Effects of Vernalization on Off–Season Flowering and Gene Expression in Sub-Tropical Strawberry cv. Pharachatan 80
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Growing Conditions
2.3. Gene Expression Analysis
2.3.1. RNA Extraction
2.3.2. Treating DNase I
2.3.3. cDNA Synthesis
2.3.4. Gene Expression by Semi-Quantitative RT-PCR Analysis
2.4. Data Analysis
3. Results
3.1. Effect of Vernalization and Flowering
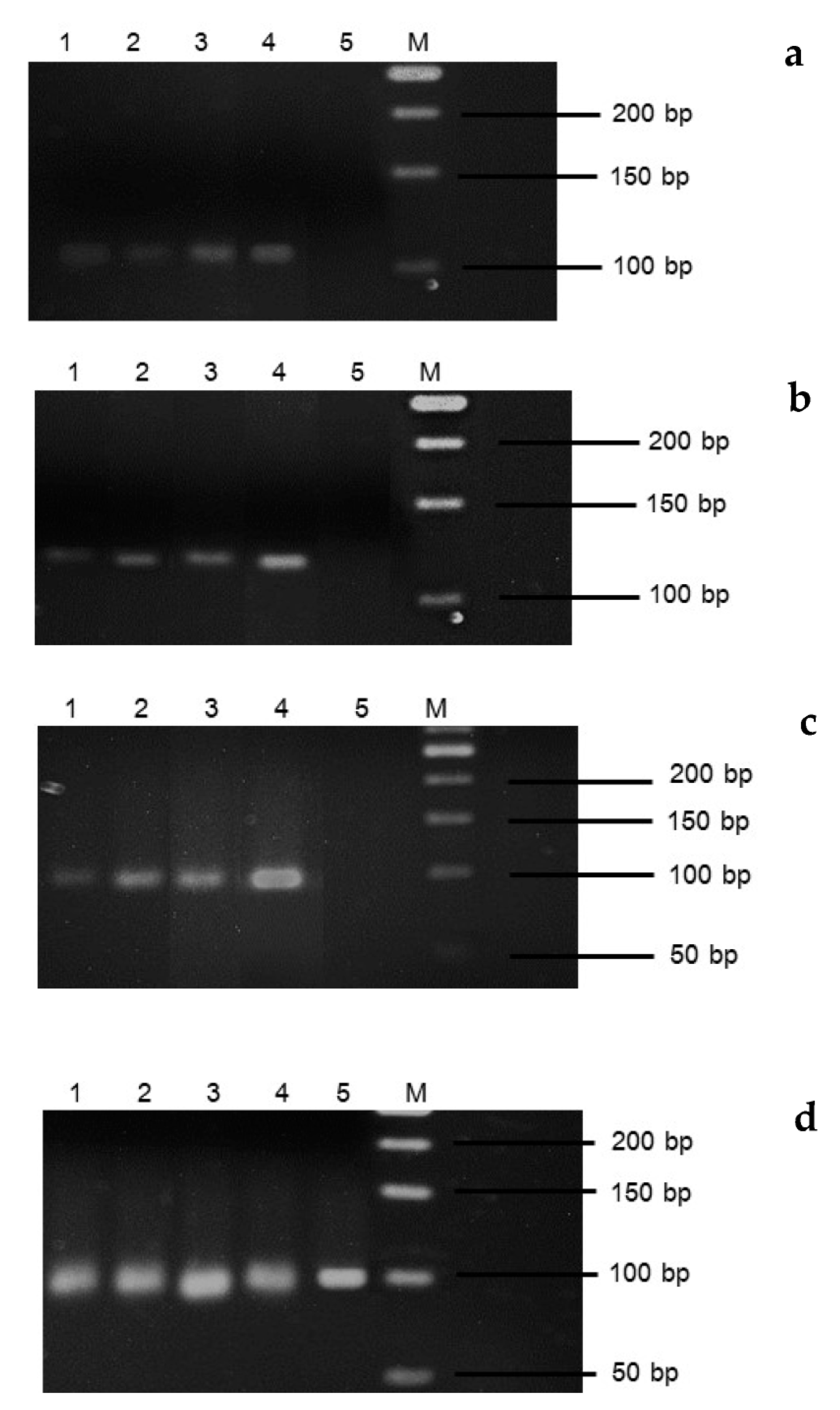
3.2. Vernalization and Gene Expression
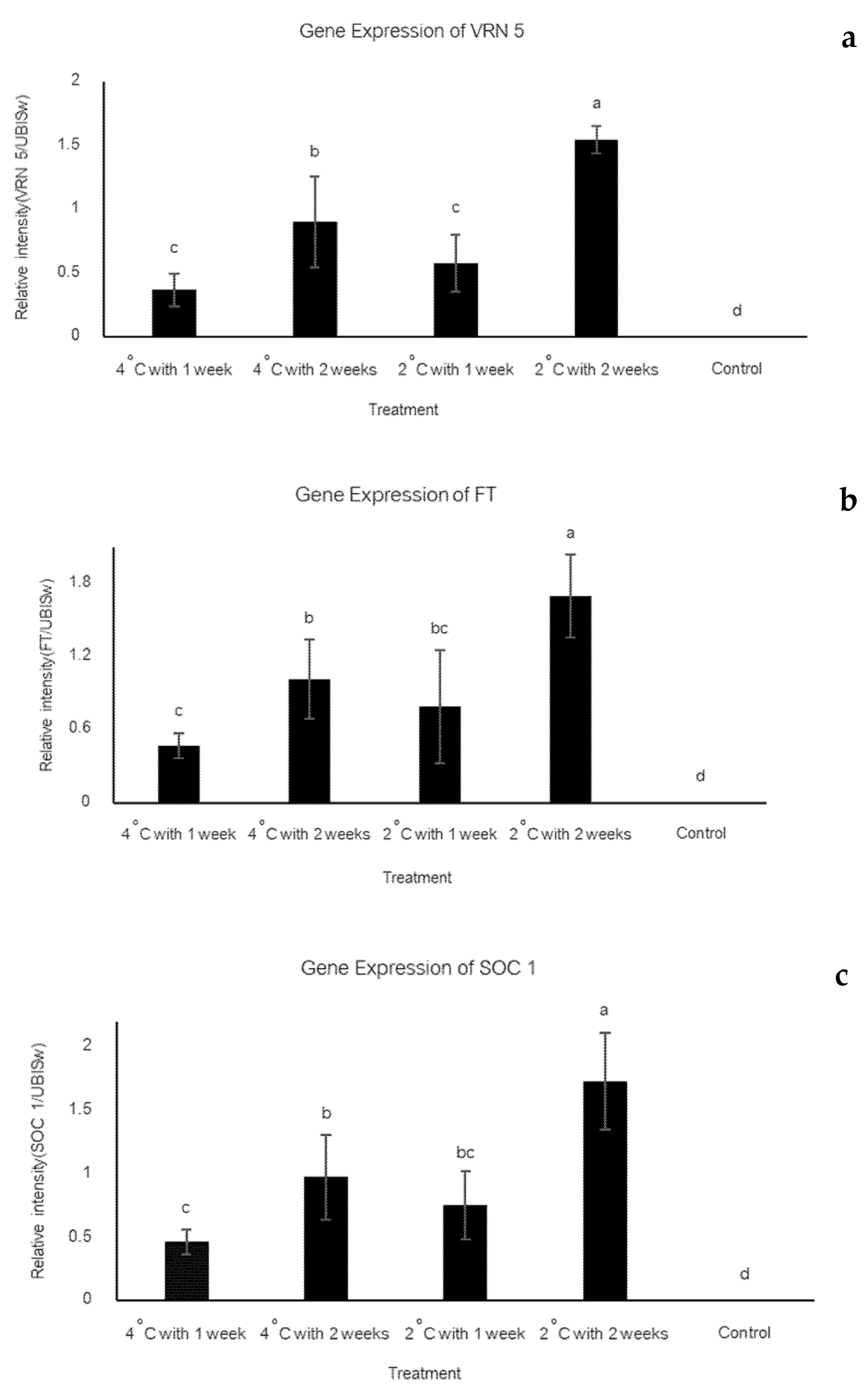
3.3. Expression Levels of VRN5 FT and SOC1 Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phiphatthanawong, N. Strawberry New Economic Crops; Kasetsart University: Bangkok, Thailand, 2007; p. 158. [Google Scholar]
- Meteorological Department. Seasons of Thailand. 2020. Available online: https://www3.tmd.go.th/info/ (accessed on 1 December 2020).
- Department of Agriculture Extension. Agricultural Production Information. 2021. Available online: https://production.doae.go.th/service/site/index (accessed on 17 December 2021).
- Oviedo, V.R.S.; Enciso-Garay, C.; Figueredo, E. Vernalizing pre-transplants improved theagronomic characteristics of strawberry genotypes under tropical conditions. Rev. Caatinga 2020, 33, 653–659. [Google Scholar] [CrossRef]
- Aukerman, M.J.; Sakai, H. Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 2003, 15, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Seo, Y.H.; Seo, P.J.; Reyes, J.L.; Yun, J.; Chua, N.H.; Parka, C.M. The GIGANTEA-Regulated MicroRNA172 Mediates Photoperiodic Flowering Independent of CONSTANS in Arabidopsis. Plant Cell 2007, 19, 2736–2748. [Google Scholar] [CrossRef] [PubMed]
- Corbesier, L.; Vincent, C.; Jang, S.; Fornara, F.; Fan, Q.; Searle, I.; Giakountis, A.; Farrona, S.; Gissot, L.; Turnbull, C.; et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 2007, 316, 1030–1033. [Google Scholar] [CrossRef]
- Mathieu, J.; Yant, L.J.; Murdter, F.; Kuttner, F.; Schmid, M. Repression of flowering by the miR172 target SMZ. PLoS Biol. 2009, 7, e1000148. [Google Scholar] [CrossRef]
- Wang, C.Q.; Guthrie, C.; Sarmast, M.K.; Dehesh, K. BBX19 inter- acts with CONSTANS to repress FLOWERING LOCUS T transcription, defining a flowering time checkpoint in Arabidopsis. Plant Cell 2014, 26, 3589–3602. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Wu, M.F.; Yang, L.; Wu, G.; Poethig, R.S.; Wagner, D. The MicroRNA-Regulated SBP-Box Transcription Factor SPL3 Is a Direct Upstream Activator of LEAFY, FRUITFULL, and APETALA1. Dev. Cell 2009, 17, 268–278. [Google Scholar] [CrossRef]
- Causier, B.; Castillo, R.; Zhou, J.; Ingram, R.; Xue, Y.; Schwarz-Sommer, Z.; Davies, B. Evolution in action: Following function in duplicated floral homeotic genes. Curr. Biol. 2005, 15, 1508–1512. [Google Scholar] [CrossRef]
- Lee, J.H.; Honga, S.; Yooa, S.; Parka, O.; Leeb, J.; Ahna, J. Integration of floral inductive signals by flowering locus T and suppressor of overexpression of Constans. Physiol. Plant. 2006, 126, 475–483. [Google Scholar] [CrossRef]
- Searle, I.; He, Y.; Turck, F.; Vincent, C.; Fornara, F.; Krober, S.; Amasino, R.A.; Coupland, G. The transcription factor FLC confers a flowering response to vernalization be repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev. 2006, 20, 898–912. [Google Scholar] [CrossRef]
- Sung, S.; Amasino, R.M. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 2004, 427, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.C.; Robertson, M.; Tanner, G.; Peacock, W.J.; Dennis, E.S.; Helliwell, C.A. The Arabidopsis thaliana vernalization response requires a Polycomb-like protein complex that also includes Vernalization Insensitive 3. Proc. Natl. Acad. Sci. USA 2006, 103, 14631–14636. [Google Scholar] [CrossRef] [PubMed]
- Mouhu, K.; Hytönen, T.; Folta, K.; Rantanen, M.; Paulin, L.; Auvinen, P.; Elomaa, P. Identification of flowering genes in strawberry, a perennial SD plant. BMC Plant Biol. 2009, 9, 122. [Google Scholar] [CrossRef]
- Badex, B.; Napiorkowska, B.; Masny, A.; Korbin, M. Changes in The Expression of Three Cold-Regulated Genes in ‘ELSANTA’ and ‘SELVIK’ Strawberry (Fragaria × Ananassa) Plants Exposed to Freezing. J. Hortic. Res. 2014, 22, 53–61. [Google Scholar]
- Jiang, P. Temperature Regulation of Flowering in Woodland Strawberry. Master’s Thesis, University of Helsinki, Helsinki, Finland, 2013; p. 49. [Google Scholar]
- Costa, A.F.; Leal, N.; Ventura, J.; Gonçalves, L.; Júnior, A.; Costa, H. Adaptability and stability of strawberry cultivars using a mixed model. Acta Sci. Agron. 2015, 37, 435–440. [Google Scholar] [CrossRef]
- Diel, M.I.; Marcos, V.; Pinheiro, V.; Cocco, C.; Thiesen, L.; Altíssimo, B.; Fontana, D.; Schmidt, D. Artificial vernalization in strawberry plants: Phyllochron, production and quality. Aust. J. Crop Sci. 2017, 11, 1315–1319. [Google Scholar] [CrossRef]
- Al-madhagi, I.A.H.; Al-Munibary, M.; Al-Doubibi, M. Effect of chilling and accumulative photo-thermal units on flowering of strawberry (Fragaria × Ananassa Duch.). J. Hortic. Res. 2018, 26, 25–35. [Google Scholar] [CrossRef]
- Sønsteby, A.; Heide, O.M. Effects of photoperiod and temperature on growth, flowering, and fruit yield in annual-fruiting red raspberry cultivars (Rubus idaeus L.). Eur. J. Hort. Sci. 2012, 77, 97–108. [Google Scholar]
- Husaini, A.M.; Neri, D. Strawberry Growth, Development and Diseases; CPI Group (UK) Ltd.: London, UK, 2016; p. 323. [Google Scholar]
- Tagong, A. Effect of Low Temperature, Short Day Conditions and Cytokinin on Flowering of Strawberry. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 2011; p. 132. [Google Scholar]
- Grez, J.; Contreras, E.; Sanchez, S.; Alcalde, A.; Gambardella, M. Floral induction and dormancy behaviour in ‘Chilean white strawberry’ (Fragaria chiloensis (L.) Mill. subsp. chiloensis f. chiloensis). Sci. Hortic. 2020, 274, 109648. [Google Scholar] [CrossRef]
- Greb, T.; Mylne, J.; Crevillen, P.; Geraldo, N.; An, H.; Gendall, A.; Dean, C. The PHD finger protein VRN5 functions in the epigenetic silencing of Arabidopsis FLC. Curr. Biol. 2007, 17, 73–78. [Google Scholar] [CrossRef]
- Koskela, E. Genetic and Environmental Control of Flowering in Wild and Cultivated Strawberries. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2016; p. 55. [Google Scholar]
- Koskela, E.A.; Kurokura, T.; Toivainen, T.; Sønsteby, A.; Heide, O.; Sargent, D.; Isobe, S.; Jaakola, L.; Hilmarsson, H.; Elomaa, P.; et al. Altered regulation of TERMINAL FLOWER 1 causes the unique vernalisation response in an arctic woodland strawberry accession. New Phytol. 2017, 216, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Suh, S.; Lee, H.; Choi, K.; Hong, C.; Paek, N.; Kim, S.; Lee, L. The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J. 2003, 35, 613–623. [Google Scholar] [CrossRef] [PubMed]

| Primer | Annealing Temperature (°C) | DNA Sequence (5′ to 3′) | Reference |
|---|---|---|---|
| SOC1 | 49 | F: ACTTGCTGGGTTCATTTTCC | [18] |
| R: GAGCTTTCCTCTGGGAGAGA | |||
| VRN5 | 48 | F: AGCCCTTGATGTCATCAGCTG | [16] |
| R: CCGATGAATGGTTGGCTAATG | |||
| FT | 53 | F: CAATCTCTTGGCCGAAAACT | [18] |
| R: TGAGGCTCAAACCTTCCCAAG | |||
| UBISw | 53 | F: CAGACCAGCAGAGGCTTATCTT | [16] |
| R: TCTGGATATTGTAGTCTGCTAGGG |
| Treatments | Number of Days to Flowering | Percentage of Flowering | Number of Days to the First Bloom |
|---|---|---|---|
| Vernalization at 4 °C for 1 week | 25.7 b | 60.0 c | 16.9 |
| Vernalization at 4 °C for 2 weeks | 24.9 b | 80.0 b | 17.4 |
| Vernalization at 2 °C for 1 week | 21.4 c | 80.0 b | 17.4 |
| Vernalization at 2 °C for 2 weeks | 23.1 bc | 92.0 a | 17.2 |
| Non-vernalization | 62.2 a | 10.0 d | 18.5 |
| Factor 1 | * | * | ns |
| Factor 2 | ns | * | ns |
| Factor 1 × 2 | * | * | ns |
| Treatment | Number of Inflorescences per Plant | Number of Flowers per Inflorescence | Number of Flowers per Plant |
|---|---|---|---|
| Vernalization at 4 °C for 1 week | 2.6 b | 2.2 b | 6.1 c |
| Vernalization at 4 °C for 2 weeks | 2.9 b | 2.6 b | 7.9 b |
| Vernalization at 2 °C for 1 week | 2.7 b | 2.2 b | 6.2 bc |
| Vernalization at 2 °C for 2 weeks | 3.6 a | 3.4 a | 12.3 a |
| Non-vernalization | 1.0 c | 1.2 c | 1.2 d |
| Factor 1 | * | * | * |
| Factor 2 | * | * | * |
| Factor 1 × 2 | * | * | * |
| Treatments | Inflorescence Length (cm.) | Flower Size (cm) | |
|---|---|---|---|
| Length | Width | ||
| vernalization at 4 °C for 1 week | 6.9 a | 2.1 a | 2.1 a |
| vernalization at 4 °C for 2 weeks | 8.3 a | 2.2 a | 2.2 a |
| vernalization at 2 °C for 1 week | 7.8 a | 2.3 a | 2.2 a |
| vernalization at 2 °C for 2 weeks | 10.3 a | 2.1 a | 2.3 a |
| Non-vernalization | 3.1 b | 1.7 b | 1.6 b |
| Factor 1 | * | ns | ns |
| Factor 2 | * | ns | ns |
| Factor 1 × 2 | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thammasophon, T.; Pusadee, T.; Bundithya, W.; Naphrom, D. Effects of Vernalization on Off–Season Flowering and Gene Expression in Sub-Tropical Strawberry cv. Pharachatan 80. Horticulturae 2023, 9, 87. https://doi.org/10.3390/horticulturae9010087
Thammasophon T, Pusadee T, Bundithya W, Naphrom D. Effects of Vernalization on Off–Season Flowering and Gene Expression in Sub-Tropical Strawberry cv. Pharachatan 80. Horticulturae. 2023; 9(1):87. https://doi.org/10.3390/horticulturae9010087
Chicago/Turabian StyleThammasophon, Thanyarat, Tonapha Pusadee, Weenun Bundithya, and Daruni Naphrom. 2023. "Effects of Vernalization on Off–Season Flowering and Gene Expression in Sub-Tropical Strawberry cv. Pharachatan 80" Horticulturae 9, no. 1: 87. https://doi.org/10.3390/horticulturae9010087
APA StyleThammasophon, T., Pusadee, T., Bundithya, W., & Naphrom, D. (2023). Effects of Vernalization on Off–Season Flowering and Gene Expression in Sub-Tropical Strawberry cv. Pharachatan 80. Horticulturae, 9(1), 87. https://doi.org/10.3390/horticulturae9010087








