Abstract
The application of garden waste compost teas (CTs) in sustainable agriculture constitutes a biostimulant and environmentally friendly alternative. The purpose of this work was to study the physicochemical properties of three CTs prepared with different brewing processes (CT1, CT2, and CT3) immediately after extraction and six months later to determine whether those properties changed over time and evaluate the effect of CT application on tomato (Solanum lycopersicum L.) plant growth. The brewing process had a significant effect on the extracts’ chemical composition, while long-term storage did not lead to significant differences. The most energy-efficient CT was evaluated in a pot and in vitro assays by measuring plant growth parameters and root traits. CT1 directly supplied to the substrate increased the leaf number, plant height, and dry weight of tomato plants compared to the control and foliar treatments, whereas no significant differences were found among foliar treatments. In terms of the effects of CT application on root development, the results of the in vitro assays showed that CT supply enhanced the primary root length, lateral root number, and root fresh weight while decreasing shoot height and weight in 10-day-old tomato seedlings. From an agronomic standpoint, this study contributes new insights regarding the storage stability of CT and its impact on tomato plant growth.
1. Introduction
Tomato (Solanum lycopersicum L.) is the second most important vegetable crop in the world after potato (Solanum tuberosum L.) and is cultivated for consumption as fresh and processed products. The world production and consumption of tomatoes have increased rapidly over the last 30 years. The current global production rate is around 186.82 million tons of fresh fruit produced on 5.05 million hectares in over 165 countries [1]. Conventional agriculture is typically characterized by the use of a significant amount of synthetic fertilizers, pesticides, and growth regulators, leading to a heavy reliance on non-renewable resources, reduced biodiversity, and chemical residues in food, among others [2]. Thus, the trend toward organic agriculture to avoid the issues caused by conventional agriculture has led to the necessity of finding new environmentally friendly compounds to promote plant growth. Following this line of thought, compost is an organic product that results from a controlled bio-oxidative process that requires proper conditions for obtaining a high-quality humified product, including adequate humidity, aeration, or heterogeneous organic materials [3]. The starting materials can be derived from a variety of sources, including green wastes and manures. Compared to organic compost, compost prepared from green waste seems to pose a lower risk of toxicity, since organic composts contain different compounds or microorganisms such as heavy metals, pollutants, viruses, or fecal coliforms [3,4,5]. Moreover, mature compost could be mixed with running water in 1:5 or 1:10 (v/v) ratios during a specific time varying between 2 and 15 days, resulting in an organic liquid product called compost tea (CT) [6,7]. CTs are mainly composed of soluble nutrients and beneficial compounds and microorganisms (filamentous fungi, oomycetes, actinomycetes, yeasts, and bacteria) that have a synergic effect on suppressing disease and promoting plant growth [8,9,10]. CTs can be used as biostimulant agents for promoting plant growth. However, several authors have described that the physicochemical and microbiological properties of the CTs depend on the characteristics of the starting compost, the compost-to-water ratio, temperature, and aeration [11,12,13].
The application of aerated CTs from organic compost restored soil health and increased plant growth and the yield of red leaf lettuce (Lactuca sativa L.), sweet corn (Zea mays L.), and soybean (Glycine max L.) [14,15]. Following this line of investigation, a positive effect of CT application was observed on pepper (Capsicum annuum L.), potato, and tomato production [7,16,17]. Furthermore, the application of CT increased the yield and improved the quality of the baby spinach (Spinacia oleracea L.) [18]. Nevertheless, in recent years, several researchers have focused their research on the influence of CT applications on root growth and development in different horticultural crops, since the root system plays a relevant role in plant anchorage, metabolites storage, and biosynthesis, as well as water and nutrient uptake. Reeve et al. [19] reported a synergistic effect when a CT was used in combination with an inorganic fertilizer, resulting in a higher wheat (Triticum aestivum L.) root (40–66%) biomass than that observed when the inorganic fertilizer was applied alone. Moreover, CTs stimulate root growth in sweet pepper and pak choi (Brasica rapa var. Chinensis) [12,20]. One of the previous studies focused on the effects and relationships of compost type, aeration, and brewing time on the properties of different CTs [12]. The authors studied aerated and non-aerated CTs prepared from banana (Musa sp.) leaf and lawn clipping composts and showed that the above-stated factors significantly affected the microbial and chemical properties of CTs. Furthermore, Kim et al. [14] also observed that the application of a mixture of an oriental medicinal herb compost and vermicompost tea significantly increased root growth in red leaf lettuce, sweet corn, and soybean. Finally, the total root length and fine roots were higher in lettuce plants, confirming the importance of having a healthy root system for plants to use the water and nutrients efficiently, increasing crop yield [21]. This beneficial effect of compost extracts could be due to the production of auxin or auxin-like components from humic substances, which would promote root growth. Altogether, these findings lead us to the hypothesis that the application of CT obtained from green waste mature compost would improve plant growth by enhancing root growth development. Hence, the main purpose of this work is to study the different physicochemical properties of garden-waste-based CTs in order to assess whether these properties are retained over time and investigate the influence of their application on the plant growth and root development of tomato plants by measuring growth parameters and root traits. These objectives can provide new insights into the questions proposed by Hu et al., namely how to enhance the efficiency, eco-friendliness, and sustainability of fertilization inputs and how to improve the utilization of agricultural waste [22].
2. Materials and Methods
2.1. Compost Tea Preparation, Analytical Characterization, and Selection
The compost was based on green and pruning residues (mainly composed of a mixture of grass cuttings and pruning debris, i.e., leaves and branches of mainly cypress (Cupressus sp.), willow (Salix sp.), and poplar trees (Populus alba L.), reaching a C/N ratio of 30), which were obtained from public gardens of the Province of Salamanca (Spain). The composting process was carried out in a garden center located in Salamanca (40°57′23″ N; 5°41′8″ W; 775 m above sea level) using aerated piles with a length of 15 m, a width of 2 m, and a height of 2 m. The piles were turned twice per week over eight weeks, and once a week during the whole bio-oxidative process. Pile moisture and temperature were controlled weekly, and the composting process lasted 180 days. The main physicochemical characteristics of the compost were as follows: 49% humidity; 1.74 dS/m electrical conductivity (EC); 7.5 pH; 47.5% organic matter (OM); 2% N; 4185 mg/kg P; 12054 mg/kg K; and 10.9% humic acids. Regarding micronutrients, the following contents were identified: 6549 ppm Fe; 57 ppm Zn; 100 ppm B; 286 ppm Mn; 14 ppm Pb; 73 ppm Ni; and Cu, Hg, and Cd were not detected. The whole composition of this compost was previously described by Morales-Corts et al. [23]. Thus, this compost revealed excellent physicochemical characteristics that allow for its use as a substrate, ensuring that the values do not exceed the indicated limits by legislation. Furthermore, the germination index values showed the absence of phytotoxicity [23].
At the composting plant, the compost was mixed with running water in a ratio of 1:5 (v/v) in 1000 L polyethylene non-degradable containers. Water had been previously aerated for 8 h to reduce chloride concentration. To determine the effect of the brewing period and the extraction temperature on the final composition of CT, three different CTs were prepared as follows:
- -
- CT1: the brewing period lasted 5 days, with 5 h agitation per day; temperature: 20 °C;
- -
- CT2: the brewing period lasted 15 days with 5 h agitation per day; temperature: 20 °C;
- -
- CT3: the brewing period lasted 5 days, with 5 h agitation per day; temperature: 15 °C.
The three CTs were tested in order to study the effect of agitation time and temperature on CT characteristics. Thus, CT2 was prepared to study the effect of the brewing and agitation time on CT composition and CT3 was used to analyze the temperature effect.
In all cases, the mixture was aerated by applying circular stirring and making fine bubbles of air with a pump (750 W, 300 rpm). After natural decantation, the liquid was transferred to 2 L PET bottles (6 bottles for each CT) while being filtered through a double-layered cheesecloth. CT bottles were closed, and their contents were analyzed immediately (CT1, CT2, and CT3); six months after storage at room temperature (CT1-6, CT2-6, and CT3-6), the samples were again analyzed in order to investigate whether their characteristics had been retained over time.
The pH and EC were determined by using a CRISON pH meter (Hach, Ames, IA, USA) and a CRISON EC meter (dS/m) (Hach, Ames, IA, USA), respectively. Assimilable nutrient concentrations of NO3−, P2O5, K2O, S, Ca, and Mg were analyzed by using a HANNA HI 993310 photometer (HANNA instruments, Smithfield, RI, USA). Humic acids were determined using the alkali–acid fractionation method following the procedure indicated by Pant et al. [20].
2.2. Pot Assays
A growth test was carried out in 1.5 L pots with ten plants per treatment and replicate. Tomato seeds cv. Marglobe (commercial variety) were germinated in sterile substrates composed of vermiculite. Seedlings with one true leaf were transferred to pots containing blond peat as the substrate. The main characteristics of the substrate were pH 5.8; N 60 mg/L; P2O5 40 mg/L; K2O 50 mg/L; organic matter 85%; and EC 0.1 dS/m. The experiment was carried out in a greenhouse (temperature of 21 °C during the day and 13 °C at night, and 60% relative humidity) for twelve weeks during October, November, and December of 2017 and 2018.
Control plants were irrigated with 60 mL of running water per pot and week, which was previously aerated to reduce the chlorine content. Two doses of CT were used, 40 mL and 60 mL per pot and week. The total volume applied to the different treatments was the same, 60 mL per pot weekly, adding running water to reach this volume when necessary (Table 1). These volumes were applied in two different ways: foliar application (spraying with a COFAN manual device) and root application (directly on the substrate via drenching) in order to evaluate the effects of the manner of application and both dosages. For this assay, CT1 was used since the differences between CT1 and CT2 were not so significant, and the process implies high energy savings (from 5 to 15 days brewing with an agitation of 5 h per day). The employment of CT3 was also discarded in this assay due to the effect of temperature on nutrient extraction. The tested treatments, which were assessed immediately after transplantation, are shown in Table 1. The extract supplies were divided into three weekly applications using water or CT depending on the treatment. Plants were watered via subirrigation when needed, applying the same volume to all the pots via drenching. The application of subirrigation was only applied three times during the whole experiment ensuring that more than two days had passed after CT application to avoid dilution effect. No fertilizers, pesticides, or phytosanitary products were applied to the plants. The experiment was conducted in a completely randomized design. Ten plants per treatment in two independent replicates (season 2017 and 2018) were considered (n = 20).

Table 1.
Description of the different treatments used in the pot assays.
To evaluate the effect of CT application, the following plant growth parameters were determined: the height of the plants (cm) with a meter stick; the number of leaves; chlorophyll content with a chlorophyll meter Minolta SPAD-502 (Spectrum Technologies, Aurora, IL, USA); the dry weight (DW) of the root system and aerial part (before weighting the plants, they were dried in a P-Selecta-210 (J.P. Selecta, Barcelona, Spain) oven at 65 °C for 48 h). Weights were determined by using a Sartorius BL150S precision balance (Sartorius, Göttingen, Germany).
2.3. Determination of Root Development
The results obtained in Section 2.2 led to an in vitro study of the shoot and root development induced by the application of CT in tomato seedlings. To this end, tomato cv. Marglobe seeds were sterilized following the protocol described by González-Hernández [24]. Briefly, the seeds were cleaned with sodium hypochlorite (75% v/v) containing 0.1% of Tween 20 (Sigma-Aldrich, San Luis, MO, USA) for 8 min and then washed with sterilized distilled water for 5 min four times. Then, they were transferred to agar plates (1.5% w/v), and they were kept in the dark for 72 h. The homogeneously germinated seeds were transferred to plates 15 cm in diameter containing two treatments: (1) a sterilized modified Hoagland solution medium composed of 400 ppm KNO3, 820 ppm Ca(NO3)2, 400 ppm MgSO4 7H2O, 2.68 ppm H3BO4, 0.07 mL/L H3PO4, 2.20 ppm ZnSO4 7H2O, 0.09 ppm MoO3, 0.11 ppm CuSO4 5H2O, 9.15 ppm MnSO4, 67 ppm Sequestrene, and agar (1.5% w/v) (control) or (2) six-month-old filtered compost tea (CT1-6). The filtration procedure was carried out with a 0.22 µm filter in order to remove the microorganisms. The final dilution of CT was 1:5 (CT:water, v:v). The pH of the different media was adjusted to compost tea pH. Seedlings were grown in the treatment plates for 7 days, and the plates were placed in a growth chamber at 26/18 °C temperature (day/night), 16/8 h photoperiod, and 60% relative humidity, maintaining the roots in the dark. After this time, the primary root (PR) length, lateral root (LR) number, and shoot height were determined through images obtained with ImageJ software (National Institutes of Health, Bethesda, MD, USA). Finally, fresh and dry weights (FW and DW) were measured with an analytical balance. These measurements were carried out in at least eight seedlings of each treatment of three independent replicates.
2.4. Statistical Analyses
Statistical analyses were carried out via one-way analysis of variance to analyze CT properties and plant parameters with Statgraphics Centurion XVIII software (Statistical Graphics Corp., Rockville, MD, USA). The results are presented as means with standard errors, and Tukey’s honest significant difference (HSD) post hoc test with a 95% confidence interval (p < 0.05) was performed to compare the individual means of the different treatments. Pairwise comparisons were performed between CT1-6 treatment and control plants grown under in vitro conditions using Student’s t-test (p < 0.05).
3. Results
3.1. Chemical Properties of Compost Teas
As shown in Table 2, the pH of the different CTs ranged between 7.11 and 7.33, indicating that they were neutral-basic in all cases. No significant differences in EC were observed between the different CTs (1.2–1.48). NO3− and K2O were the most important nutrients identified in garden-waste-based-CTs, ranging from 2741 for CT3 to 4700 ppm NO3− for CT1-6, and 2782 ppm K2O for CT3-6 and 4123 ppm in CT2. No differences in nutrient content, except for P2O5, were found between the CTs that underwent 5 days of brewing and those subjected to 15 days brewing. The total quantity of the extracted assimilable elements was superior for CT1 and CT2 prepared at 20 °C compared with CT3 produced at 15 °C, except for Ca. As regards K2O and Mg nutrients, significant differences were found between CT1 and CT2, compared with CT3. Differences in chemical composition 6 months later in relation to recent CTs were only found for NO3− in the case of CT1 and CT1-6, and for Ca in CT2 and CT2-6 as well as CT3 and CT3-6. Clear differences were observed for the extracted humic acids between the different extraction temperatures, which were markedly higher at 20 °C. Finally, it should be noted that micronutrients were not analyzed in these CTs since, in the previous analysis of compost and CT, phytotoxicity was not detected. Thus, the chemical sensitivity analysis ensured that Zn, Mn, Cu, Pb, Hg, Cr, Ni, and Cd levels were clearly below the allowable limits according to legislation [7,23].

Table 2.
Physicochemical composition of CTs.
Considering the nutritional values, humic content, and the obtaining time, which represent considerable energy savings, CT1 (based on the 5-day brewing period) was selected for subsequent experiments, and it was used in pot and in vitro assays.
3.2. Pot Experiment
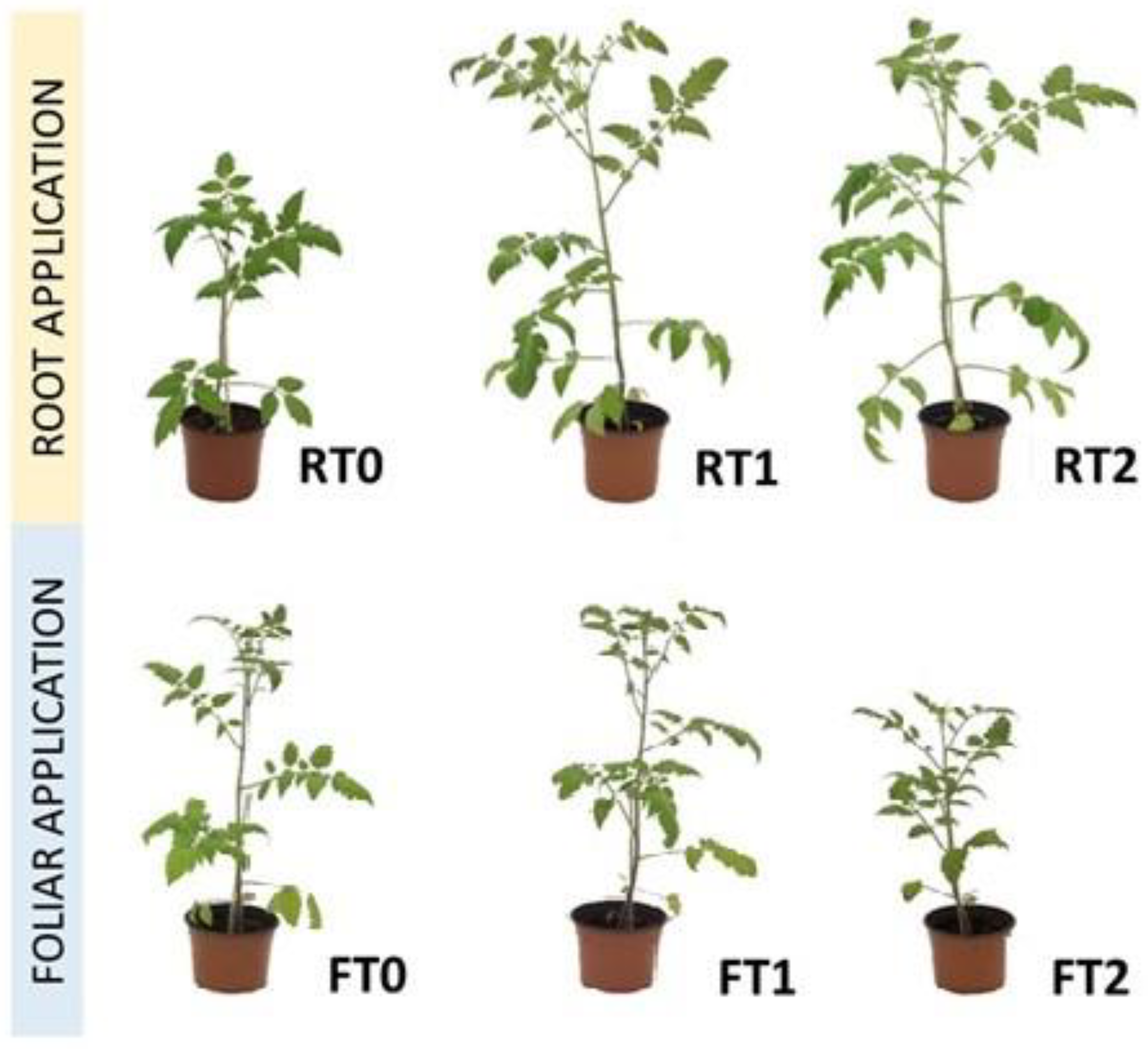
The treatments with CT supplied directly to the substrate (RT1 and RT2) led to an increase in the number of leaves; plant height; and aerial, root, and total dry weights compared with the control RT0 and foliar treatments (FT0, FT1, and FT2) (Table 3 and Figure 1). The dosages of the different treatments are described in Table 1. Nevertheless, no significant differences were found among the different foliar treatments except for plant height, which was higher in FT1 than in FT2. The chlorophyll content was not significantly different among the treatments. The increase in the weekly quantity of the applied CT did not lead to improvements in any parameter, even causing a significant decrease in plant height with foliar treatment.

Table 3.
Growth parameters of tomato seedlings with CT supply via irrigation or foliar application.
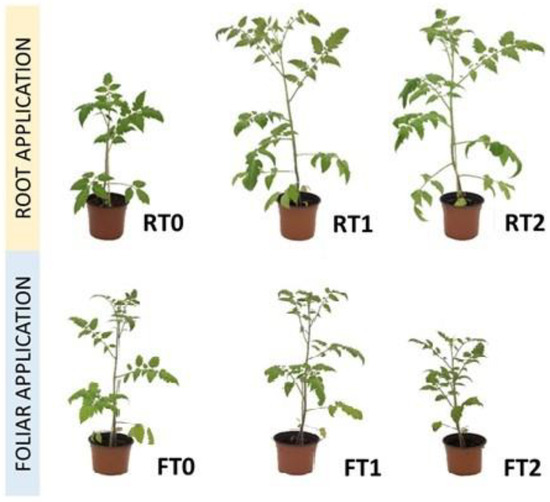
Figure 1.
Plant development under the different root and foliar treatments.
3.3. In Vitro CT Effect on Root and Shoot Development
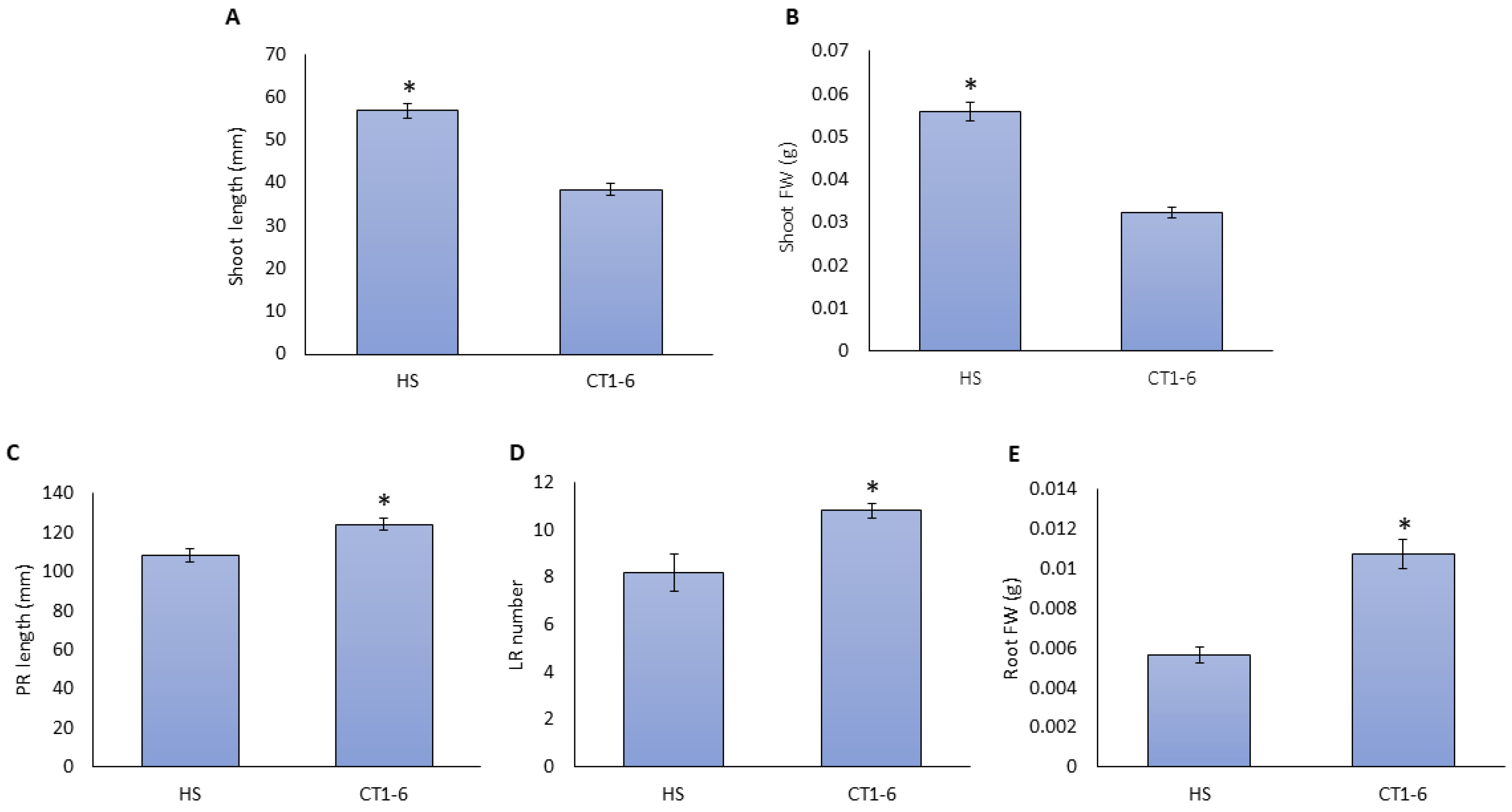
To confirm the observed root and shoot development in response to CT1-6, different plates containing filtered CT1-6 were evaluated (Figure 2). CT supply decreased shoot height and fresh weight (Figure 2A,B) and increased PR length, LR number, and root FW (Figure 2C–E) in 10-day-old tomato seedlings compared to the Hoagland solution (HS).
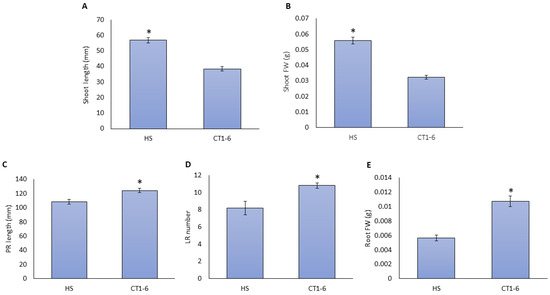
Figure 2.
Changes in root and shoot development with the addition of CT. The seedlings of tomato plants were grown with a tomato Hoagland solution (HS) or filtered compost tea (CT1-6). Shoot length (A), shoot FW (B), the primary root (PR) length (C), the lateral root (LR) number (D), and root FW (E) were measured. The presented data are the mean of at least three independent experiments ± standard error (SE). The asterisk indicates statistically significant differences among treatments as determined by Student’s t-test (p < 0.05).
4. Discussion
CTs are considered good alternatives in sustainable agriculture since they have been shown to influence plant growth and protection. The quality of composting products determines their use and is affected by the waste material and process parameters [25]. The concentration of extractable compounds in CT seems to depend on compost substrates, composting methods, and extraction techniques [13]. Thus, this work mainly involves the study of the physicochemical properties of different CTs in order to assess whether these properties are preserved over time. Then, the CT with the most optimal balance between energy savings and mineral content was chosen for the assessment of tomato growth using a pot experiment, which was supported with an in vitro experiment to confirm the better root development induced by CT application.
When applied to crops, CT provides nutrients, organic matter, and a wide range of microorganisms that influence plant growth, resistance, and soil health [7]. These positive effects on plants could be direct or indirect through chemical and/or biological mechanisms [26]. As mentioned above, this work is mainly focused on the direct mode, which involves increased nutrient supply and increased activity of microbial bioactive compounds, including humic acids. Thus, the physicochemical properties of the different CTs were first assessed. Extract analyses revealed a relevant concentration of nitrogen (N), which was higher 6 months later (CT1-6) compared with the analysis of CT immediately after brewing (CT1). This fact could be due to the conversion of NH4+ to NO3−. The content of K2O was also high, especially in the CTs brewed at 20 °C (CT1 and CT2). However, the other nutrients (P2O5, SO42−, Ca, and Mg) were maintained or slightly decreased, indicating that the storage time did not affect these CT properties much. The decrease in Ca in CT1-6, CT2-6, and CT3-6 can be due to the precipitation phenomena. Furthermore, the enhancement in the brewing time from 5 to 15 days resulted in a significant increase in P2O5 levels (CT1 versus CT2) due to further solubilization caused by a higher brewing time and agitation, while the other nutrients and humic acids were almost similar. Fluctuation in pH values was low, and the EC and nitrate content followed a similar trend, as previously shown by Kiss et al. [27]. Moreover, the EC slightly increased with the brewing time and after 6 months of CT preparation, but it was especially noted in CTs subjected to higher temperatures during the brewing time (CT1 and CT2). In accordance with these results, other authors found that an increase in extraction time leads to a higher EC value [14,25]. This effect could be explained by the increase in the total dissolved solids resulting from the increase in extraction time [27]. It should be noted that aeration and temperature, together with the stability of the composts from which CTs are produced, contribute to greater phytotoxicity [28]. Nevertheless, the CT of this study was prepared from gardening wastes, and the obtained compost was a stable product since no phytotoxicity was observed [23], and it presented stability over time despite microorganism enrichment.
CTs supplied as the sole nutrient source can provide the proper nutrients necessary for maintaining plant growth and development, which not only depends on the application rate and frequency, concentration, and crop species but also on the compost source, the brewing method, and the applied dilution [26]. Several works have studied CT application through either soil drenching or foliar application, showing its positive effect on plant growth and quality. Previous studies carried out by the authors revealed that an aerated CT induced a positive effect on the growth of different plant species when it was applied via soil drenching. For example, CT supply increased shoot and root DW, chlorophyll content, and stem diameter in tomato plants [7]; reduced the days to flowering after transplantation and enhanced the stem diameter and the mean of fruit weight of pepper [16]; boosted the yield, shoot number, tuber weight and tuber size and advanced potato sprouting [17]. In accordance with these results, Pant et al. [20] revealed that vermicompost tea induced pak choi growth and yield. In the present work, CTs directly supplied to the substrate increased the leaf number; plant height; and aerial, root, and total DW compared to the control, while foliar application did not yield any positive and/or negative growth effect. On the other hand, the effect of CTs applied as foliar spray and via drenching on kohlrabi and lettuce cultivation was also evaluated by Pane et al. [29], who showed that both treatments considerably improved crop yields. Moreover, Mahmoud et al. [30] indicated that nutrient availability was greater as a result of foliar application than drench application, leading to the hypothesis that CT increases the length of time that stomata stay open, thus reducing leaf loss. This was supported by Kaya et al. [31], indicating that CTs enhanced the cellular membrane’s permeability to nutrients. Here, it could be suggested that stomata blockage might occurred, thus inhibiting the uptake of nutrients via foliar application since no dilution of CT was carried out before application, and the volume was too high to be evaluated as a biostimulant. Therefore, this effect highlights the relevance of the dilution and applied volume of CT, especially in foliar application. However, this requires further experimentation.
Interestingly, applying CT directly to the substrate doubled the root’s and aerial part’s DW. This led to an in vitro study of the shoot and root development induced by the application of CT in tomato seedlings. The CT was filtered before application to remove microorganisms and determine whether growth promotion is mainly due to microorganisms since it is already known that CT contains different metabolites or different species of the genera Penicillium, Trichoderma, Bacillus, Aspergillus, Rhizobacteria, Enterobacter, or Pseudomonas spp., among others, which could stimulate plant growth [11,32,33,34]. CT supply led to an increase in PR length, LR number, and root FW, while it decreased shoot height and shoot FW in 10-day-old tomato seedlings. The promotion of root development has been previously linked to better nutrient uptake by CT-treated plants than by those treated with mineral fertilizers [16,35], which is mainly related to the water-extractable nutrients and other metabolites such as humic acids or other biostimulants. The high content of humic acids could explain why CT1-6 induced a higher rate of root development than the Hoagland solution. Keeling et al. [36] identified different low-molecular-weight organic compounds that could play a role in plant growth. Moreover, Spaccini et al. [37] found different bioactive compounds of microbial origin in aerated CTs. Several metabolites such as gibberellins, indoleacetic acid, and cytokinins, which are involved in plant growth, have been previously identified in CTs [7,38,39]. It is already known that the application of humic acids extracted from vermicompost induces the metabolic pathways related to plant stress response and cellular growth in sugarcane [40]. Humic acids induced phenolic and flavonoid accumulation, improving Cichorium intybus yield [41], which are present in plants and their derived products and provide antioxidant effects [42]. In addition, these compounds have been previously found to have an auxin-like activity, inducing nitrate metabolism and therefore improving plant growth [43,44,45,46]. Plant growth is enhanced using humic acids by reinforcing nutrient uptake and extending LR due to the induction of ATPase activity in the plasma membrane [47]. Humic-like substances from CTs enhanced growth and increased chlorophyll content in cucumber plants [48]. In addition, an increase in melon biomass was observed when treated with CTs carrying auxin- and cytokinin-like compounds [49]. Altogether, the findings indicate that there is a greater impact on roots, resulting in increased root hair proliferation and root initiation [50]. This suggests that during the first days, CT induced root development instead of aerial part growth, and this promoting effect is mainly due to the chemical properties of CT because no microorganisms were present in the media. In addition, Olaetxea et al. [51] indicated that the shoot-promoting effect of sedimentary humic acids is dependent on their ability to increase root hydraulic conductivity through signaling pathways related to abscisic acid (ABA). They also suggested that humic acids have a physical action in roots as well, resulting from the transient mild stress caused by humic acids. This mild stress could explain the detrimental effect observed in shoots in ten-day-old tomato plants grown under in vitro conditions. Further research into the biochemical properties of CTs is required to determine the most predominant compounds and elucidate how they mediate plant growth responses.
Compost tea constitutes an excellent alternative source of liquid organic fertilizers for horticultural and agricultural use, and it is an effective substitute for synthetic fertilizers. By using CTs more frequently, horticultural supply chains may become more productive and provide higher quality, since this leads to less agricultural waste, mineral fertilization, plant fungicide application, and soil fumigation, and the outcomes may be better integrated into industrial agricultural production systems [52,53]. In addition, on-farm composting meets the demands of the bioeconomy by improving agricultural waste or biomass unsuitable for energy generation and resolving the challenges associated with the disposal of agricultural waste [54,55].
5. Conclusions
In the current work, we examined the physicochemical characteristics of three garden-waste-based CTs prepared with different brewing times and temperatures and investigated how these properties were preserved over time. In terms of the sample with optimal balance between energy savings and mineral content, the CT prepared with a brewing period of 5 days, an agitation of 5 h per day, and a temperature of 20 °C was the most energy-saving alternative to be used in tomato growth in the pot experiment. CT directly supplied to the substrate increased the growth parameters of tomato plants compared to the control and foliar treatments, but no differences were found among the different foliar treatments. Moreover, CT supply enhanced root traits while decreasing shoot height and weight, which could be related to the transient mild stress caused by humic acids. Thus, we conclude that CTs are very stable over time, and their application reinforces tomato root growth at the initial developmental stages, thereby reducing the application of synthetic fertilizers.
Author Contributions
Conceptualization, A.I.G.-H., M.Á.G.-S., R.P.-S. and M.R.M.-C.; methodology, A.I.G.-H., M.Á.G.-S. and M.R.M.-C.; software, A.I.G.-H. and M.R.M.-C.; validation, A.I.G.-H. and M.R.M.-C.; investigation, A.I.G.-H., M.Á.G.-S. and M.R.M.-C.; data curation, A.I.G.-H., R.P.-S. and M.R.M.-C.; writing—original draft preparation, A.I.G.-H., M.Á.G.-S., R.P.-S. and M.R.M.-C.; writing—review and editing, A.I.G.-H., M.Á.G.-S., R.P.-S. and M.R.M.-C.; project administration, A.I.G.-H. and M.R.M.-C.; funding acquisition, M.R.M.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad de Salamanca, grant number USAL2015-10.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food and Agriculture Organization (FAO). Faostat: Agriculture Data. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 15 October 2022).
- Pimentel, D.; Hepperly, P.; Hanson, J.; Douds, D.; Seidel, R. Environmental, energetic, and economic comparisons of organic and conventional farming systems. BioScience 2005, 55, 573–582. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Benito, M.; Masaguer, A.; De Antonio, R.; Moliner, A. Use of pruning waste compost as a component in soilless growing media. Bioresour. Technol. 2005, 96, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.M.L.; Bertoncini, I.B.; Abreu-Junior, C.H. Composting sewage sludge with green waste from tree pruning. Sci. Agric. 2015, 72, 432–439. [Google Scholar] [CrossRef]
- Al-Dahmani, J.H.; Abbasi, P.A.; Miller, S.A.; Hoitink, H.A.J. Suppression of bacterial spot of tomato with foliar sprays of compost extracts under greenhouse and field conditions. Plant Dis. 2003, 87, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Morales-Corts, M.R.; Pérez-Sánchez, R.; Gómez-Sánchez, M.A. Efficiency of garden waste compost teas on tomato growth and its suppressiveness against soilborne pathogens. Sci. Agric. 2018, 75, 400–409. [Google Scholar] [CrossRef]
- Castaño, R.; Borrero, C.; Trillas, M.I.; Avilés, M. Selection of biological control agents against tomato Fusarium wilt and evaluation in greenhouse conditions of two selected agents in three growing media. BioControl 2013, 58, 105–116. [Google Scholar] [CrossRef]
- De Corato, U. Agricultural waste recycling in horticultural intensive farming systems by on-farm composting and compost-based tea application improves soil quality and plant health: A review under the perspective of a circular economy. Sci. Total Environ. 2020, 738, 139840. [Google Scholar] [CrossRef]
- Zaccardelli, M.; Sorrentino, R.; Caputo, M.; Scotti, R.; De Falco, E.; Pane, C. Stepwise-selected Bacillus amyloliquefaciens and B. subtilis strains from composted aromatic plant waste able to control soil-borne diseases. Agriculture 2020, 10, 30. [Google Scholar] [CrossRef]
- Scheuerell, S.J.; Mahaffee, W.F. Compost tea: Principles and prospects for plant disease control. Compost. Sci. Util. 2002, 10, 313–338. [Google Scholar] [CrossRef]
- Martin, C.C.G.; Dorinvil, W.; Brathwaite, R.A.I.; Ramsubhag, A. Effects and relationships of compost type, aeration and brewing time on compost tea properties, efficacy against Pythium ultimum, phytotoxicity and potential as a nutrient amendment for seedling production. Biol. Agric. Hortic. 2012, 28, 185–205. [Google Scholar] [CrossRef]
- Islam, M.K.; Yaseen, T.; Traversa, A.; Ben Kheder, M.; Brunetti, G.; Cocozza, C. Effects of the main extraction parameters on chemical and microbial characteristics of compost tea. Waste Manag. 2016, 52, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Shim, C.K.; Kim, Y.K.; Hong, S.J.; Park, J.H.; Han, E.J.; Kim, J.H.; Kim, S.C. Effect of aerated compost tea on the growth promotion of lettuce, soybean, and sweet corn in organic cultivation. Plant Pathol. J. 2015, 31, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Giller, K.E.; Hijbeek, R.; Andersson, J.A.; Sumberg, J. Regenerative Agriculture: An agronomic perspective. Outlook Agric. 2021, 50, 13–25. [Google Scholar] [CrossRef] [PubMed]
- González-Hernández, A.I.; Suárez-Fernández, M.B.; Pérez-Sánchez, R.; Gómez-Sánchez, M.Á.; Morales-Corts, M.R. Compost tea induces growth and resistance against Rhizoctonia solani and Phytophthora capsici in pepper. Agronomy 2021, 11, 781. [Google Scholar] [CrossRef]
- González-Hernández, A.I.; Pérez-Sánchez, R.; Plaza, J.; Morales-Corts, R. Compost tea as a sustainable alternative to promote plant growth and resistance against Rhizoctonia solani in potato plants. Sci. Hortic. 2022, 300, 111090. [Google Scholar] [CrossRef]
- Ros, M.; Hurtado-Navarro, M.; Giménez, A.; Fernández, J.A.; Egea-Gilabert, C.; Lozano-Pastor, P.; Pascual, J.A. Spraying agro-industrial compost tea on baby spinach crops: Evaluation of yield, plant quality and soil health in field experiments. Agronomy 2020, 10, 440. [Google Scholar] [CrossRef]
- Reeve, J.R.; Carpenter-Boggs, L.; Reganold, J.P.; York, A.L.; Brinton, W.F. Influence of biodynamic preparations on compost development and resultant compost extracts on wheat seedling growth. Bioresour. Technol. 2010, 101, 5658–5666. [Google Scholar] [CrossRef]
- Pant, A.P.; Radovich, T.J.K.; Hue, N.V.; Paull, R.E. Biochemical properties of compost tea associated with compost quality and effects on pak choi growth. Sci. Hortic. 2012, 148, 138–146. [Google Scholar] [CrossRef]
- Giménez, A.; Fernández, J.A.; Pascual, J.A.; Ros, M.; Egea-Gilabert, C. Application of directly brewed compost extract improves yield and quality in baby leaf lettuce grown hydroponically. Agronomy 2020, 10, 370. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, T.; Guo, Y.; Wang, M.; Brachhold, K.; Chu, C.; Hanson, A.; Kumar, S.; Lin, R.; Long, W.; et al. 100 essential questions for the future of agriculture. Mod. Agric. 2023, 1, 4–12. [Google Scholar] [CrossRef]
- Morales-Corts, M.R.; Gómez-Sánchez, M.A.; Pérez-Sánchez, R. Evaluation of green/pruning wastes compost and vermicompost, slumgum compost and their mixes as growing media for horticultural production. Sci. Hort. 2014, 172, 155–160. [Google Scholar] [CrossRef]
- González-Hernández, A.I.; Scalschi, L.; García-Agustín, P.; Camañes, G. Exogenous carbon compounds modulate tomato root development. Plants 2020, 9, 837. [Google Scholar] [CrossRef] [PubMed]
- Milinković, M.; Lalević, B.; Jovičić-Petrović, J.; Golubović-Ćurguz, V.; Kljujev, I.; Raičević, V. Biopotential of compost and compost products derived from horticultural waste—Effect on plant growth and plant pathogens’ suppression. Process. Saf. Environ. Prot. 2019, 121, 299–306. [Google Scholar] [CrossRef]
- Eudoxie, G.; Martin, M. Compost Tea Quality and Fertility. In Organic Fertilizers—History, Production and Applications; Larramendy, M., Soloneski, S., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Kiss, N.E.; Gorliczay, E.; Nagy, P.T.; Tamás, J. Effect of compost/water ratio on some main parameter of compost solutions. Acta Agrar. Debreceniensis 2021, 1, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Carballo, T.; Gil, M.V.; Calvo, L.F.; Morán, A. The influence of aeration system, temperature and compost origin on the phytotoxicity of compost tea. Compost Sci. Util. 2009, 17, 127–139. [Google Scholar] [CrossRef]
- Pane, C.; Palese, A.M.; Celano, G.; Zaccardelli, M. Effects of compost tea treatments on productivity of lettuce and kohlrabi systems under organic cropping management. Ital. J. Agron. 2014, 9, 153–156. [Google Scholar] [CrossRef]
- Mahmoud, E.; El-Gizawy, E.; Geries, L. Effect of compost extract, N2-fixing bacteria and nitrogen levels applications on soil properties and onion crop. Arch. Agron. Soil Sci. 2014, 61, 185–201. [Google Scholar] [CrossRef]
- Kaya, M.; Atak, M.; Khawar, K.M.; Çiftçi, C.Y.; Özcan, S. Effect of pre-sowing seed treatment with zinc and foliar spray of humic acids on yield of common bean (Phaseolus vulgaris L.). Int. J. Agri. Biol. 2005, 7, 875–878. [Google Scholar]
- Mahmoud, E.K.; Salem, H.A. The compost qualityand used as a growing media. J. Soil. Sci. Agric. Eng. 2005, 30, 3469–3477. [Google Scholar] [CrossRef]
- Phae, C.G.; Shoda, M. Expression of the suppresive effects of Bacillus subtilis on phytopathogens in inoculated compost. J. Ferment. Bioeng. 1990, 70, 409–414. [Google Scholar] [CrossRef]
- Sylvia, E.W. The effect of compost extract on the yield of strawberries and severity of Botrytis cinerea. J. Sustain. Agr. 2004, 25, 57–68. [Google Scholar] [CrossRef]
- Ingham, E.R. The Compost Tea Brewing Manual, 5th ed.; Soil Food International Inc.: Corvallis, OR, USA, 2005. [Google Scholar]
- Keeling, A.A.; McCallum, K.R.; Beckwith, C.P. Mature green waste compost enhances growth and nitrogen uptake in wheat (Triticum aestivum L.) and oilseed rape (Brassica napus L.) through the action of water-extractable factors. Bioresour. Technol. 2003, 90, 127–132. [Google Scholar] [CrossRef]
- Spaccini, R.; Baiano, S.; Gigliotti, G.; Piccolo, A. Molecular characterization of a compost and its water-soluble fractions. J. Agric. Food Chem. 2008, 56, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Pizzeghello, D.; Baglieri, A.; Cadili, V.; Tambone, F.; Gennari, M.; Nardi, S. Humic-like substances from agro-industrial residues affect growth and nitrogen assimilation in maize (Zea mays L.) plantlets. J. Geochem. Explor. 2013, 129, 103–111. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, S.N.; Wong, W.S.; Ng, C.Y.L.; Teo, C.H.; Ge, L.; Chen, X.; Yong, J.W.H. Mass spectrometric evidence for the occurrence of plant growth promoting cytokinins in vermicompost tea. Biol. Fertil. Soils 2014, 50, 401–403. [Google Scholar] [CrossRef]
- Othibeng, K.; Nephali, L.; Ramabulana, A.T.; Steenkamp, P.; Petras, D.; Kang, K.B.; Opperman, H.; Huyser, J.; Tugizimana, F. A metabolic choreography of maize plants treated with a humic substance-based biostimulant under normal and starved conditions. Metabolites 2021, 11, 403. [Google Scholar] [CrossRef]
- Gholami, H.; Saharkhiz, M.J.; Fard, F.R.; Ghani, A.; Nadaf, F. Humic acid and vermicompost increased bioactive components, antioxidant activity and herb yield of Chicory (Cichorium intybus L.). Biocatal. Agric. Biotechnol. 2018, 14, 286–292. [Google Scholar] [CrossRef]
- Čakar, U.; Petrović, A.; Janković, M.; Pejin, B.; Vajs, V.; Čakar, M.; Djordjević, B. Differentiation of wines made from berry and drupe fruits according to their phenolic profiles. Eur. J. Hortic. Sci. 2018, 83, 49–61. [Google Scholar] [CrossRef]
- Cacco, G.; Attinà, E.; Gelsomino, A.; Sidari, M. Effect of nitrate and humic substances of different molecular size on kinetic parameters of nitrate uptake in wheat seedlings. J. Plant Nutr. Soil. Sci. 2000, 163, 313–320. [Google Scholar] [CrossRef]
- Eyheraguibel, B.; Silvestre, J.; Morard, P. Effects of humic substances derived from organic waste enhancement on the growth and mineral nutrition of maize. Bioresour. Technol. 2008, 99, 4206–4212. [Google Scholar] [CrossRef] [PubMed]
- Muscolo, A.; Sidari, M. Carboxyl and phenolic humic fractions affect callus growth and metabolism. Soil Sci. Soc. Am. J. 2009, 73, 1119–1129. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef]
- Jindo, K.; Lopes-Olivares, F.; da Paixao Malcher, D.J.; Sánchez-Monedero, M.A.; Kempenaar, C.; Canellas, L.P. From lab to field: Role of humic substances under open-field and greenhouse conditions as biostimulant and biocontrol agent. Front. Plant Sci. 2020, 11, 426. [Google Scholar] [CrossRef]
- Xu, D.B.; Wang, Q.J.; Wu, Y.C.; Yu, G.H.; Sheng, Q.R.; Huang, Q.W. Humic-like substances from different compost extracts could significantly promote cucumber growth. Pedosphere 2012, 22, 815–824. [Google Scholar] [CrossRef]
- Bernal-Vicente, A.; Ros, M.; Tittarelli, F.; Intrigliolo, F.; Pascual, J.A. Citrus compost and its water extract for cultivation of melon plants in greenhouse nurseries. Bioresour. Technol. 2008, 99, 8722–8728. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Aviad, T. Effects of humic substances on plant growth. In Humic Substances in Soil and Crop Sciences; MacCarthy, P., Clapp, C., Malcolm, R., Bloom, P.R., Eds.; SSSA: Madison, WI, USA, 1990; pp. 161–186. [Google Scholar]
- Olaetxea, M.; Mora, V.; Calderin-García, A.; Azevedo-Santos, L.; Baigorri, R.; Fuentes, M.; Garnica, M.; Louro-Berbara, R.L.; Zamarreño, A.M.; García-Mina, J.M. Root-shoot signaling crosstalk involved in the shoot growth promoting action of rhizospheric humic acids. Plant Signal. Behav. 2016, 11, e1161878. [Google Scholar] [CrossRef] [PubMed]
- Pilla, N.; Tranchida-Lombardo, V.; Gabrielli, P.; Aguzzi, A.; Caputo, M.; Lucarini, M.; Durazzo, A.; Zaccardelli, M. Effect of compost tea in horticulture. Horticulturae 2023, 9, 984. [Google Scholar] [CrossRef]
- Gamage, A.; Gangahagedara, R.; Gamage, J.; Jauasinghe, N.; Kodikara, N.; Suraweera, P.; Merah, O. Role of organic farming for achieving sustainability in agriculture. Farming Syst. 2023, 1, 100005. [Google Scholar] [CrossRef]
- De Corato, U. Compost and Compost Tea from On-Farm Composted Agro-Wastes Improve the Sustainability of Horticultural Organic Cropping Systems. In Agri-Based Bioeconomy; CRC Press: Boca Raton, FL, USA, 2021; pp. 143–162. [Google Scholar]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Plaza-Úbeda, J.A.; Camacho-Ferre, F. The management of agricultural waste biomass in the framework of circular economy and bioeconomy: An opportunity for greenhouse agriculture in southeast Spain. Agronomy 2020, 10, 489. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).