Interspecific Hybridization between Ganoderma lingzhi and G. resinaceum by PEG-Induced Double-Inactivated Protoplast Fusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Media
2.2. Protoplasm Preparation and Inactivation
2.3. Protoplast Fusion
2.4. Preliminary Screening of Clamp Connection and Antagonism Test
2.5. Nuc-ITS and cox2 Gene Amplification
2.6. ISSR Profiling
2.7. Determination of the Mycelial Growth Rate and Fruiting Experiment
2.8. Determination of Polysaccharides and Triterpenes in Fruiting Bodies
2.9. Statistical Analysis
3. Results
3.1. Protoplast Inactivation Conditions
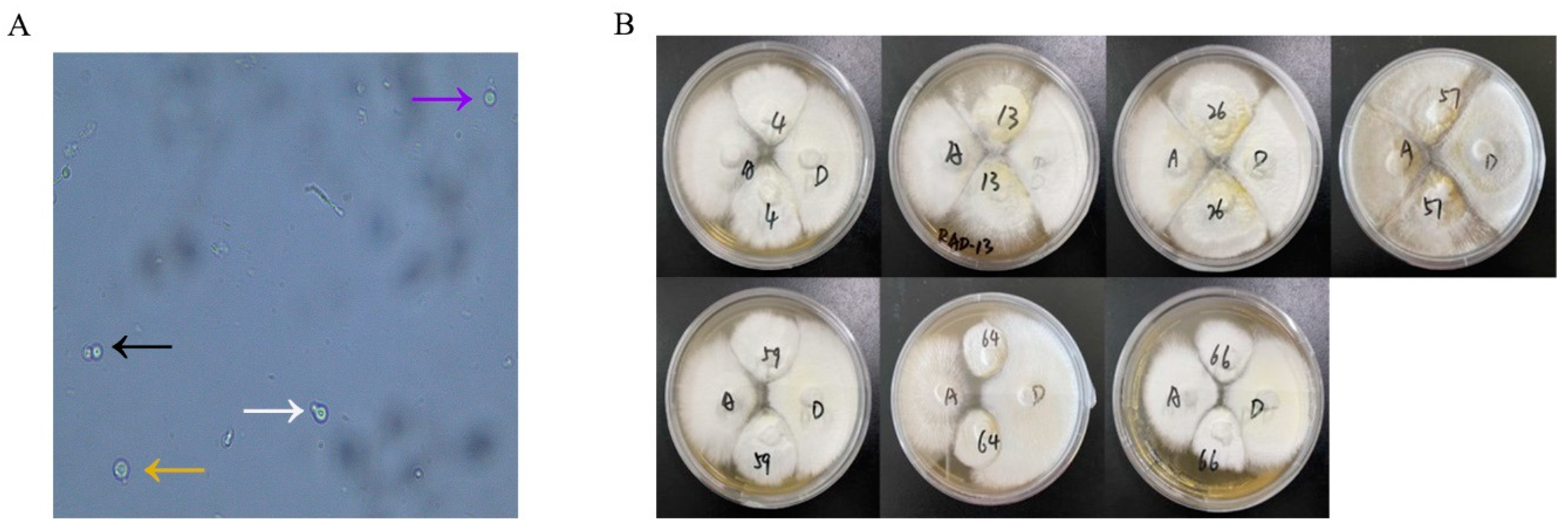
3.2. Protoplast Fusion and Antagonism Test
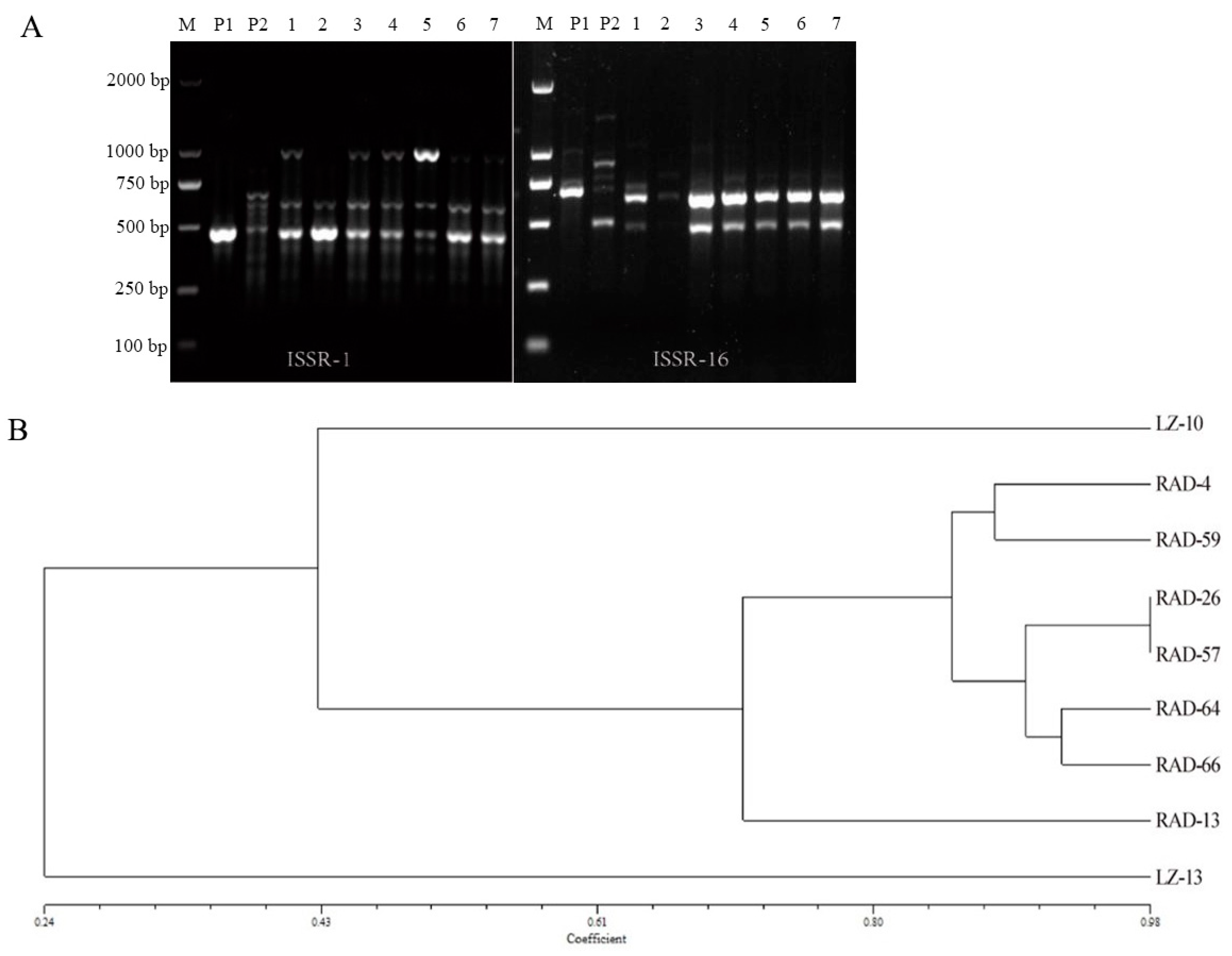
3.3. Nuc-ITS and Mitochondrial DNA Identification
3.4. ISSR Analysis
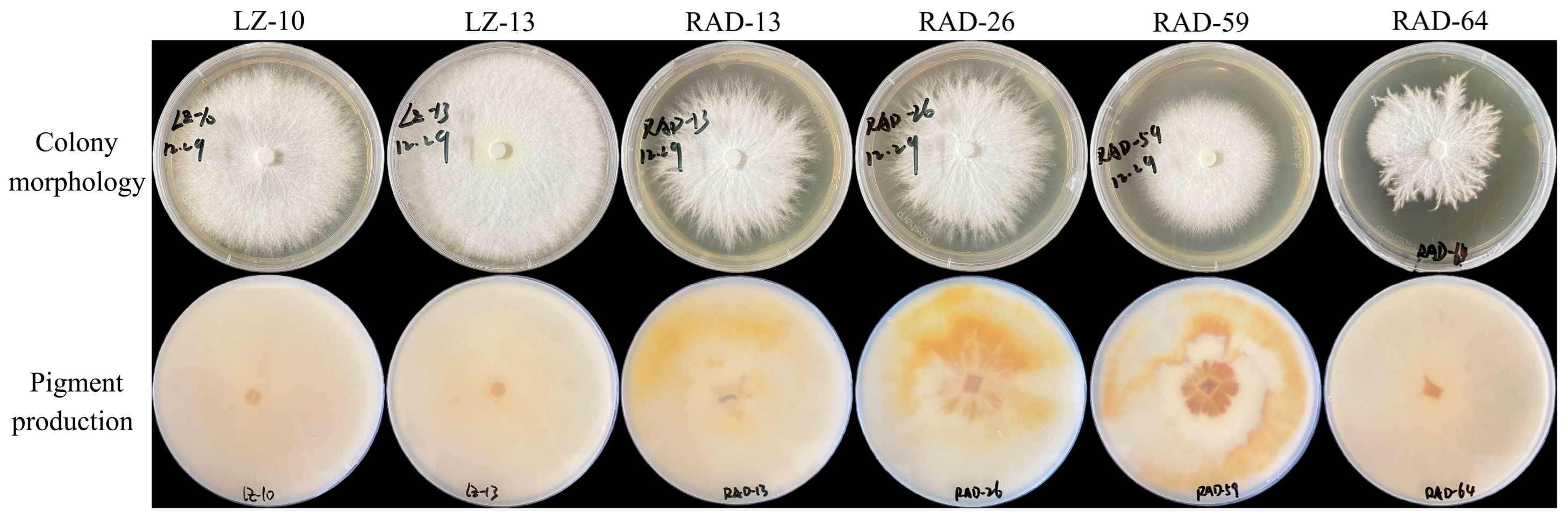
3.5. Colony Morphology
3.6. Growth Characteristics and Agronomic Traits
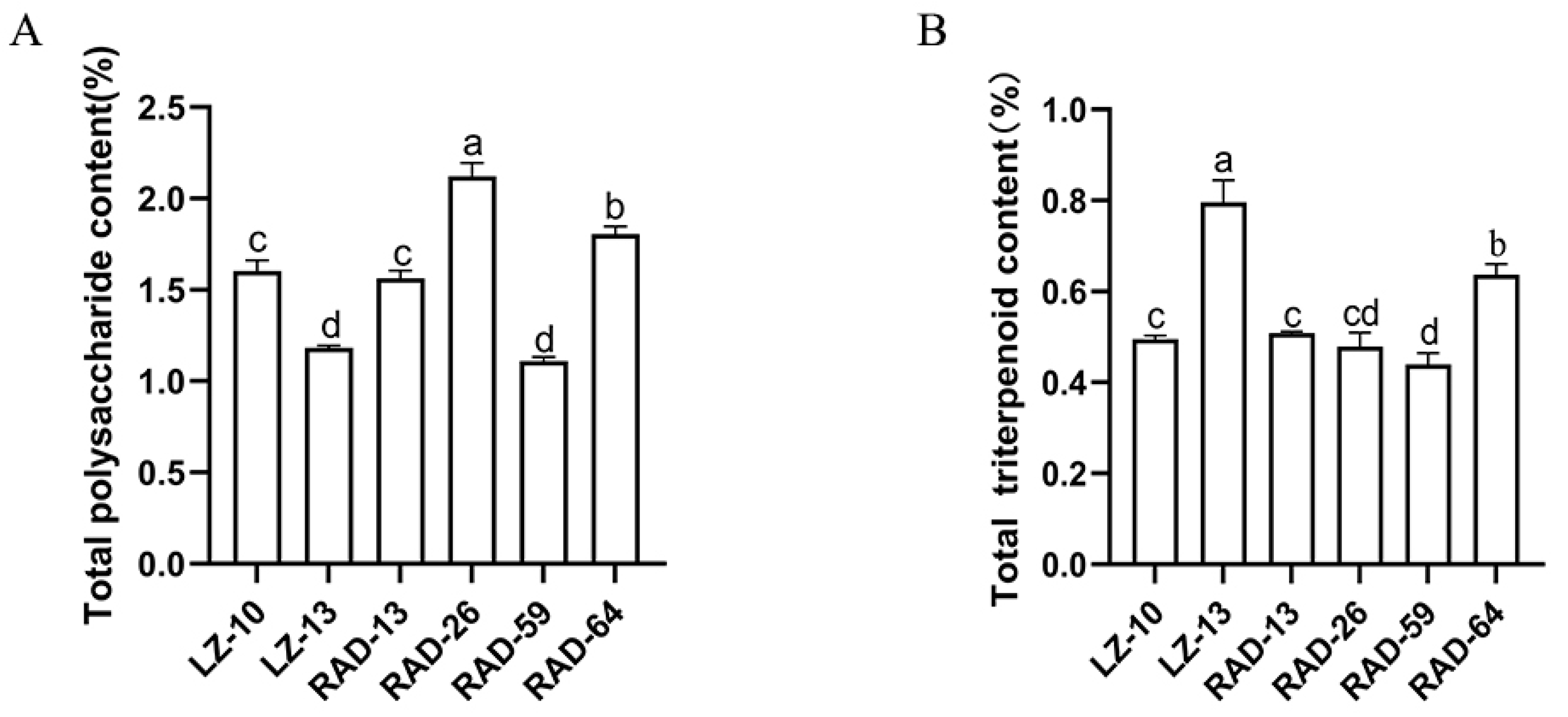
3.7. Contents of Polysaccharides and Triterpenes in Fruiting Bodies
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, Y.; Wu, S.H.; Dai, Y.C. Species clarification of the prize medicinal Ganoderma mushroom “Lingzhi”. Fungal Divers. 2012, 56, 49–62. [Google Scholar] [CrossRef]
- Cui, B.K.; Pan, X.H.; Pan, F.; Sun, Y.F.; Xing, J.H.; Dai, Y.C. Species diversity and resources of Ganoderma in China. Mycosystema 2023, 40, 49–62. [Google Scholar] [CrossRef]
- Nakagawa, T.; Zhu, Q.C.; Tamrakar, S.; Amen, Y.; Mori, Y.; Suhara, H.; Kaneko, S.; Kawashima, H.; Okuzono, K.; Inoue, Y.; et al. Changes in content of triterpenoids and polysaccharides in Ganoderma lingzhi at different growth stages. J. Nat. Med. 2018, 72, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.H.; Qin, X.; Zhang, J.S.; Yan, M.Q.; Zhou, S.; Feng, J.; Zhou, J.; Liu, Y.F. β-glucooligosaccharide separated and purified from Ganoderma lingzhi and its in vitro anti-inflammation activities. Mycosystema 2023, 42, 1360–1369. [Google Scholar] [CrossRef]
- Feng, N.; Yue, Y.W.; Cheng, C.L.; Yang, M.; Wang, C.; Zhang, J.S. Research progress of triterpenes from mycelia of Ganoderma lingzhi and its pharmacological effects. Mycosystema 2022, 41, 1341–1353. [Google Scholar] [CrossRef]
- Zhu, J.; Shi, L.; Ren, A.; Liu, R.; Zhao, M.W. Research progress on the biosynthesis of ganoderic acid. J. Nanjing Agric. Univ. 2022, 45, 981–989. [Google Scholar] [CrossRef]
- Chen, B.Z.; Ke, B.R.; Ye, L.Y.; Jin, S.S.; Jie, F.; Zhao, L.L.; Wu, X.P. Isolation and varietal characterization of Ganoderma resinaceum from areas of Ganoderma lucidum production in China. Sci. Hortic. 2017, 224, 109–114. [Google Scholar] [CrossRef]
- Huang, Y.J.; Li, X.; Peng, X.R.; Adegoke, A.T.; Chen, J.C.; Su, H.G.; Hu, G.L.; Wei, G.; Qiu, M.H. NMR-based structural classification, Identification, and Quantification of triterpenoids from edible mushroom Ganoderma resinaceum. J. Agric. Food Chem. 2020, 68, 2816–2825. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Huang, Y.J.; Su, H.G.; Gao, Y.; Lu, S.Y.; Peng, X.R.; Li, X.N.; Zhou, L.; Qiu, M.H. Structurally diverse lanostane triterpenoids from medicinal and edible mushroom Ganoderma resinaceum Boud. Bioorganic Chem. 2020, 100, 103871. [Google Scholar] [CrossRef]
- Kou, R.W.; Xia, B.; Wang, Z.J.; Li, J.N.; Yang, J.R.; Gao, Y.Q.; Yin, X.; Gao, J.M. Triterpenoids and meroterpenoids from the edible Ganoderma resinaceum and their potential anti-inflammatory, antioxidant and anti-apoptosis activities. Bioorganic Chem. 2022, 121, 105689. [Google Scholar] [CrossRef]
- Chen, X.Q.; Zhao, J.; Chen, L.X.; Wang, S.F.; Wang, Y.; Li, S.P. Lanostane triterpenes from the mushroom Ganoderma resinaceum and their inhibitory activities against alpha-glucosidase. Phytochemistry 2018, 149, 103–115. [Google Scholar] [CrossRef]
- Huang, Y.J.; Wei, G.; Peng, X.R.; Hu, G.L.; Su, H.G.; Liu, J.L.; Chen, X.; Qiu, M.H. Triterpenoids from functional mushroom Ganoderma resinaceum and the novel role of Resinacein S in enhancing the activity of brown/beige adipocytes. Food Res. Int. 2020, 136, 109303. [Google Scholar] [CrossRef] [PubMed]
- Adaskaveg, J.E.; Gilbertson, R.L. Cultural Studies and genetics of sexuality of Ganoderma lucidum and G. tsugae in relation to the taxonomy of the G. lucidum complex. Mycologia 2018, 78, 694–705. [Google Scholar] [CrossRef]
- Raman, J.; Jang, K.Y.; Oh, Y.L.; Oh, M.; Im, J.H.; Lakshmanan, H.; Kong, W.S. Interspecific hybridization between Ganoderma lingzhi and G. applanatum through protoplast fusion. World J. Microbiol. Biotechnol. 2021, 37, 114. [Google Scholar] [CrossRef]
- Dong, Y.T.; Miao, R.Y.; Feng, R.C.; Wang, T.; Yan, J.J.; Zhao, X.; Han, X.; Gan, Y.; Lin, J.B.; Li, Y.J.; et al. Edible and medicinal fungi breeding techniques, a review: Current status and future prospects. Curr. Res. Food Sci. 2022, 5, 2070–2080. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Yao, L.; Chen, S.J. Study on inactivation conditions of protoplasts of vincamine indole alkaloids producing endophytic fungus CH1. Guangdong Chem. Ind. 2021, 48, 11–12+19. [Google Scholar]
- Pan, M. Studies on Protoplast Fusion and Synthetic Complete Medium of Mortierella alpina. Master’s Thesis, University of Science and Technology of China, Hefei, China, 2017. [Google Scholar]
- Wang, K. Study on Morphology of Sclerotial Development, IMV Detection and Protoplast Fusion of Morchella Mushrooms. Master’s Thesis, Zhengzhou University of Light Industry, Zhengzhou, China, 2019. [Google Scholar]
- Zhao, K.; Ping, W.X.; Zhang, L.N.; Liu, J.; Lin, Y.; Jin, T.; Zhou, D.P. Screening and breeding of high taxol producing fungi by genome shuffling. Sci. China C Life Sci. 2008, 51, 222–231. [Google Scholar] [CrossRef]
- He, J.C.; He, Y.X. Research on inactivated protoplast fusion breeding of Pleurotus mushroom. Edible Fungi 1999, 6, 10–11. [Google Scholar]
- Sun, J.J. Preliminary Study on Protoplast Fusion of Lactarius delicious and Pleurotus ostreatus. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2012. [Google Scholar]
- Jiang, L.; Huang, J.W.; Ci, L.K.; Lu, Y.F. Protoplast fusion and regeneration of Agrocybe aegerita and Copyinds comatus. Food Sci. 2011, 32, 141–144. [Google Scholar]
- Xu, L.; Zhang, X.C.; Zhou, Y.J.; Yan, M.X. Research progress of Ganoderma lucidum breeding. Spec. Wild Econ. Anim. Plant Res. 2022, 1–7. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Zhang, X.C.; Hu, Q.X.; Yan, M.X. Optimization of preparation and regeneration conditions of two kinds of Ganoderma lucidum protoplasts. J. Jilin Agric. Univ. 2022, 1–10. [Google Scholar] [CrossRef]
- Quan, W.F.; He, J.X.; Wang, J.; Liu, G.J.; Ji, H.G.; Xue, J. Study on selection and breeding of selenium-rich Ganoderma lucidum by protoplast fusion. Edible Fungi 2012, 34, 19–21+31. [Google Scholar]
- Chen, Q.; Zhang, J.X. An improved method to characterize fungal vegetative compatible group. J. Microbiol. 2010, 30, 45–47. [Google Scholar]
- Meng, G.L.; Zeng, P.M.; Wang, Z.Y.; Cheng, B.; Ye, L.Y.; Wu, X.P. Application of the Ganoderma multipileum mitogenome to identification of Ganoderma species. Mycosystema 2020, 39, 23–33. [Google Scholar] [CrossRef]
- Meng, G.L.; Cheng, B.; Hao, J.B.; Li, S.Y.; Ye, L.Y.; Wu, X.P. Analyses of mitochondrial genome and its influence on mycelial growth and active component of Ganoderma lingzhi. Mycosystema 2020, 39, 42–56. [Google Scholar] [CrossRef]
- Ye, L.Y.; Meng, G.L.; He, X.F.; Ma, S.L.; Wu, X.P. Comparative analysis of mitochondrial genomes among Ganoderma Species. GAB 2022, 41, 1723–1731. [Google Scholar] [CrossRef]
- Xu, K. Based on Molecular Markers Assisted Breeding for Ganoderma lucidum. Master’s Thesis, Nanjing Agriculture University, Nanjing, China, 2014. [Google Scholar]
- Xing, Z.W. Germplasm Identification of Different Varieties and Screening of Excellent Strains of Ganoderma lucidum. Master’s Thesis, Hebei University of Engineering, Handan, China, 2016. [Google Scholar]
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China (Part 1), 2020th ed.; China Medical Science and Technology Press: Beijing, China, 2020; pp. 195–196. [Google Scholar]
- He, P.X.; Yu, M.; Wang, K.; Cai, Y.L.; Li, B.; Liu, W. Interspecific hybridization between cultivated morels Morchella importuna and Morchella sextelata by PEG-induced double inactivated protoplast fusion. World J. Microbiol. Biotechnol. 2020, 36, 58. [Google Scholar] [CrossRef]
- Lai, W.K.; Wu, Y.C.; Yeh, T.S.; Hsieh, C.R.; Tsai, Y.H.; Wei, C.K.; Li, C.Y.; Lu, Y.C.; Chang, F.R. The protoplast two-way fusions and fusant characteristics of Antrodia cinnamomea and Cordyceps militaris. Food Sci. Hum. Wellness 2022, 11, 1240–1251. [Google Scholar] [CrossRef]
- Huo, G.H.; Sun, J.J.; Luo, G.X.; Han, Q.C. Protoplast Hybridization between Lactarius deliciosus and Pleurotus ostreatus and Characteristic of Fusant Strains. J. Chin. Inst. Food Sci. Technol. 2014, 14, 39–46. [Google Scholar] [CrossRef]
- Sun, X.R. Technical Research of Protoplast Fusion between Pleurotus geesterani and pleurotus citrinopileatus Sing. Master’s Thesis, Shanxi Agricultural University, Taigu, Cina, 2018. [Google Scholar]
- Guo, C.J.; Zhao, R.; Zhu, W.B. Protoplast Fusion between Cordyceps sinensis and Cordyceps militaris. Food Sci. 2010, 31, 165–171. [Google Scholar]
- Li, Y.J.; Sun, G.Q.; Guo, J.F.; Wang, H.Y.; Yu, C.Z.; Pang, J. Research progress on the protoplast fusion technique in edible mushroom. J. North Agric. 2021, 49, 121–127. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.Z.; Zhu, S.F.; Zhang, D.M.; Han, S.J.; Li, Z.G.; Lu, J.H.; Chu, H.W.; Xiao, J.M.; Li, S.L. Integrated flow cytometric and proteomics analyses reveal the regulatory network underlying sugarcane protoplast responses to fusion. Plant Physiol. Biochem. 2023, 202, 107918. [Google Scholar] [CrossRef] [PubMed]
- He, J.C.; He, C.Y.; Lu, M.H.; Ma, Y.H.; Wang, M.L. Study on the breeding of Agaricus bisporus and Agaricus blazei by inactivated protoplast fusion. Edible Fungi China 2014, 33, 9–10. [Google Scholar] [CrossRef]
- Bornet, B.; Branchard, M. Nonanchored inter simple sequence repeat (ISSR) markers: Reproducible and specific tools for genome fingerprinting. Plant Mol. Biol. Rep. 2012, 19, 209–215. [Google Scholar] [CrossRef]
- Song, X.X.; Zhang, L.J.; Song, C.Y.; Zhao, Y.; Fei, R.G.; Tan, Q.; Chen, M.J.; Wang, Q.; Zha, L. Effect of alloplasmic cytoplasm on agronomic traits of Lentinula edodes L808. Acta Edulis Fungi 2020, 27, 1–7. [Google Scholar] [CrossRef]
- Zheng, C.W.; Rangsinth, P.; Shiu, P.H.T.; Wang, W.; Li, R.K.; Li, J.J.; Kwan, Y.W.; Leung, G.P.H. A review on the sources, structures, and pharmacological activities of lucidenic Acids. Molecules 2023, 28, 1756. [Google Scholar] [CrossRef]
- Lu, X. Pleurotus pulmonarius Crossbreeding and Their Alloplasm-Homokaryon Identification. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2017. [Google Scholar]
- Ye, L.Y.; He, X.F.; Su, C.B.; Feng, H.Y.; Meng, G.L.; Chen, B.Z.; Wu, X.P. The Effect of mitochondria on Ganoderma lucidum growth and bioactive components based on transcriptomics. J. Fungi 2022, 8, 1182. [Google Scholar] [CrossRef]
- Li, N.; Lu, J.; Wang, Z.R.; Du, P.; Li, P.W.; Su, J.; Xiao, J.; Wang, M.; Wang, J.Q.; Wang, R.M. Improving the regeneration rate of deep lethal mutant protoplasts by fusion to promote efficient L-lysine fermentation. BMC Biotechnol. 2023, 23, 22. [Google Scholar] [CrossRef]
- Asitok, A.; Ekpenyong, M.; Akwagiobe, E.; Asuquo, M.; Rao, A.; Ubi, D.; Lheanacho, J.; Ikharia, E.; Antai, A.; Essien, J. Interspecific protoplast fusion of atmospheric and room-temperature plasma mutants of Aspergillus generates an L-asparaginase hyper-producing hybrid with techno-economic benefits. Prep. Biochem. Biotechnol. 2023, 53, 827–840. [Google Scholar] [CrossRef]

| Primer Code | Sequence (5′–3′) | Annealing Temperature (°C) |
|---|---|---|
| ISSR-1 | CACCACACACACACACA | 46 |
| ISSR-3 | GAGAGAGAGAGAGAGACC | 46 |
| ISSR-4 | AGCAGCAGCAGCAGCAGCG | 49 |
| ISSR-5 | TGCACACACACACAC | 46 |
| ISSR-6 | GAGAGAGAGAGAGAGAT | 43 |
| ISSR-7 | AGAGAGAGAGAGAGAGC | 46 |
| ISSR-8 | CACACACACACACACAT | 46 |
| ISSR-9 | GAGAGAGAGAGAGAGACT | 52 |
| ISSR-10 | TTCCCTTCCCTTCCC | 50 |
| ISSR-11 | GTGACACACACACAC | 45 |
| ISSR-12 | AGTGTGTGTGTGTGT | 45 |
| ISSR-16 | GGATGCAACACACACACAC | 56 |
| Strains | Temperature (°C) | Time (min) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | ||
| G. lingzhi | 40 | 40.4 | 45.2 | 86.1 | 97.8 | 98.7 | 99.6 | 100 | 100 |
| 45 | 92.2 | 99.1 | 100 | 100 | 100 | 100 | 100 | 100 | |
| 50 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Strains | Time (s) | |||||||
|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | |
| G. resinaceum | 31.5 | 41.9 | 58.9 | 66.1 | 78.2 | 89.5 | 100 | 100 |
| Strains | LZ-10 | LZ-13 | RAD-4 | RAD-13 | RAD-26 | RAD-57 | RAD-59 | RAD-64 | RAD-66 |
|---|---|---|---|---|---|---|---|---|---|
| LZ-10 | 100% | 94.79% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| LZ-13 | 94.79% | 100% | 94.79% | 94.79% | 94.79% | 94.79% | 94.79% | 94.79% | 94.79% |
| Strains | LZ-10 | LZ-13 | RAD-4 | RAD-13 | RAD-26 | RAD-57 | RAD-59 | RAD-64 | RAD-66 |
|---|---|---|---|---|---|---|---|---|---|
| LZ-10 | 100% | 95.32% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| LZ-13 | 95.32% | 100% | 95.32% | 95.32% | 95.32% | 95.32% | 95.32% | 95.32% | 95.32% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liu, L.; Xu, L.; Wang, S.; Zhang, N.; Sun, C.; Yan, M. Interspecific Hybridization between Ganoderma lingzhi and G. resinaceum by PEG-Induced Double-Inactivated Protoplast Fusion. Horticulturae 2023, 9, 1129. https://doi.org/10.3390/horticulturae9101129
Li J, Liu L, Xu L, Wang S, Zhang N, Sun C, Yan M. Interspecific Hybridization between Ganoderma lingzhi and G. resinaceum by PEG-Induced Double-Inactivated Protoplast Fusion. Horticulturae. 2023; 9(10):1129. https://doi.org/10.3390/horticulturae9101129
Chicago/Turabian StyleLi, Jintao, Linling Liu, Lin Xu, Sheng Wang, Nan Zhang, Changwei Sun, and Meixia Yan. 2023. "Interspecific Hybridization between Ganoderma lingzhi and G. resinaceum by PEG-Induced Double-Inactivated Protoplast Fusion" Horticulturae 9, no. 10: 1129. https://doi.org/10.3390/horticulturae9101129
APA StyleLi, J., Liu, L., Xu, L., Wang, S., Zhang, N., Sun, C., & Yan, M. (2023). Interspecific Hybridization between Ganoderma lingzhi and G. resinaceum by PEG-Induced Double-Inactivated Protoplast Fusion. Horticulturae, 9(10), 1129. https://doi.org/10.3390/horticulturae9101129





