The Application of Nitrogen, Phosphorus, and Potassium Regulate the Growth and Morphological Development of Torreya grandis (Taxaceae) Saplings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Materials
2.3. Experimental Design
2.4. Measured Sapling Attributes
2.4.1. Height and Root Collar Diameter of Saplings
2.4.2. Determination of Biomass
2.4.3. Determination of N, P, and K
2.5. Data Processing and Analysis
2.5.1. Calculation of Comprehensive Index
- Sapling root–shoot ratio (RS) = root biomass (g)/(stem biomass + leaves biomass) (g);
2.5.2. Data Analysis
3. Results
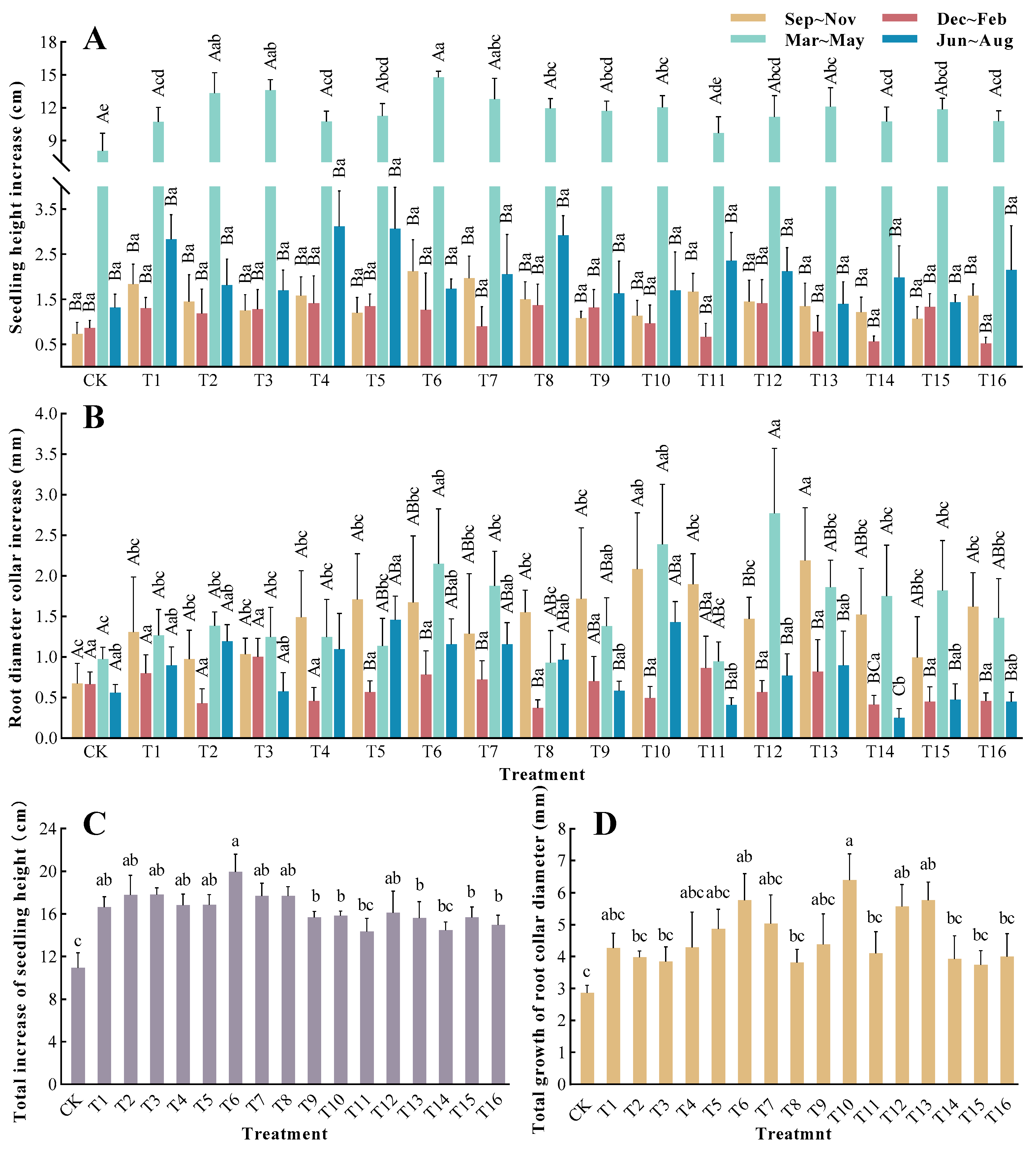
3.1. Effects of Different Fertilization Treatments on the Growth of Torreya grandis
3.2. Impacts of Different Fertilization Treatments on Root–Shoot Ratio, Sapling Biomass and Seedling Quality Index
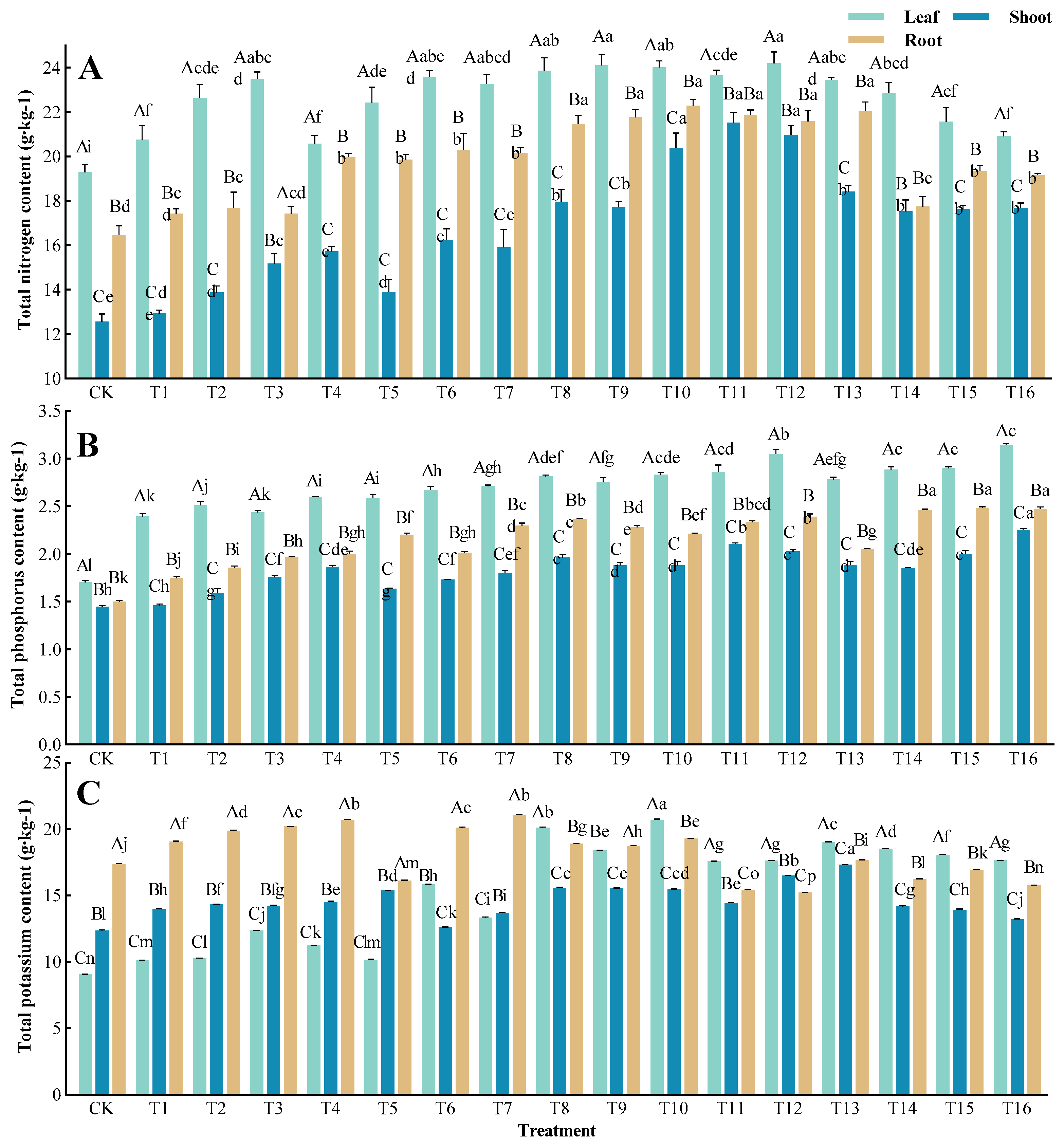
3.3. Impacts of Different Fertilization Treatments on the Nutrient Content of Sapling Organs
3.4. Comprehensive Analysis
4. Discussion
4.1. Fertilization Promotes Plant Growth
4.2. Effects of Fertilization on the Biomass and Nutrient Status of Torreya grandis
4.3. Effects of Fertilization on the Quality Index of T. grandis Saplings
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, S.; Shi, K.; Ma, J.; Zhang, L.; Ma, L.; Jia, Z. Effects of Fertilization Ratios and Frequencies on the Growth and Nutrient Uptake of Magnolia wufengensis (Magnoliaceae). Forests 2019, 10, 65. [Google Scholar] [CrossRef]
- Asante, M.O.O.; Ahiakpa, J.K.; Amoatey, C.; Adjei-Nsiah, S. Effect of shade and level of fertilizer application on nutrient uptake and dry matter partitioning in cocoyam (Xanthosoma sagittifolium L.). J. Plant Nutr. 2017, 40, 2312–2325. [Google Scholar] [CrossRef]
- Haque, M.M.; Biswas, J.C.; Islam, M.R.; Islam, A.; Kabir, M.S. Effect of long-term chemical and organic fertilization on rice productivity, nutrient use-efficiency, and balance under a rice-fallow-rice system. J. Plant Nutr. 2019, 42, 2901–2914. [Google Scholar] [CrossRef]
- Pennazio, S. Mineral nutrition of plants: A short history of plant physiology. Riv. Di. Biol. 2005, 98, 215–236. [Google Scholar]
- Meharg, A. Marschner′s Mineral Nutrition of Higher Plants. 3rd edition. Edited by P. Marschner. Amsterdam, Netherlands: Elsevier/Academic Press (2011), pp. 684, USA124.95. ISBN 978-0-12-384905-2. Exp. Agr. 2012, 48, 305. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin, F.S. The Mineral Nutrition of Wild Plants Revisited: A Re-evaluation of Processes and Patterns. In Advances in Ecological Research; Academic Press: Cambridge, MA, USA, 1999; Volume 30, pp. 1–67. [Google Scholar]
- Luo, J.; Qin, J.; He, F.; Li, H.; Liu, T.; Polle, A.; Peng, C.; Luo, Z.B. Net fluxes of ammonium and nitrate in association with H+ fluxes in fine roots of Populus popularis. Planta 2013, 237, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Gusewell, S. N: P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Warren, C.R. How does P affect photosynthesis and metabolite profiles of Eucalyptus globulus? Tree Physiol. 2011, 31, 727–739. [Google Scholar] [CrossRef]
- Marschner, C. Book Review. J. Plant Physiol. 1995, 148, 765. [Google Scholar] [CrossRef]
- Gan, H.; Jiao, Y.; Jia, J.; Wang, X.; Li, H.; Shi, W.; Peng, C.; Polle, A.; Luo, Z.B. Phosphorus and nitrogen physiology of two contrasting poplar genotypes when exposed to phosphorus and/or nitrogen starvation. Tree Physiol. 2016, 36, 22–38. [Google Scholar] [CrossRef]
- Burman, U.; Garg, B.K.; Kathju, S. Effect of Phosphorus Application on Clusterbean under Different Intensities of Water Stress. J. Plant Nutr. 2009, 32, 668–680. [Google Scholar] [CrossRef]
- Dos Santos, M.G.; Ribeiro, R.V.; de Oliveira, R.F.; Machado, E.C.; Pimentel, C. The role of inorganic phosphate on photosynthesis recovery of common bean after a mild water deficit. Plant Sci. 2006, 170, 659–664. [Google Scholar] [CrossRef]
- Li, X.; Mu, C.; Lin, J.; Wang, Y.; Li, X. Effect of Alkaline Potassium and Sodium Salts on Growth, Photosynthesis, Ions Absorption and Solutes Synthesis of Wheat Seedlings. Exp. Agr. 2013, 50, 144–157. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Kronzucker, H.J. The physiology of channel-mediated K+ acquisition in roots of higher plants. Physiol. Plant 2014, 151, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Mäser, P.; Gierth, M.; Schroeder, J.I. Molecular mechanisms of potassium and sodium uptake in plants. Plant Soil 2002, 247, 43–54. [Google Scholar] [CrossRef]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Glass, A.D.M. Nitrogen Use Efficiency of Crop Plants: Physiological Constraints upon Nitrogen Absorption. Criti. Rev. Plant Sci. 2010, 22, 453–470. [Google Scholar] [CrossRef]
- Raun, W.R.; Solie, J.B.; Johnson, G.V.; Stone, M.L.; Mullen, R.W.; Freeman, K.W.; Thomason, W.E.; Lukina, E.V. Improving Nitrogen Use Efficiency in Cereal Grain Production with Optical Sensing and Variable Rate Application. Agron. J. 2002, 94, 815–820. [Google Scholar] [CrossRef]
- Bussi, C.; Smith, M.A.L. Effects of nitrogen and potassium fertilization on the growth, yield and pitburn of apricot (cv. Bergeron). J. Hort. Sci. Bio. 1998, 73, 387–392. [Google Scholar] [CrossRef]
- Bussi, C.; Besset, J.; Girard, T. Effects of fertilizer rates and dates of application on apricot (cv Bergeron) cropping and pitburn. Sci. Hortic. 2003, 98, 139–147. [Google Scholar] [CrossRef]
- Fox, T.R.; Lee Allen, H.; Albaugh, T.J.; Rubilar, R.; Carlson, C.A. Tree Nutrition and Forest Fertilization of Pine Plantations in the Southern United States. S. J. Appl. Fores. 2007, 31, 5–11. [Google Scholar] [CrossRef]
- Wertz, S.; Leigh, A.K.K.; Grayston, S.J. Effects of long-term fertilization of forest soils on potential nitrification and on the abundance and community structure of ammonia oxidizers and nitrite oxidizers. FEMS Microbiol. Ecol. 2012, 79, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Gough, C.M.; Seiler, J.R.; Maier, C.A. Short-term effects of fertilization on loblolly pine (Pinus taeda L.) physiology. Plant Cell Environ. 2004, 27, 876–886. [Google Scholar] [CrossRef]
- King, N.T.; Seiler, J.R.; Fox, T.R.; Johnsen, K. Post-fertilization physiology and growth performance of loblolly pine clones. Tree Physiol. 2008, 28, 703–711. [Google Scholar] [CrossRef]
- FAO. Kuaijishan Ancient Chinese Torreya, China. Available online: http://www.fao.org/giahs/giahsaroundtheworld/designated-sites/asia-and-the-acific/kuajishan-ancient-chinese-torreya/en (accessed on 10 May 2021).
- MOA. Available online: http://www.moa.gov.cn/ztzl/zywhycsl/dypzgzywhyc/ (accessed on 10 May 2021).
- Huang, Y.; Wang, J.; Li, G.; Zheng, Z.; Su, W. Antitumor and antifungal activities in endophytic fungi isolated from pharmaceutical plants Taxus mairei, Cephalataxus fortunei and Torreya grandis. FEMS Immunol. Med. Microbiol. 2001, 31, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Q.; Cui, X.Y.; Zhao, X.; Zhang, Y.H.; Piao, H.S.; Kim, J.H.; Lee, B.C.; Pyo, H.B.; Yun, Y.P. Antioxidative and acute antiinflammatory effects of Torreya grandis. Fitoterapia 2006, 77, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Hu, Y.-Y.; Yu, W.-W.; Song, L.-L.; Wu, J.-S. Growth, photosynthetic and physiological responses of Torreya grandis seedlings to varied light environments. Trees 2015, 29, 1011–1022. [Google Scholar] [CrossRef]
- Han, Y.; Wang, G.G.; Wu, T.; Chen, W.; Ji, Y.; Jin, S. Fertilization Failed to Make Positive Effects on Torreya grandis in Severe N-Deposition Subtropics. Sustainability 2021, 13, 9736. [Google Scholar] [CrossRef]
- Chu, H.; Lin, X.; Fujii, T.; Morimoto, S.; Yagi, K.; Hu, J.; Zhang, J. Soil microbial biomass, dehydrogenase activity, bacterial community structure in response to long-term fertilizer management. SBB 2007, 39, 2971–2976. [Google Scholar] [CrossRef]
- Kanchikerimath, M.; Singh, D. Soil organic matter and biological properties after 26 years of maize–wheat–cowpea cropping as affected by manure and fertilization in a Cambisol in semiarid region of India. Agr. Eco. Env. 2001, 86, 155–162. [Google Scholar] [CrossRef]
- Cai, Z.C.; Qin, S.W. Dynamics of crop yields and soil organic carbon in a long-term fertilization experiment in the Huang-Huai-Hai Plain of China. Geoderma 2006, 136, 708–715. [Google Scholar] [CrossRef]
- Osmond, D.L. Nitrogen fertilizer requirements for maize produced in the tropics: A comparison of three computer-based recommendation systems. Agric. Syst. 1996, 50, 37–50. [Google Scholar] [CrossRef]
- Konde, N.M.; Kanase, N.M.; Jadhao, S.M.; Goud, V.V. Yield targetting equation for soybean with conjoint use of manure and chemical fertilizer based on fertility gradient approach. Ann. Plant Physiol. 2009, 23, 210–214. [Google Scholar]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Quality Appraisal of White Spruce and White Pine Seedling Stock in Nurseries. For. Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Seedling Quality—Soil Fertility Relationship of White Spruce, and Red and Whit Pine iin Nurseries. For. Chron. 1960, 36, 48–52. [Google Scholar]
- Puttonen, P. Criteria for using seedling performance potential tests. New For. 1989, 3, 67–87. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Wu, X.-H.; Grossnickle, S.C.; Chen, L.-H.; Yu, X.-X.; El-Kassaby, Y.A.; Feng, J.-L. Formula Fertilization Promotes Phoebe bournei Robust Seedling Cultivation. Forests 2020, 11, 781. [Google Scholar] [CrossRef]
- Valinger, E.; Sjögren, H.; Nord, G.; Cedergren, J. Effects on stem growth of Scots pine 33 years after thinning and/or fertilization in northern Sweden. Sca. J. For. Res. 2018, 34, 33–38. [Google Scholar] [CrossRef]
- Guo, J.; Wu, Y.; Wang, B.; Lu, Y.; Cao, F.; Wang, G. The Effects of Fertilization on the Growth and Physiological Characteristics of Ginkgo biloba L. Forests 2016, 7, 293. [Google Scholar] [CrossRef]
- Thomas, A.; Priault, P.; Piutti, S.; Dallé, E.; Marron, N. Growth dynamics of fast-growing tree species in mixed forestry and agroforestry plantations. For. Ecol. Manag. 2021, 480, 118672. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Wu, X.-H.; Chen, L.-H.; Huang, L.-M.; Chen, Y.; Wu, J.; El-Kassaby, Y.A.; Grossnickle, S.C.; Feng, J.-L. Fertilization Regulates Accumulation and Allocation of Biomass and Nutrients in Phoebe bournei Seedlings. Agriculture 2021, 11, 1187. [Google Scholar] [CrossRef]
- López-Sandoval, D.C.; Duarte, C.M.; Agustí, S. Nutrient and temperature constraints on primary production and net phytoplankton growth in a tropical ecosystem. Limnol. Oceanogr. 2021, 66, 2923–2935. [Google Scholar] [CrossRef]
- Jankovska-Bortkevič, E.; Gavelienė, V.; Koryznienė, D.; Jankauskienė, J.; Mockevičiūtė, R.; Jurkonienė, S. Response of winter oilseed rape to imitated temperature fluctuations in autumn-winter period. Environ. Exp. Bot. 2019, 166, 103801. [Google Scholar] [CrossRef]
- Kamil, K.R.; Anita, K. Evaluation of a different fertilisation in technology of corn for silage, sugar beet and meadow grasses production and their impact on the environment in Poland. Afr. J. Agr. Res. 2015, 10, 1351–1358. [Google Scholar] [CrossRef]
- Hedwall, P.-O.; Gong, P.; Ingerslev, M.; Bergh, J. Fertilization in northern forests—Biological, economic and environmental constraints and possibilities. Sca. J. For. Res. 2014, 29, 301–311. [Google Scholar] [CrossRef]
- Dinesh, T.; Zuzana, M. Rhizome trait scaling relationships are modulated by growth conditions and are linked to plant fitness. Ann. Bot. 2022, 137, 116253. [Google Scholar]
- Zaczek, J.J.; Baer, S.G.; Dalzotto, D.J. Fire and Fertilization Effects on the Growth and Spread of Rhizome-Transplanted Giant Cane (Arundinaria gigantea). Restor. Ecol. 2010, 18, 462–468. [Google Scholar] [CrossRef]
- Guarise, M.; Borgonovo, G.; Bassoli, A.; Ferrante, A. Effect of Fertilization on Yield and Quality of Sisymbrium officinale (L.) Scop. Grown as Leafy Vegetable Crop. Agronomy 2019, 9, 401. [Google Scholar] [CrossRef]
- Soratto, R.P.; Fernandes, A.M. Phosphorus Effects on Biomass Accumulation and Nutrient Uptake and Removal in Two Potato Cultivars. Agron. J. 2016, 108, 1225–1236. [Google Scholar] [CrossRef]
- Malhi, S.S.; Johnston, A.M.; Schoenau, J.J.; Wang, Z.L.; Vera, C.L. Seasonal biomass accumulation and nutrient uptake of wheat, barley and oat on a Black Chernozem Soil in Saskatchewan. Can. J. Plant Sci. 2006, 86, 1005–1014. [Google Scholar] [CrossRef]
- Khalofah, A.; Ghramh, H.A.; Al-Qthanin, R.N.; L′Taief, B. The impact of NPK fertilizer on growth and nutrient accumulation in juniper (Juniperus procera) trees grown on fire-damaged and intact soils. PLoS ONE 2022, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Guo, J.; Shu, M.; Wang, P.; Hu, S. Impacts of drought and nitrogen enrichment on leaf nutrient resorption and root nutrient allocation in four Tibetan plant species. Sci. Total Env. 2020, 723, 138106. [Google Scholar] [CrossRef]
- Sardans, J.; Grau, O.; Chen, H.Y.H.; Janssens, I.A.; Ciais, P.; Piao, S.; Peñuelas, J. Changes in nutrient concentrations of leaves and roots in response to global change factors. Glob. Chang. Biol. 2017, 23, 3849–3856. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, H.; Xia, J.; Hou, F.; Shi, X.; Hao, X.; Hafeez, A.; Han, H.; Luo, H. Optimal pre-plant irrigation and fertilization can improve biomass accumulation by maintaining the root and leaf productive capacity of cotton crop. Sci. Rep. 2017, 7, 17168. [Google Scholar] [CrossRef]
- Wang, X.; Bian, S.; Chang, P.; Wang, N.; Xuan, L.; Zhang, M.; Dong, B.; Zhang, C.; Wu, J.; Ying, Y.; et al. The effects of different nitrogen sources on camptothecin content and related gene expression in Camptotheca acuminata seedlings. J. For. Res. 2019, 31, 1347–1357. [Google Scholar] [CrossRef]
- Barunawati, N.; Giehl, R.F.; Bauer, B.; von Wiren, N. The influence of inorganic nitrogen fertilizer forms on micronutrient retranslocation and accumulation in grains of winter wheat. Front. Plant Sci. 2013, 4, 320. [Google Scholar] [CrossRef] [PubMed]
- Caroline, D.T.; Bart, V.; Bruno, V.; Sarah, O.; Tim, D.M.; Jill, D.V.; Peter, D.; Lieven, C.; Tina, K.; Jane, D. Chitin in strawberry cultivation: Foliar growth and defense response promotion, but reduced fruit yield and disease resistance by nutrient imbalances. MPMI 2020, 34, 227–239. [Google Scholar]
- Saloner, A.; Bernstein, N. Effect of Potassium (K) Supply on Cannabinoids, Terpenoids and Plant Function in Medical Cannabis. Agronomy 2022, 12, 1242. [Google Scholar] [CrossRef]
- Ordóñez, R.A.; Castellano, M.J.; Danalatos, G.N.; Wright, E.E.; Hatfield, J.L.; Burras, L.; Archontoulis, S.V. Insufficient and excessive N fertilizer input reduces maize root mass across soil types. Field Crops Res. 2021, 267, 108142. [Google Scholar] [CrossRef]
- Ou, X.; Cui, X.; Zhu, D.; Guo, L.; Liu, D.; Yang, Y. Lowering Nitrogen and Increasing Potassium Application Level Can Improve the Yield and Quality of Panax notoginseng. Front. Plant Sci. 2020, 11, 595095. [Google Scholar] [CrossRef] [PubMed]
- Daoud, B.; Pawelzik, E.; Naumann, M. Different potassium fertilization levels influence water-use efficiency, yield, and fruit quality attributes of cocktail tomato—A comparative study of deficient-to-excessive supply. Sci. Hortic. 2020, 272, 109562. [Google Scholar] [CrossRef]


| pH | NH4-N (mg·kg−1) | NO3-N (mg·kg−1) | AP (mg·kg−1) | TN (g·kg−1) | TP (g·kg−1) | TK (g·kg−1) |
|---|---|---|---|---|---|---|
| 6.15 ± 0.05 | 0.99 ± 0.14 | 0.14 ± 0.02 | 16.96 ± 0.13 | 1.31 ± 0.05 | 0.46 ± 0.01 | 7.10 ± 0.16 |
| No. | Treatment | N (g · Sapling−1) | P2O5 (g · Sapling−1) | K2O (g · Sapling−1) |
|---|---|---|---|---|
| CK | N0P0K0 | 0 | 0 | 0 |
| T1 | N1P1K1 | 1.0 (0.46) | 1.0 (0.16) | 1.0 (0.52) |
| T2 | N1P2K2 | 1.0 | 2.0 (0.32) | 2.0 (1.04) |
| T3 | N1P3K3 | 1.0 | 3.0 (0.48) | 3.0 (1.56) |
| T4 | N1P4K4 | 1.0 | 4.0 (0.64) | 4.0 (2.08) |
| T5 | N2P1K2 | 2.0 (0.92) | 1.0 | 2.0 |
| T6 | N2P2K1 | 2.0 | 2.0 | 1.0 |
| T7 | N2P3K4 | 2.0 | 3.0 | 4.0 |
| T8 | N2P4K3 | 2.0 | 4.0 | 3.0 |
| T9 | N3P1K3 | 3.0 (1.38) | 1.0 | 3.0 |
| T10 | N3P2K4 | 3.0 | 2.0 | 4.0 |
| T11 | N3P3K1 | 3.0 | 3.0 | 1.0 |
| T12 | N3P4K2 | 3.0 | 4.0 | 2.0 |
| T13 | N4P1K4 | 4.0 (1.84) | 1.0 | 4.0 |
| T14 | N4P2K3 | 4.0 | 2.0 | 3.0 |
| T15 | N4P3K2 | 4.0 | 3.0 | 2.0 |
| T16 | N4P4K1 | 4.0 | 4.0 | 1.0 |
| Indicator | N | P | K |
|---|---|---|---|
| THI (cm) | 2.86 | 0.8 | 0.18 |
| TDI (mm) | 1.02 | 0.83 | 1.38 |
| LB (g) | 20.54 | 5.46 | 4.56 |
| SB (g) | 11.4 | 4.02 | 3.34 |
| RB (g) | 43.55 | 17.39 | 17.06 |
| TB (g) | 75.49 | 24.02 | 11.29 |
| RS | 0.18 | 0.19 | 0.3 |
| QI | 1.93 | 0.36 | 0.32 |
| Indicator | N | P | K | |||
|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| THI | 5.365 | 0.002 | 0.343 | 0.794 | 0.016 | 0.997 |
| TDI | 1.771 | 0.159 | 1.165 | 0.328 | 2.636 | 0.055 |
| LB | 11.568 | <0.001 | 1.128 | 0.350 | 0.714 | 0.550 |
| SB | 5.737 | 0.002 | 1.028 | 0.391 | 0.597 | 0.621 |
| RB | 9.750 | <0.001 | 1.656 | 0.193 | 1.393 | 0.260 |
| TB | 14.926 | <0.001 | 1.531 | 0.222 | 0.360 | 0.782 |
| RS | 1.376 | 0.265 | 1.748 | 0.174 | 3.587 | 0.022 |
| QI | 10.023 | <0.001 | 0.542 | 0.657 | 0.263 | 0.851 |
| Principal Component | Score Formula |
|---|---|
| 1 | Y = −0.128x1 + 0.106x2 + 0.147x3 + 0.345x4 + 0.244x5 + 0.292x6 + 0.324x7 + 0.280x8 + 0.342x9 + 0.222x10 − 0.264x11 − 0.261x12 − 0.223x13 − 0.218x14 − 0.24x15 − 0.071x16 − 0.188x17 |
| 2 | Y = 0.315x1 + 0.249x2 + 0.323x3 + 0.169x4 + 0.253x5 + 0.218x6 + 0.124x7 + 0.187x8 + 0.145x9 + 0.125x10 + 0.155x11 + 0.260x12 + 0.235x13 + 0.326x14 + 0.320x15 + 0.219x16 + 0.323x17 |
| 3 | Y = 0.042x1 + 0.346x2 + 0.203x3 + 0.020x4 + 0.129x5 − 0.256x6 − 0.299x7 − 0.391x8 + 0.046x9 + 0.390x10 + 0.297x11 − 0.219x12 − 0.368x13 − 0.000x14 − 0.116x15 + 0.267x16 − 0.045x17 |
| 4 | Y = −0.072x1 − 0.346x2 − 0.000x3 + 0.104x4 − 0.260x5 + 0.001x6 + 0.170x7 + 0.109x8 + 0.175x9 − 0.313x10 + 0.141x11 − 0.268x12 − 0.267x13 + 0.242x14 + 0.044x15 + 0.635x16 + 0.023x17 |
| Treatments | F1 | F2 | F3 | F4 | F | Ranking |
|---|---|---|---|---|---|---|
| CK | −2.63 | −6.52 | 0.63 | 0.49 | −3.55 | 17 |
| T1 | −3.40 | −1.34 | 0.15 | −0.76 | −1.99 | 16 |
| T2 | −2.44 | −0.94 | 0.21 | −0.81 | −1.43 | 15 |
| T3 | −2.53 | 0.96 | 0.91 | 2.02 | −0.39 | 12 |
| T4 | −1.53 | −0.03 | 0.47 | 0.55 | −0.55 | 13 |
| T5 | −1.43 | 0.08 | −1.05 | −2.91 | −0.93 | 14 |
| T6 | −2.32 | 2.98 | 0.20 | 0.25 | 0.26 | 8 |
| T7 | −2.74 | 3.42 | −1.00 | −0.08 | 0.11 | 10 |
| T8 | 1.18 | 1.54 | 0.07 | 0.59 | 1.16 | 3 |
| T9 | 0.08 | 2.41 | −0.08 | 0.12 | 0.99 | 6 |
| T10 | 2.16 | 1.63 | 1.79 | −0.33 | 1.69 | 2 |
| T11 | 2.61 | 0.17 | −1.27 | −0.24 | 1.00 | 5 |
| T12 | 2.75 | 2.04 | 0.54 | 0.10 | 2.00 | 1 |
| T13 | 3.75 | −1.61 | 2.48 | −1.58 | 1.02 | 4 |
| T14 | 2.09 | −1.70 | −0.18 | 1.93 | 0.36 | 7 |
| T15 | 1.74 | −1.21 | −1.29 | 0.66 | 0.17 | 9 |
| T16 | 2.66 | −1.90 | −2.58 | 0.00 | 0.10 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Huang, D.; Huang, C.; Tong, Y.; Yuan, F.; Ma, X.; Liu, H.; Fu, S. The Application of Nitrogen, Phosphorus, and Potassium Regulate the Growth and Morphological Development of Torreya grandis (Taxaceae) Saplings. Horticulturae 2023, 9, 1203. https://doi.org/10.3390/horticulturae9111203
Ma X, Huang D, Huang C, Tong Y, Yuan F, Ma X, Liu H, Fu S. The Application of Nitrogen, Phosphorus, and Potassium Regulate the Growth and Morphological Development of Torreya grandis (Taxaceae) Saplings. Horticulturae. 2023; 9(11):1203. https://doi.org/10.3390/horticulturae9111203
Chicago/Turabian StyleMa, Xiaomin, Dongchen Huang, Cheng Huang, Yinhao Tong, Feiyang Yuan, Xiaoxiang Ma, Hua Liu, and Songling Fu. 2023. "The Application of Nitrogen, Phosphorus, and Potassium Regulate the Growth and Morphological Development of Torreya grandis (Taxaceae) Saplings" Horticulturae 9, no. 11: 1203. https://doi.org/10.3390/horticulturae9111203
APA StyleMa, X., Huang, D., Huang, C., Tong, Y., Yuan, F., Ma, X., Liu, H., & Fu, S. (2023). The Application of Nitrogen, Phosphorus, and Potassium Regulate the Growth and Morphological Development of Torreya grandis (Taxaceae) Saplings. Horticulturae, 9(11), 1203. https://doi.org/10.3390/horticulturae9111203





