Morphological Characteristics and Occurrence of an Important Stem-Boring Pest, Nassophasis sp. (Coleoptera: Rhynchophorinae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Colony
2.2. Anatomy and Observations on the External Morphology and Genitalia of Nassophasis sp.
2.3. Larval Instar Determination
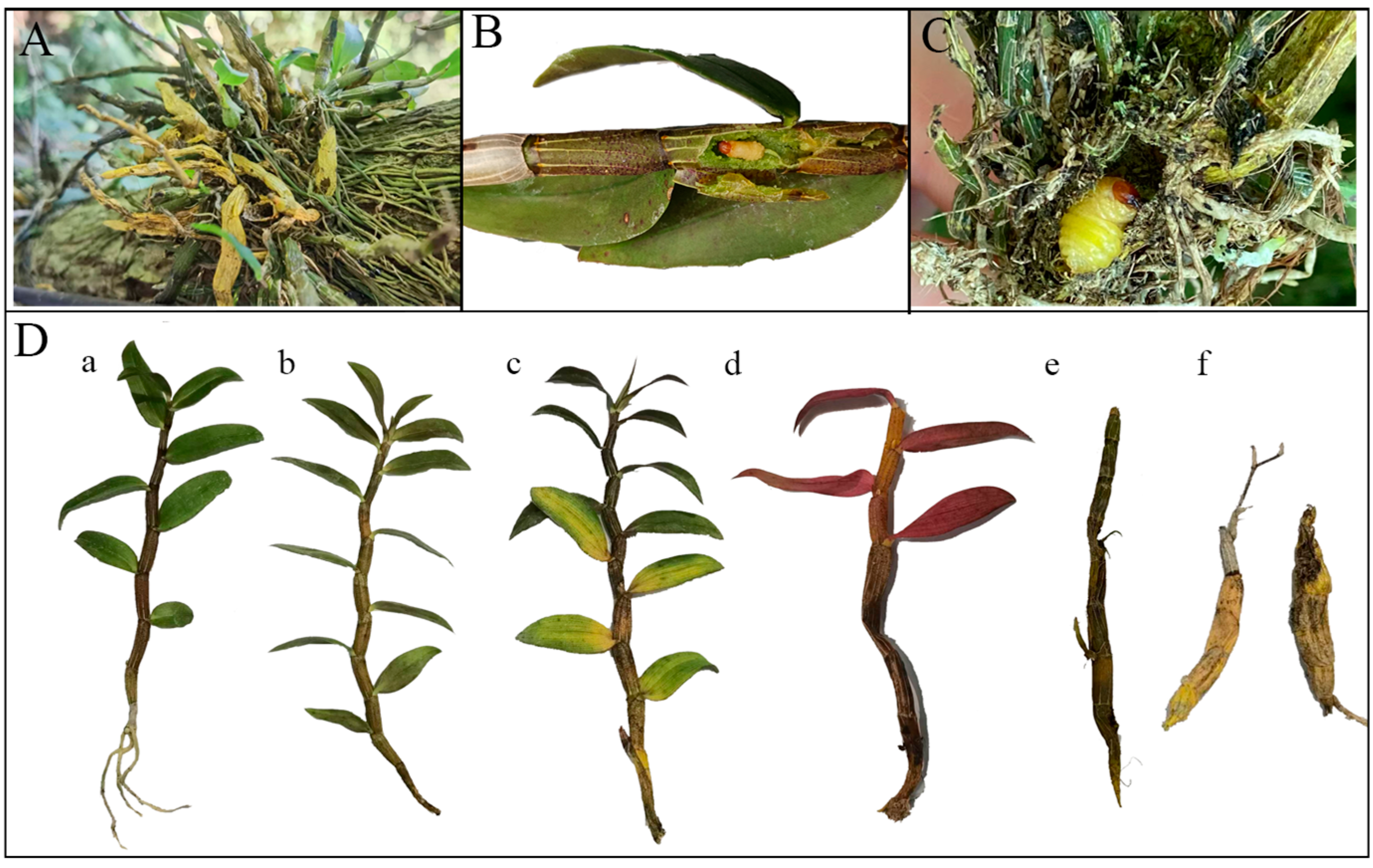
2.4. Determination of Damage, Life History, and Spatial Distribution
2.5. Statistical Analysis
3. Results
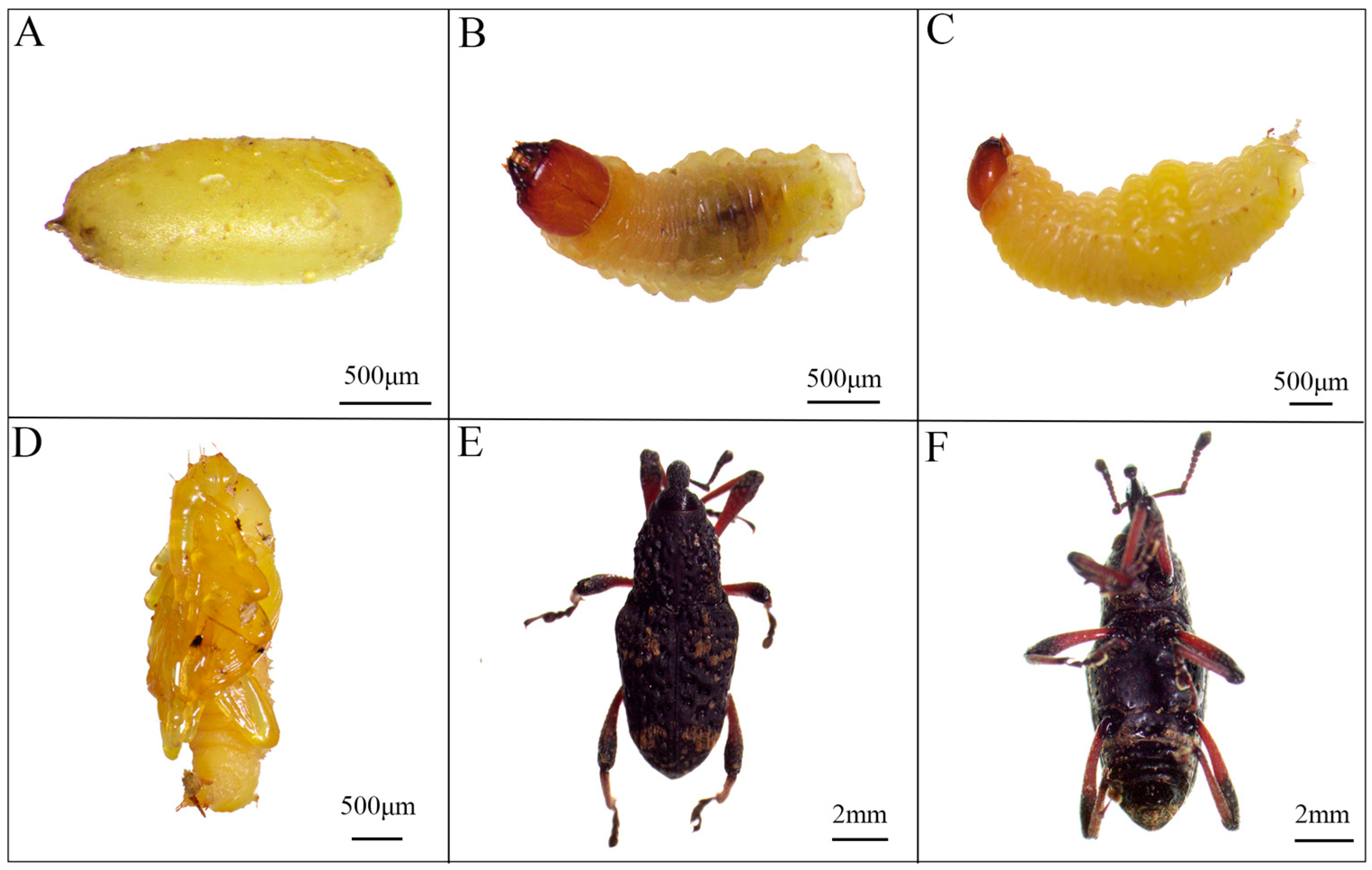
3.1. Morphological Characteristics of the Nassophasis sp.
3.1.1. External Morphological Characteristics
3.1.2. Morphological Differences between Male and Female Adults
3.1.3. Morphological Characteristics of Male and Female External Genitalia
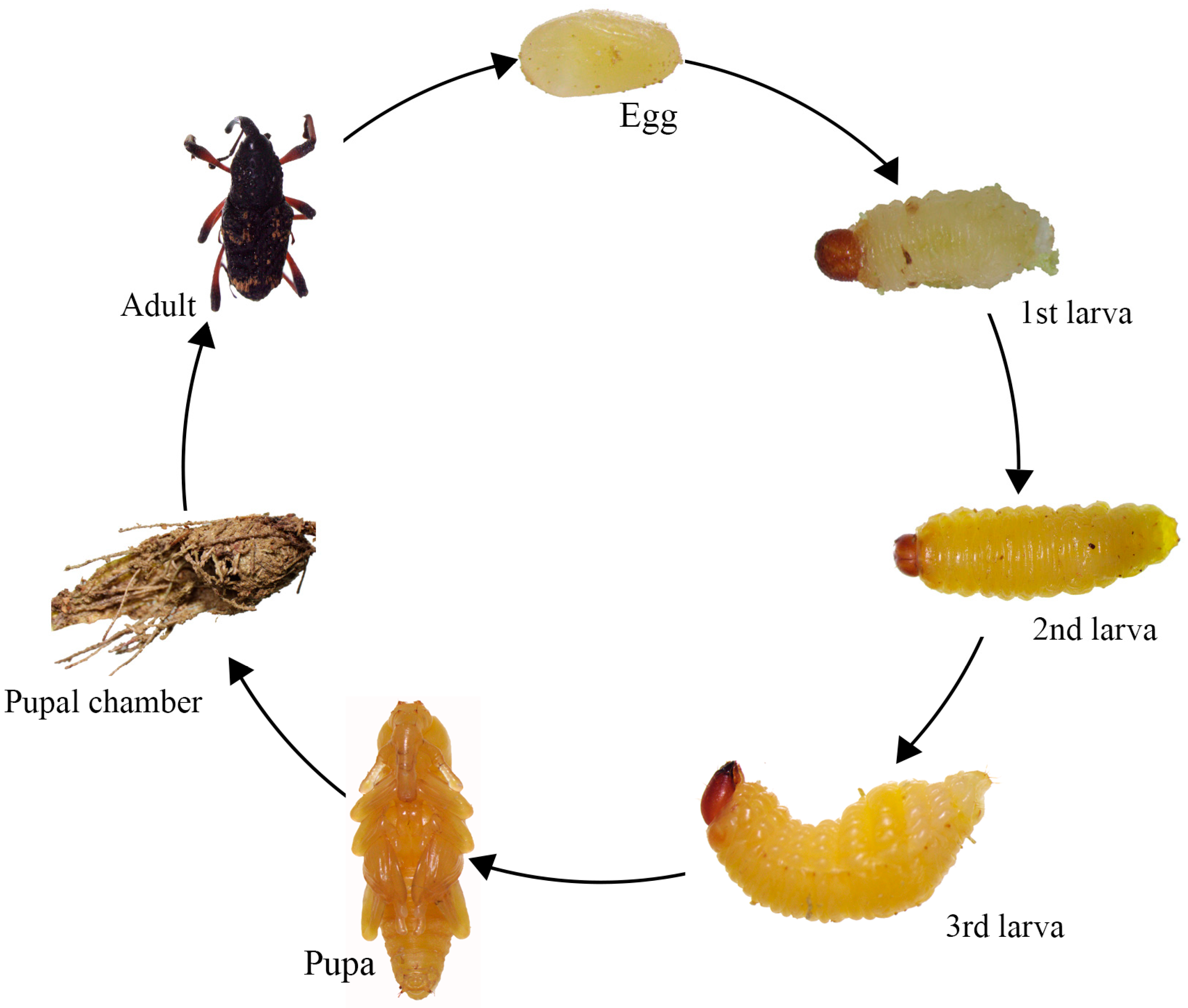
3.2. Biological Characteristics of the Nassophasis sp.
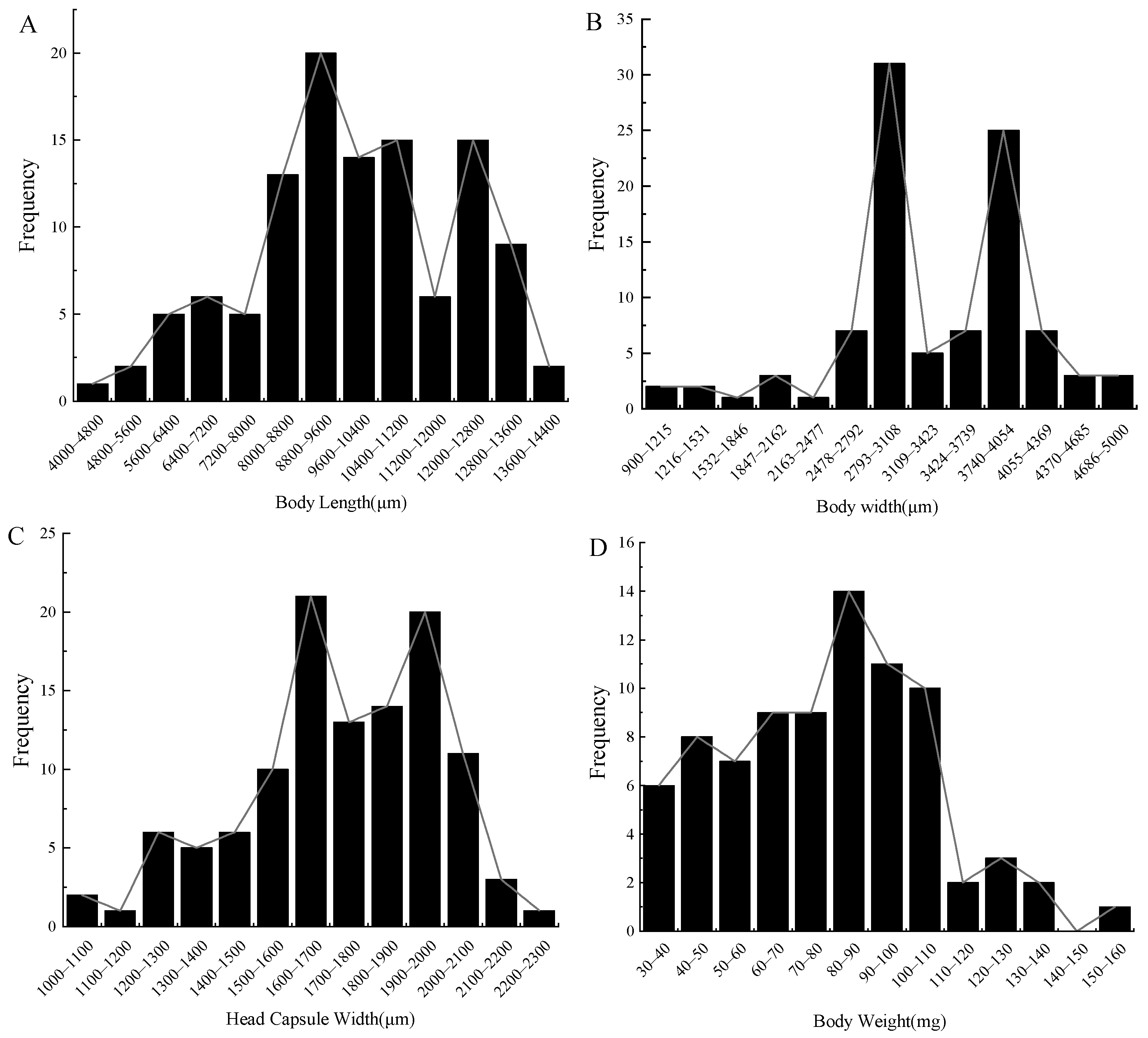
3.2.1. Larval Instar Determination
3.2.2. Life History
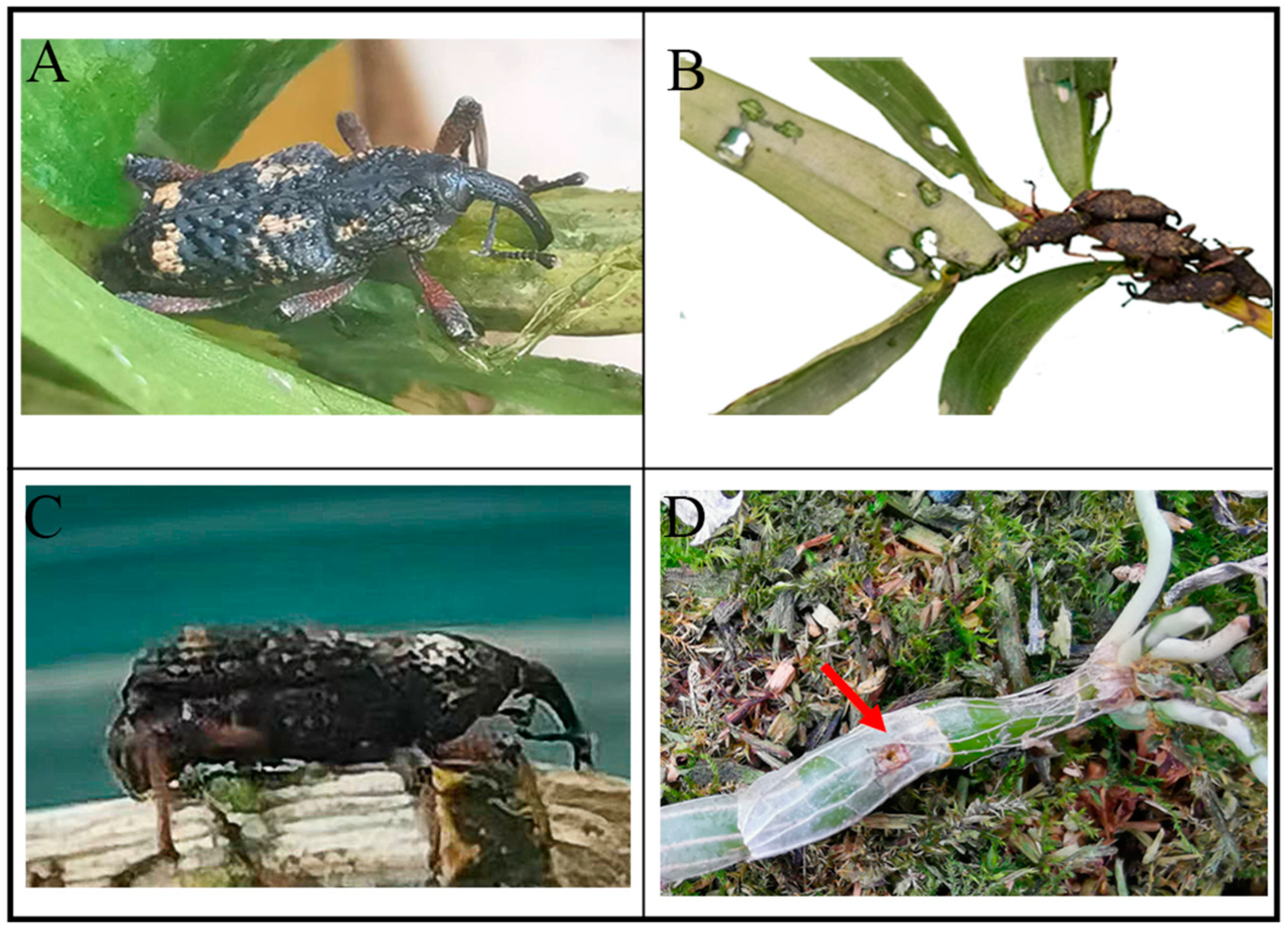
3.3. Infestation Traits and Spatial Distribution of the Nassophasis sp.
3.3.1. Infestation Traits
3.3.2. Spatial Distribution
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ketsa, S.; Warrington, I.J. The Dendrobium Orchid: Botany, horticulture, and utilization. Crop Sci. 2023, 63, 1829–1888. [Google Scholar] [CrossRef]
- Chen, W.H.; Lu, J.M.; Zhang, J.H.; Wu, J.J.; Yu, L.L.; Qin, L.P.; Zhu, B. Traditional Uses, Phytochemistry, Pharmacology, and Quality Control of Dendrobium officinale Kimura et. Migo. Front. Pharmacol. 2021, 12, 24. [Google Scholar] [CrossRef]
- Prena, J. Orchid weevils (Coleoptera: Curculionidae) in Canada. Can. Entomol. 2017, 149, 38–47. [Google Scholar] [CrossRef]
- Gutting, A.; Zettler, J.A.; Zettler, L.W.; Richardson, L.W. An update on mealybugs and scale insects (Hemiptera) on native epiphytic orchids in South Florida, including a new record for Pseudococcus microcirculus (Pseudococcidae). Fla. Entomol. 2015, 98, 401–404. [Google Scholar] [CrossRef]
- Whitehead, D.R.; Chamorro, M.L.; Anderson, R.S. An Illustrated Key to the Species of Curculio Linnaeus (Coleoptera: Curculionidae) of North America East of the Mississippi River. Proc. Entomol. Soc. Wash. 2018, 120, 616–641. [Google Scholar] [CrossRef]
- Morales-Baez, M.; Salinas-Castro, A.; Bello, D.E.; Cadena, M.G.L.; Fernandez, A.R.; Trigos, A. Stethobaroides nudiventris (Coleoptera: Curculionidae), the Curculionid Cause of Petal Wilting on the Catasetum integerrimum Orchid. Ann. Entomol. Soc. Am. 2016, 109, 845–849. [Google Scholar] [CrossRef]
- Milosavljevic, I.; El-Shafie, H.A.F.; Faleiro, J.R.; Hoddle, C.D.; Lewis, M.; Hoddle, M.S. Palmageddon: The wasting of ornamental palms by invasive palm weevils, Rhynchophorus spp. J. Pest Sci. 2019, 92, 143–156. [Google Scholar] [CrossRef]
- Pope, T.W.; Roberts, J.M. Vine Weevil, Otiorhynchus sulcatus (Coleoptera: Curculionidae), Management: Current State and Future Perspectives. Annu. Rev. Entomol. 2022, 67, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhao, Y.; Zhu, J.; Lu, L.; He, Y.; Zeng, F.; Yang, L.; Xiao, C. Biological characteristics of Nassophasis sp. Chin. Bull. Entomol. 2010, 47, 82–85. (In Chinese) [Google Scholar]
- Gao, X.; He, Y.; Zhao, Y.; Zeng, F.; Yang, L.; Xiao, C. Behavioural responses of Nassophasis sp. (Coleoptera: Dryophthoridae) adults to different odor sources. Acta Entomol. Sin. 2009, 52, 814–819. (In Chinese) [Google Scholar] [CrossRef]
- Yan, Z.; XiaoYu, G.; Ji, A.; YueQiu, H.; Chun, X. Selection Behavior of Nassophasis sp.Adults (Coleoptera:Dryophthoridae) among Host Plants. Southwest China J. Agric. 2018, 31, 742–747. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, Y.; XiaoYu, G.; Gao, X.; YueQiu, H.; Zeng, F.; Xiao, C. Study on the feeding behavior and control measures of Nassophasis sp. GuangDong Agric. Sci. 2013, 40, 68–70+237. (In Chinese) [Google Scholar] [CrossRef]
- Valente, R.M.; Da Silva, P.A.L.; De Medeiros, B.A.S. The first species of Cotithene Voss (Coleoptera: Curculionidae: Curculioninae) from Amazonian Brazil, with notes on its role as a pollinator of Evodianthus funifer (Poit.) Lindm. (Cyclanthaceae). Zootaxa 2019, 4576, 461–482. [Google Scholar] [CrossRef]
- Friedman, A.L.L. A new species of Brachycerus from Socotra Island (Coleoptera: Curculionoidea: Brachyceridae). Acta Entomol. Musei Nat. Pragae 2017, 57, 173–182. [Google Scholar] [CrossRef][Green Version]
- Panzavolta, T. Instar Determination for Pissodes castaneus (Coleoptera: Curculionidae) Using Head Capsule Widths and Lengths. Environ. Entomol. 2007, 36, 1054–1058. [Google Scholar] [CrossRef]
- Iwao, S.I. A new regression method for analyzing the aggregation pattern of animal populations. Res. Popul. Ecol. 1968, 10, 1–20. [Google Scholar] [CrossRef]
- Taylor, L.R. Aggregation, Variance and the Mean. Nature 1961, 189, 732–735. [Google Scholar] [CrossRef]
- Blackith, R.E. Nearest-Neighbour Distance Measurements for the Estimation of Animal Populations. J. Ecol. 1958, 39, 147–150. [Google Scholar] [CrossRef]
- Li, P.Y.; Li, L.; Wang, Y.Z. Traditional uses, chemical compositions and pharmacological activities of Dendrobium: A review. J. Ethnopharmacol. 2023, 310, 27. [Google Scholar] [CrossRef]
- Pan, C.X.; Chen, S.R.; Chen, Z.M.; Li, Y.M.; Liu, Y.K.; Zhang, Z.J.; Xu, Y.N.; Liu, G.T.; Yang, K.Y.; Liu, G.R.; et al. Assessing the geographical distribution of 76 Dendrobium species and impacts of climate change on their potential suitable distribution area in China. Environ. Sci. Pollut. Res. 2022, 29, 20571–20592. [Google Scholar] [CrossRef]
- Mulcahy, M.M.; Wilson, B.E.; Reagan, T.E. Spatial Distribution of Lissorhoptrus oryzophilus (Coleoptera: Curculionidae) in Rice. Environ. Entomol. 2022, 51, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Grigolli, J.F.J.; Souza, L.A.; Mota, T.A.; Fernandes, M.G.; Busoli, A.C. Sequential Sampling Plan of Anthonomus grandis (Coleoptera: Curculionidae) in Cotton Plants. J. Econ. Entomol. 2017, 110, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, W.; Barbosa, J.C.; Pavarini, R.; Maruyama, W.I.; Oliveira, R.A. Spatial Distribution and Sequential Sampling of the Banana Root Borer. Agron. J. 2016, 108, 1030–1040. [Google Scholar] [CrossRef]
- Silk, P.J.; Mayo, P.D.; LeClair, G.; Brophy, M.; Pawlowski, S.; MacKay, C.; Hillier, N.K.; Hughes, C.; Sweeney, J.D. Semiochemical attractants for the beech leaf-mining weevil, Orchestes fagi. Entomol. Exp. Appl. 2017, 164, 102–112. [Google Scholar] [CrossRef]
- Petersson, M.; Örlander, G. Effectiveness of combinations of shelterwood, scarification, and feeding barriers to reduce pine weevil damage. Can. J. Forest Res. 2003, 33, 64–73. [Google Scholar] [CrossRef]
- Castaneda-Vildozola, A.; Gonzalez-Hernandez, H.; Equihua-Martinez, A.; Valdez-Carrasco, J.; Pena, J.E.; Cazado, L.E.; Franco-Mora, O. Head capsule width is useful for determining larval instar in Heilipus lauri (Coleoptera: Curculionidae). Fla. Entomol. 2016, 99, 822–825. [Google Scholar] [CrossRef]
- Gomez, J.; Chavez, B.Y.; Castillo, A.; Valle, F.J.; Vega, F.E. The Coffee Berry Borer (Coleoptera: Curculionidae): How Many Instars are There? Ann. Entomol. Soc. Am. 2015, 108, 311–315. [Google Scholar] [CrossRef]
- Castaneda-Vildozola, A.; Illescas-Riquelme, C.P.; Valdez-Carrasco, J.; Cazado, L.E.; Sanchez-Pale, J.R.; Lopez-Martinez, V. Recognition of Five Larval Instars in Conotrachelus perseae (Coleoptera: Curculionidae). J. Entomol. Sci. 2019, 54, 1–8. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, H.; Haack, R.A.; Langor, D.W. Biology of Pissodes yunnanensis (Coleoptera: Curculionidae), a pest of Yunnan pine in southwestern China. Can. Entomol. 2004, 136, 719–726. [Google Scholar] [CrossRef]
- Milovac, Z.; Zoric, M.; Franeta, F.; Terzic, S.; Obradovic, O.P.; Jeromela, A.M. Analysis of oilseed rape stem weevil chemical control using a damage rating scale. Pest Manag. Sci. 2017, 73, 1962–1971. [Google Scholar] [CrossRef]
- Solano-Rojas, Y.; Gamez, M.; Lopez, I.; Garay, J.; Varga, Z.; Cabello, T. Conservation Strategy for Palm Groves: Optimal Chemical Control Model for Red Palm Weevil, Rhynchophorus ferrugineus. Agronomy 2021, 11, 920. [Google Scholar] [CrossRef]




| Distribution Pattern | C | I | Ca | K | m* | m*/m |
|---|---|---|---|---|---|---|
| Random distribution | =1 | =0 | =0 | =0 | =m | =1 |
| Uniform distribution | <1 | <0 | <0 | <0 | <m | <1 |
| Aggregate distribution | >1 | >0 | >0 | 0 > K > 8 | >m | >1 |
| α | Distribution of Individuals | β | Distribution Pattern |
|---|---|---|---|
| =0 | Single | =1 | Random distribution |
| <0 | Mutual exclusion | <1 | Uniform distribution |
| >0 | Mutual attraction | >1 | Aggregate distribution |
| Distribution Pattern | Determination Criteria |
|---|---|
| Random distribution | lga = 0, b = 1 |
| Uniform distribution | lga < 0, b < 1 |
| Aggregate distribution, no density dependence between individuals | lga > 0, b = 1 |
| Aggregate distribution, with density dependence between individuals | lga > 0, b > 1 |
| Variables | Larval Instar | Range | Mean ± SE | Variation Coefficient (%) | Brooks Index | Crosby Index |
|---|---|---|---|---|---|---|
| Body length | 1st | 4500–7744 μm | 6429.15 ± 201.74c | 3.14 | — | — |
| 2nd | 7745–10,905 μm | 9143.77 ± 117.46b | 1.28 | 1.41 ± 0.35 | — | |
| 3rd | 10,906–14,100 μm | 12,049.28 ± 130.46a | 1.08 | 1.31 ± 0.20 | 0.07 ± 0.02 | |
| Body width | 1st | 990–2324 μm | 1656.26 ± 159.05c | 9.60 | — | — |
| 2nd | 2325–3658 μm | 3045.68 ± 38.61b | 1.27 | 1.83 ± 0.28 | — | |
| 3rd | 3659–5000 μm | 4385.30 ± 63.06a | 1.44 | 1.43 ± 0.03 | 0.23 ± 0.11 | |
| Head capsule width | 1st | 1000–1428 μm | 1260.23 ± 30.95c | 2.46 | — | — |
| 2nd | 1429–1856 μm | 1665.80 ± 15.01b | 0.90 | 1.31 ± 0.02 | — | |
| 3rd | 1857–2300 μm | 1992.21 ± 14.74a | 0.74 | 1.19 ± 0.01 | 0.09 ± 0.01 | |
| Body weight | 1st | 0.030–0.072 g | 0.054 ± 0.002c | 3.70 | — | — |
| 2nd | 0.073–0.113 g | 0.092 ± 0.002b | 2.17 | 1.73 ± 0.15 | — | |
| 3rd | 0.114–0.160 g | 0.130 ± 0.005a | 3.85 | 1.43 ± 0.04 | 0.16 ± 0.05 |
| Variables | Fitted Model | Regression Equation | Regression Coefficient |
|---|---|---|---|
| Head capsule width | Linear | y = 937.35 + 355.61x | 0.83 |
| Quadratic | y = 755.51 + 524.29x2 − 39.58x | 0.83 | |
| Exponential | y = 3228.25 − 2581.93 × 0.80x | 0.83 | |
| Body length | Linear | y = 3526.26 + 2831.62x | 0.84 |
| Quadratic | y = 3905.44 + 2428.26x2 + 95.45x | 0.84 | |
| Exponential | y = 4765.84e0.32x | 0.85 |
| Month | 1~3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | M | L | E | M | L | E | M | L | E | M | L | E | M | L | E | M | L | E | M | L | E | M | L | E | M | L | E | M | L | |
| Overwintering generation | (+) | (+) | (+) | |||||||||||||||||||||||||||
| · | · | · | · | · | ||||||||||||||||||||||||||
| 1st generation | - | - | - | - | - | - | ||||||||||||||||||||||||
| ▲ | ▲ | ▲ | ▲ | ▲ | ||||||||||||||||||||||||||
| + | + | + | + | + | + | + | + | |||||||||||||||||||||||
| 2nd generation | · | · | · | · | · | · | · | · | ||||||||||||||||||||||
| - | - | - | - | - | - | - | - | - | ||||||||||||||||||||||
| ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ||||||||||||||||||||||||
| + | + | + | + | + | + | + | + | + | + | + | (+) | (+) | (+) | (+) | ||||||||||||||||
| 3rd generation | · | · | · | · | · | · | · | |||||||||||||||||||||||
| - | - | - | - | - | - | - | ||||||||||||||||||||||||
| ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | |||||||||||||||||||||||
| + | + | + | + | + | (+) | (+) | (+) | (+) | (+) | |||||||||||||||||||||
| No. | Mean Insect Population Density | Standard Deviation | C | I | CA | K | m* | m*/m | Spatial Distribution Type |
|---|---|---|---|---|---|---|---|---|---|
| Forest-1 | 1.05 | 33.70 | 1078.16 | 1077.16 | 1022.62 | 0.0010 | 1078.22 | 1023.62 | Aggregation |
| Forest-2 | 0.67 | 19.11 | 547.97 | 546.97 | 820.45 | 0.0012 | 547.64 | 821.45 | Aggregation |
| Forest-3 | 0.10 | 3.96 | 151.03 | 150.03 | 1442.59 | 0.0007 | 150.13 | 1443.59 | Aggregation |
| shed-1 | 0.17 | 6.67 | 256.38 | 255.38 | 1473.37 | 0.0007 | 255.56 | 1474.37 | Aggregation |
| shed-2 | 2.64 | 71.98 | 1960.33 | 1959.33 | 741.23 | 0.0013 | 1961.97 | 742.23 | Aggregation |
| shed-3 | 1.10 | 20.21 | 372.64 | 371.64 | 339.08 | 0.0029 | 372.73 | 340.08 | Aggregation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Li, L.; Tang, M. Morphological Characteristics and Occurrence of an Important Stem-Boring Pest, Nassophasis sp. (Coleoptera: Rhynchophorinae). Horticulturae 2023, 9, 1089. https://doi.org/10.3390/horticulturae9101089
Zhang M, Li L, Tang M. Morphological Characteristics and Occurrence of an Important Stem-Boring Pest, Nassophasis sp. (Coleoptera: Rhynchophorinae). Horticulturae. 2023; 9(10):1089. https://doi.org/10.3390/horticulturae9101089
Chicago/Turabian StyleZhang, Mengmeng, Li Li, and Ming Tang. 2023. "Morphological Characteristics and Occurrence of an Important Stem-Boring Pest, Nassophasis sp. (Coleoptera: Rhynchophorinae)" Horticulturae 9, no. 10: 1089. https://doi.org/10.3390/horticulturae9101089
APA StyleZhang, M., Li, L., & Tang, M. (2023). Morphological Characteristics and Occurrence of an Important Stem-Boring Pest, Nassophasis sp. (Coleoptera: Rhynchophorinae). Horticulturae, 9(10), 1089. https://doi.org/10.3390/horticulturae9101089






