Effect of Humidity-Triggered Controlled-Release 1-Methylcyclopropene (1-MCP) on Postharvest Quality of Papaya Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit and Treatments
2.2. Color
2.3. Weight Loss
2.4. Firmness
2.5. Total Soluble Solid Content and Titratable Acidity
2.6. Decay Rate
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results and Discussion
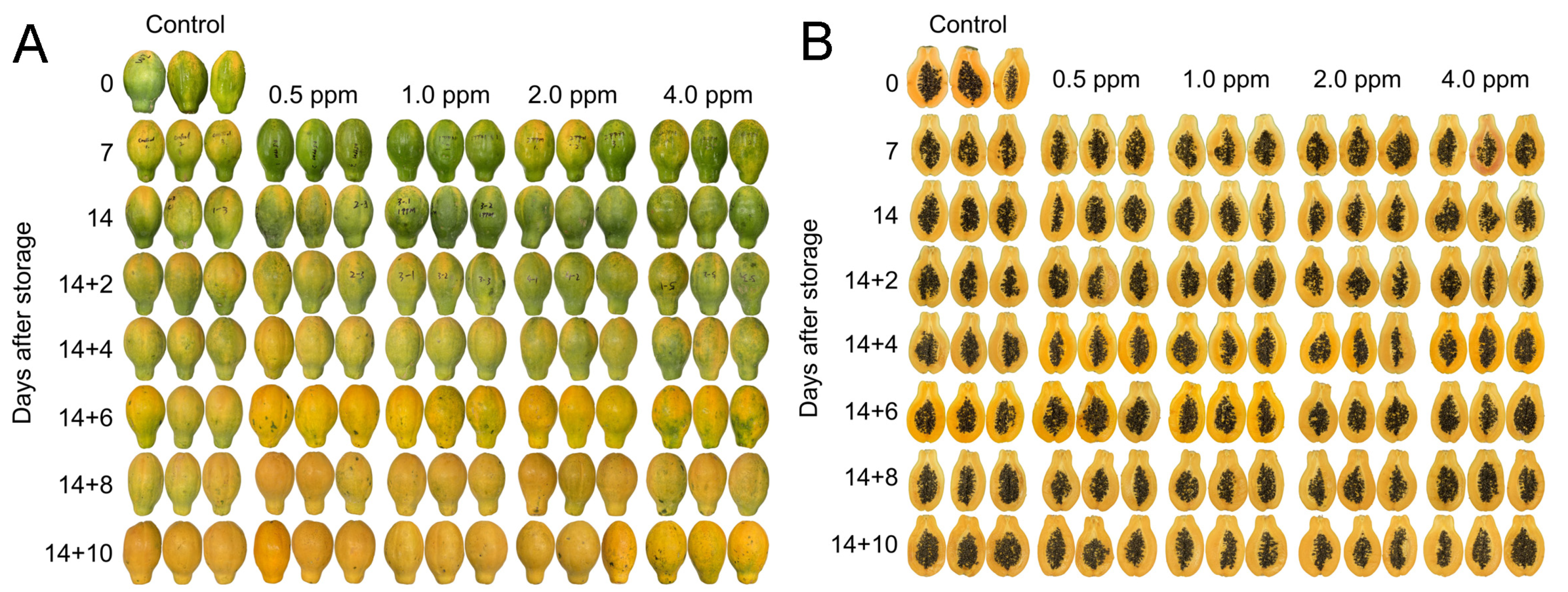
3.1. Fruit Appearance and Color Change
3.2. Weight Loss
3.3. Firmness
3.4. Soluble Solid Content and Titratable Acidity
3.5. Decay Rate
3.6. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wall, M.M. Ascorbic Acid, Vitamin A, and Mineral Composition of Banana (Musa Sp.) and Papaya (Carica Papaya) Cultivars Grown in Hawaii. J. Food Compos. Anal. 2006, 19, 434–445. [Google Scholar] [CrossRef]
- Tripathi, S.; Suzuki, J.Y.; Carr, J.B.; McQuate, G.T.; Ferreira, S.A.; Manshardt, R.M.; Pitz, K.Y.; Wall, M.M.; Gonsalves, D. Nutritional Composition of Rainbow Papaya, the First Commercialized Transgenic Fruit Crop. J. Food Compos. Anal. 2011, 24, 140–147. [Google Scholar] [CrossRef]
- McGregor, B.M. Tropical Products Transport Handbook; US Department of Agriculture, Office of Transportation: Washington, DC, USA, 1989.
- Watkins, C.B. The Use of 1-Methylcyclopropene (1-MCP) on Fruits and Vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
- Li, R.; Ma, J.; Gu, H.; Jia, W.; Shao, Y.; Li, W. 1-Methylcyclopropene Counteracts Ethylene Promotion of Fruit Softening and Roles of MiERF2/8 and MiPG in Postharvest Mangoes. Front. Plant Sci. 2022, 13, 971050. [Google Scholar] [CrossRef] [PubMed]
- Falagán, N.; Terry, L. 1-Methylcyclopropene Maintains Postharvest Quality in Norwegian Apple Fruit. Food Sci. Technol. Int. 2020, 26, 420–429. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, C.; Masum, M.I.; Cheng, Y.; Wei, C.; Guan, Y.; Guan, J. Dynamic Microbiome Changes Reveal the Effect of 1-Methylcyclopropene Treatment on Reducing Post-Harvest Fruit Decay in “Doyenne Du Comice” Pear. Front. Microbiol. 2021, 12, 729014. [Google Scholar] [CrossRef]
- Fabi, J.P.; Cordenunsi, B.R.; de Mattos Barreto, G.P.; Mercadante, A.Z.; Lajolo, F.M.; Oliveira do Nascimento, J.R. Papaya Fruit Ripening: Response to Ethylene and 1-Methylcyclopropene (1-MCP). J. Agric. Food Chem. 2007, 55, 6118–6123. [Google Scholar] [CrossRef]
- De Souza, M.S.; De Azevedo, I.G.; Corrêa, S.F.; Da Silva, M.G.; Pereira, M.G.; De Oliveira, J.G. Responses of 1-MCP applications in ‘Golden’ papaya fruits on different maturation stages. Rev. Bras. Frutic. 2009, 31, 693–700. [Google Scholar] [CrossRef]
- Bai, J.; Mattheis, J.P.; Reed, N. Re-Initiating Softening Ability of 1-Methylcyclopropene-Treated ‘Bartlett’ and ‘d’Anjou’ Pears after Regular Air or Controlled Atmosphere Storage. J. Hortic. Sci. Biotechnol. 2006, 81, 959–964. [Google Scholar] [CrossRef]
- Bai, J.; Chen, P.M. Extending Shelf-Life of Partially Ripened “d’anjou” Pears by 1-Methylcyclopropene Treatment. Acta Hortic. 2005, 671, 325–331. [Google Scholar] [CrossRef]
- Lartey, E.N.; Appiah, F. Effect of Different Concentrations of 1-MCP and Varied Storage Environments on Physical Characteristics and Consumer Acceptability of Solo Papaya (Carica Papaya L.) Fruits. Asian J. Adv. Res. Rep. 2023, 17, 41–57. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Li, W.; Shao, Y. Comparative Analyses of Ripening, Texture Properties and Cell Wall Composition in Three Tropical Fruits Treated with 1-Methylcyclopropene during Cold Storage. Horticulturae 2023, 9, 126. [Google Scholar] [CrossRef]
- Zheng, S.; Hao, Y.; Fan, S.; Cai, J.; Chen, W.; Li, X.; Zhu, X. Metabolomic and Transcriptomic Profiling Provide Novel Insights into Fruit Ripening and Ripening Disorder Caused by 1-MCP Treatments in Papaya. Int. J. Mol. Sci. 2021, 22, 916. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.; Sarageno, J.F.; Keute, J.; Lundgren, A. Compositions and Methods for Differential Release of 1-Methylcyclopropene. U.S. Patent 11492419B2, 28 October 2021. [Google Scholar]
- Blankenship, S.M.; Dole, J.M. 1-Methylcyclopropene: A Review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Sun, X.; Wall, M.; Follett, P.; Liang, P.; Xu, S.; Zhong, T. Effect of Pectin Coatings Containing Trans-Cinnamaldehyde on the Postharvest Quality of Rambutan. HortScience 2023, 58, 11–15. [Google Scholar] [CrossRef]
- Shen, Y.H.; Yang, F.Y.; Lu, B.G.; Zhao, W.W.; Jiang, T.; Feng, L.; Chen, X.J.; Ming, R. Exploring the Differential Mechanisms of Carotenoid Biosynthesis in the Yellow Peel and Red Flesh of Papaya. BMC Genom. 2019, 20, 49. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, M.; Bai, L.; Han, X.; Ge, Y.; Wang, W.; Li, J. Effects of 1-Methylcyclopropene (1-MCP) on the Expression of Genes Involved in the Chlorophyll Degradation Pathway of Apple Fruit during Storage. Food Chem. 2020, 308, 125707. [Google Scholar] [CrossRef]
- Gómez-Lobato, M.E.; Hasperué, J.H.; Civello, P.M.; Chaves, A.R.; Martínez, G.A. Effect of 1-MCP on the Expression of Chlorophyll Degrading Genes during Senescence of Broccoli (Brassica Oleracea L.). Sci. Hortic. 2012, 144, 208–211. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, L.; Chen, Y.; Liao, L.; Li, R.; Wang, H.; Mo, Y.; Lin, L.; Liu, K. The Combined Effect of Ascorbic Acid and Chitosan Coating on Postharvest Quality and Cell Wall Metabolism of Papaya Fruits. LWT 2022, 171, 114134. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Steingass, C.B.; Mora, E.; Esquivel, P.; Carle, R. Carotenogenesis and Physico-Chemical Characteristics during Maturation of Red Fleshed Papaya Fruit (Carica Papaya L.). Food Res. Int. 2011, 44, 1373–1380. [Google Scholar] [CrossRef]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of Chitosan Coatings on the Physicochemical Characteristics of Eksotika II Papaya (Carica Papaya L.) Fruit during Cold Storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef]
- Wang, H.; Qan, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Manenoi, A.; Bayogan, E.R.V.; Thumdee, S.; Paull, R.E. Utility of 1-Methylcyclopropene as a Papaya Postharvest Treatment. Postharvest Biol. Technol. 2007, 44, 55–62. [Google Scholar] [CrossRef]
- Bron, I.U.; Jacomino, A.P. Ripening and Quality of “Golden” Papaya Fruit Harvested at Different Maturity Stages. Braz. J. Plant Physiol. 2006, 18, 389–396. [Google Scholar] [CrossRef]
- Hua, X.; Li, T.; Wu, C.; Zhou, D.; Fan, G.; Li, X.; Cong, K.; Yan, Z.; Cheng, X. Pulsed Light Improved the Shelf Life of Apricot (after Simulated Long-Distance Air Transportation) by Regulating Cell Wall Metabolism. Postharvest Biol. Technol. 2023, 196, 112187. [Google Scholar] [CrossRef]
- Tatsuki, M.; Endo, A.; Ohkawa, H. Influence of Time from Harvest to 1-MCP Treatment on Apple Fruit Quality and Expression of Genes for Ethylene Biosynthesis Enzymes and Ethylene Receptors. Postharvest Biol. Technol. 2007, 43, 28–35. [Google Scholar] [CrossRef]
- Ohashi, T.L.; Foukaraki, S.; Corrêa, D.S.; Ferreira, M.D.; Terry, L. Influence of 1-Methylcyclopropene on the Biochemical Response and Ripening of ‘Solo’ Papayas. Rev. Bras. Frutic. 2016, 38, e791. [Google Scholar] [CrossRef][Green Version]
- Bai, J.; Baldwin, E.A.; Goodner, K.L.; Mattheis, J.P.; Brecht, J.K. Response of Four Apple Cultivars to 1-Methylcyclopropene Treatment and Controlled Atmosphere Storage. HortScience 2005, 40, 1534–1538. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, D.J.; Hurr, B.M.; Rao, J. Delay of Tomato Fruit Ripening in Response to 1-Methylcyclopropene is Influenced by Internal Ethylene Levels. Postharvest Biol. Technol. 2009, 54, 1–8. [Google Scholar] [CrossRef]
- Moreira, M.d.R.; Ponce, A.G.; del Valle, C.E.; Ansorena, R.; Roura, S.I. Effects of Abusive Temperatures on the Postharvest Quality of Lettuce Leaves: Ascorbic Acid Loss and Microbial Growth. J. Appl. Hortic. 2006, 8, 109–113. [Google Scholar] [CrossRef]
- Ding, P.; Bee, N.S. Effects of 1-Methylcyclopropene on the Postharvest Life of Eksotika Papaya. J. Appl. Hortic. 2008, 10, 123–128. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Sivakumar, D.; Bello-Pérez, A.; Villanueva-Arce, R.; Hernández-López, M. A Review of the Management Alternatives for Controlling Fungi on Papaya Fruit during the Postharvest Supply Chain. Crop Prot. 2013, 49, 8–20. [Google Scholar] [CrossRef]
- Li, J.; Lei, H.; Song, H.; Lai, T.; Xu, X.; Shi, X. 1-Methylcyclopropene (1-MCP) Suppressed Postharvest Blue Mold of Apple Fruit by Inhibiting the Growth of Penicillium Expansum. Postharvest Biol. Technol. 2017, 125, 59–64. [Google Scholar] [CrossRef]
- Alkan, N.; Fortes, A.M. Insights into Molecular and Metabolic Events Associated with Fruit Response to Post-Harvest Fungal Pathogens. Front. Plant Sci. 2015, 6, 889. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, T.; Di, J.; Liu, Y.; Wang, Y. Involvement of Ethylene in Glutamate-Mediated Tomato Fruit Resistance to Alternaria Alternata. Postharvest Biol. Technol. 2022, 190, 111940. [Google Scholar] [CrossRef]
- Wang, X.; Meng, H.; Tang, Y.; Zhang, Y.; He, Y.; Zhou, J.; Meng, X. Phosphorylation of an Ethylene Response Factor by MPK3/MPK6 Mediates Negative Feedback Regulation of Pathogen-Induced Ethylene Biosynthesis in Arabidopsis. J. Genet. Genom. 2022, 49, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Mata, C.I.; Van de Poel, B.; Hertog, M.L.A.T.M.; Tran, D.; Nicolai, B.M. Transcription Analysis of the Ethylene Receptor and CTR Genes in Tomato: The Effects of on and off-Vine Ripening and 1-MCP. Postharvest Biol. Technol. 2018, 140, 67–75. [Google Scholar] [CrossRef]
- Bai, J.; Ueda, Y.; Iwata, T. Effect of Packaging with Polyethylene Bags on Shelf Life and Volatiles Production of Ripening-Initiated Bananas. Nippon. Shokuhin Kogyo Gakkaishi 1990, 37, 971–977. [Google Scholar] [CrossRef][Green Version]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, Flavor, Texture, and Nutritional Quality of Fresh-Cut Fruits and Vegetables: Desirable Levels, Instrumental and Sensory Measurement, and the Effects of Processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef]
- Brewer, S.; Plotto, A.; Bai, J.; Crane, J.; Chambers, A. Evaluation of 21 Papaya (Carica Papaya L.) Accessions in Southern Florida for Fruit Quality, Aroma, Plant Height, and Yield Components. Sci. Hortic. 2021, 288, 110387. [Google Scholar] [CrossRef]
- Gomez, M.; Lajolo, F.; Cordenunsi, B. Evolution of Soluble Sugars During Ripening of Papaya Fruit and its Relation to Sweet Taste. J. Food Sci. 2002, 67, 442–447. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Qin, G.; Tian, S. Molecular Basis of 1-Methylcyclopropene Regulating Organic Acid Metabolism in Apple Fruit during Storage. Postharvest Biol. Technol. 2016, 117, 57–63. [Google Scholar] [CrossRef]
- Zhou, Z.; Ford, R.; Bar, I.; Kanchana-udomkan, C. Papaya (Carica Papaya L.) Flavour Profiling. Genes 2021, 12, 1416. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Group | Storage Time (Day) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 14 + 2 | 14 + 4 | 14 + 6 | 14 + 8 | 14 + 10 | ||
| L* | Control | 62.42 ± 2.16 | 60.83 ± 1.83 | 65.33 ± 1.99 a | 66.08 ± 0.38 a | 69.39 ± 0.65 a | 69.86 ± 0.32 a | 70.06 ± 0.19 a | 67.28 ± 0.41 ab |
| 0.5 ppm | 62.42 ± 2.16 | 52.08 ± 1.83 | 60.58 ± 2.73 ab | 62.00 ± 1.19 ab | 71.28 ± 0.93 a | 65.50 ± 0.74 ab | 62.42 ± 0.48 b | 66.67 ± 0.78 b | |
| 1.0 ppm | 62.42 ± 2.16 | 51.25 ± 2.77 | 50.42 ± 1.77 b | 62.47 ± 1.13 ab | 70.22 ± 0.83 a | 64.75 ± 0.77 ab | 59.86 ± 0.92 b | 70.94 ± 0.13 a | |
| 2.0 ppm | 62.42 ± 2.16 | 49.83 ± 2.91 | 52.17 ± 1.29 b | 58.00 ± 1.23 b | 62.00 ± 1.65 b | 62.75 ± 0.31 bc | 61.92 ± 0.76 b | 65.03 ± 1.14 b | |
| 4.0 ppm | 62.42 ± 2.1 a | 54.58 ± 2.26 | 52.50 ± 0.66 b | 58.06 ± 1.00 b | 57.08 ± 0.96 b | 57.92 ± 1.95 c | 59.81 ± 0.61 b | 63.86 ± 0.68 b | |
| a* | Control | −16.17 ± 0.77 | −7.67 ± 2.00 a | −6.00 ± 4.22 | 9.94 ± 1.05 a | 3.47 ± 2.02 a | 8.75 ± 0.31 a | 14.69 ± 1.41 a | 22.28 ± 0.62 a |
| 0.5 ppm | −16.17 ± 0.77 | −22.83 ± 2.19 b | −11.42 ± 1.58 | −4.53 ± 1.54 b | 4.39 ± 0.85 a | 5.94 ± 1.41 ab | 13.00 ± 2.58 a | 24.44 ± 0.08 a | |
| 1.0 ppm | −16.17 ± 0.77 | −23.67 ± 1.20 b | −17.92 ± 0.18 | −10.03 ± 1.08 bc | −5.19 ± 0.23 b | 3.19 ± 0.82 ab | 7.28 ± 0.33 ab | 17.19 ± 0.84 b | |
| 2.0 ppm | −16.17 ± 0.77 | −20.08 ± 2.79 ab | −17.25 ± 0.82 | −12.89 ± 0.65 c | −5.81 ± 2.12 b | 1.81 ± 1.29 b | 12.89 ± 1.56 a | 15.81 ± 0.75 b | |
| 4.0 ppm | −16.17 ± 0.77 | −15.75 ± 2.60 ab | −17.75 ± 1.63 | −11.50 ± 1.93 bc | −11.11 ± 0.92 b | −5.03 ± 0.94 c | 1.53 ± 1.19 b | 4.61 ± 0.83 c | |
| b* | Control | 66.33 ± 1.09 | 75.75 ± 1.36 a | 73.67 ± 2.98 a | 74.36 ± 1.11 a | 76.06 ± 1.07 a | 76.53 ± 0.49 | 70.06 ± 0.72 ab | 78.22 ± 0.35 a |
| 0.5 ppm | 66.33 ± 1.09 | 62.33 ± 1.77 ab | 56.58 ± 3.13 b | 66.69 ± 2.83 ab | 72.94 ± 1.36 ab | 73.78 ± 3.29 | 69.89 ± 0.99 ab | 79.11 ± 0.53 a | |
| 1.0 ppm | 66.33 ± 1.09 | 60.33 ± 1.88 b | 49.83 ± 4.72 b | 62.89 ± 1.14 b | 70.58 ± 0.38 ab | 75.17 ± 1.19 | 64.14 ± 1.16 c | 76.53 ± 0.47 ab | |
| 2.0 ppm | 66.33 ± 1.09 | 62.75 ± 3.89 ab | 47.83 ± 0.92 b | 58.50 ± 0.31 b | 64.78 ± 3.19 bc | 74.00 ± 1.12 | 71.28 ± 1.32 a | 70.75 ± 2.36 bc | |
| 4.0 ppm | 66.33 ± 1.09 | 69.08 ± 3.18 ab | 58.42 ± 1.41 ab | 60.44 ± 1.76 b | 55.17 ± 1.91 c | 70.17 ± 2.45 | 65.31 ± 0.66 bc | 68.03 ± 1.01 c | |
| Firmness (N) | Control | 55.49 ± 10.57 | 24.86 ± 6.65 b | 27.48 ± 4.74 | 11.51 ± 1.72 c | 4.52 ± 0.39 b | 7.81 ± 1.83 bc | 3.96 ± 1.11 b | 1.97 ± 0.26 c |
| 0.5 ppm | 55.49 ± 10.57 | 36.61 ± 2.54 ab | 43.66 ± 10.85 | 11.85 ±2.91 c | 11.61 ± 2.49 ab | 4.38 ±0.35 c | 8.40 ±2.03 ab | 5.56 ± 2.70 b | |
| 1.0 ppm | 55.49 ± 10.57 | 74.86 ± 6.09 a | 67.14 ± 12.23 | 22.12 ± 1.03 bc | 39.29 ± 9.86 a | 9.97 ± 3.13 abc | 15.83 ± 3.67 ab | 8.76 ± 1.13 b | |
| 2.0 ppm | 55.49 ± 10.57 | 33.91 ± 14.59 ab | 56.48 ± 12.75 | 29.97 ± 1.56 b | 26.99 ± 5.35 ab | 40.97 ± 11.25 a | 33.98 ± 8.98 a | 20.48 ± 7.29 a | |
| 4.0 ppm | 55.49 ± 10.57 | 36.61 ± 2.54 ab | 51.97 ± 8.81 | 46.69 ± 3.22 a | 39.37 ±3.11 a | 40.26 ±5.25 ab | 22.73 ±5.58 ab | 14.99 ±3.07 a | |
| SSC (%) | Control | 15.17 ± 0.26 | 14.83 ± 0.29 | 15.07 ± 0.09 | 14.90 ± 0.22 | 14.37 ± 0.22 ab | 13.00 ± 0.27 a | 14.25 ± 0.37 | 14.10 ± 0.10 |
| 0.5 ppm | 15.17 ± 0.26 | 14.05 ± 0.10 | 15.55 ± 0.36 | 13.85 ± 0.38 | 13.78 ± 0.06 ab | 11.80 ± 0.31 b | 12.87 ± 0.28 | 13.82 ± 0.22 | |
| 1.0 ppm | 15.17 ± 0.26 | 14.53 ± 0.09 | 15.30 ± 0.18 | 14.23 ± 0.18 | 13.50 ± 0.19 b | 12.58 ± 0.13 ab | 13.17 ± 0.47 | 12.78 ± 0.42 | |
| 2.0 ppm | 15.17 ± 0.26 | 15.67 ± 0.33 | 15.75 ± 0.70 | 14.88 ± 0.70 | 14.92 ± 0.35 ab | 12.22 ± 0.09 ab | 13.75 ± 0.18 | 13.90 ± 0.47 | |
| 4.0 ppm | 15.17 ± 0.26 | 14.87 ± 0.62 | 14.32 ± 0.58 | 14.32 ± 0.58 | 15.18 ± 0.31 a | 12.82 ± 0.08 ab | 13.73 ± 0.18 | 13.35 ± 0.40 | |
| Titratable acidity (%) | Control | 0.31 ± 0.06 | 0.29 ± 0.01 | 0.28 ± 0.00 | 0.32 ± 0.03 | 0.34 ± 0.01 | 0.27 ± 0.01 b | 0.27 ± 0.01 b | 0.31 ± 0.01 ab |
| 0.5 ppm | 0.31 ± 0.06 | 0.32 ± 0.01 | 0.33 ± 0.01 | 0.30 ± 0.03 | 0.29 ± 0.03 | 0.40 ± 0.06 a | 0.29 ± 0.02 b | 0.26 ± 0.01 b | |
| 1.0 ppm | 0.31 ± 0.06 | 0.31 ± 0.02 | 0.29 ± 0.00 | 0.24 ± 0.01 | 0.36 ± 0.01 | 0.38 ± 0.04 a | 0.40 ± 0.02 a | 0.26 ± 0.01 b | |
| 2.0 ppm | 0.31 ± 0.06 | 0.25 ± 0.01 | 0.28 ± 0.04 | 0.26 ± 0.01 | 0.33 ± 0.05 | 0.29 ± 0.04 ab | 0.36 ± 0.02 ab | 0.27 ± 0.00 b | |
| 4.0 ppm | 0.31 ± 0.06 | 0.31 ± 0.03 | 0.26 ± 0.02 | 0.24 ± 0.02 | 0.29 ± 0.00 | 0.32 ± 0.01 ab | 0.32 ± 0.03 ab | 0.34 ± 0.02 a | |
| Group | Cold Storage | Shelf Life | ||
|---|---|---|---|---|
| Control | y = 0.135x + 0.0102 | R2 = 0.9997 | y = 0.541x + 1.5358 | R² = 0.9998 |
| 0.5 ppm | y = 0.0825x + 0.0145 | R² = 0.9981 | y = 0.4175x + 0.8318 | R² = 0.9952 |
| 1.0 ppm | y = 0.0666x + 0.083 | R² = 0.9132 | y = 0.4595x + 0.6469 | R² = 0.9968 |
| 2.0 ppm | y = 0.0869x + 0.037 | R² = 0.9890 | y = 0.475x + 1.0472 | R² = 0.9807 |
| 4.0 ppm | y = 0.0852x + 0.0512 | R² = 0.9783 | y = 0.4464x + 1.092 | R² = 0.9488 |
| Dose of 1-MCP (ppm) | Edible Stage | Unmarketable * |
|---|---|---|
| 0 (Control) | 7 | 14 + 2 |
| 0.5 | 14 + 2 | 14 + 4 |
| 1.0 | 14 + 2 | 14 + 6 |
| 2.0 | 14 + 4 | >14 + 10 |
| 4.0 | 14 + 8 | >14 + 10 |
| Firmness | SSC | TA | Sweetness | Sourness | Off-Flavor | Papaya Flavor | Hardness | Overall Score | |
|---|---|---|---|---|---|---|---|---|---|
| Firmness | 1 | ||||||||
| SSC | −0.168 | 1 | |||||||
| TA | 0.238 | 0.106 | 1 | ||||||
| Sweetness | −0.331 | 0.714 | 0.309 | 1 | |||||
| Sourness | 0.399 | −0.160 | 0.865 | 0.335 | 1 | ||||
| Off-flavor | −0.554 | 0.731 | 0.464 | −0.044 | 0.123 | 1 | |||
| Papaya flavor | −0.405 | −0.773 | −0.118 | 0.883 | −0.118 | −0.255 | 1 | ||
| Hardness | 0.968 | 0.022 | 0.198 | −0.485 | 0.253 | −0.441 | −0.509 | 1 | |
| Overall score | 0.691 | 0.615 | 0.542 | 0.431 | 0.623 | −0.454 | 0.268 | 0.578 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, C.; Wall, M.M.; Follett, P.A.; Sugimoto, N.; Bai, J.; Sun, X. Effect of Humidity-Triggered Controlled-Release 1-Methylcyclopropene (1-MCP) on Postharvest Quality of Papaya Fruit. Horticulturae 2023, 9, 1062. https://doi.org/10.3390/horticulturae9101062
Shu C, Wall MM, Follett PA, Sugimoto N, Bai J, Sun X. Effect of Humidity-Triggered Controlled-Release 1-Methylcyclopropene (1-MCP) on Postharvest Quality of Papaya Fruit. Horticulturae. 2023; 9(10):1062. https://doi.org/10.3390/horticulturae9101062
Chicago/Turabian StyleShu, Chang, Marisa M. Wall, Peter A. Follett, Nobuko Sugimoto, Jinhe Bai, and Xiuxiu Sun. 2023. "Effect of Humidity-Triggered Controlled-Release 1-Methylcyclopropene (1-MCP) on Postharvest Quality of Papaya Fruit" Horticulturae 9, no. 10: 1062. https://doi.org/10.3390/horticulturae9101062
APA StyleShu, C., Wall, M. M., Follett, P. A., Sugimoto, N., Bai, J., & Sun, X. (2023). Effect of Humidity-Triggered Controlled-Release 1-Methylcyclopropene (1-MCP) on Postharvest Quality of Papaya Fruit. Horticulturae, 9(10), 1062. https://doi.org/10.3390/horticulturae9101062










