Macronutrient Fertilization and Cadmium Absorption in Two Cocoa Clones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Conditions and Plant Material
2.2. Physical–Chemical Characteristics of the Substrate
2.3. Experimental Design
2.4. Macronutrient Fertilization
2.5. Physiological Variables
2.5.1. Leaf Gas Exchange
2.5.2. Chlorophyll Index (CI)
2.5.3. Determination of Cd in Tissues
2.5.4. Cd Content of Shoot and Root
2.5.5. Cadmium Extracting Capacity
2.5.6. Macronutrient Concentration in Leaf Tissue
2.5.7. Soil pH and Electrical Conductivity
2.5.8. Shoot and Root Dry Biomass
2.6. Statistical Analysis
3. Results
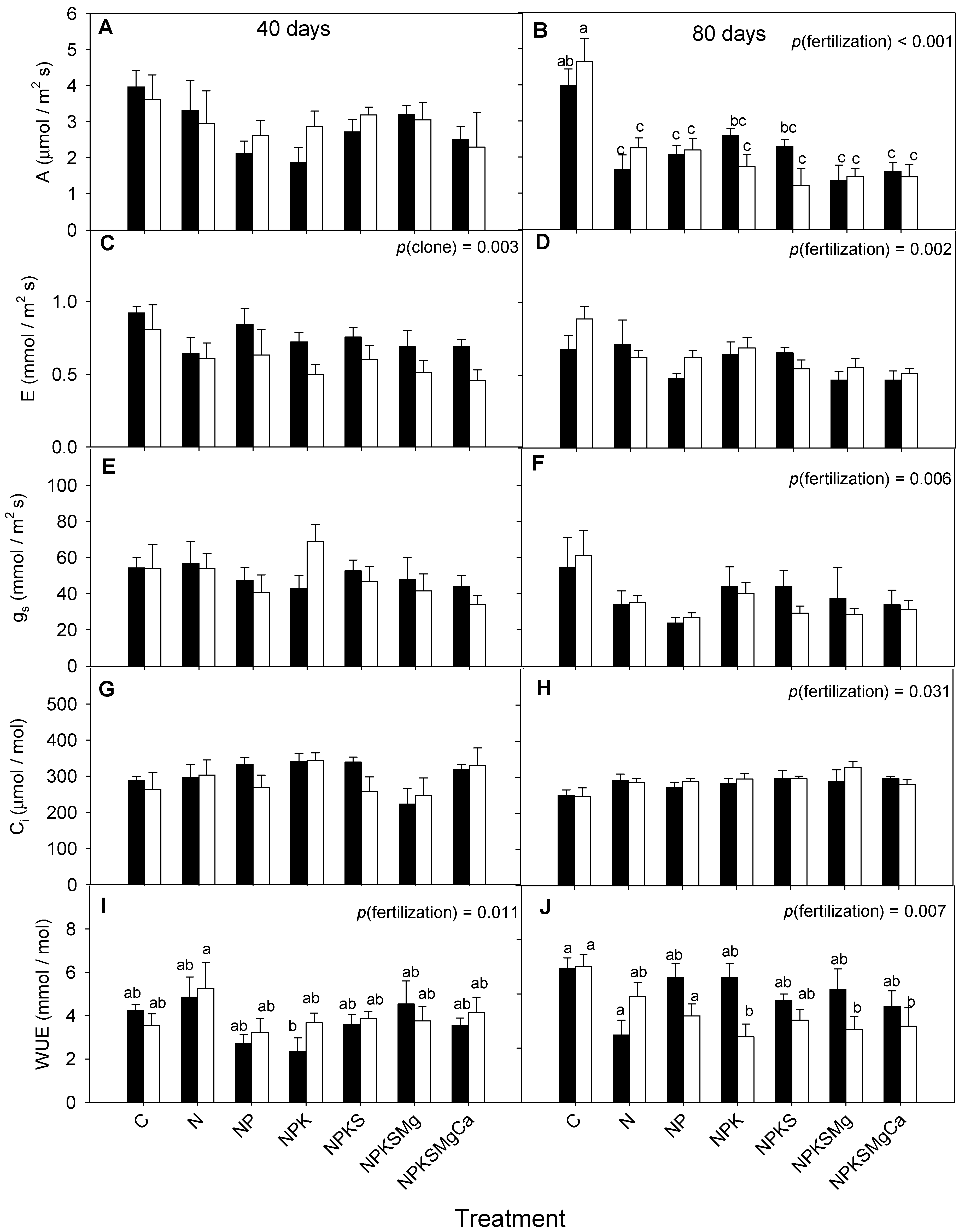
3.1. Leaf Gas Exchange
3.2. Chlorophyll Index
3.3. Cadmium Accumulation and Extraction Capacity
3.4. Nutrient Application
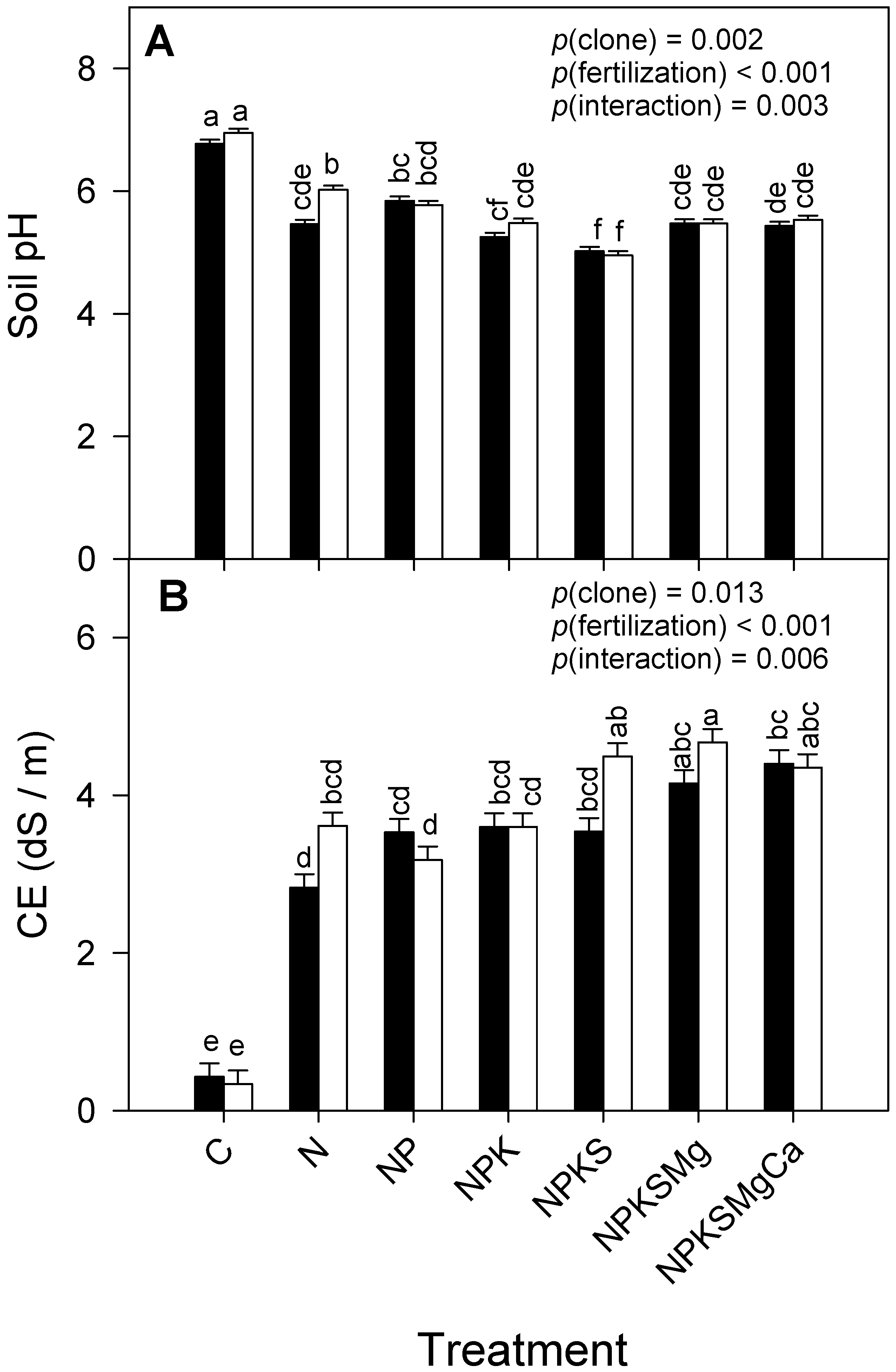
3.5. Soil pH and Electrical Conductivity
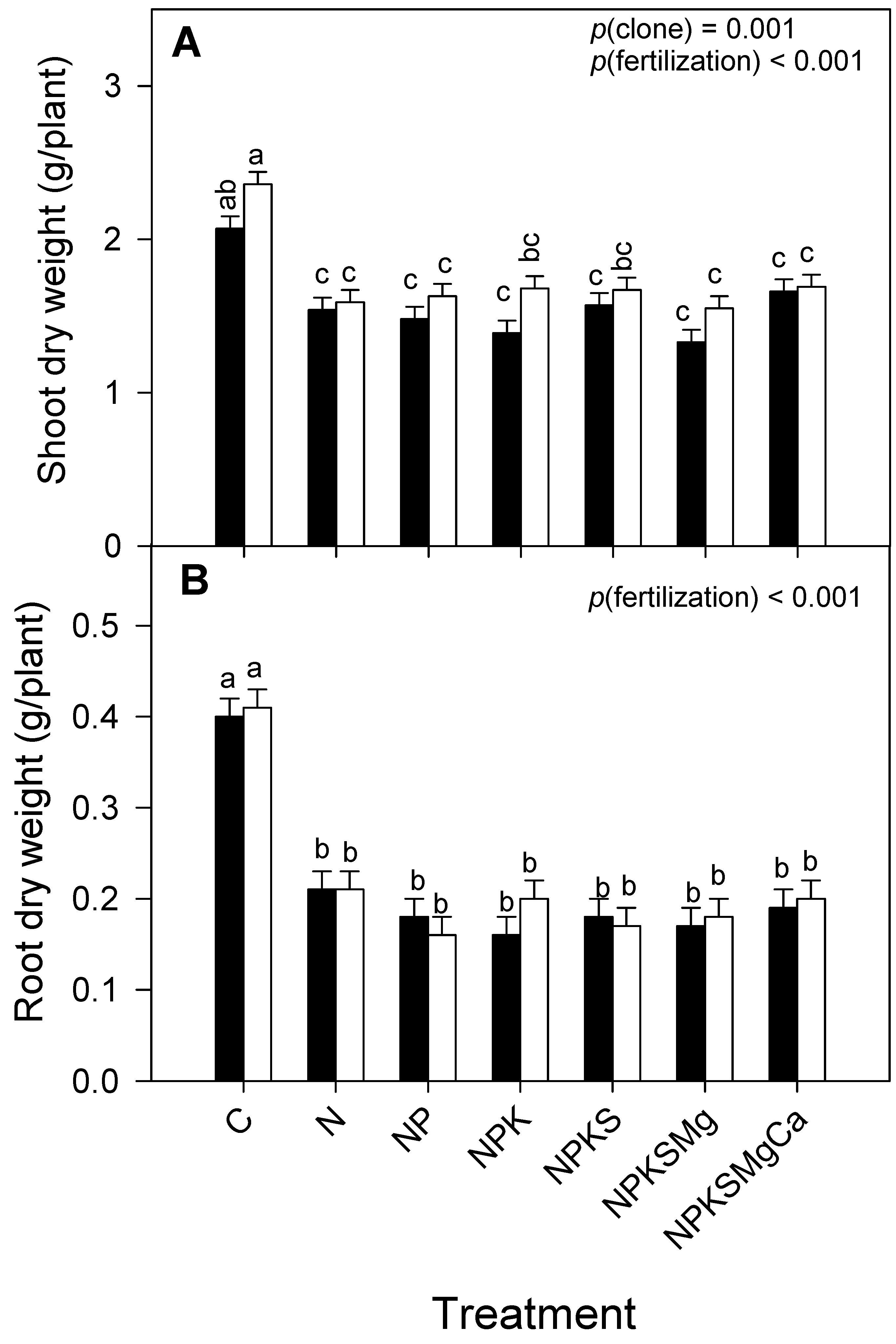
3.6. Shoot and Root Dry Biomass
3.7. Pearson’s Correlation Matrix
3.8. Nutrient Interactions with Cd
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chavez, E.; He, Z.L.; Stoffella, P.J.; Mylavarapub, R.S.; Li, Y.C.; Moyanod, B.; Baligar, V.C. Concentration of cadmium in cacao beans and its relationship with soil cadmium in southern Ecuador. Sci. Total Environ. 2015, 533, 205–214. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Liu, L.; Cao, X.; Ulhassan, Z.; Bilal, M.; Yang, X. Cadmium mobility in three contaminated soils amended with different additives as evaluated by dynamic flow-through experiments. Chemosphere 2020, 261, 127763. [Google Scholar] [CrossRef]
- Santander Ruiz, W.; Garay Montesa, R.; Verde Girbaub, C.; Mendieta Taboadaa, O. Determinación del contenido de cadmio en suelos, frutos, granos fermentados y secos, licor de cacao y chocolate en zonas productoras de la región San Martín. Rev. Soc. Quím. Per. 2021, 87, 39–49. [Google Scholar] [CrossRef]
- De Almeida, N.M.; de Almeida, A.-A.F.; de Almeida Santos, N.; do Nascimento, J.L.; de Carvalho Neto, C.H.; Pirovani, C.P.; Ahnert, D.; Baligar, V.C. Scion-rootstock interaction and tolerance to cadmium toxicity in juvenile Theobroma cacao plants. Sci. Hortic. 2022, 300, 111086. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Sun, X.; Yang, J.; Wang, D.; Shen, L.; Zhao, Y. Effects of sulfur application on cadmium bioaccumulation in tobacco and its possible mechanisms of rhizospheric microorganisms. J. Hazard. Mater. 2019, 368, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.R.M.; de Almeida, A.-A.F.; de Almeida Santos, N.; Pirovani, C.P. Tolerance strategies and factors that influence the cadmium uptake by cacao tree. Sci. Hortic. 2022, 293, 110733. [Google Scholar] [CrossRef]
- ICCO. Production of Cocoa Beans. Quarterly Bulletin of Cocoa Statistics. Cocoa Year 455 2020/21, 2022, (Vol. XLVI). Available online: https://www.icco.org/about-us/icco-news/398-456quarterly-bulletin-of-cocoa-statistics-november-2018.htm (accessed on 17 September 2022).
- Instituto Nacional de Estadística y Censos (INEC). Encuesta Sobre la Superficie y la Continua Producción Agrícola 2020; Government of Ecuador: Quito, Ecuador, 2021; 36p.
- Mite, F.; Carrillo, M.; Durango, W. Advances in monitoring the presence of cadmium in cocoa beans, soils, and water in Ecuador. In Proceedings of the 2010, XII Ecuadorian Congress of Soil Science, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Meter, A.; Atkinson, R.J.; Laliberte, B. Cadmio en el Cacao de América Latina y el Caribe—Análisis de la Investigación y Soluciones Potenciales para la Mitigación; Bioversity International: Roma, Italy, 2019; ISBN 978-92-9255-136-0. [Google Scholar]
- Argüello, D.; Chavez, E.; Lauryssen, F.; Vanderschueren, R.; Smolders, E.; Montalvo, D. Soil properties and agronomic factors affecting cadmium concentrations in cocoa beans: A nationwide survey in Ecuador. Sci. Total Environ. 2019, 649, 120–127. [Google Scholar] [CrossRef]
- Gramlich, A.; Tandy, S.; Gauggel, C.; López, M.; Perla, D.; Gonzalez, V.; Schulin, R. Soil cadmium uptake by cocoa in Honduras. Sci. Total Environ. 2018, 612, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.; Basu, S. Regulation of boron, iron, and cadmium transporters in maintaining proper balance between their deficiency and excess in plants (Chapter 12). In Metal and Nutrient Transporters in Abiotic Stress; Roychoudhury, A., Kumar, D., Deshmuk, R., Eds.; Academic Press: London, UK, 2021; pp. 237–250. ISBN 978-0-12-817955-0. [Google Scholar]
- Maksymiec, W.; Wojcik, M.; Krupa, Z. Variation in oxidative stress and photochemical activity in Arabidopsis thaliana leaves subjected to cadmium and excess copper in the presence or absence of jasmonate and ascorbate. Chemosphere 2007, 66, 421–427. [Google Scholar] [CrossRef]
- Liu, M.; Huang, Z.; Xie, K.; Guo, C.; Wang, Y.; Wang, X. Mitostasis is the central biological hub underlying the response of plants to cadmium stress. J. Hazard. Mater. 2023, 41, 129930. [Google Scholar] [CrossRef]
- Younis, U.; Malik, S.A.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Shah, M.H.R.; Rehman, R.A.; Ahmad, N. Biochar enhances the cadmium tolerance in spinach (Spinacia oleracea) through modification of Cd uptake and physiological and biochemical attributes. Environ. Sci. Pollut. Res. 2016, 23, 21385–21394. [Google Scholar] [CrossRef]
- Pereira de Araújo, R.; Furtado de Almeida, A.A.; Silva Pereira, L.; Mangabeira, P.A.O.; Olimpio Souza, J.; Pirovani, C.P.; Ahnert, D.; Baligar, V.C. Photosynthetic, antioxidant, molecular and ultrastructural responses of young cocoa plants to Cd toxicity in the soil. Ecotoxicol. Environ. Saf. 2017, 144, 148–157. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Zia-Ur-Rehman, M.; Farid, M.; Asma, M. Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Vera-Chang, J.; Cabrera-Verdezoto, R.; Morán-Morán, J.; Neira-Rengifo, K.; Haz-Burgos, R.; Vera-Barahona, J.; Molina-Triviño, H.; Moncayo-Carreño, O.; Díaz-Ocampo, E.; Cabrera-Verdesoto, C. Evaluación de tres métodos de polinización artificial en clones de cacao (Theobroma cacao L.) CCN-51. Idesia 2016, 34, 35–40. [Google Scholar] [CrossRef]
- Paladines-Rezabala, A.; Moreira-Morrillo, A.A.; Mieles, A.E.; Garcés-Fiallos, F.R. Avances en la comprensión de la interacción entre Ceratocystis cacaofunesta y Xyleborus ferrugineus (Coleoptera: Curculionidae: Scolytinae) en árboles de cacao. Sci. Agropecu. 2022, 13, 43–52. [Google Scholar] [CrossRef]
- Vera Barahona, J.; Suárez Capello, C.; Mogrovejo, E. Technical Description of Some Cocoa Hybrids and Clones Recommended by the National Institute of Agricultural Research (INIAP); INIAP, Pichilingue Tropical Experimental Station, Cocoa Program: Quevedo, Ecuador, 1984; (Technical Communication No. 12). Available online: https://repositorio.iniap.gob.ec/handle/41000/1602 (accessed on 7 May 2022).
- Quezada Crespo, C.J.; Carrillo Zenteno, M.D.; Morales Intriago, F.L.; Carrillo Alvarado, R.A. Nutrient critical levels and availability in soils cultivated with peach palm (Bactris gasipaes Kunth.) in Santo Domingo de Los Tsáchilas, Ecuador. Acta Agron. 2017, 66, 235–240. [Google Scholar] [CrossRef]
- Carrillo, M. Characterization of the Forms of Heavy Metals, Their Bioavailability and Their Adsorption and Mobility Dynamics in Ecuador Alone. Doctoral Thesis, Federal University of Viçosa, Minas Gerais, Brazil, 2003. [Google Scholar]
- Tezara, W.; De Almeida, J.; Valencia, E.; Cortes, J.; Bolaños, M. Actividad fotoquímica de clones élites de cacao Theobroma cacao L, ecuatoriano en el norte de la provincia Esmeraldas. Rev. Cient. Inter. Investig. Sab. 2015, 4, 37–52. Available online: http://revistasdigitales.utelvt.edu.ec/revista/index.php/investigacion_y_saberes/article/view/90 (accessed on 3 July 2022).
- Wang, F.Y.; Lin, X.G.; Yin, R. Inoculation with arbuscular mycorrhizal fungus Acaulospora mellea decreases Cu phytoextraction by maize from Cu-contaminated soil. Pedobiologia 2007, 51, 99–109. [Google Scholar] [CrossRef]
- Minitab. Userߣs Guide: Statistical Software; Version 19; Minitab Inc.: State College, PA, USA, 2021. [Google Scholar]
- Engbersen, N.; Gramlich, A.; Lopez, M.; Schwarz, G.; Hattendorf, B.; Gutierrez, O.; Schulin, R. Cadmium accumulation and allocation in different cacao cultivars. Sci. Total Environ. 2019, 678, 660–670. [Google Scholar] [CrossRef]
- Zhang, D.; Du, G.; Chen, D.; Shi, G.; Rao, W.; Li, X.; Jianga, Y.; Liua, S.; Wang, D. Effect of elemental sulfur and gypsum application on the bioavailability and redistribution of cadmium during rice growth. Sci. Total Environ. 2019, 657, 1460–1467. [Google Scholar] [CrossRef]
- García, G.; García, M.; Ramírez, H. Comportamiento de siete cultivares de Allium cepa L. ante diferentes niveles de estrés salino. Bioagro 2015, 27, 93–102. Available online: http://www.redalyc.org/articulo.oa?id=85741585005 (accessed on 27 May 2022).
- García Lozano, J.; Moreno Fonseca, L.P. Respuestas fisiológicas de Theobroma cacao L. en etapa de vivero a la disponibilidad de agua en el suelo. Acta Agron. 2016, 65, 44–50. Available online: https://www.redalyc.org/articulo.oa?id=169943143008 (accessed on 7 June 2022). [CrossRef]
- Almeida, A.A.-F.; Valle, R. Ecophysiology of the cacao tree. Braz. J. Plant Physiol. 2007, 19, 425–448. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca de Raton, FL, USA, 2001; p. 331. ISBN 0-8493-1575-1. [Google Scholar]
- Wan, G.; Najeeb, U.; Jilani, G.; Naeem, M.S.; Zhou, W. Calcium invigorates the cadmium-stressed Brassica napus L. plants by strengthening their photosynthetic system. Environ. Sci. Pollut. Res. 2011, 18, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Jalloh, M.A.; Chen, J.; Zhen, F.; Zhang, G. Effect of different N fertilizer forms on anti-oxidant capacity and grain yield of rice growing under Cd stress. J. Hazard. Mater. 2009, 162, 1081–1085. [Google Scholar] [CrossRef]
- Wang, K.; Fu, G.; Yu, Y.; Wan, Y.; Liu, Z.; Wang, Q.; Li, H. Effects of different potassium fertilizers on cadmium uptake by three crops. Environ. Sci. Pollut. Res. 2019, 26, 27014–27022. [Google Scholar] [CrossRef]
- Toledo, M. Management of Acid Soils in the Highlands of Honduras: Concepts and Methods; Inter-American Institute for Cooperation on Agriculture (IICA): Tegucigalpa, Honduras, 2016; 151p, ISBN 978-99979-55-01-2. [Google Scholar]
- Torres, D.; Mendoza, B.; Meru, M.L.; Gómez, C. Riesgos de salinización y sodificación por el uso de abonos orgánicos en la depresión de Quíbor-Venezuela. Multiciencias 2016, 16, 133–142. [Google Scholar]
- Martínez-Villavicencio, N.; López-Alonzo, C.V.; Pérez-Leal, R.; Basurto-Sotelo, M. Efectos por salinidad en el desarrollo vegetativo: Effects of salinity on vegetative growth. Tecnociencia Chihuahua 2011, 5, 156–161. [Google Scholar] [CrossRef]
- Nawaz, K.; Husain, K.; Majeed, A.; Khan, F.; Afghan, S.; Ali, K. Fatality of salt stress to plants: Morphological, physiological and biochemical aspects. Afr. J. Biotechnol. 2010, 9, 5475–5480. [Google Scholar]
- Rodríguez-Pérez, L. Implicaciones fisiológicas de la osmorregulación en plantas. Agron. Colomb. 2006, 24, 28–37. Available online: https://www.redalyc.org/articulo.oa?id=180316238004 (accessed on 3 October 2022).
- Sadeghian Khalajabadi, S.; Zapata, R.D. Crecimiento de café (Coffea arabica L.) durante la etapa de almácigo en respuesta a la salinidad generada por fertilizantes. Revis Cienc. Agríc. 2014, 31, 40–50. [Google Scholar] [CrossRef]
- Cai, W.; Navarro, D.A.; Du, J.; Ying, G.; Yang, B.; McLaughlin, M.J.; Kookana, R.S. Increasing ionic strength and valency of cations enhance sorption through hydrophobic interactions of PFAS with soil surfaces. Sci. Total Environ. 2022, 817, 152975. [Google Scholar] [CrossRef]
- Cardero Llopiz, Y.; Garcell Puyáns, L.R. Efecto del potencial iónico sobre la adsorción específica de cationes en suspensiones de laterita y de cieno carbonatado. Tecnología Química 2010, 30, 78–86. Available online: https://www.redalyc.org/articulo.oa?id=445543771009 (accessed on 7 March 2023).
- Carbonel Ramos, D. Adsorción de Cadmio, Cobre y Plomo en Bentonita, Caolinita y Zeolita Naturales y Modificadas: Una Revisión de los Parámetros de Operación, Isotermas y Cinética. Ingeniería 2018, 23, 252–273. [Google Scholar] [CrossRef]
- He, L.; Dai, Z.; Liu, X.; Tang, C.; Xu, J. Effect of alkaline lignin on immobilization of cadmium and lead in soils and the associated mechanisms. Chemosphere 2021, 281, 130969. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, D.; Liu, X.; Meng, J.; Tang, C.; Xu, J. Remediation of As(III) and Cd(II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. J. Hazard. Mater. 2018, 348, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Lovera, E.; Coronel, I.; Jaimez, R.; Urich, R.; Pereyra, G.; Araque, O.; Tezara, W. Ecophysiological traits of adult trees of Criollo cocoa cultivars (Theobroma cocoa L.) from a germplasm bank in Venezuela. Exp. Agric. 2016, 52, 137–153. [Google Scholar] [CrossRef]
- Héctor Ardisana, E.F.; Torres García, A.; Fosado Téllez, O.; Álava Álava, J.; Sancán Pin, G.T.; León Aguilar, R. Contenido de clorofilas totales en doce clones de cacao (Theobroma cacao L.). Rev. Agroc. 2018, 20, 11–18. [Google Scholar] [CrossRef]
- Ahmad, A. Salinity in soil increased cadmium uptake and accumulation potential of two terrestrial plants. Int. J. Biol. Sci. 2017, 10, 132–142. [Google Scholar]
- Akhter, S.M.; Noreen, S.; Mahmood, S.; Athar, H.-R.; Ashraf, M.; Abdullah Alsahli, A.; Ahmad, P. Influence of salinity stress on PSII in barley (Hordeum vulgare L.) Genotypes, probed by chlorophyll-a fluorescence. J. King Saud Univ. Sci. 2021, 33, 101239. [Google Scholar] [CrossRef]
- Gonçalves, A.Z.; Latansio, S.; Detmann, K.C.; Marabesi, M.A.; Neto, A.A.C.; Aidar, M.P.M.; Mercier, H. What does the RuBisCO activity tell us about a C3-CAM plant? Plant Physiol. Biochem. 2020, 147, 172–180. [Google Scholar] [CrossRef]
- Sage, R.F.; Stata, M. Photosynthetic diversity meets biodiversity: The C4 plant example. J. Plant Physiol. 2015, 172, 104–119. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, J.; Tezara, W.; Herrera, A. Physiological responses to drought and experimental water deficit and waterlogging of four clones of cocoa (Theobroma cacao L.) selected for cultivation in Venezuela. Agric. Water Manag. 2016, 171, 80–88. [Google Scholar] [CrossRef]
- Souza dos Santos, M.L.; de Almeida, A.-A.F.; da Silva, N.M.; Machado, B.; Oliveira, B.R.M.; Silva, J.V.S.; Souza, J.O., Jr.; Ahnert, D.; Baligar, V.C. Mitigation of cadmium toxicity by zinc in juvenile cacao: Physiological, biochemical, molecular and micromorphological responses. Environ. Exp. Bot. 2020, 179, 104201. [Google Scholar] [CrossRef]
- Hao, X.Z.; Zhou, D.M.; Li, D.D.; Jiang, P. Cadmium and Zinc Accumulation of Ornamental Sunflower (Helianthus annuus L.) in Contaminated Soil with Different Amendments. Pedosphere 2012, 22, 631–639. [Google Scholar] [CrossRef]
- Lyčka, M.; Barták, M.; Helia, O.; Kopriva, S.; Moravcová, D.; Hajek, J.; Fojt, L.; Cmelíl, R.; Fajkus, J.; Fojtová, M. Sulfate supplementation affects nutrient and photosynthetic status of Arabidopsis thaliana and Nicotiana tabacum differently under prolonged exposure to cadmium. J. Hazard. Mater. 2023, 445, 130527. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Castro, A.V.; de Almeida, A.-A.F.; Pirovani, C.P.; Reis, G.S.M.; Almeida, N.M.; Mangabeira, P.A.O. Morphological, biochemical, molecular and ultrastructural changes induced by Cd toxicity in seedlings of Theobroma cacao L. Ecotoxicol. Environ. Saf. 2015, 115, 174–186. [Google Scholar] [CrossRef]
- Llatance, W.; Gonza, C.; Guzmán, W.; Pariente, E. Bioacumulación de cadmio en el cacao (Theobroma cacao) en la comunidad nativa de Pakun, Perú. Rev. For. Perú. 2018, 33, 63–75. [Google Scholar] [CrossRef]
- Tantalean Pedraza, E.; Huauya Rojas, M.A. Distribución del contenido de cadmio en los diferentes órganos del cacao CCN-51 en suelo aluvial y residual en las localidades de Jacintillo y Ramal de Aspuzana. Rev. Inv. Agrop. Sust. 2017, 1, 69–78. [Google Scholar] [CrossRef]
- Barraza, F.; Schreck, E.; Uzu, G.; Lévêque, T.; Zouiten, C.; Boidot, M.; Maurice, L. Beyond cadmium accumulation: Distribution of other trace elements in soils and cacao beans in Ecuador. Environ. Res. 2021, 192, 110241. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-Hernández, C.O.; Arévalo-Gardini, E.; Barraza, F.; Farfán, A.; He, Z.; Baligar, V.C. Growth and nutritional responses of wild and domesticated cocoa clones to soil cd stress. Sci. Total Environ. 2021, 763, 144021. [Google Scholar] [CrossRef]
- Ullah, I.; Wang, Y.; Eide, D.J.; Dunwell, J.M. Evolution, and functional analysis of Natural Resistance-Associated Macrophage Proteins (NRAMPs) from Theobroma cocoa and their role in cadmium accumulation. Sci. Rep. 2018, 8, 14412. [Google Scholar] [CrossRef] [PubMed]
- Puentes-Páramo, Y.; Menjivar-Flores, J.; Aranzazu-Hernández, F. Eficiencias en el uso de nitrógeno, fósforo y potasio en clones de cacao (Theobroma cacao L.). Bioagro 2014, 26, 99–106. Available online: https://www.redalyc.org/articulo.oa?id=85731100004 (accessed on 22 March 2023).
- Rosas-Patiño, G.; Puentes-Páramo, Y.J.; Menjivar-Flores, J.C. Efecto del pH sobre la concentración de nutrientes en cacao (Theobroma cacao L.) en la Amazonia Colombiana. Rev. U.D.C.A Act. Div. Cient. 2021, 24, 1–10. [Google Scholar] [CrossRef]
- López, M.; López de Rojas, I.; España, M.; Izquierdo, A.; Herrera, L. Efecto de la fertilización inorgánica sobre la disponibilidad de nutrimentos en el suelo, nivel nutricional de la planta y hongos micorrícicos arbusculares en plantaciones de Theobroma cacao. Agronomía Trop. 2007, 57, 31–43. Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0002192X2007000100005&lng=es&tlng=es (accessed on 21 June 2022).
- Quinteiro, M.; Furtado de Almeida, A.-A.; Schramm, M.; Pinto Gomes, F.; Viana Pires, M.; Baligar, V.C. Aluminum effects on growth photosynthesis and mineral nutrition of cocoa clones. J. Plant Nutr. 2013, 36, 1161–1179. [Google Scholar] [CrossRef]
- Correa, J.E.; Ramírez, R.; Ruíz, O.; Leiva, E.I. Effect of soil characteristics on cadmium absorption and plant growth of Theobroma cacao L. seedlings. J. Sci. Food Agric. 2021, 101, 5437–5445. [Google Scholar] [CrossRef]
- Liu, J.G.; Liang, J.S.; Li, K.Q.; Zhang, Z.J.; Yu, B.Y.; Lu, X.L.; Yang, J.C.; Zhu, Q.S. Correlations between cadmium and mineral nutrients in absorption and accumulation in various clones of rice under cadmium stress. Chemosphere 2003, 52, 1467–1473. [Google Scholar] [CrossRef]
- Tezara, W.; Coronel, I.; Dzib, G.; Calvo Irabien, L.M. Relación entre la capacidad fotosintética y el contenido de aceites esenciales de Lippia graveolens (Verbenaceae) en dos localidades con diferencias en precipitación anual. Interciencia 2013, 38, 669–675. Available online: https://www.redalyc.org/articulo.oa?id=33929480010 (accessed on 4 May 2022).
- Oliva, M.; Rubio, K.; Epquin, M.; Marlo, G.; Leiva, S. Cadmium Uptake in Native Cacao Trees in Agricultural Lands of Bagua, Peru. Agronomy 2020, 10, 1551. [Google Scholar] [CrossRef]
- Scaccabarozzi, D.; Castillo, L.; Aromatisi, A.; Milne, L.; Búllon Castillo, A.; Muñoz-Rojas, M. Soil, Site, and Management Factors Affecting Cadmium Concentrations in Cacao-Growing Soils. Agronomy 2020, 10, 806. [Google Scholar] [CrossRef]


| Nutrients | Fertilizer Content | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P2O5 | K2O | S | MgO | CaO | Urea | (NH₄)₂SO₄ | Ca(NO₃)₂ | DAP | MgSO₄ | KCl | |
| Treatment | g (700 g Soil)−1 | |||||||||||
| Control | - | - | - | - | - | - | - | - | - | - | - | - |
| N | 2.15 | - | - | - | - | - | 4.71 | - | - | - | - | - |
| N-P | 2.15 | 1.81 | - | - | - | - | 3.15 | - | - | 3.94 | - | - |
| N-P-K | 2.15 | 1.81 | 1.18 | - | - | - | 3.15 | - | - | 3.94 | - | 1.96 |
| N-P-K-S | 2.15 | 1.81 | 1.18 | 0.88 | - | - | 1.52 | 3.6 | - | 3.94 | - | 1.96 |
| N-P-K-S-Mg | 2.15 | 1.81 | 1.18 | 0.88 | 0.29 | - | 1.81 | 2.94 | - | 3.94 | 1.00 | 1.96 |
| N-P-K-S-Mg-Ca | 2.15 | 1.81 | 1.18 | 0.88 | 0.29 | 0.6 | 1.04 | 2.94 | 2.32 | 3.94 | 1.00 | 1.96 |
| Physiological Traits | DAS | p (Clone) | p (Fertilization) | p (Interaction) |
|---|---|---|---|---|
| A (μmol m−2 s−1) | 40 days | 0.710 | 0.098 | 0.844 |
| E (mmol m−2 s−1) | 0.003 | 0.088 | 0.961 | |
| gs (mmol m−2 s−1) | 0.593 | 0.266 | 0.573 | |
| Ci (μmol mol−1) | 0.334 | 0.061 | 0.647 | |
| WUE (mmol mol−1) | 0.074 | 0.011 | 0.310 | |
| A (μmol m−2 s−1) | 80 days | 0.639 | <0.001 | 0.086 |
| E (mmol m−2 s−1) | 0.149 | <0.001 | 0.331 | |
| gs (mmol m−2 s−1) | 0.953 | <0.001 | 0.597 | |
| Ci (μmol mol−1) | 0.450 | 0.031 | 0.032 | |
| WUE (mmol mol−1) | 0.120 | 0.007 | 0.202 | |
| Absorption Cd (mg kg−1) | <0.001 | <0.001 | 0.660 | |
| Translocation Cd | <0.001 | <0.001 | <0.001 | |
| Shoot Cd content (mg kg−1) | <0.001 | <0.001 | <0.001 | |
| Root Cd content (mg kg−1) | <0.001 | <0.001 | 0.070 | |
| N (dag kg−1) | <0.001 | <0.001 | <0.001 | |
| P (dag kg−1) | <0.001 | <0.001 | <0.001 | |
| K (dag kg−1) | 0.764 | 0.002 | 0.052 | |
| S (dag kg−1) | <0.001 | <0.001 | <0.001 | |
| Mg (dag kg−1) | 0.751 | <0.001 | 0.236 | |
| Ca (dag kg−1) | 0.831 | <0.001 | <0.001 | |
| Soil pH | 0.002 | <0.001 | 0.003 | |
| CE (dS m−1) | 0.013 | <0.001 | 0.006 | |
| Shoot dry weight (g plant−1) | 0.001 | <0.001 | 0.557 | |
| Root dry weight (g plant−1) | 0.558 | <0.001 | 0.758 |
| Chlorophyll Index (SPAD) | |||||
|---|---|---|---|---|---|
| Clone | 45 DAS | 60 DAS | 75 DAS | 90 DAS | |
| CCN-51 | Control | 43.32 ± 1.73 | 45.59 ± 1.42 | 49.07 ± 0.59 | 46.76 ± 2.86 a |
| N | 40.70 ± 0.48 | 45.49 ± 1.02 | 49.2 ± 1.55 | 46.64 ± 2.29 a | |
| N-P | 42.28 ± 1.14 | 44.51 ± 1.74 | 45.5 ± 1.17 | 42.08 ± 2.25 ab | |
| N-P-K | 42.06 ± 1.26 | 44.55 ± 0.96 | 48.5 ± 2.09 | 40.18 ± 2.08 abc | |
| N-P-K-S | 42.84 ± 0.82 | 45.97 ± 1.17 | 46.81 ± 4.21 | 45.68 ± 0.36 a | |
| N-P-K-S-Mg | 41.24 ± 1.06 | 42.98 ± 1.27 | 45.41 ± 1.63 | 34.07 ± 0.12 bcd | |
| N-P-K-S-Mg-Ca | 43.17 ± 0.81 | 43.88 ± 2.19 | 56.84 ± 1.01 | 37.98 ± 3.46 abcd | |
| EET-103 | Control | 39.28 ± 2.00 | 42.33 ± 1.85 | 45.33 ± 1.20 | 43.52 ± 1.29 ab |
| N | 40.00 ± 0.83 | 43.81 ± 0.45 | 44.87 ± 1.13 | 39.01 ± 3.01 abcd | |
| N-P | 40.27 ± 1.48 | 42.30 ± 1.38 | 43.06 ± 1.92 | 35.69 ± 0.60 abcd | |
| N-P-K | 40.72 ± 0.98 | 43.29 ± 1.41 | 44.88 ± 1.40 | 28.22 ± 0.51 d | |
| N-P-K-S | 41.46 ± 0.90 | 43.53 ± 1.05 | 45.27 ± 2.43 | 39.49 ± 1.17 abc | |
| N-P-K-S-Mg | 39.77 ± 0.59 | 43.56 ± 0.63 | 43.27 ± 1.56 | 30.88 ± 2.55 cd | |
| N-P-K-S-Mg-Ca | 39.22 ± 1.02 | 42.56 ± 1.35 | 43.23 ± 0.52 | 39.55 ± 3.08 abc | |
| p(clone) | 0.003 | 0.033 | 0.014 | <0.001 | |
| p(fertilization) | 0.808 | 0.876 | 0.589 | 0.002 | |
| p(interaction) | 0.721 | 0.859 | 0.542 | 0.005 | |
| Organ Mass | Cd Content | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Root | Shoot | CI 90 | Shoot | Root | Plant | AE | TE | pH | EC | A at 40 DAS | E at 40 DAS | A at 80 DAS | N | P | S | Mg |
| Shoot mass | 0.83 | ||||||||||||||||
| Shoot Cd content | NS | NS | NS | ||||||||||||||
| Root Cd content | NS | NS | NS | NS | |||||||||||||
| Whole-plant Cd content | NS | NS | NS | NS | 0.52 | ||||||||||||
| Absorption efficiency | −0.82 | −0.58 | NS | 0.80 | NS | 0.78 | |||||||||||
| Translocation efficiency | −0.54 | NS | NS | NS | −0.52 | NS | 0.60 | ||||||||||
| Soil pH | 0.82 | 0.67 | NS | −0.65 | NS | −0.64 | −0.81 | −0.50 | |||||||||
| Soil EC | −0.86 | −0.65 | −0.55 | 0.67 | NS | 0.67 | 0.85 | NS | −0.83 | ||||||||
| Ci at 40 DAS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | −0.62 | ||||||
| A at 80 DAS | 0.77 | 0.71 | NS | NS | NS | NS | −0.65 | NS | 0.69 | −0.80 | NS | 0.57 | |||||
| N | −0.86 | −0.77 | NS | 0.64 | NS | 0.62 | 0.83 | 0.59 | −0.84 | 0.80 | NS | NS | −0.65 | ||||
| P | −0.69 | −0.55 | NS | 0.78 | NS | 0.78 | 0.87 | NS | −0.82 | 0.83 | NS | NS | −0.64 | 0.79 | |||
| S | −0.50 | NS | −0.63 | 0.64 | 0.63 | 0.66 | 0.70 | NS | −0.56 | 0.76 | NS | NS | −0.60 | NS | 0.79 | ||
| Mg | −0.74 | −0.66 | NS | 0.54 | NS | 0.55 | 0.69 | NS | −0.67 | 0.81 | NS | NS | −0.77 | 0.65 | 0.67 | 0.64 | |
| Ca | −0.52 | NS | NS | NS | NS | NS | NS | NS | NS | 0.53 | NS | NS | −0.54 | NS | NS | NS | 0.62 |
| Plant Species | Purpose of the Research | Nutrient Interactions with Cd | Authors |
|---|---|---|---|
| Theobroma cacao (54 genotype) 13 genotype of Wild cacao from river basins of Peruvian Amazon Peruvian farmers’ 13 cacao genotypes, ICT collection Brazilian cacao, 13 genotypes National and international 14 cacao genotypes (from Peru, Ecuador, Trinidad and Tobago, and Costa Rica) | Determine the interactions between macro and micronutrients with Cd uptake and bioaccumulation. Identify cocoa genetic materials with high and low Cd extraction capacity. | Negative, N increased the concentration of Cd in the shoots. | [27] |
| Theobroma cacao Cocoa progeny: Catongo × Catongo CCN-10 × SCA-6 | Identify mechanisms of tolerance or resistance to Cd stress in cocoa progenies of high heterozygosity and of homozygotes. | Negative, S benefits the increase in Cd concentration in leaf tissues. | [28] |
| Oryza sativa (20 cultivars) Origen (genotypes) Philippines (NPT) China (Indica, Hybrid Indica, Indica, Japonica) Japan (Japonica) Korea (Japonica) | Genotypic differences in Cd accumulation and relationships between Cd and mineral nutrients. | Negative, Fe, Zn, and Cu cations present a significant correlation with Cd concentrations in shoots and roots. | [29] |
| Theobroma cacao CCN-51 | To evaluate whether zinc has a beneficial effect on reducing physiological, anatomical, and molecular disorders caused by Cd. | Positive, the addition of Zn decreases the uptake, transport, and accumulation of Cd in shoots. | [30] |
| Brasica Napus | To evaluate the role of calcium in reducing Cd-induced toxicity. | Positive, Ca competes with Cd and reduces its transport and accumulation in shoots. | [31] |
| Oryza sativa | To study whether nitrogen forms (NO3−, NH4+) have an antagonistic or synergistic effect on Cd absorption and accumulation. | Negative, nitrate has a synergistic effect on increasing Cd concentrations in leaves, stems, and panicles. | [32] |
| Helianthus annuus | To examine the benefits of bovine manure, muriate of potassium (K), and salicylic acid on Cd accumulation. | Negative, K application increases the translocation of Cd from the root to aerial parts. | [33] |
| Oryza sativa (cv. Yuanyouyihao) Triticum aestivum (cv. 051), Pak choi (cv. Wuyueman) | To examine the influence of three different K fertilizers (KCl, K2SO4, and KNO3) on Cd bioaccumulation. | Negative, KCl application significantly increases Cd bioaccumulation in pak Choi sprouts and in rice and wheat grains. | [34] |
| Oryza sativa (cv. Y-liangyou 1) | Investigate the effects of different forms and concentrations of S on Cd accumulation. | Positive, the application of S reduces the concentration of Cd in rice grains. | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Pérez, J.J.; Pincay-Ganchozo, R.A.; Carrillo-Zenteno, M.D.; Reynel, V.; Peña-Salazar, K.; Tezara, W. Macronutrient Fertilization and Cadmium Absorption in Two Cocoa Clones. Horticulturae 2023, 9, 1223. https://doi.org/10.3390/horticulturae9111223
Reyes-Pérez JJ, Pincay-Ganchozo RA, Carrillo-Zenteno MD, Reynel V, Peña-Salazar K, Tezara W. Macronutrient Fertilization and Cadmium Absorption in Two Cocoa Clones. Horticulturae. 2023; 9(11):1223. https://doi.org/10.3390/horticulturae9111223
Chicago/Turabian StyleReyes-Pérez, Juan J., Roger A. Pincay-Ganchozo, Manuel D. Carrillo-Zenteno, Víctor Reynel, Karina Peña-Salazar, and Wilmer Tezara. 2023. "Macronutrient Fertilization and Cadmium Absorption in Two Cocoa Clones" Horticulturae 9, no. 11: 1223. https://doi.org/10.3390/horticulturae9111223
APA StyleReyes-Pérez, J. J., Pincay-Ganchozo, R. A., Carrillo-Zenteno, M. D., Reynel, V., Peña-Salazar, K., & Tezara, W. (2023). Macronutrient Fertilization and Cadmium Absorption in Two Cocoa Clones. Horticulturae, 9(11), 1223. https://doi.org/10.3390/horticulturae9111223





