Role of Humic Acid on Inducing Salt Tolerance of Ivy Geranium (Pelargonium peltatum L.) Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Layout
2.2. Vegetative Growth
2.3. Flowering Attributes
2.4. Physiological Growth Characteristics
2.5. Statistical Analysis
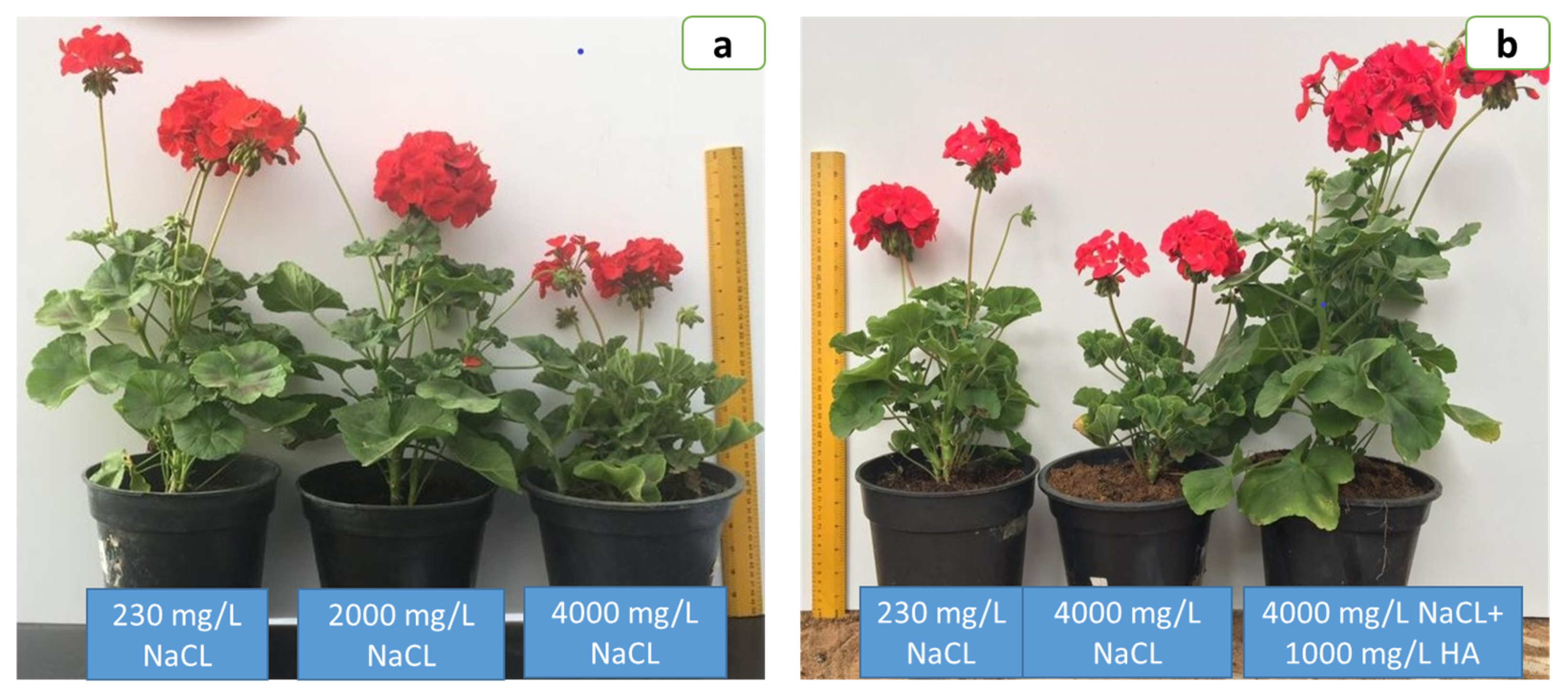
3. Results
3.1. Growth Attributes
3.2. Flowering Attributes
3.3. Chlorophyll
3.4. Proline
3.5. Ion Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Keles, B.; Ertürk, Y. Advantages of microorganism containing biological fertilizers and evaluation of their use in ornamental plants. Int. J. Agric. For. Life Sci. 2021, 5, 189–197. Available online: http://dergipark.gov.tr/ijafls (accessed on 23 July 2021).
- Rocha, C.S.; Rocha, D.C.; Kochi, L.Y.; Carneiro, D.N.M.; Dos Reis, M.V.; Gomes, M.P. Phytoremediation by ornamental plants: A beautiful and ecological alternative. Environ. Sci. Pollut. Res. 2022, 29, 3336–3354. [Google Scholar] [CrossRef] [PubMed]
- Chandler, S.F.; Sanchez, C. Genetic modification; the development of transgenic ornamental plant varieties. Plant Biotech. J. 2012, 10, 891–903. [Google Scholar] [CrossRef]
- Azadi, P.; Bagheri, H.; Nalousi, A.M.; Nazari, F.; Chandler, S.F. Current status and biotechnological advances in genetic engineering of ornamental plants. Biotechnol. Adv. 2016, 34, 1073–1090. [Google Scholar] [CrossRef]
- Mamba, B.; Wahome, P.K. Propagation of geranium (Pelargonium hortorum) using different rooting medium components. Am.-Eurasian J. Agric. Environ. Sci. 2010, 7, 497–500. [Google Scholar] [CrossRef]
- Taylor, M.D.; Nelson, P.V.; Frantz, J.M.; Rufty, T.W. Phosphorus deficiency in Pelargonium: Effects on nitrate and ammonium uptake and acidity generation. J. Plant Nut. 2010, 33, 701–712. [Google Scholar] [CrossRef]
- Krishnaraj, S.; Dan, T.; Saxena, P. A fragrant solution to soil remediation. Inter. J. Phytoremed. 2000, 2, 117–132. [Google Scholar] [CrossRef]
- Fornes, F.; Belda, R.M.; Carrion, C.; Noguera, V.; García-Agustín, P.; Abad, M. Pre-conditioning ornamental plants to drought by means of saline water irrigation as related to salinity tolerance. Sci. Hort. 2007, 113, 52–59. [Google Scholar] [CrossRef]
- Al-Omran, A.M.; Aly, A.A.; Halim, M.K. Status and New Development on the Use of Brackish Water for Agricultural Production in the Near East Saudi Arabia Country Report; United Nations, Food and Agriculture (FAO), Regional Office for the Near East (RNF): Rome, Italy, 2012; Available online: https://www.fao.org/neareast/events/view/ru/c/249312/2012 (accessed on 23 July 2021).
- Niu, G.; Rodriguez, D. Response of bedding plants to saline water irrigation. HortSci 2010, 45, 628–636. [Google Scholar] [CrossRef]
- Elhindi, K.M.; Al-Mana, F.A.; Algahtani, A.M.; Alotaibi, M.A. Effect of irrigation with saline magnetized water and different soil amendments on growth and flower production of Calendula officinalis L. plants. Saudi J. Bio. Sci. 2020, 27, 3072–3078. [Google Scholar] [CrossRef]
- Amarin, R.; Kafawin, O.; Ayad, J.; Al-Zyoud, F.; Ghidan, A. Effect of saline water irrigation and growing media on growth, physiological and mineral parameters of clove pink Dianthus caryophyllus. Jor. J. Agric. Sci. 2020, 16, 55–62. [Google Scholar] [CrossRef]
- Anny Mrudhula, K.; Venkata Subbaiah, G.; Sambaiah, A.; Sunil Kumar, M. Performance of flower and medicinal plants with saline irrigation water through drip system. Pharma Innov. J. 2021, 10, 1514–1519. Available online: https://www.thepharmajournal.com/archives/2021/vol10issue8/PartU/10-7-439-247.pdf (accessed on 23 July 2021).
- Banon, D.; Lorente, B.; Ortuño, M.F.; Bañón, S.; Sanchez-Blanco, M.J.; Alarcon, J. Effects of saline irrigation on the physiology and ornamental quality of Euphorbia Ascot Rainbow and its relationship with salinity indexes based on the bulk electrical conductivity. Sci. Hort. 2022, 305, 111406. [Google Scholar] [CrossRef]
- Kim, H.; Fonseca, J.M.; Choi, J.; Kubota, C.; Kwon, D.Y. Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.; AL-Huqail, A.A. Sustainable biochar and/or melatonin improve salinity tolerance in borage plants by modulating osmotic adjustment, antioxidants, and ion homeostasis. Plants 2022, 11, 765. [Google Scholar] [CrossRef]
- Farouk, S.; Arafa, S.A. Mitigation of salinity stress in canola plants by sodium nitroprusside application. Span. J. Agric. Res. 2018, 16, e0802. [Google Scholar] [CrossRef]
- Helaly, M.N.; Farouk, S.; Arafa, S.A.; Amhimmid, N.B.I.A. Inducing salinity tolerance of rosemary (Rosmarinus officinalis L.) plants by chitosan or zeolite application. Asian J. Adv. Agric. Res. 2018, 5, 1–20. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Amri, S.M. Exogenous zinc forms counteract NaCl-induced damage by regulating the antioxidant system, osmotic adjustment substances, and ions in canola (Brassica napus L. cv. Pactol) plants. J. Soil Sci. Plant Nutr. 2019, 19, 887–899. [Google Scholar] [CrossRef]
- Sofy, M.R.; Elhindi, K.M.; Farouk, S.; Alotaibi, M.A. Zinc and paclobutrazol mediated regulation of growth, upregulating antioxidant aptitude and plant productivity of pea plants under salinity. Plants 2020, 9, 1197. [Google Scholar] [CrossRef]
- El-Banna, M.F.; AL-Huqail, A.A.; Farouk, S.; Belal, B.E.A.; El-kenawy, M.A.; Abd El-Khalek, A. Morpho-physiological and anatomical alterations of salt-affected thompson seedless grapevine (Vitis vinifera L.) to brassinolide spraying. Horticulturae 2022, 8, 568. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L. Physiological responses to humic substances as plant growth promoter. Chem. Biol. Technol. Agric. 2014, 1, 3. [Google Scholar] [CrossRef]
- Olaetxea, M.; De Hita, D.; García, A.C.; Fuentes, M.; Baigorri, R.; Mora, V.; García Mina, J.M. Hypothetical framework integrating the main mechanism involved in the promoting action of rhizospherichumic substances on plant root- and shoot-growth. App. Soil Ecol. 2018, 123, 521–537. [Google Scholar] [CrossRef]
- Baigorri, R.; Fuentes, M.; Gonzalez-Gaitano, G.; Garcia-Mina, J.M. Simultaneous presence of diverse molecular patterns in humic substances in solution. J. Phys. Chem. B 2007, 111, 10577–10582. [Google Scholar] [CrossRef] [PubMed]
- Baigorri, R.; Fuentes, M.; Gonzalez-Gaitano, G.; García-Mina, J.M. Analysis of molecular aggregation in humic substances in solution. Colloids Surf. A 2007, 302, 301–306. [Google Scholar] [CrossRef]
- Nyoman Rupiasih, N.; Vidyasagar, P.B. A review: Compositions, structures, properties and applications of humic substances. J. Adv. Inter. Sci. Technol. 2005, 8, 16–25. Available online: https://www.researchgate.net/publication/236347209_A_Review_Compositions_Structures_Properties_and_Applications_of_Humic_Substances (accessed on 23 July 2021).
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzedbased products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18. [Google Scholar] [CrossRef]
- Amir, H.S.; Hani, A. Effect of ethanol and humic acid foliar spraying on morphological traits, photosynthetic pigments and quality and quantity of essential oil content of Dracocephalummoldavica L. Iran. J. Plant Physiol. 2017, 8, 2299–2306. [Google Scholar]
- Nofal, E.M.S.; Menesi, F.A.; EL-Bably, S.Z.; Abd EL Rahman, M. Effect of NPK and humic acid on growth, flowering and chemical composition of (blue sake) Erantheumumpulchellum Andrews plant. App. Ecol. Environ. Res. 2020, 18, 2555–2567. [Google Scholar] [CrossRef]
- Hammam, K.A.; AwadAlla, S.S.S.; Noreldin, T. Response of growth, yield and essential oil of geranium plants to surface irrigation and humic acid treatments. Asian Plant Res. J. 2021, 7, 39–56. [Google Scholar] [CrossRef]
- Hagagg, L.F.; Shahin, M.F.M.; Mustafa, N.S.; Merwad, M.A.; Khalil, F.H. Influence of using humic acid during full bloom and fruit set stages on productivity and fruit quality of ‘Kalamata’ olive trees. J. App. Sci. Res. 2013, 9, 2287–2292. Available online: https://www.aensiweb.com/old/jasr/jasr/2013/2287-2292.pdf (accessed on 1 March 2013).
- Bohme, M.; Thilua, H. Influence of mineral and organic treatments in the rhizosphere on the growth of tomato plants. Acta Hortic. 1997, 450, 161–168. [Google Scholar] [CrossRef]
- Ennab, H.A.; Mohamed, A.H.; El-Hoseiny, H.M.; Omar, A.A.; Hassan, I.F.; Gaballah, M.S.; Khalil, S.E.; Mira, A.M.; Abd El-Khalek, A.F.; Alam-Eldein, S.M. Humic acid improves the resilience to salinity stress of drip-irrigated Mexican lime trees in saline clay soils. Agronomy 2023, 13, 1680. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Cooper, T.G. The Tools of Biochemistry; A Wiley-Interscience Publication John Wiley and Sons: New York, NY, USA, 1977. [Google Scholar]
- Costa, J.M.; Ortuno, M.F.; Chaves, M.M. Deficit irrigation as a strategy to save water: Physiology and potential application to horticulture. J. Integr. Plant Biol. 2007, 49, 1421–1434. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; Pascale, S.D.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Ennab, H.; Alam-Eldein, S.M. Biostimulants foliar application to improve growth, yield, and fruit quality of ‘Valencia’ orange trees under deficit irrigation conditions. J. Am. Pom. Soc. 2020, 74, 118–134. Available online: https://www.pubhort.org/aps/74/v74_n3_a1.htm (accessed on 1 July 2020).
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Hashem, A.; Abd Allah, E.F.; Ahmad, P. Arbuscular mycorrhiza in crop improvement under environmental stress. In Emerging Technologies and Management of Crop Stress Tolerance; Ahmad, P., Rasool, S., Eds.; Academic Press: Cambridge, MA, USA; London, UK, 2014; pp. 69–95. [Google Scholar] [CrossRef]
- Kozminska, A.; Al Hassan, M.; Kumar, D.; Oprica, L.; Martinelli, F.; Grigore, M.N.; Vicente, O.; Boscaiu, M. Characterizing the effects of salt stress in Calendula officinalis L. J. Appl. Bot. Food Qual. 2017, 90, 323–329. [Google Scholar] [CrossRef]
- Li, G.; Evens, M.R. Humic acid substrate treatments and foliar spray application effects on root growth and development of seedlings. HortSci 2000, 35, 434. [Google Scholar] [CrossRef]
- Baldotto, M.A.; Baldotto, L.E.B. Gladiolus development in response to bulb treatment with different concentrations of humic acids. Rev. Ceres Viçosa 2013, 60, 138–142. [Google Scholar] [CrossRef]
- Aydine, A.; Kant, C.; Turan, M. Humic acid application alleviates salinity stress of bean (Phaseolus vulgaris L.) plants decreasing membrane leakage. Afr. J. Agric. Res. 2012, 7, 1073–1086. [Google Scholar] [CrossRef]
- Can, W.; Kafi, M.; Babalar, M.; Xia, Y. Effect of humic acid on plant growth, nutrient uptake, and postharvest life of gerbera. J. Plant Nutr. 2008, 31, 2155–2167. [Google Scholar] [CrossRef]
- Li, H.; Kong, F.; Tang, T.; Luo, Y.; Gao, H.; Xu, J.; Xing, G.; Li, L. Physiological and transcriptomic analyses revealed that humic acids improve low-temperature stress tolerance in zucchini (Cucurbita pepo L.) seedlings. Plants 2023, 12, 548. [Google Scholar] [CrossRef]
- Trevisan, S.; Botton, A.; Vaccaro, S.; Vezzaro, A.; Quaggiotti, S.; Nardi, S. Humic substances affect Arabidopsis physiology altering the expression of genes involved in primary metabolism, growth and development. Envir. Exp. Bot. 2011, 74, 45–55. [Google Scholar] [CrossRef]
- Savy, D.; Canellas, L.; Vinci, G.; Cozzolino, V.; Piccolo, A. Humic-like water-soluble lignins from giant reed (Arundo donax L.) display hormone-like activity on plant growth. J. Plant Growth Regul. 2017, 36, 995–1001. [Google Scholar] [CrossRef]
- Xiong, H.; Lu, D.; Li, Z.; Wu, J.; Ning, X.; Lin, W.; Bai, Z.; Zheng, C.; Sun, Y.; Chi, W.; et al. The DELLA-ABI4-HY5 module integrates light and gibberellin signals to regulate hypocotyl elongation. Plant Comm. 2023, 4, 100597. [Google Scholar] [CrossRef]
- Zandonadi, D.; Santos, M.; Dobbss, L.; Fb, O.; Canellas, L.; Binzel, M.; Façanha, A. Nitric oxide mediates humic acids-induced root development and plasma membrane H+-ATPase activation. Planta 2010, 231, 1025–1036. [Google Scholar] [CrossRef]
- Jindo, K.; Canellas, L.P.; Albacete, A.; Figueiredo dos Santos, L.; Frinhani Rocha, R.L.; Carvalho Baia, D.; Oliveira Aguiar Canellas, N.; Goron, T.L.; Olivares, F.L. Interaction between humic substances and plant hormones for phosphorous acquisition. Agronomy 2020, 10, 640. [Google Scholar] [CrossRef]
- Ashour, H.A.; El-Attar, A.B. Morphological and physiological responses of silvery (Leucophyllum frutescens) to water deficient and irrigation water salinity stresses. J. Hort. Sci. Ornamen. Plants 2017, 9, 1–16. [Google Scholar]
- Kucukahmetler, O. The effects of salinity on yield and quality of ornamental plants and cut flowers. Acta Hortic. 2002, 573, 407–414. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.A.; Qasim, M.; Ahmad, R. Growth, yield and quality of Rosa hybrida L. as influenced by NaCl salinity. J. Ornam. Hortic. Plants 2013, 3, 143–153. Available online: https://jornamental.rasht.iau.ir/article_513390_8543f77e6319e13c7dee80836060c0e7.pdf (accessed on 23 July 2021).
- Lyengar, E.R.; Reddy, M.P. Photosynthesis in highly salt tolerant plants. In Handbook of Photosynthesis; Pesserkali, M., Ed.; Marshal Dekar: Baten Rose, LA, USA, 1996; pp. 897–909. [Google Scholar]
- Sairam, R.K.; Tyagi, A. Physiology and molecular biology of salinity stress tolerance in plants. Current Sci. 2004, 86, 408–421. [Google Scholar] [CrossRef]
- Rogers, H.J. From models to ornamentals: How is flower senescence regulated? Plant Mol. Biol. 2013, 82, 563–574. [Google Scholar] [CrossRef]
- Atiyeh, R.M.; Edwards, C.A.; Metzger, J.D.; Lee, S.; Arancon, N.Q. The influence of humic acids derived from earthworm-processed organic wastes on plant growth. Biores. Technol. 2002, 84, 7–14. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Lee, S.; Edwards, C.A.; Atiyeh, R. Effect of humic acids derived from cattle, food and paper-waste vermicompost on growth of green house plants. Pedobiologia 2003, 47, 741–744. [Google Scholar] [CrossRef]
- Turan, S.; Tripathy, B.C. Salt-stress induced modulation of chlorophyll biosynthesis during de-etiolation of rice seedlings. Physiol. Plant. 2015, 153, 477–491. [Google Scholar] [CrossRef]
- Siddiqui, M.; Alamri, S.; Al-Khaishany, M.; Khan, M.; Al-Amri, A.; Ali, H.; Alaraidh, I.; Alsahli, A. Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int. J. Mol. Sci. 2019, 20, 353. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Ghamdi, A.A.M. Sodium nitroprusside application enhances drought tolerance in marjoram herb by promoting chlorophyll biosynthesis, sustaining ion homeostasis, and enhancing osmotic adjustment capacity. Arab. J. Geosci. 2021, 14, 430. [Google Scholar] [CrossRef]
- Farouk, S.; AL-Huqail, A.A.; El-Gamal, S.M.A. Potential role of biochar and silicon in improving physio-biochemical and yield characteristics of borage plants under different irrigation regimes. Plants 2023, 12, 1605. [Google Scholar] [CrossRef]
- Hassanein, R.A.; El Khawas, S.A.; Khafaga, H.S.; Abd El-Nabe, A.S.; Abd Elrady, A.S. Amelioration of drought stress on physiological performance of pearl millet (Pennisetum americanum) plant grown under saline condition using potassium humate and silicon source. Egypt J. Exp. Biol. 2017, 13, 57–68. [Google Scholar] [CrossRef]
- El-Hoseiny, H.M.; Helaly, M.N.; Elsheery, N.I.; Alam-Eldein, S.M. Humic acid and boron to minimize the incidence of alternate bearing and improve the productivity and fruit quality of mango trees. HortSci 2020, 55, 1026–1037. [Google Scholar] [CrossRef]
- Olaetxea, M.; Mora, V.; Bacaicoa, E.; Baigorri, R.; Garnica, M.; Fuentes, M.; Zamarreño, A.M.; Spíchal, L.; García-Mina, J.M. Root ABA and H+ -ATPase are key players in the root and shoot growth-promoting action of humic acids. Plant Direct. 2019, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, M.; Kafi, M.; Fang, P. Photosynthetic activity and N metabolism of lettuce as affected by humic acid. Inter. J. Veg. Sci. 2012, 18, 182–189. [Google Scholar] [CrossRef]
- Berbara, R.L.; García, A.C. Humic substances and plant defense metabolism. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Parvaiz, A., Mohd, R.W., Eds.; Springer: New York, NY, USA, 2014; pp. 297–319. [Google Scholar]
- Hammam, K.A.; AwadAlla, S.S.S. Mitigation of saline water stress on french lavender (Lavandula dentata L.) plants. J. Hort. Sci. Ornamen. Plants 2020, 12, 8–16. [Google Scholar]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Amini, S.; Ghobadi, C.; Yamchi, A. Proline accumulation and osmotic stress: An overview of P5CS gene in plants. J. Plant Mol. Biol. Breed. 2015, 3, 44–55. [Google Scholar] [CrossRef]
- Bellinger, Y.; Bensaoud, A.; Larher, F. Physiological significance of proline accumulation, a trait of use to breeding for stress tolerance. In Physiology–Breeding of Winter Cereals for Stressed Mediterranean Environment; Acevedo, E., Conesa, A.P., Monneveux, P., Srivastava, J.P., Eds.; INRA: Paris, France, 1991; pp. 449–458. [Google Scholar]
- Fichman, Y.; Gerdes, S.Y.; Kovács, H.; Szabados, L.; Zilberstein, A.; Csonka, L.N. Evolution of proline biosynthesis: Enzymology, bioinformatics, genetics, and transcriptional regulation. Biol. Rev. 2015, 90, 1065–1099. [Google Scholar] [CrossRef]
- Nardi, S.; Muscolo, A.; Vaccaro, S.; Baiano, S.; Spaccini, R.; Piccolo, A. Relationship between molecular characteristics of soil humic fractions and glycolytic pathway and krebs cycle in maize seedlings. Soil Biol. Biochem. 2007, 39, 3138–3146. [Google Scholar] [CrossRef]
- Boehme, M.; Schevtschenko, J.; Pinker, I. Iron supply of cucumbers in substrate culture with humate. Acta Hort. 2005, 41, 329–335. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Gilliham, M. Salinity tolerance of crops–what is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Benito, B.; Haro, R.; Amtmann, A.; Cuin, T.A.; Dreyer, I. The twins K+ and Na+ in plants. J. Plant Physiol. 2014, 171, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M. Interactions between Ca, Mg, Na and K: Alleviation of toxicity in saline solutions. Plant Soil 2012, 352, 353–362. [Google Scholar] [CrossRef]
- Sahar, M.Z.; El-Quesni, F.E.M.; Mazhar, A.A.M. Influence of potassium humate on growth and chemical constituents of Thuja orientalis L. seedlings. Ozean J. App. Sci. 2009, 2, 73–78, CorpusID: 99039569. Available online: https://api.semanticscholar.org/ (accessed on 1 November 2015).
- Malcum, R.L.; Vaughum, D. Humic substances and phosphatase activities in plant tissues. Soil Biochem. 1999, 11, 253–259. [Google Scholar] [CrossRef]
- Mesut, C.K.; Onder, T.; Metin, T.; Burcu, T. Phosphorus, and humic acid application alleviate salinity stress of pepper seedling. Afr. J. Biotech. 2010, 9, 5845–5851. Available online: http://www.academicjournals.org/AJB (accessed on 21 August 2013).
- Nabati, D.A. Responses of two grass species to plant regulators, fertilizer N, chelated Fe, salinity and water stress. Ph.D. Thesis, Crop and Soil Environmental Science Deptrtment, Virginia Tech, Blacksburg, VA, USA, 1994. [Google Scholar]
- Demir, D.; Günes, A.; Inal, A.; Alpaslan, M. Effects of humic acids on the yield and mineral nutrition of cucumber (Cucumis sativus L.) grown with different salinity levels. Acta Hort. 2014, 492, 95–104. [Google Scholar] [CrossRef]
- Vaughan, D.; Macdonald, I.R. Effects of humic acid on protein synthesis and ion uptake in beet. J. Exp. Bot. 2005, 22, 400–410. [Google Scholar] [CrossRef]
- Fernandez, R.E.; Benlock, M.; Barranco, D.; Duenas, A.; Ganan, J.A.G. Response of olive trees to foliar application of humic substances extracted from leonardite. Sci. Hortic. 1996, 66, 191–200. [Google Scholar] [CrossRef]
- Schmidt, W.; Santi, S.; Pinton, R.; Varanini, Z. Water-extractable humic substances alter root development and epidermal cell pattern in Arabidopsis. Plant Soil 2007, 300, 259–267. [Google Scholar] [CrossRef]
- Vaccaro, S.; Ertani, A.; Nebbioso, A.; Muscolo, A.; Quaggiotti, S.; Piccolo, A.; Nardi, S. Humic substances stimulate maize nitrogen assimilation and amino acid metabolism at physiological and molecular level. Chem. Biol. Technol. Agric. 2015, 2, 5. [Google Scholar] [CrossRef]
| Treatments | Stem Length (cm) | Stem Diameter (mm) | Leaf Number plant−1 | Leaf Area (cm2) | Shoot FW (g) | Shoot Dry Weight (g) |
|---|---|---|---|---|---|---|
| NaCl salinity (mg/L) | ||||||
| 230 tap water (S0) | 32.2 ± 2.8a | 29.1 ± 1.1a | 55.2 ± 4.3a | 655.2 ± 51.4a | 195.0 ± 8.2a | 92.1 ± 6.8a |
| 2000 (S1) | 15.8 ± 0.9b | 20.7 ± 1.1b | 45.4 ± 3.0b | 322.0 ± 26.0b | 166.0 ± 8.0b | 39.4 ± 2.7b |
| 4000 (S2) | 12.4 ± 0.9b | 18.8 ± 1.1c | 21.5 ± 1.7c | 253.4 ± 17.4b | 116.8 ± 3.5c | 26.5 ± 1.8c |
| ANOVA p values | *** | *** | *** | *** | *** | |
| HA (mg/L) | ||||||
| 0 (H0) | 13.5 ± 1.7b | 17.8 ± 1.6d | 25.6 ± 3.0c | 290.8 ± 43.6c | 130.0 ± 10.1d | 38.5 ± 7.4c |
| 500 (H1) | 22.9 ± 3.8a | 24.1 ± 1.6b | 47.7 ± 6.4a | 441.2 ± 75.9ab | 169.3 ± 12.3b | 58.6 ± 11.5b |
| 1000 (H2) | 26.8 ± 4.2a | 28.1 ± 1.5a | 50.6 ± 5.8a | 528.5 ± 93.0a | 186.2 ± 14.9a | 70.0 ± 13.5a |
| 2000 (H3) | 17.4 ± 2.4b | 21.4 ± 1.6c | 38.7 ± 4.9b | 380.2 ± 44.3bc | 151.5 ± 9.4c | 43.5± 7.7c |
| ANOVA p values | *** | *** | *** | *** | *** | *** |
| Interaction effects | ||||||
| S0H0 | 20.0 ± 0.5d | 24.0 ± 0.5de | 34.0 ± 0.5d | 451.7 ± 18.8cd | 167.3 ± 5.8d | 67.6 ± 1.4c |
| S0H1 | 38.4 ± 0.2b | 30.3 ± 0.8b | 68.3 ± 0.8a | 719.3 ± 15.3b | 205.0 ± 4.0b | 104.0 ± 4.0b |
| S0H2 | 43.6 ± 0.7a | 34.0 ± 0.5a | 68.6 ± 0.0a | 893.8 ± 39.0a | 233.0 ± 4.5a | 123.0 ± 0.5a |
| S0H3 | 27.0 ± 0.6c | 28.0 ± 0.5bc | 50.0 ± 0.5bc | 556.0 ± 3.53bc | 174.6 ± 6.8d | 74.0 ± 0.5c |
| S1H0 | 12.0 ± 0.5fg | 16.3 ± 0.3hi | 29.0 ± 0.5de | 266.4 ± 0.8ef | 123.6± 0.8e | 30.0 ± 0.5fg |
| S1H1 | 16.6 ± 0.7e | 22.0 ± 0.5ef | 51.0 ± 1.1bc | 318.6 ± 103.3d–f | 180.6 ± 1.2cd | 42.0 ± 0.5e |
| S1H2 | 20.2 ± 0.4d | 26.3 ± 0.8cd | 54.3 ± 2.4b | 393.0 ± 2.9c–e | 194.6 ± 2.6bc | 53.0 ± 1.1d |
| S1H3 | 14.3 ± 0.6ef | 18.3 ± 0.3gh | 47.3 ± 0.8c | 310.2 ± 8.2d–f | 165.0 ± 0.5d | 32.6 ± 0.3f |
| S2H0 | 8.5 ± 0.5h | 13.3 ± 0.3i | 14.0 ± 0.5g | 154.4 ± 0.8f | 99.0 ± 0.5f | 18.0 ± 0.5h |
| S2H1 | 13.9 ± 0.5e–g | 20.0 ± 0.5fg | 24.0 ± 0.5ef | 285.7 ± 0.5d–f | 122.3 ± 0.8e | 30.0 ± 0.5fg |
| S2H2 | 16.5 ± 0.5e | 24.0 ± 0.5de | 29.0 ± 0.5de | 298.8 ± 0.5d–f | 131.0 ± 0.5e | 34.0 ± 0.5f |
| S2H3 | 11.0 ± 0.5gh | 18.0 ± 0.5gh | 19.0 ± 0.5fg | 274.6 ± 0.5ef | 115.0 ± 1.0ef | 24.0 ± 0.5gh |
| ANOVA p values | *** | *** | *** | *** | *** | *** |
| Treatments | Inflorescence No/Plant | Inflorescence Stalk Length (cm) | Inflorescence Diameter (mm) | Inflorescence Fresh Weight (g) | Inflorescence Dry Weight (g) |
|---|---|---|---|---|---|
| NaCl salinity (mg/L) | |||||
| 230 tap water (S0) | 68.0 ± 2.8a | 23.5 ± 1.9a | 50.0 ± 2.4a | 57.5 ± 6.4a | 27.5±2.9a |
| 2000 (S1) | 43.0 ± 1.6b | 10.0 ± 0.7b | 36.1 ± 2.2b | 33.7 ± 1.8b | 8.3 ± 0.5b |
| 4000 (S2) | 34.4 ± 2.2c | 7.7 ± 0.7b | 18.5 ± 1.4c | 22.5 ± 1.8c | 7.0 ± 0.7b |
| ANOVA p values | *** | *** | *** | *** | *** |
| HA (mg/L) | |||||
| 0 (H0) | 37.4 ± 4.2d | 9.8 ± 1.8c | 25.1 ± 3.9d | 23.7 ± 2.4c | 8.9 ± 1.6c |
| 500 (H1) | 52.0 ± 5.7b | 15.2 ± 2.7a | 37.7 ± 4.8b | 40.7 ± 6.0b | 15.4 ± 3.6b |
| 1000 (H2) | 56.7 ± 4.9a | 18.8 ± 3.3b | 43.4 ± 5.3a | 54.0 ± 8.8a | 21.1 ± 5.1a |
| 2000 (H3) | 47.8 ± 5.4c | 11.0 ± 1.9c | 33.3 ± 4.0c | 33.1 ± 3.9bc | 11.6 ± 2.8bc |
| ANOVA p values | *** | *** | *** | *** | *** |
| Interaction effects | |||||
| S0H0 | 52.6 ± 1.2c | 17.3 ± 0.3c | 38.6 ± 0.3d | 30.6 ± 0.3de | 15.3 ± 0.3d |
| S0H1 | 74.3 ± 0.3ab | 26.0 ± 1.0b | 53.3 ± 0.8b | 64.0 ± 1.5b | 30.0 ± 1.1b |
| S0H2 | 76.3 ± 0.8a | 32.3 ± 0.6a | 61.0 ± 0.5a | 88.0 ± 5.1a | 41.6 ± 1.7a |
| S0H3 | 69.0 ± 0.5b | 18.3 ± 1.8c | 47.0 ± 0.5c | 47.3 ± 1.4c | 23.0 ± 0.5c |
| S1H0 | 35.2 ± 2.1g | 7.0 ± 0.5f | 25.3 ± 0.3f | 26.0 ± 1.1d-f | 6.8 ± 0.1gh |
| S1H1 | 45.0 ± 0.5de | 11.0 ± 0.5de | 40.0 ± 0.5d | 34.0 ± 0.5d | 8.6 ± 0.3e-g |
| S1H2 | 50.0 ± 0.5cd | 13.3 ± 0.3d | 45.3 ± 0.3c | 43.0 ± 0.5c | 11.0 ± 0.5e |
| S1H3 | 42.0 ± 0.5ef | 8.8 ± 0.6ef | 34.0 ± 0.5e | 32.0 ± 0.5de | 7.0 ± 0.5f–h |
| S2H0 | 24.4 ± 2.1h | 5.3 ± 0.3f | 11.3 ± 0.3h | 14.6 ± 0.3g | 4.8 ± 0.1h |
| S2H1 | 36.6 ± 2.8fg | 8.6 ± 0.3ef | 20.0 ± 0.5g | 24.3 ± 1.6ef | 7.6 ± 0.6e–h |
| S2H2 | 44.0 ± 0.5de | 11.0 ± 0.5de | 24.0 ± 0.5f | 31.0 ± 0.0de | 10.6 ± 0.3ef |
| S2H3 | 32.6 ± 0.3g | 6.0 ± 0.5f | 19.0 ± 1.1g | 20.0 ± 0.5fg | 5.0 ± 0.5gh |
| ANOVA p values | *** | *** | *** | *** | *** |
| Treatments | Chlorophyll | Proline |
|---|---|---|
| NaCl salinity (mg/L) | ||
| 230 tap water (S0) | 22.0 ± 1.9a | 147.4 ± 5.9c |
| 2000 (S1) | 15.5 ± 0.7b | 228.0 ± 3.7b |
| 4000 (S2) | 13.9 ± 0.6b | 246.7 ± 3.3a |
| ANOVA p values | *** | *** |
| HA (mg/L) | ||
| 0 (H0) | 12.5 ± 0.5c | 188.4 ± 17.7d |
| 500 (H1) | 18.1 ± 1.5b | 213.7 ± 15.1b |
| 1000 (H2) | 22.6 ± 2.2a | 226.1 ± 13.5a |
| 2000 (H3) | 15.4 ± 0.8bc | 201.3 ± 14.5c |
| ANOVA p values | *** | *** |
| Interaction effects | ||
| S0H0 | 14.0 ± 0.5ef | 118.7 ± 0.5j |
| S0H1 | 24.0 ± 0.5b | 154.0 ± 0.9h |
| S0H2 | 31.6 ± 0.8a | 172.9 ± 0.6g |
| S0H3 | 18.6 ± 0.3c | 144.2 ± 0.5i |
| S1H0 | 12.6 ± 0.3fg | 212.3 ± 0.6f |
| S1H1 | 16.0 ± 0.0de | 234.5 ± 0.6d |
| S1H2 | 19.3 ± 0.3c | 244.3 ± 0.6c |
| S1H3 | 14.3 ± 0.3ef | 221.0 ± 0.4e |
| S2H0 | 11.0 ± 0.5g | 234.3 ± 0.4d |
| S2H1 | 14.3 ± 0.3ef | 252.7 ± 0.7b |
| S2H2 | 17.0 ± 0.5cd | 261.1 ± 0.7a |
| S2H3 | 13.3 ± 0.3fg | 238.8 ± 0.1cd |
| ANOVA p values | *** | *** |
| Treatments | Nitrogen | Phosphorus | Potassium | Calcium | Magnesium |
|---|---|---|---|---|---|
| NaCl salinity (mg/L) | |||||
| 230 tap water (S0) | 372.2 ± 12.1a | 81.7 ± 3.0a | 502.1 ± 6.8a | 48.7 ± 3.0a | 47.6 ± 3.0a |
| 2000 (S1) | 264.1 ± 4.8b | 76.7 ± 2.9b | 398.0 ± 15.8b | 33.1 ± 2.1b | 32.1 ± 2.1b |
| 4000 (S2) | 168.0 ± 9.2c | 40.2 ± 1.7c | 259.7 ± 8.5c | 26.7 ± 2.4c | 25.7 ± 2.5c |
| ANOVA p values | *** | *** | *** | *** | *** |
| HA (mg/L) | |||||
| 0 (H0) | 243.9 ± 32.5c | 55.7 ± 5.4c | 340.5 ± 38.0c | 23.1 ± 3.3c | 22.0 ± 3.3c |
| 500 (H1) | 289.7 ± 32.2a | 73.0 ± 6.9a | 410.8 ± 35.5a | 38.9 ± 3.1b | 42.3 ± 5.0a |
| 1000 (H2) | 285.1 ± 33.5ab | 75.9 ± 7.3a | 424.6 ± 36.4a | 45.6 ± 4.5a | 40.5 ± 2.5a |
| 2000 (H3) | 253.7 ± 22.9bc | 60.3 ± 6.4b | 370.6 ± 31.8b | 37.0 ± 2.1b | 35.7 ± 2.2b |
| ANOVA p values | *** | *** | *** | *** | *** |
| Interaction effects | |||||
| S0H0 | 330.9 ± 2.7c | 69.4 ± 0.6e | 475.9 ± 0.37c | 35.5 ± 0.3e | 34.2 ± 0.5e |
| S0H1 | 408.3 ± 0.6b | 88.8 ± 0.3b | 513.5 ± 0.6b | 50.9 ± 0.4b | 62.2 ± 0.5a |
| S0H2 | 416.3 ± 0.6a | 94.4 ± 0.4a | 533.3 ± 0.6a | 63.2 ± 0.5a | 50.0 ± 0.4b |
| S0H3 | 333.3 ± 0.4c | 74.3 ± 0.5d | 485.8 ± 0.2c | 45.2 ± 0.5c | 44.2 ± 0.5c |
| S1H0 | 284.3 ± 0.5d | 63.2 ± 0.6f | 333.4 ± 0.6f | 21.1 ± 0.4g | 20.1 ± 0.4h |
| S1H1 | 274.4 ± 0.7e | 85.2 ± 0.5c | 444.4 ± 0.6d | 35.9 ± 0.3e | 34.9 ± 0.3e |
| S1H2 | 244.3 ± 0.5g | 86.5 ± 0.7bc | 454.6 ± 0.7d | 40.2 ± 0.6d | 39.3 ± 0.6d |
| S1H3 | 253.4 ± 0.6f | 71.9 ± 0.1de | 359.8 ± 7.4e | 35.0 ± 0.4e | 34.0 ± 0.4e |
| S2H0 | 116.5 ± 0.6k | 34.4 ± 0.6h | 212.3 ± 0.6i | 12.6 ± 0.7h | 11.6 ± 0.7i |
| S2H1 | 186.5 ± 0.5i | 45.1± 0.4g | 274.4 ± 0.6h | 30.0 ± 0.3f | 29.9 ± 0.1fg |
| S2H2 | 194.5 ± 0.6h | 46.7 ± 0.6g | 285.9 ± 0.3g | 33.4 ± 0.5e | 32.4 ± 0.5ef |
| S2H3 | 174.6 ± 0.6j | 34.6 ± 0.5h | 266.2 ± 0.5h | 30.9 ± 0.1f | 29.0 ± 0.3g |
| ANOVA p values | *** | *** | *** | *** | *** |
| Treatments | Manganese | Copper | Iron | Zinc | Sodium |
|---|---|---|---|---|---|
| NaCl salinity (mg/L) | |||||
| 230 tap water (S0) | 0.95 ± 0.00a | 0.18 ± 0.01a | 1.55 ± 0.03b | 0.37 ± 0.00a | 47.8 ± 1.3c |
| 2000 (S1) | 0.82 ± 0.02b | 0.20 ± 0.00b | 2.24 ± 0.10a | 0.30 ± 0.02b | 67.5 ± 3.2b |
| 4000 (S2) | 0.60 ± 0.02c | 0.12 ± 0.00c | 2.25 ± 0.07a | 0.24 ± 0.01c | 77.1 ± 3.0a |
| ANOVA p values | *** | *** | *** | *** | *** |
| HA (mg/L) | |||||
| 0 (H0) | 0.71 ± 0.06c | 0.13 ± 0.01c | 1.65 ± 0.05c | 0.24 ± 0.02d | 77.9 ± 5.8a |
| 500 (H1) | 0.81 ± 0.05b | 0.17 ± 0.01b | 2.07 ± 0.14b | 0.34 ± 0.01b | 60.4 ± 3.9b |
| 1000 (H2) | 0.87 ± 0.04a | 0.20 ± 0.01a | 2.28 ± 0.13a | 0.37 ± 0.01a | 55.7 ± 3.5c |
| 2000 (H3) | 0.78 ± 0.04b | 0.16 ± 0.01b | 2.06 ± 0.14b | 0.27 ± 0.02c | 62.4 ± 4.0b |
| ANOVA p values | *** | *** | *** | *** | *** |
| Interaction effects | |||||
| S0H0 | 0.92± 0.00ab | 0.14 ± 0.00d | 1.47 ± 0.04g | 0.34 ± 0.00c | 55.1± 0.0g |
| S0H1 | 0.97 ± 0.00a | 0.20 ± 0.00b | 1.51 ± 0.06g | 0.38 ± 0.00b | 44.9± 0.3hi |
| S0H2 | 0.98 ± 0.00a | 0.23 ± 0.00a | 1.74 ± 0.00ef | 0.42 ± 0.00a | 44.3 ± 0.61i |
| S0H3 | 0.95 ± 0.00a | 0.17 ± 0.00c | 1.50 ± 0.00g | 0.37 ± 0.00bc | 46.8 ± 0.2h |
| S1H0 | 0.74 ± 0.00de | 0.17 ± 0.00c | 1.66 ± 0.00f | 0.23 ± 0.00f | 84.2 ± 0.5b |
| S1H1 | 0.85 ± 0.03bc | 0.19 ± 0.00bc | 2.33 ± 0.00c | 0.35 ± 0.00c | 65.2 ± 0.5f |
| S1H2 | 0.92 ± 0.00ab | 0.25 ± 0.00a | 2.56 ± 0.00a | 0.38 ± 0.00b | 54.0± 0.7g |
| S1H3 | 0.80 ± 0.00cd | 0.19 ± 0.00bc | 2.44 ± 0.00bc | 0.24 ± 0.00f | 66.4 ± 0.3ef |
| S2H0 | 0.49 ± 0.03g | 0.10 ± 0.00e | 1.84 ± 0.00e | 0.15 ± 0.00g | 94.5 ± 0.6e |
| S2H1 | 0.61 ± 0.00f | 0.13 ± 0.00d | 2.38 ± 0.00c | 0.28 ± 0.00e | 71.1 ± 0.4d |
| S2H2 | 0.71 ± 0.01e | 0.13 ± 0.00d | 2.54 ± 0.00ab | 0.32 ± 0.00d | 68.8 ± 0.1de |
| S2H3 | 0.61 ± 0.00f | 0.12 ± 0.00de | 2.24 ± 0.00d | 0.22 ± 0.00f | 74.0 ± 0.3c |
| ANOVA p values | *** | *** | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhindi, K.M.; Almana, F.A.; Al-Yafrsi, M.A. Role of Humic Acid on Inducing Salt Tolerance of Ivy Geranium (Pelargonium peltatum L.) Plants. Horticulturae 2023, 9, 1012. https://doi.org/10.3390/horticulturae9091012
Elhindi KM, Almana FA, Al-Yafrsi MA. Role of Humic Acid on Inducing Salt Tolerance of Ivy Geranium (Pelargonium peltatum L.) Plants. Horticulturae. 2023; 9(9):1012. https://doi.org/10.3390/horticulturae9091012
Chicago/Turabian StyleElhindi, Khalid M., Fahed A. Almana, and Mohammed A. Al-Yafrsi. 2023. "Role of Humic Acid on Inducing Salt Tolerance of Ivy Geranium (Pelargonium peltatum L.) Plants" Horticulturae 9, no. 9: 1012. https://doi.org/10.3390/horticulturae9091012
APA StyleElhindi, K. M., Almana, F. A., & Al-Yafrsi, M. A. (2023). Role of Humic Acid on Inducing Salt Tolerance of Ivy Geranium (Pelargonium peltatum L.) Plants. Horticulturae, 9(9), 1012. https://doi.org/10.3390/horticulturae9091012







