRef]
- Almagro, L.; Calderón, A.A.; Pedreño, M.A.; Ferrer, M.A. Differential Response of Phenol Metabolism Associated with Antioxidative Network in Elicited Grapevine Suspension Cultured Cells under Saline Conditions. Antioxidants 2022, 11, 388. [Google Scholar] [CrossRef]
- Czarnocka, W.; Karpiński, S. Friend or Foe? Reactive Oxygen Species Production, Scavenging and Signaling in Plant Response to Environmental Stresses. Free. Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-Related Redox Regulation and Signaling in Plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive Oxygen Species, Abiotic Stress and Stress Combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant Properties of Phenolic Compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Pannala, A.S.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavonoid B-Ring Chemistry and Antioxidant Activity: Fast Reaction Kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Kiani, R.; Arzani, A.; Maibody, S.A.M.M. Polyphenols, Flavonoids, and Antioxidant Activity Involved in Salt Tolerance in Wheat, Aegilops cylindrica and Their Amphidiploids. Front. Plant Sci. 2021, 12, 646221. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of Flavonoids and Effects of Stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- López-Orenes, A.; Bueso, M.C.; Párraga-Aguado, I.M.; Calderón, A.A.; Ferrer, M.A. Coordinated Role of Soluble and Cell Wall Bound Phenols Is a Key Feature of the Metabolic Adjustment in a Mining Woody Fleabane (Dittrichia viscosa L.) Population under Semi-Arid Conditions. Sci. Total Environ. 2018, 618, 1139–1151. [Google Scholar] [CrossRef]
- López-Orenes, A.; Ros-Marín, A.F.; Ferrer, M.A.; Calderón, A.A. Antioxidant Capacity as a Marker for Assessing the In Vitro Performance of the Endangered Cistus Heterophyllus. Sci. World J. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Ha, P.T.T.; Tran, N.T.B.; Tram, N.T.N.; Kha, V.H. Total Phenolic, Total Flavonoid Contents and Antioxidant Potential of Common Bean (Phaseolus vulgaris L.) in Vietnam. AIMS Agric. Food 2020, 5, 635–648. [Google Scholar] [CrossRef]
- Pérez, F. Ascorbic Acid and Flavonoid-Peroxidase Reaction as a Detoxifying System of H2O2 in Grapevine Leaves. Phytochemistry 2002, 60, 573–580. [Google Scholar] [CrossRef]
- Ferreres, F.; Figueiredo, R.; Bettencourt, S.; Carqueijeiro, I.; Oliveira, J.; Gil-Izquierdo, A.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Duarte, P.; et al. Identification of Phenolic Compounds in Isolated Vacuoles of the Medicinal Plant Catharanthus roseus and Their Interaction with Vacuolar Class III Peroxidase: An H2O2 Affair? J. Exp. Bot. 2011, 62, 2841–2854. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Munné-Bosch, S. Rapid and Sensitive Hormonal Profiling of Complex Plant Samples by Liquid Chromatography Coupled to Electrospray Ionization Tandem Mass Spectrometry. Plant Method. 2011, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Latef, A.A.H.A.; Akter, A.; Tahjib-Ul-Arif, M. Foliar Application of Auxin or Cytokinin Can Confer Salinity Stress Tolerance in Vicia faba L. Agronomy 2021, 11, 790. [Google Scholar] [CrossRef]
- Shaki, F.; Maboud, H.E.; Niknam, V. Effects of Salicylic Acid on Hormonal Cross Talk, Fatty Acids Profile, and Ions Homeostasis from Salt-Stressed Safflower. J. Plant Interact. 2019, 14, 340–346. [Google Scholar] [CrossRef]
- Torun, H.; Novák, O.; Mikulík, J.; Strnad, M.; Ayaz, F.A. The Effects of Exogenous Salicylic Acid on Endogenous Phytohormone Status in Hordeum vulgare L. under Salt Stress. Plants 2022, 11, 618. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Abdoli, S.; Ghassemi-Golezani, K. Salicylic Acid: An Effective Growth Regulator for Mitigating Salt Toxicity in Plants. J. Plant Physiol. Breed. 2021, 11, 1–15. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, S.M.; López-Orenes, A.; Ferrer, M.A.; Calderón, A.A. Foliar Application of Salicylic Acid Enhances the Endogenous Antioxidant and Hormone Systems and Attenuates the Adverse Effects of Salt Stress on Growth and Yield of French Bean Plants. Horticulturae 2023, 9, 75. https://doi.org/10.3390/horticulturae9010075
Youssef SM, López-Orenes A, Ferrer MA, Calderón AA. Foliar Application of Salicylic Acid Enhances the Endogenous Antioxidant and Hormone Systems and Attenuates the Adverse Effects of Salt Stress on Growth and Yield of French Bean Plants. Horticulturae. 2023; 9(1):75. https://doi.org/10.3390/horticulturae9010075
Chicago/Turabian StyleYoussef, Sabry M., Antonio López-Orenes, María A. Ferrer, and Antonio A. Calderón. 2023. "Foliar Application of Salicylic Acid Enhances the Endogenous Antioxidant and Hormone Systems and Attenuates the Adverse Effects of Salt Stress on Growth and Yield of French Bean Plants" Horticulturae 9, no. 1: 75. https://doi.org/10.3390/horticulturae9010075
APA StyleYoussef, S. M., López-Orenes, A., Ferrer, M. A., & Calderón, A. A. (2023). Foliar Application of Salicylic Acid Enhances the Endogenous Antioxidant and Hormone Systems and Attenuates the Adverse Effects of Salt Stress on Growth and Yield of French Bean Plants. Horticulturae, 9(1), 75. https://doi.org/10.3390/horticulturae9010075





