Physicochemical Properties of Geographical Indication (GI) Sweet Cherries in China and Their Influencing Factors of Cultivar, Climate Type, and Soil Condition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit and Soil Materials
2.2. Chemicals and Reagents
2.3. Weight and Size Measurements
2.4. Juice Yield and Edible Rate Measurements
2.5. Soluble Solids Content, Titratable Acidity, Maturity Index, Sugar Composition, and Total Sugar
2.6. Total Phenolics, Total Anthocyanins, Total Flavonoids, and Procyanidin
2.7. β-Carotene and Ascorbic Acid
2.8. Mineral Content of Cherry Fruits
2.9. Parameters of Planted Soil
2.10. Statistics Analysis
3. Results and Discussions
3.1. Variability in Physicochemical Properties of GI Cherries
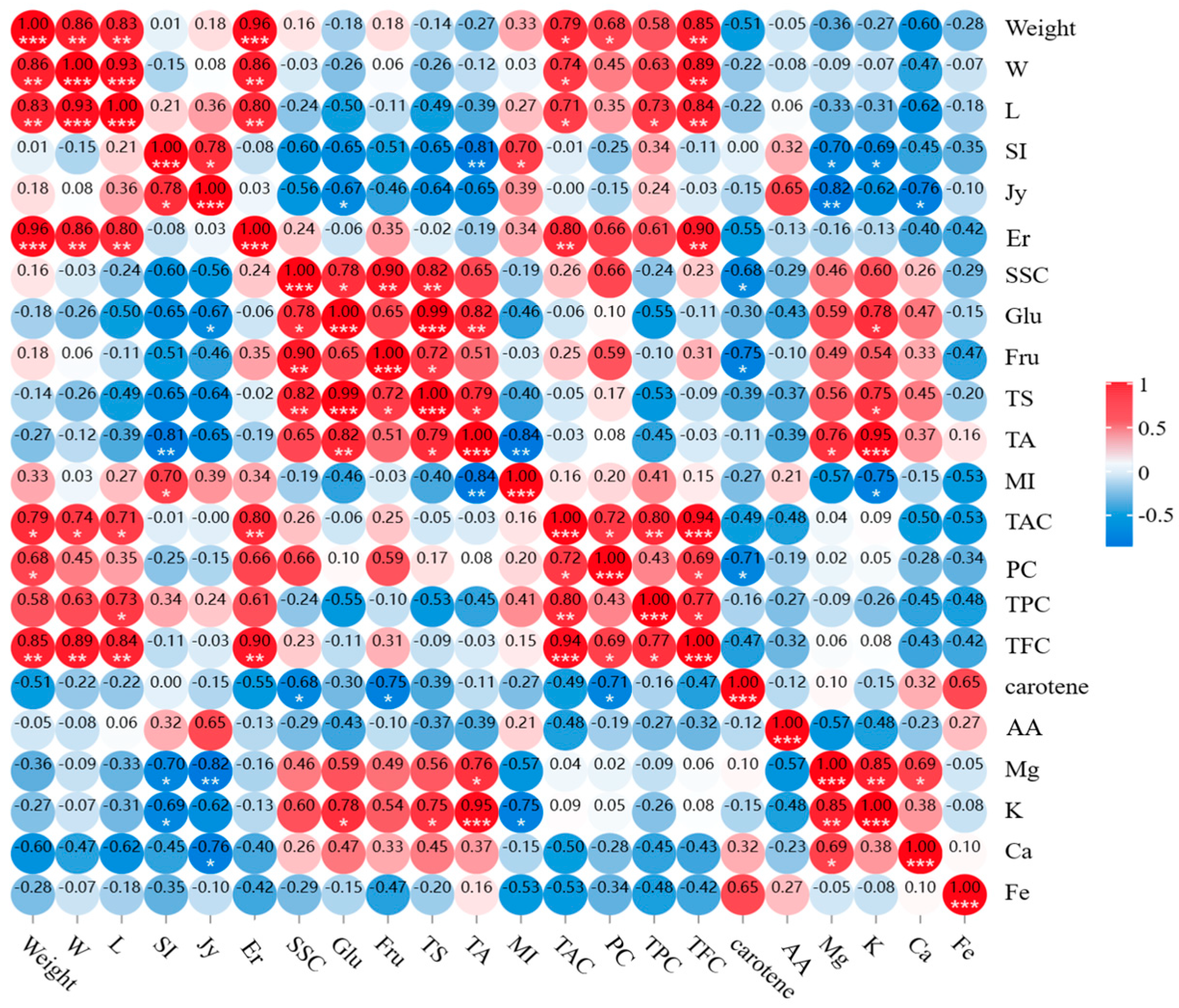
3.2. Correlation Analysis of Quality Parameters for GI Cherries
3.3. Effect of Cultivar and Climate Type on the Properties of GI Cherries
3.4. The Relationship between Soil Conditions and Properties of GI Cherries
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marinella, D.L.; Anna, M.I.; Maria, P.G.; Valeria, D.; Fabiano, C.; Filippo, S.; Gianfranco, D.; Nunziatina, D.T.; Alessandra, B. Comparative chemical analysis of six ancient Italian sweet cherry (Prunus avium L.) varieties showing antiangiogenic activity. Food Chem. 2021, 360, 129999. [Google Scholar]
- Manuel, J.S.; Alberto, M.; Santiago, R.M.; Alejandro, H.; Margarita, L.C.; María, G.C. Physicochemical and sensorial characterisation of four sweet cherry cultivars grown in Jerte Valley (Spain). Food Chem. 2012, 133, 1551–1559. [Google Scholar]
- Szilágyi, S.; Horváth-Kupi, T.; Desiderio, F.; Bekefi, Z. Evaluation of sweet cherry (Prunus avium L.) cultivars for fruit size by FW_G2a QTL analysis and phenotypic characterization. Sci. Hortic. 2022, 292, 110656. [Google Scholar] [CrossRef]
- 07-1537; United States Standards for Grades of Sweet Cherries. Agricultural Marketing Service. United States Department of Agriculture: Washington, DC, USA, 2007.
- GB/T 26901-2011; Grades of Cherries. State Administration for Market Regulation. Standardization Administration: Beijing, China, 2011.
- Recommended Quality Guide for Export Cherries. Chilean Cherry Committee: Chile, 2021. Available online: https://fructidor.com/newsdetail.aspx?idn=58303 (accessed on 23 August 2023).
- Ana, C.G.; Gonçalo, C.; Gilberto, A.; Cristina, G.V.; Diego, A.M.; Luís, R.S. Physical and phytochemical composition of 23 Portuguese sweet cherries as conditioned by variety (or genotype). Food Chem. 2021, 335, 127637. [Google Scholar]
- Kubilay, V.; Hasim, K.; Serkan, S. A study on some chemical and physico-mechanic properties of three sweet cherry varieties (Prunus avium L.) in Turkey. J. Food. Eng. 2006, 74, 568–575. [Google Scholar]
- Federica, B.; Oomah, B.D. Sweet and sour cherries: Origin, distribution, nutritional composition and health benefits. Trends Food Sci. Technol. 2019, 86, 517–529. [Google Scholar]
- Kim, D.O.; Heo, H.J.; Kim, Y.J.; Hyun, S.Y.; Chang, Y.L. Sweet and Sour Cherry Phenolics and Their Protective Effects on Neuronal Cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef]
- Guillermo, C.; Francisco, L.; Maria, P.G.; Carine, S.; Víctor, L. Bioactive and functional properties of sour cherry juice (Prunus cerasus). Food Funct. 2016, 7, 4675–4682. [Google Scholar]
- Fatih, M.Y. Sour Cherry By-products: Compositions, Functional Properties and Recovery Potentials. Crit. Rev. Food Sci. Nutr. 2019, 59, 3549–3563. [Google Scholar]
- Pissard, A.; Lateur, M.; Baeten, V.; Magein, H.; Dupont, P.; Tabart, J.; Pincemail, J.; Kevers, C. Determination of total phenolic compound content and antioxidant activity in cherry species and cultivars. J. Berry Res. 2016, 6, 81–91. [Google Scholar] [CrossRef]
- Ewa, S.; Tomasz, K.; Karolina, M.K.; Sebastian, P. Fruit quality and contents of some bioactive compounds in selected Czech sweet cherry (Prunus avium L.) cultivars under conditions of Central Poland. Agriculture 2022, 12, 1859. [Google Scholar]
- Sofia, C.; Filipa, Q.; Carlos, R.; Alice, V.; Alfredo, A.; Ana, I.B.; Rob, S.; Ana, P.S.; Berta, G. Effects of calcium and growth regulators on sweet cherry (Prunus avium L.) quality and sensory attributes at harvest. Sci. Hortic. 2019, 248, 231–240. [Google Scholar]
- Giménez, M.J.; María, S.; Juan, M.V.; Domingo, M.R.; Salvador, C.; Daniel, V.; Fabián, G. Preharvest salicylic acid and acetylsalicylic acid treatments preserve quality and enhance antioxidant systems during postharvest storage of sweet cherry cultivars. J. Sci. Food Agric. 2017, 97, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Mehmet, A.O.; Yusuf, C.G.; Husnu, D.; Leyla, D.; Idris, M.; Gul, C.O. Comparative evaluation of phenolic profile and antioxidant activity of new sweet cherry (Prunus avium L.) genotypes in Turkey. Phytochem. Anal. 2022, 33, 564–576. [Google Scholar]
- Thouraya, A.G.; Tahar, S.; Youssef, A. Behavior and morphometric characterization of local and introduced cultivars of sweet cherries (Prunus avium), tested in a multi-site trial in Tunisia. Sci. Hortic. 2020, 270, 109455. [Google Scholar]
- Li, B.; Xie, Z.; Zhang, A.; Xu, W.; Zhang, C.; Liu, Q.; Liu, C.; Wang, S. Tree growth characteristics and flower bud differentiation of sweet cherry (Prunus avium L.) under different climate conditions in China. Hortic. Sci. 2010, 37, 6–13. [Google Scholar] [CrossRef]
- Tristan, T.W.; Louise, M.N.; Denise, N.; Gerry, H.N.; Tom, A.F. Soil amendments influence Pratylenchus penetrans populations, beneficial rhizosphere microorganisms, and growth of newly planted sweet cherry. Appl. Soil Ecol. 2017, 117, 212–220. [Google Scholar]
- Anna, B.; Zdzisław, K.; Jan, K.; Jerzy, Z. Yielding and fruit quality of Lithuanian sweet cherry cultivars grown under the climatic and soil conditions of Warmia. Folia Hortic. 2011, 23, 101–106. [Google Scholar]
- Mohd, H.I.; Hawa, Z.E.J.; Ehsan, K.; Ali, G. Primary, secondary metabolites, photosynthetic capacity and antioxidant activity of the malaysian herb kacip fatimah (Labisia Pumila Benth) exposed to potassium fertilization under greenhouse conditions. Int. J. Mol. Sci. 2012, 13, 15321–15342. [Google Scholar]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Christian, Z.; Mehmet, S.; Edgar, P. Potassium in agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar]
- Bai, Q.; Shen, Y.; Huang, Y. Advances in mineral nutrition transport and signal transduction in Rosaceae fruit quality and postharvest storage. Front. Plant Sci. 2021, 12, 620018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yan, G.; Zhang, X.; Wang, J.; Duan, X. Sweet cherry growing in China. Acta Hortic. 2019, 1235, 133–140. [Google Scholar] [CrossRef]
- FAS Beijing. Stone Fruit Annual (Report No. CH2022-0078). USDA Foreign Agriculture Service. 2022. Available online: https://www.fas.usda.gov/data/china-stone-fruit-annual-6 (accessed on 23 August 2023).
- World Trade Organization. Uruguay Round Agreement: Trips. 2016. Available online: https://www.wto.org/english/docs_e/legal_e/27-trips_04b_e.htm#3 (accessed on 23 August 2023).
- Angela, G.P.; Antonio, F.M.; Maria, R.F.; Giuseppe, S.; Giacomo, D.; Vincenzo, L.T.; Rosaria, C.F.C.; Marcella, D.B.; Giuseppa, D.B. Multielement and chemometric analysis for the traceability of the Pachino Protected Geographical Indication (PGI) cherry tomatoes. Food Chem. 2022, 386, 132746. [Google Scholar]
- Longobardi, F.; Ventrella, A.; Bianco, A.; Catucci, L.; Cafagna, I.; Gallo, V.; Mastrorilli, P.; Agostiano, A. Non-targeted 1H NMR fingerprinting and multivariate statistical analyses for the characterization of the geographical origin of Italian sweet cherries. Food Chem. 2013, 141, 3028–3033. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, R.R.; Chen, Y.F.; Xing, J.G. Discrimination of geographical indication of Chinese green teas using an electronic nose combined with quantum neural networks: A portable strategy. Sens. Actuators B-Chem. 2023, 375, 132946. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Han, G.; Liu, Y.; Jiang, J.J.; Jia, Y.Y.; Li, X.G. Nutrient composition and quality traits of dried jujube fruits in seven producing areas based on metabolomics analysis. Food Chem. 2022, 385, 132627. [Google Scholar] [CrossRef]
- Su, S.; Wu, J.; Peng, X.Y.; Li, B.; Li, Z.J.; Wang, W.; Ni, J.W.; Xu, X.Q. Genetic and agro-climatic variability in seed fatty acid profiles of Akebia trifoliata (Lardizabalaceae) in China. J. Food Compos. Anal. 2021, 102, 104064. [Google Scholar] [CrossRef]
- Palma, M.; Sepúlveda, Á.; Yuri, J.A. Effect of plastic roof and high tunnel on microclimate, physiology, vegetative growth and fruit characteristics of ’Santina’ sweet cherry. Sci. Hortic. 2023, 317, 112037. [Google Scholar] [CrossRef]
- Rutkowski, K.; Łysiak, G.P. Weather conditions, orchard age and nitrogen fertilization influences yield and quality of ‘Łutówka’ sour cherry fruit. Agriculture 2022, 12, 2008. [Google Scholar] [CrossRef]
- Victor, B.; Pedro, J.B.R.; Roque, T.S.; Rafael, D. Influence of regulated deficit irrigation and environmental conditions on reproductive response of sweet cherry trees. Plants 2020, 9, 94. [Google Scholar]
- Uçgun, K. Effects of nitrogen and potassium fertilization on nutrient content and quality attributes of sweet cherry fruits. Not. Bot. Horti Agrobot. 2019, 47, 114–118. [Google Scholar] [CrossRef]
- Hüseyin, Y.; Ömer, A. Effects of potassium fertilization on leaf nutrient content and quality attributes of sweet cherry fruits (Prunus avium L.). J. Plant Nutr. 2020, 44, 946–957. [Google Scholar]
- Jia, C.S.; Geoffrey, I.N.; Sun, D.X.; Sun, Y.G.; Wu, P. Variety compound quality relationship of 12 sweet cherry varieties by HPLC-chemometric analysis. Int. J. Food Sci. Technol. 2019, 54, 2897–2914. [Google Scholar] [CrossRef]
- Elena, C.C.; Mireia, C.; Alfonso, M.; Dolores, H.; Carla, M.S.; Paula, M.B.; Antonio, J.M.M. Effect of regulated deficit irrigation on commercial quality parameters, carotenoids, phenolics and sugars of the black cherry tomato (Solanum lycopersicum L.) ‘Sunchocola’. J. Food Compos. Anal. 2022, 105, 104220. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela–Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteau reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Kim, M.K.; Kim, H.; Kim, H.S.; Youngsang, L.; Yongho, K. Identification and quantification of anthocyanin pigments in colored rice. Nutr. Res. Pract. 2008, 2, 46–49. [Google Scholar] [CrossRef]
- Olszewska, M.A. Variation in the phenolic content and in vitro antioxidant activity of Sorbus aucuparia leaf extracts during vegetation. Acta Pol. Pharm. 2011, 68, 937–944. [Google Scholar]
- GB 5099.83-2016; National Food Safety Standards, Determination of Carotene in Food China. Food and Drug Administration, National Health and Family Planning Commission of the PRC: Beijing, China, 2017.
- GB 5099.86-2016; National Food Safety Standards, Determination of Ascorbic Acid in Food. China Food and Drug Administration, National Health and Family Planning Commission of the PRC: Beijing, China, 2017.
- Matos-Reyes, M.N.; Simonot, J.; Lopez-Salazar, O.; Cervera, M.L.; Guardia, M. Authentication of Alicante’s mountain cherries protected designation of origin by their mineral profile. Food Chem. 2013, 141, 2191–2197. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, J.; Zeng, S.; Wu, D.; Jacobs, D.F.; Sloan, J.L. Soil pH, organic matter, and nutrient content change with the continuous cropping of Cunninghamia lanceolata plantations in South China. J. Soils Sediments 2017, 17, 2230–2238. [Google Scholar] [CrossRef]
- Skjemstad, J.O.; Krull, E.S.; Swift, R.S.; Szarvas, S. Mechanisms of protection of soil organic matter under pasture following clearing of rainforest on an Oxisol. Geoderma 2008, 143, 231–242. [Google Scholar] [CrossRef]
- Tekalign, M.; Ritcher, C.; Heiligtag, B. Comparison of extractants for the determination of available phosphorus, potassium, calcium, magnesium and sodium in some Ethiopian and German soils. Commun. Soil Sci. Plant Anal. 1996, 27, 2197–2212. [Google Scholar]
- Ballistreri, G.; Continella, A.; Gentile, A.; Amenta, M.; Fabroni, S.; Rapisarda, P. Fruit quality and bioactive compounds relevant to human health of sweet cherry (Prunus avium L.) cultivars grown in Italy. Food Chem. 2013, 140, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Kalyoncu, I.H.; Ersoy, N.; Yilmaz, M. Contents of sweet cherry (Prunus avium L.) type grown in Konya. Afr. J. Biotechnol. 2009, 8, 2744–2749. [Google Scholar]
- Marlon, J.R.S.; Ana, P.M.P.; Adilson, P.J.; Camilo, A.P.C.; Daniel, C.; Mara, F.M.; Sarita, L.; Marco, A.T. Yield performance of new juice grape varieties grafted onto different rootstocks under tropical conditions. Sci. Hortic. 2018, 241, 194–200. [Google Scholar]
- Suwimol, C.; John, B.G.; Quan, V.V.; Konstantinos, P.; Costas, E.S. Sweet cherry: Composition, postharvest preservation, processing and trends for its future use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Ozgur, C.; Cemile, E.O.; Atakan, G.; İsa, E.; İsmail, D. Determination of optimum harvest date of sweet cherry cv. Lapins grown in Isparta. Turk. J. Agric. For. 2014, 2, 1905–1910. [Google Scholar]
- Wani, A.A.; Singh, P.; Gul, K.; Wani, M.H.; Langowski, H.C. Sweet cherry (Prunus avium): Critical factors affecting the composition and shelf life. Food Packag. Shelf Life 2014, 1, 86–99. [Google Scholar] [CrossRef]
- Kappel, F.; Granger, A.; Hrotkó, K.; Schuster, M. Fruit Breeding; Springer: Berlin, Germany, 2011; pp. 459–504. [Google Scholar]
- Ooraikul, B.; Stiles, M.E. Modified Atmosphere Packaging of Food; Ellis Horwood Ltd.: Chichester, UK, 1991; pp. 169–242. [Google Scholar]
- Sakshi, P.; Rachel, A.I.; Carlos, R.C.; Luis, F.L.; Veronica, C.S.; Juan, C.C.; Dario, J.C. Fruit Characterization of Prunus serotina subsp. capuli. Horticulturae 2022, 8, 838. [Google Scholar] [CrossRef]
- Valentina, U.; Jerneja, F.; Franci, Š. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar]
- Ali, A.H.; Nurullah, D. Physicochemical characteristics, antioxidant activity, organic acid and sugar contents of 12 sweet cherry (Prunus avium L.) cultivars grown in Turkey. J. Food Sci. 2015, 80, 564–570. [Google Scholar]
- Girard, B.; Kopp, T.G. Physicochemical Characteristics of Selected Sweet Cherry Cultivars. J. Agric. Food Chem. 1998, 46, 471–476. [Google Scholar] [CrossRef]
- Gloria, D.R.; Daniel, R.V.; Patricia, M.; Merichel, P.; Maria, L.M. Composition of nonextractable polyphenols from sweet cherry pomace determined by dart-orbitrap-hrms and their in vitro and in vivo potential antioxidant, antiaging, and neuroprotective activities. J. Agric. Food Chem. 2002, 70, 7993–8009. [Google Scholar]
- Nuria, A.; Ana, G.; Marta, B.; Antonia, G.; Dolores, M.M. Comparison of phenolic compounds profile and antioxidant properties of different sweet cherry (Prunus avium L.) varieties. Food Chem. 2019, 279, 260–271. [Google Scholar]
- Dominika, Ś.T.; Alicja, P.; Ewelina, H.; Agnieszka, G.; Elżbieta, R. The profile and content of polyphenols and carotenoids in local and commercial sweet cherry fruits (Prunus avium L.) and their antioxidant activity in vitro. Antioxidants 2019, 8, 534. [Google Scholar]
- Chaovanalikit, A.; Wrolstad, R.E. Anthocyanin and phenolic composition of fresh and processed cherries. J. Food Sci. 2004, 69, 173–183. [Google Scholar]
- Tahir, M.; Farooq, A.; Ijaz, A.B.; Tahira, I. Effect of maturity on proximate composition, phenolics and antioxidant attributes of cherry fruit. Pak. J. Bot. 2013, 45, 909–914. [Google Scholar]
- Cao, J.P.; Jiang, Q.; Lin, J.Y. Physicochemical characterization of four cherry species (Prunus spp.) grown in China. Food Chem. 2015, 173, 855–863. [Google Scholar] [CrossRef]
- Aneta, W.; Paulina, N.; Piotr, L.; Jan, O. Evaluation of Sour Cherry (Prunus cerasus L.) Fruits for Their Polyphenol Content, Antioxidant Properties, and Nutritional Components. J. Agric. Food Chem. 2014, 62, 12332–12345. [Google Scholar]
- Sílvia, A.; Ivo, V.O.; Anne, S.M.; Alfredo, A.; Maria, J.S.; Berta, G. Phenolic Profile and Bioactive Potential of Stems and Seed Kernels of Sweet Cherry Fruit. Antioxidants 2020, 9, 1295. [Google Scholar]
- Taki, D. Determination of carotenoid, organic acid and sugar content in some sweet cherry cultivars grown in Sakarva, Turkey. J. Food. Agric. Environ. 2013, 11, 73–75. [Google Scholar]
- Mauro, C.; Martino, B.; Flavia, D.C.; Stefania, P.; Alessandra, B.; Francesca, M.; Stefano, N.; Matteo, S.; Stefania, C.; Linda, A.; et al. Multi-approach metabolomics analysis and artificial simplified phytocomplexes reveal cultivar-dependent synergy between polyphenols and ascorbic acid in fruits of the sweet cherry (Prunus avium L.). PLoS ONE 2017, 12, e0180889. [Google Scholar] [CrossRef]
- Zan, S.Y.; Wang, R.; Zhang, F.; Zhang, D.Y.; Liu, B.J.; Meng, X.H. Composition analysis of rootstock cherry (Prunus mahaleb L.), a potential source of human nutrition and dietary supplements. Eur. Food Res. Technol. 2022, 248, 1421–1435. [Google Scholar] [CrossRef]
- Ali, K.; Mahsa, M.; Kimiya, A. Morphological and pomological characterizations of sweet cherry (Prunus avium L.), sour cherry (Prunus cerasus L.) and duke cherry (Prunus ×gondouinii Rehd.) to choose the promising selections. Sci. Hortic. 2019, 257, 108719. [Google Scholar]
- Mirko, S.; Grafe, C.; Wolfram, B.; Schmidt, H. Cultivars resulting from cherry breeding in Germany. Erwerbs-obstbau 2014, 56, 67–72. [Google Scholar]
- Julia, P.; Craig, H.; Cai, L.C.; Zhao, Y.Y.; Amy, I.; Cameron, P. Genomic heritability estimates in sweet cherry reveal non-additive genetic variance is relevant for industry-prioritized traits. BMC Genet. 2018, 19, 23. [Google Scholar]
- Erdal, A.; Onur, S.; Orhan, K.; Burhan, O.; Sefa, G. The relationship between fruit color and fruit quality of sweet cherry (Prunus avium L. cv. ‘0900 Ziraat’). Turk. J. Food Agric. Sci. 2019, 1, 1–5. [Google Scholar]
- Chen, C.Q.; Chen, H.X.; Yang, W.L.; Li, J.; Tang, W.J.; Gong, R.G. Transcriptomic and metabolomic analysis of quality changes during sweet cherry fruit development and mining of related genes. Int. J. Mol. Sci. 2022, 23, 7402. [Google Scholar] [CrossRef]
- Verena, O.; Michaela, S.; Michael, B. Non-destructive sensor-based prediction of maturity and optimum harvest date of sweet cherry fruit. Sensors 2017, 17, 277. [Google Scholar]
- Tahir, M.; Farooq, A.; Mateen37 Mary, C.B.; Nazamid, S. Compositional variation in sugars and organic acids at different maturity stages in selected small fruits from Pakistan. Int. J. Mol. Sci. 2012, 13, 1380–1392. [Google Scholar]
- María, S.; Fabián, G.; Domingo, M.R.; Salvador, C.; Daniel, V. Chemical constituents and antioxidant activity of sweet cherry at different ripening stages. J. Agric. Food Chem. 2005, 53, 2741–2745. [Google Scholar]
- Marco, B.; Ariel, M. Impact of potassium pre-harvest applications on fruit quality and condition of sweet cherry (Prunus avium L.) cultivated under plastic covers in southern Chile orchards. Plants 2021, 10, 2778. [Google Scholar]
- Özgür, A.; Volkan, A.; Ece, T.; Gülser, Y. Effects of potassium fertilization on sweet cherry fruit (Prunus avium L.) quality and mineral content. Commun. Soil Sci. Plant Anal. 2022, 53, 1777–1782. [Google Scholar]
- Kadir, U.; Mesut, A. Effects of increasing doses of nitrogen, phosphorus, and potassium on the uptake of other nutrients in sweet cherry trees. Commun. Soil Sci. Plant Anal. 2021, 52, 1248–1255. [Google Scholar]
- Deniz, E. Effect of Preharvest Calcium Treatments on Sweet Cherry Fruit Quality. Not. Bot. Horti Agrobot. 2014, 42, 150–153. [Google Scholar]
- Andreas, W.; Bennet, F.; Moritz, K. Calcium physiology of sweet cherry fruits. Trees 2020, 34, 1157–1167. [Google Scholar]
- Felicia, C.; Sina, N.C. Morphological and biochemical characteristics of fruits of different cornelian cherry (Cornus mas L.) genotypes from spontaneous flora. Not. Sci. Biol. 2017, 9, 577–581. [Google Scholar]
- James, W.O.; Amy, F.I.; Matthew, D.W. Genotypic differences in sweet cherry fruit size are primarily a function of cell number. J. Am. Soc. Hortic. Sci. 2007, 132, 697–703. [Google Scholar]
- Sansavini, S.; Lugli, S. Sweet cherry breeding programs in Europe and Asia. Acta Hortic. 2008, 795, 41–58. [Google Scholar] [CrossRef]
- Chen, L. A new middle ripening sweet cherry cultivar “Linglongcui”. Acta Hortic. Sin. 2018, 45, 1419–1420. [Google Scholar]
- Diana, N.; Rim, M.; Walid, E.K. Quality and phytochemical composition of sweet cherry cultivars can be influenced by altitude. Plants 2023, 12, 2254. [Google Scholar]
- Danilo, C.; Francesca, A.; Carolina, T.; Corrado, C.; Emilia, C.; Roberto, C. Can environment and genotype influence sweet cherry qualitative parameters? Plant Biosyst. 2022, 156, 581–589. [Google Scholar]
- Iryna, I.; Maryna, S.; Vira, M.; Iryna, B.; Ihor, K.; Tetiana, T.; Oksana, T.; Oleksandr, T.; Mikhailo, M.; Alina, O. The study of soluble solids content accumulation dynamics under the influence of weather factors in the fruits of cherries. Slovak J. Food Sci. 2021, 15, 350–359. [Google Scholar]
- Legua1, P.; Domenech, A.; Martínez, J.J.; Sánchez-Rodríguez, L.; Hernández, F.; Carbonell-Barrachina, A.A.; Melgarejo, P. Bioactive and volatile compounds in sweet cherry cultivars. J. Food Nutr. Res. 2017, 5, 844–851. [Google Scholar] [CrossRef]
- Zhang, C.X.; Matthew, W. The occurrence of protruding pistil in sweet cherry (Prunus avium L.) and its consequence on fertilization. Sci. Hortic. 2012, 140, 149–156. [Google Scholar] [CrossRef]
- Cristóbal, P.P.; Marjorie, R.D.; Jorge, G.V.; Alejandra, R.F. The potential roles of the N and P supplies on the internal browning incidence in sweet cherries in the Southern Chile. Horticulturae 2022, 8, 1209. [Google Scholar]
- Valentinuzzi, F.; Mason, M.; Scampicchio, M.; Andreotti, C.; Cesco, S.; Mimmo, T. Enhancement of the bioactive compound content in strawberry fruits grown under iron and phosphorus deficiency. J. Sci. Food Agric. 2015, 95, 2088–2094. [Google Scholar] [CrossRef]
- William, E.C. Organic matter in the agricultural soils of Tasmania, Australia—A review. Geoderma 2018, 312, 170–182. [Google Scholar]
- Tirhas, G.; Paige, M.; Tom, A.F.; Melanie, D.J.; Louise, M.N. Mulching improved soil fertility, plant growth and productivity, and postharvest deficit irrigation reduced water use in sweet cherry orchards in a semi-arid region. Arch. Agron. Soil Sci. 2022, 69, 1419–1436. [Google Scholar]
- Cao, F.; Guan, C.; Dai, H.; Li, X.; Zhang, Z. Soluble solids content is positively correlated with phosphorus content in ripening strawberry fruits. Sci. Hortic. 2015, 195, 183–187. [Google Scholar] [CrossRef]
- Erdal, A.; Onur, S. Role of the foliar fertilization treatments on quality attributes of sweet cherry fruits (Prunus avium). Akad. Ziraat Derg. 2018, 7, 131–136. [Google Scholar]
- Andreas, W.; Moritz, K. Calcium and the physiology of sweet cherries: A review. Sci. Hortic. 2019, 245, 107–115. [Google Scholar]
- Tingzhen, M.; Renaud, R.; Franz, D.O.; Robert, T.; Sylvain, C. A nature-based negative emissions technology able to remove atmospheric methane and other greenhouse gases. Atmos. Pollut. Res. 2021, 12, 101035. [Google Scholar]
- Zhou, B.B.; Zhang, Q.; Sun, J.; Li, X.L.; Wei, Q.P. Study and application of partial least squares regression on relationship between soil nutrient and fruit quality. J. Agric. Sci. Technol. 2016, 17, 362–366. [Google Scholar]
- Liu, Z.B.; Huang, Y.; Tan, F.J.; Chen, W.C.; Ou, L.J. Effect of soil type on trace element absorption and fruit quality of pepper. Front. Plant Sci. 2021, 12, 698796. [Google Scholar] [CrossRef]
- Xu, M.G.; Zeng, X.B.; Li, J.M. Effect of pH on adsorption and desorption of Cu2+ in latersol and yellow brown earth. Chin. J. Soil Sci. 2005, 36, 349–351. [Google Scholar]
- Cassiano, N.L.; Geovani, S.L.; Evandro, M.S.; Reginaldo, G.N.; Hans, R.G.; Lauriane, A.A.S. Fruit quality of West Indian cherry under saline water irrigation and nitrogen-potassium fertilization. Braz. J. Agric. Environ. Eng. 2021, 25, 741–749. [Google Scholar]
- Yogaratnam, N.; Sharples, R.O. Supplementing the nutrition of bramley’s seedling apple with phosphorus sprays ii. effects on fruit composition and storage quality. J. Hortic. Sci. Biotech. 1982, 57, 53–59. [Google Scholar] [CrossRef]
- Johnson, C.R. Phosphorus nutrition on mycorrhizal colonization, photosynthesis, growth and nutrient composition of Citrus aurantium. Plant Soil 1984, 80, 35–42. [Google Scholar] [CrossRef]
- Fredeen, A.L.; Rao, I.M.; Terry, N. Influence of phosphorus nutrition on growth and carbon partitioning in Glycine max. Plant Physiol. 1989, 89, 225–230. [Google Scholar] [CrossRef]
- López-Arredondo, D.L.; Leyva-González, M.A.; González-Morales, S.I.; López-Bucio, J.; Herrera-Estrella, L. Phosphate nutrition: Improving low-phosphate tolerance in crops. Annu. Rev. Plant Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Peng, L.; Hong, Y.; Qiao, G. Optimizing Nitrogen, Phosphorus, and Potassium Fertilization Rates for Fruit Performance of Chinese Cherry (Prunus pseudocerasus Lindl.). Int. J. Fruit Sci. 2022, 22, 769–778. [Google Scholar] [CrossRef]
- Olivos, A.; Johnson, S.; Xiaoqiong, Q.; Crisosto, C.H. Fruit phosphorous and nitrogen deficiencies affect ‘Grand Pearl’ nectarine flesh browning. Horstscience 2012, 47, 391–394. [Google Scholar] [CrossRef]
- Hina, M.; Vandana Sandeep, D.; Renu, P. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Karl, K.; Hanne, L.P. Effects of potassium, phosphorus and nitrogen fertilization on endogenous ethylene and quality characteristics of apples (Malus domestica L.). J. Plant Nutr. 2014, 37, 1148–1155. [Google Scholar]
- Changwei, S.; Ding, Y.; Lei, X.; Zhao, P.; Wang, S.; Xu, Y.; Dong, C. Effects of foliar potassium fertilization on fruit growth rate, potassium accumulation, yield, and quality of ‘kousui’ Japanese pear. J. Am. Soc. Hortic. Sci. 2016, 26, 270–277. [Google Scholar]
- Santos, M.; Ferreira, H.; Egea-Cortines, M.; Sousa, J.R.; Raimundo, F.; Matos, M.; Gonçalves, B. Evaluation of fruit quality, chromatic parameters and anthocyanin’s content under foliar application of magnesium and potassium on sweet cherry (Prunusavium L.) cv. Burlat. Biol. Life Sci. Forum 2021, 3, 47. [Google Scholar]
- Pant, R.C.; Singh, B. Potassium Level and Physiological Response and Fruit Quality in Hydroponically Grown Tomato. Int. J. Veg. Sci. 2009, 16, 85–99. [Google Scholar]
- Liu, J.; Hu, T.; Feng, P.; Yao, D.; Gao, F.; Hong, X. Effect of potassium fertilization during fruit development on tomato quality, potassium uptake, water and potassium use efficiency under deficit irrigation regime. Agric. Water Manag. 2021, 250, 106831. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Roberts, W. Can potassium application affect the mineral and antioxidant content of horticultural crops? In Proceedings of the Symposium on Fertilizing Crops for Functional Food, Indianapolis, IN, USA, 11 November 2002. [Google Scholar]
- José, G.V.; Sonia, O. Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2019; pp. 207–244. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Elevated Carbon Dioxide Increases Contents of flavonoids and Phenolic Compounds, and Antioxidant Activities in Malaysian Young Ginger (Zingiber officinale Roscoe.) Varieties. Molecules 2010, 15, 7907–7922. [Google Scholar] [CrossRef]
- Wei, H.; Tye, L.; Bresnick, E.; Birt, D.F. Inhibitory effect of epigenin, a plant flavonoid, on epidermal ornithine decarboxylase and skin tumor promotion in mice. Cancer Res. 1990, 50, 499–502. [Google Scholar]
| GI Name | Origin | Variety | Climate Type | Picking Time |
|---|---|---|---|---|
| Baqiao | Xi’an, Shanxi | Hongdeng | Temperate monsoon | Mid May |
| Tianbao | Taian, Shandong | Huangmi | Temperate monsoon | Mid May |
| Jiangxian | Yuncheng, Shanxi | Tieton | Temperate monsoon | Mid May |
| Wenchuan | Aba Tibetan and Qiang Autonomous Prefecture, Sichuan | Hongdeng | Subtropical monsoon | Mid May |
| Xiehu | Lianyungang, Jiangsu | Brooks | Subtropical monsoon | Mid May~Early June |
| Tongzhou | Tongzhou, Beijing | Hongdeng | Temperate monsoon | Early June |
| Qinzhou | Tianshui, Gansu | Tieton | Temperate monsoon | Mid June |
| Ledu | Haidong, Qinghai | Tieton | Plateau mountain | Mid June |
| Dalian | Dalian, Liaoning | Russian NO.8 | Temperate monsoon | Late June |
| Sample | Width (mm) | Length (mm) | Shape Index | Weight (g) | Juice Yield (%) | Edible Rate (%) |
|---|---|---|---|---|---|---|
| Baqiao | 28.66 ± 0.98 d | 24.53 ± 0.83 c | 0.86 ± 0.03 bc | 10.24 ± 0.09 d | 60.47 ± 1.29 c | 92.50 ± 0.36 b |
| Tianbao | 21.61 ± 1.65 a | 21.38 ± 0.95 ab | 0.99 ± 0.04 e | 5.00 ± 0.23 a | 63.63 ± 1.10 d | 90.93 ± 0.75 a |
| Jiangxian | 24.08 ± 1.24 b | 21.05 ± 5.67 ab | 0.91 ± 0.18 bcd | 11.00 ± 0.04 e | 66.08 ± 1.63 e | 93.37 ± 0.31 c |
| Wenchuan | 24.23 ± 1.35 b | 20.33 ± 1.20 a | 0.84 ± 0.04 ab | 10.40 ± 0.01 d | 49.09 ± 1.30 b | 92.93 ± 0.54 bc |
| Xiehu | 24.41 ± 0.68 b | 22.23 ± 0.67 b | 0.91 ± 0.04 d | 6.71 ± 0.11 b | 68.20 ± 0.23 f | 90.73 ± 0.15 a |
| Tongzhou | 26.93 ± 2.68 c | 21.27 ± 1.48 ab | 0.79 ± 0.04 a | 6.97 ± 0.21 b | 39.70 ± 0.61 a | 92.67 ± 0.42 bc |
| Qinzhou | 31.77 ± 1.34 e | 29.45 ± 1.05 d | 0.93 ± 0.03 d | 14.76 ± 0.24 f | 71.10 ± 0.82 g | 95.20 ± 0.27 d |
| Ledu | 26.80 ± 1.43 c | 23.60 ± 0.90 c | 0.88 ± 0.03 bcd | 8.22 ± 0.25 c | 66.17 ± 0.35 e | 92.50 ± 0.36 b |
| Dalian | 34.78 ± 1.26 f | 31.15 ± 1.24 e | 0.90 ± 0.03 cd | 17.92 ± 0.10 g | 59.97 ± 0.35 c | 96.23 ± 0.25 e |
| Mean | 27.03 | 23.89 | 0.89 | 10.14 | 60.49 | 93.01 |
| Std. | 4.15 | 3.88 | 0.06 | 4.10 | 10.05 | 1.78 |
| CV% | 15.37 | 16.27 | 6.41 | 40.47 | 16.61 | 1.92 |
| Sample | Soluble Solids (Brix°) | Titratable Acidity (g/kg) | Maturity Index | Total Sugar (g/100 g) | Glucose (g/100 g) | Fructose (g/100 g) |
|---|---|---|---|---|---|---|
| Baqiao | 12.65 ± 0.49 a | 4.9 ± 0.00 c | 25.65 ± 1.10 b | 9.09 ± 0.16 a | 4.89 ± 0.09 a | 4.20 ± 0.01 a |
| Tianbao | 12.64 ± 0.10 a | 3.3 ± 0.01 a | 38.56 ± 1.82 e | 9.83 ± 0.01 a | 5.33 ± 0.26 a | 4.50 ± 0.01 a |
| Jiangxian | 17.47 ± 1.15 c | 5.4 ± 0.00 c | 32.59 ± 2.16 d | 15.69 ± 0.05 e | 10.30 ± 0.45 d | 5.39 ± 0.11 b |
| Wenchuan | 23.67 ± 1.00 e | 8.0 ± 0.00 e | 29.52 ± 0.96 c | 18.75 ± 0.36 f | 12.38 ± 0.64 e | 6.37 ± 0.41 d |
| Xiehu | 15.29 ± 0.17 b | 8.1 ± 0.00 e | 18.82 ± 0.10 a | 13.85 ± 0.79 d | 9.42 ± 0.40 c | 4.43 ± 0.18 a |
| Tongzhou | 19.08 ± 0.61 d | 9.9 ± 0.01 f | 19.21 ± 0.84 a | 19.70 ± 0.22 g | 13.79 ± 0.50 f | 5.91 ± 0.27 c |
| Qinzhou | 15.69 ± 0.06 b | 4.5 ± 0.00 b | 35.22 ± 0.30 e | 12.79 ± 0.13 c | 7.28 ± 0.34 b | 5.51 ± 0.18 bc |
| Ledu | 17.72 ± 0.28 c | 6.9 ± 0.02 d | 25.72 ± 0.37 b | 12.73 ± 0.02 c | 6.83 ± 0.08 b | 5.90 ± 0.08 c |
| Dalian | 17.22 ± 0.28 c | 5.5 ± 0.01 c | 31.13 ± 0.78 cd | 11.86 ± 0.06 b | 6.67 ± 0.09 b | 5.19 ± 0.11 b |
| Mean | 16.54 | 6.10 | 28.49 | 13.81 | 8.11 | 5.24 |
| Std. | 3.15 | 2.00 | 6.77 | 3.65 | 3.08 | 0.68 |
| CV% | 19.01% | 32.94% | 23.77% | 26.45% | 37.93% | 12.93% |
| Sample | TPC (mg/100 g) | TAC (mg/kg) | TFC (mg/100 g) | Procyanidin (mg/100 g) | β-Carotene (ug/100 g) | Ascorbic Acids (mg/100 g) | Potassium (mg/kg) | Magnesium (mg/kg) | Calcium (mg/kg) | Iron (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Baqiao | 123.04 ± 2.23 bc | 5.79 ± 0.27 a | 11.59 ± 0.67 bc | 16.35 ± 0.86 c | 187.86 ± 4.93 f | 6.62 ± 0.13 c | 1840.91 ± 77.73 a | 104.22 ± 4.41 b | 105.66 ± 3.01 d | 9.14 ± 0.38 f |
| Tianbao | 143.10 ± 0.60 d | 6.78 ± 0.42 a | 7.86 ± 0.49 a | 2.98 ± 1.34 a | 147.85 ± 9.44 e | 5.48 ± 0.13 b | 1883.01 ± 117.06 a | 114.47 ± 1.89 c | 126.93 ± 3.90 e | 3.66 ± 0.10 ab |
| Jiangxian | 125.65 ± 1.44 c | 17.60 ± 0.57 de | 12.23 ± 0.56 c | 28.70 ± 1.74 e | 42.94 ± 2.91 bc | 5.59 ± 0.17 b | 2144.99 ± 65.46 bc | 100.27 ± 1.56 b | 91.85 ± 2.51 c | 3.01 ± 0.14 a |
| Wenchuan | 100.04 ± 3.88 a | 15.76 ± 1.06 cd | 14.54 ± 0.11 d | 57.91 ± 1.67 g | 17.00 ± 0.95 a | 5.55 ± 0.17 b | 2459.53 ± 33.57 d | 128.82 ± 1.48 e | 125.23 ± 2.04 e | 5.05 ± 0.34 d |
| Xiehu | 104.71 ± 5.68 a | 10.75 ± 0.36 b | 9.54 ± 0.08 ab | 4.31 ± 0.06 a | 115.07 ± 7.86 d | 5.74 ± 0.23 b | 2591.51 ± 47.16 d | 112.76 ± 1.46 c | 79.10 ± 2.96 b | 6.50 ± 0.30 e |
| Tongzhou | 115.10 ± 7.18 b | 14.74 ± 0.06 c | 16.06 ± 0.44 de | 11.36 ± 0.28 b | 127.26 ± 8.60 d | 3.81 ± 0.04 a | 3090.44 ± 114.08 e | 184.10 ± 2.91 g | 155.01 ± 5.40 f | 4.82 ± 0.33 d |
| Qinzhou | 132.65 ± 0.14 c | 18.81 ± 1.00 e | 21.80 ± 0.90 f | 22.28 ± 1.46 d | 30.94 ± 1.06 b | 7.61 ± 0.42 d | 2016.18 ± 31.19 ab | 92.31 ± 0.85 a | 89.83 ± 0.39 c | 3.89 ± 0.27 bc |
| Ledu | 149.06 ± 6.31 d | 19.04 ± 0.95 e | 17.82 ± 0.76 e | 39.69 ± 2.24 f | 40.00 ± 1.59 bc | 6.96 ± 0.26 c | 2529.14 ± 0.81 d | 138.92 ± 2.21 f | 100.93 ± 0.82 d | 4.25 ± 0.11 cd |
| Dalian | 197.72 ± 3.94 e | 53.82 ± 2.31 f | 35.64 ± 2.22 g | 61.77 ± 1.85 h | 52.12 ± 2.42 c | 3.69 ± 0.24 a | 2268.05 ± 131.92 c | 120.96 ± 3.83 d | 70.13 ± 4.89 a | 3.03 ± 0.02 a |
| Mean | 131.5 | 18.44 | 18.69 | 25.43 | 125.94 | 6.09 | 2268.84 | 123.77 | 108.32 | 4.82 |
| Std. | 27.83 | 13.49 | 10.86 | 21.35 | 142.89 | 1.8 | 402.47 | 26.52 | 27.31 | 1.95 |
| CV% | 21.16% | 73.15% | 58.10% | 83.97% | 113.46% | 29.56% | 17.74% | 21.42% | 25.21% | 40.57% |
| Factors | Fruit Weight | Soluble Solids Content | Titratable Acidity | Maturity Index | Total Phenols |
|---|---|---|---|---|---|
| Cultivars | |||||
| Honegdeng | 9.50 ± 0.49 b | 19.77 0.90 b | 19.06 ± 0.30 b | 14.18 ± 1.43 ab | 112.72 ± 11.11 a |
| Huangmi | 5.00 ± 0.793 a | 12.64 ± 1.47 a | 27.50 ± 0.49 d | 7.86 ± 2.33 a | 143.10 ± 0.60 b |
| Tieton | 10.55 ± 0.49 c | 17.15 ± 0.90 b | 21.34 ± 0.30 c | 17.42 ± 1.43 b | 135.79 ± 5.68 b |
| Brooks | 6.71 ± 0.79 a | 15.29 ± 1.47 ab | 15.64 ± 0.49 a | 9.55 ± 2.33 a | 104.71 ± 3.94 a |
| Russia NO.8 | 17.93 ± 0.79 d | 17.22 ± 1.47 b | 18.86 ± 0.49 b | 35.64 ± 2.33 c | 197.72 ± 28.67 c |
| Climate types | |||||
| Temperate monsoon | 11.10 ± 0.34 b | 15.58 ± 0.64 a | 22.55 ± 0.21 c | 18.58 ± 1.01 b | 147.26 ± 1.53 b |
| Subtropical monsoon | 8.56 ± 0.56 a | 19.48 ± 1.04 b | 18.15 ± 0.35 b | 12.04 ± 1.65 a | 102.37 ± 2.94 a |
| Plateau Mountain | 8.22 ± 0.79 a | 17.72 ± 1.47 ab | 16.29 ± 0.49 a | 17.82 ± 2.33 b | 149.06 ± 3.53 c |
| ANOVA p-value Main Effects | |||||
| Cultivars | 0.000 *** | 0.011 ** | 0.000 *** | 0.000 *** | 0.000 *** |
| Climate types | 0.000 *** | 0.004 *** | 0.000 *** | NS | 0.000 *** |
| Samples | pH | Organic Matter g/kg | Available P mg/kg | Available K mg/kg | Available Fe mg/kg | Available Ca mg/kg |
|---|---|---|---|---|---|---|
| Baqiao | 7.85 ± 0.01 f | 14.50 ± 0.14 c | 13.90 ± 0.42 a | 237.00 ± 0.00 d | 20.90 ± 0.00 b | 335.00 ± 1.41 b |
| Tianbao | 6.65 ± 0.00 b | 18.50 ± 0.57 d | 40.00 ± 0.85 b | 128.00 ± 0.71 a | 28.10 ± 0.00 e | 392.00 ± 0.71 e |
| Jiangxian | 7.98 ± 0.00 g | 19.30 ± 0.06 e | 71.60 ± 0.90 c | 419.00 ± 1.41 f | 29.70 ± 0.21 f | 333.00 ± 2.12 b |
| Wenchuan | 7.21 ± 0.01 c | 44.10 ± 0.49 h | 542.00 ± 22.63 f | 837.00 ± 2.12 h | 25.50 ± 0.00 d | 427.00 ± 2.83 f |
| Xiehu | 6.61 ± 0.01 a | 13.40 ± 0.14 b | 49.90 ± 2.55 b | 187.00 ± 1.41 c | 54.60 ± 0.07 i | 291.00 ± 1.41 a |
| Tongzhou | 7.77 ± 0.00 e | 35.60 ± 0.21 f | 248.00 ± 4.95 d | 752.00 ± 9.19 g | 34.40 ± 0.00 h | 371.0 ± 02.12 d |
| Qinzhou | 7.98 ± 0.00 g | 19.30 ± 0.07 e | 60.84 ± 2.31 bc | 358.00 ± 0.00 e | 23.00 ± 0.00 c | 344.00 ± 1.41 c |
| Ledu | 8.26 ± 0.01 h | 10.60 ± 0.14 a | 14.35 ± 0.52 a | 173.00 ± 2.12 b | 5.37 ± 0.01 a | 438.00 ± 2.83 g |
| Dalian | 7.43 ± 0.01 d | 42.00 ± 0.35 g | 308.50 ± 13.44 e | 1760.00 ± 3.54 i | 31.80 ± 0.35 g | 461.00 ± 2.12 h |
| Mean | 7.53 | 24.14 | 149.90 | 539.00 | 28.15 | 376.89 |
| Std. | 0.59 | 12.84 | 180.81 | 523.23 | 13.04 | 56.70 |
| CV (%) | 7.90% | 53.18% | 120.62% | 97.07% | 46.30% | 15.05% |
| Quality Parameters | Influencing Factors | Regression Equation | F | Sig. | Modified R2 |
|---|---|---|---|---|---|
| SSC | available P | YSSC = 0.016X1 + 14.488 | 15.731 | 0.005 | 0.648 |
| TA | available P, available K, available Fe, available Ca, pH | YTA = 0.008X1 − 0.008X2 + 0.455X3 + 0.081X4 + 5.709X5 − 76.980 | 10.882 | 0.039 | 0.861 |
| TPC | available Fe, available P, available K | YTPC = −0.009X3 − 0.002X1 + 0.001X2 + 1.409 | 30.542 | 0.001 | 0.917 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, Y.; Huang, J.; Liu, R.; Wang, P.; Liu, P.; Lu, M.; Sun, J. Physicochemical Properties of Geographical Indication (GI) Sweet Cherries in China and Their Influencing Factors of Cultivar, Climate Type, and Soil Condition. Horticulturae 2023, 9, 1118. https://doi.org/10.3390/horticulturae9101118
Nie Y, Huang J, Liu R, Wang P, Liu P, Lu M, Sun J. Physicochemical Properties of Geographical Indication (GI) Sweet Cherries in China and Their Influencing Factors of Cultivar, Climate Type, and Soil Condition. Horticulturae. 2023; 9(10):1118. https://doi.org/10.3390/horticulturae9101118
Chicago/Turabian StyleNie, Ying, Jiazhang Huang, Rui Liu, Pei Wang, Peng Liu, Man Lu, and Junmao Sun. 2023. "Physicochemical Properties of Geographical Indication (GI) Sweet Cherries in China and Their Influencing Factors of Cultivar, Climate Type, and Soil Condition" Horticulturae 9, no. 10: 1118. https://doi.org/10.3390/horticulturae9101118
APA StyleNie, Y., Huang, J., Liu, R., Wang, P., Liu, P., Lu, M., & Sun, J. (2023). Physicochemical Properties of Geographical Indication (GI) Sweet Cherries in China and Their Influencing Factors of Cultivar, Climate Type, and Soil Condition. Horticulturae, 9(10), 1118. https://doi.org/10.3390/horticulturae9101118







