Effect of Climatic Variations in the Floral Phenology of Berberis microphylla and Its Pollinator Insects
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Climatic Conditions
3.2. Floral Phenology
3.3. Pollinator Insects
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arena, M.E.; Giordani, E.; Radice, S. Phenological growth and development stages of the native Patagonian fruit species Berberis buxifolia Lam. J. Food Agric. Environ. 2013, 11, 1323–1327. [Google Scholar]
- Arena, M.E.; Radice, S. Shoot growth and development of Berberis buxifolia Lam. in Tierra del Fuego (Patagonia). Sci. Hortic. 2014, 165, 5–12. [Google Scholar] [CrossRef]
- Radice, S.; Arena, M.E. Characterization and evaluation of Berberis microphylla G. Forst pollen grains. Adv. Hortic. Sci. 2016, 30, 31–37. [Google Scholar] [CrossRef]
- Radice, S.; Arena, M.E. Flower anatomy related to blooming development of Berberis microphylla G. Forst (Berberidaceae). Adv. Hortic. Sci. 2017, 30, 39–44. [Google Scholar] [CrossRef]
- Radice, S.; Arena, M.E. Reproductive shoots of Berberis microphylla G. Forst. in relation with the floral bud development and the fruit set. Heliyon 2018, 4, e00927. [Google Scholar] [CrossRef]
- Arena, M.E. Estudio de Algunos Fenómenos Morfofisiológicos y Cambios Bioquímicos en Berberis microphylla G. Forst. (sinónimo B. buxifolia Lam.) Asociados a la Formación y Maduración de Frutos en Tierra de Fuego y Su Relación con la Producción de Metabolitos Útiles. Ph.D. Thesis, Universidad Nacional del Sur, Bahía Blanca, Argentina, 2016. [Google Scholar]
- Pintos, F.M.; Flores, M.C.; Rodoni, L.; Radice, S.; Arena, M. Efecto de la liofilización en la retención de antocianinas de los frutos de calafate (Berberis microphyla G. Forst.) y su incorporación en cerveza. Investig. Cienc. Univ. 2019, 3, 48. Available online: http://sedici.unlp.edu.ar/handle/10915/94836 (accessed on 22 October 2023).
- Gutiérrez, R.S.; Pincheira, C.G. Description of the antioxidant capacity of Calafate berries (Berberis microphylla) collected in southern Chile. Food Sci. Technol. 2020, 41, 864–869. [Google Scholar] [CrossRef]
- Arena, M.E.; Lencinas, M.V.; Radice, S. Variability in floral traits and reproductive success among and within populations of Berberis microphylla G. Forst., an underutilized fruit species. Sci. Hortic. 2018, 241, 65–73. [Google Scholar] [CrossRef]
- Arena, M.E.; Pastur, G.M.; Lencinas, M.V.; Soler, R.; Bustamante, G. Changes in the leaf nutrient and pigment contents of Berberis microphylla G. Forst. in relation to irradiance and fertilization. Heliyon 2020, 6, e03264. [Google Scholar] [CrossRef]
- Suárez, F.J. Polinización en Berberis microphylla G. Forst. Estudio de la Participación de los Insectos en Esta Fase de Desarrollo. Graduation Thesis, Universidad de Morón, Buenos Aires, Argentina, 2015. [Google Scholar]
- Arena, M.E.; Giordani, E.; Radice, S. Flowering, fruiting and leaf and seed variability in Berberis buxifolia, a Patagonian native fruit species. In Native Species: Identification, Conservation and Restoration, 1st ed.; Marin, L., Kovaè, D., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; Chapter 4; pp. 117–136. ISBN 978-1-161470-613-7. [Google Scholar]
- Parry, M.L.; Canziani, O.F.; Palutikof, J.P.; van der Linden, P.J.; Hanson, C.E. Climate Change 2007: Impacts, Adaptation and Vulnerability; Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- McEwan, R.W.; Brecha, R.J.; Geiger, D.R.; John, G.P. Flowering phenology change and climate warming in southwestern Ohio. Plant Ecol. 2011, 212, 55–61. [Google Scholar] [CrossRef]
- Sherry, R.A.; Zhou, X.; Gu, S.; Arnone, J.A., III; Schimel, D.S.; Verburg, P.S.; Wallace, L.L.; Luo, Y. Divergence of reproductive phenology under climate warming. Proc. Natl. Acad. Sci. USA 2007, 104, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant phenology and global climate change: Current progresses and challenges. Glob. Chang. Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.E.; Both, C. Shifts in phenology due to global climate change: The need for a yardstick. Proc. R. Soc. B Biol. 2005, 1581, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Cosmulescu, S.; Ștefănescu, D.; Stoenescu, A.M. Variability of phenological behaviours of wild fruit tree species based on discriminant analysis. Plants 2021, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.R. Algunos aspectos de influencia do clima e temperatura sobre a cultura do arroz irrigado no sul do Brasil. Lavoura Arrozeira 1990, 43, 9–11. [Google Scholar]
- Giordani, E.; Muller, M.; Gambineri, M.; Paffetti, D.; Arena, M.E.; Radice, S. Genetic and morphological analysis of Berberis microphylla G. Forst. accessions in southern Tierra del Fuego. Plant Biosyst. 2017, 151, 715–728. [Google Scholar] [CrossRef]
- Radice, S. Biología Floral y Reproductiva del Cultivar Forastero [Prunus persica (L.) Batsch] Rosaceae, Prunoideae, en Estiones Crecidos Sobre Pies Francos o Clonales Macro y Micropropagados. Ph.D. Thesis, Facultad de Ciencias Exactas y Naturales UBA, Buenos Aires, Argentina, 2005. [Google Scholar]
- Radice, S.; Arena, M.E. Effect of different pollination treatments on Berberis microphylla G. Forst, a Patagonian barberry. Acta Hortic. 2019, 1231, 75–80. [Google Scholar] [CrossRef]
- Radice, S.; Alonso, M.; Arena, M.E. Berberis microphylla: A species with Phenotypic Plasticity in Different Climatic Conditions. Int. J. Agric. Biol. 2018, 20, 2221–2229. [Google Scholar]
- Keller, M.; Tarara, J.M. Warm spring temperatures induce persistent season-long changes in shoot development in grapevines. Ann. Bot. 2010, 106, 131–141. [Google Scholar] [CrossRef]
- Geissler, C.; Davidson, A.; Niesenbaum, R.A. The influence of climate warming on flowering phenology in relation to historical annual and seasonal temperatures and plant functional traits. Peer J 2023, 11, e15188. [Google Scholar] [CrossRef]
- Bradley, N.L.; Leopold, A.C.; Ross, J.; Huffaker, W. Phenological changes reflect climate change in Wisconsin. Proc. Natl. Acad. Sci. USA 1999, 17, 9701–9704. [Google Scholar] [CrossRef] [PubMed]
- Zinn, K.E.; Tunc-Ozdemir, M.; Harper, J.F. Temperature stress and plant sexual reproduction: Uncovering the weakest links. J. Exp. Bot. 2010, 61, 1959–1968. [Google Scholar] [CrossRef]
- Yu, H.; Luedeling, E.; Xu, J. Winter and spring warming result in delayed spring phenology on the Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2010, 107, 22151–22156. [Google Scholar] [CrossRef]
- Menzel, A. Phenology: Its importance to the global change community. Clim. Chang. 2002, 54, 379. [Google Scholar] [CrossRef]
- Forrest, J.R. Complex responses of insect phenology to climate change. Curr. Opin. Insect Sci. 2016, 17, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.M. Hoverfly Communities in Semi-Natural Grasslands, and Their Role in Pollination. Ph.D. Thesis, Swansea University, Swansea, UK, 2017. [Google Scholar]
- Magnusson Rundqvist, M. Examining the Link between Temperature and Flight Phenology in Hoverflies (Diptera: Syrphidae) Using Swedish Citizen Science Data. Bachelor’s Thesis, Linköpings Universitet, Linköpings, Sweden, 2021. [Google Scholar]
- Van Rijn, P.C.J.; Wäckers, F.L. Nectar accessibility determines fitness, flower choice and abundance of hoverflies that provide natural pest control. J. Appl. Ecol. 2016, 53, 925–933. [Google Scholar] [CrossRef]
- Correa, J. Syrphidae: A Guide to Natural History and Identification of Common Genera in Santa Cruz County. Bachelor’s Thesis, University of California, Santa Cruz, CA, USA, 2019. [Google Scholar]
- Graham-Taylor, L.G.; Stubbs, A.E.; Brook, M. de L. Changes in phenology of hoverflies in a central England garden. Insect Conserv. Divers. 2009, 2, 29–35. [Google Scholar] [CrossRef]
- Hassall, C.; Owen, J.; Gilbert, F. Phenological shifts in hoverflies (Diptera: Syrphidae): Linking measurement and mechanism. Ecography 2016, 40, 853–863. [Google Scholar] [CrossRef]
- Freitas, L.; Sazima, M. Daily blooming pattern and pollination by syrphids in Sisyrinchium vaginatum (Iridaceae) in southeastern Brazil. J. Torrey Bot. Soc. 2003, 130, 55–61. [Google Scholar] [CrossRef]
- Wolda, H. Altitude, habitat and tropical insect diversity. Biol. J. Linn. Soc. 1987, 30, 313–323. [Google Scholar] [CrossRef]
- Ball, S.; Morris, R. Britain’s Hoverflies; An Introduction to the Hoverflies of Britain; Princeton University Press: Princeton, NJ, USA, 2013; Volume 9, ISBN 9780691156590. [Google Scholar]
- Petanidou, T.; Smets, E. Does temperature stress induce nectar secretion in Mediterranean plants? New Phytol. 1996, 133, 513–518. [Google Scholar] [CrossRef]
- Bressin, S.; Willmer, P.G. Estimation of thermal constants: The importance of using equilibrium temperature rather than ambient temperature demonstrated with hoverflies (Diptera, Syrphidae, genus Eristalis). J. Exp. Biol. 2000, 203, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- Rojo, S.; Gilbert, F.; Marcos-García, M.A.; Nieto, J.M.; Mier, M.P. A World Review of Predatory Hoverflies (Diptera, Syrphidae: Syrphinae) and Their Prey, 1st ed.; Centro Iberoamericano de la Biodiversidad (CIBIO), Ed.; Universidad de Alicante: Alicante, Spain, 2003; 219p. [Google Scholar]
- Zheng, Z.; Liu, H.; Wang, X.; Wu, X.; Chen, Y.; Deng, J.; Chen, X.; Li, Y.; Pu, D. Development and reproduction of the hoverfly Eupeodes corollae (Diptera: Syrphidae). J. Earth Sci. Environ. Stud. 2019, 4, 654–660. [Google Scholar]
- Huang, W.; Lin, J. Morfología Comparativa de las Tibias Posteriores Modificadas de los Machos en Cuatro Géneros de Abejas Euglosinas (apinae: Euglossini) y Biología de una Larva Polinívora, Allograpta micrura (diptera: Syrphidae), en Flores de Castilleja talamancensis (Scrhophulariaceae). Master’s Thesis, Universidad de Costa Rica, San Pedro de Montes de Oca, Costa Rica, 2005. [Google Scholar]
- Ramadan, M.M.; Bayoumy, M.H.; Afifi, M. Ecological studies on the Peach green aphid, Myzus persicae and its natural enemies. J. Plant Prot. Pathol. 2022, 13, 29–35. [Google Scholar] [CrossRef]
- Bugg, R.L.; Colfer, R.G.; Chaney, W.E.; Smith, H.A.; Cannon, J. Flower Flies (Syrphidae) and Other Biological Control Agents for Aphids in Vegetable Crops; ANR Publication 8285; University of California ANR: Davis, CA, USA, 2008; pp. 1–25. [Google Scholar] [CrossRef]
- Iler, A.M.; Inouye, D.W.; Høye, T.T.; Miller-Rushing, A.J.; Burkle, L.A.; Johnston, E.B. Maintenance of temporal synchrony between syrphid flies and floral resources despite differential phenological responses to climate. Glob. Chang. Biol. 2013, 19, 2348–2359. [Google Scholar] [CrossRef] [PubMed]
- Buxton, P.A. Terrestrial insects and the humidity of the environment. Biol. Rev. 1932, 7, 275–320. [Google Scholar] [CrossRef]

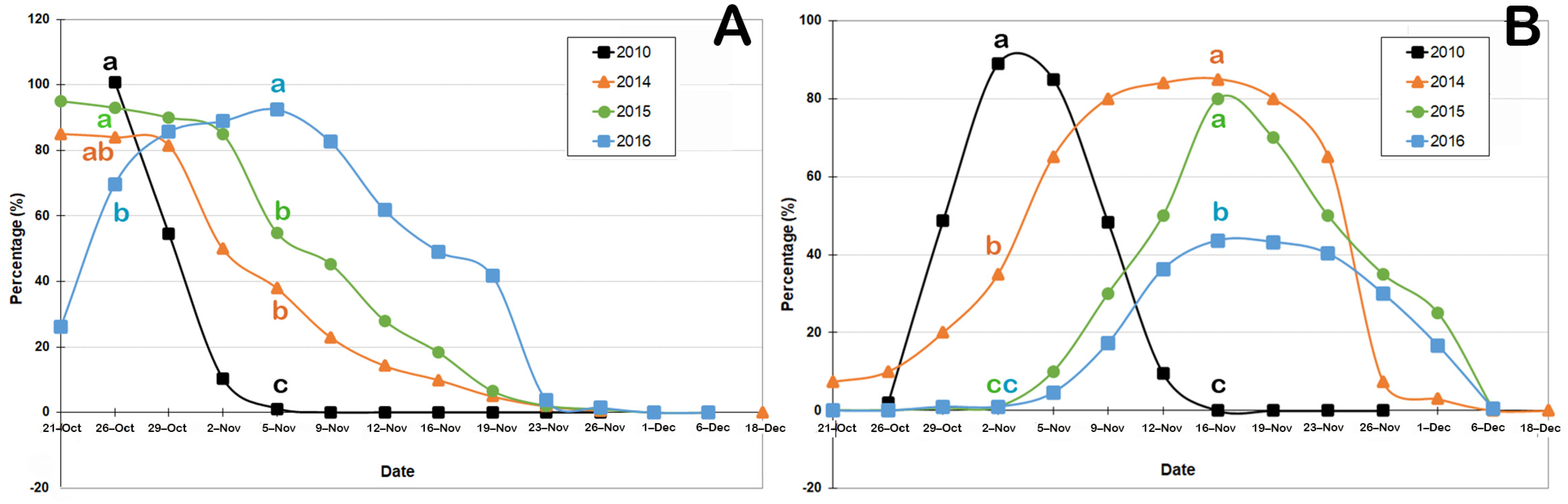
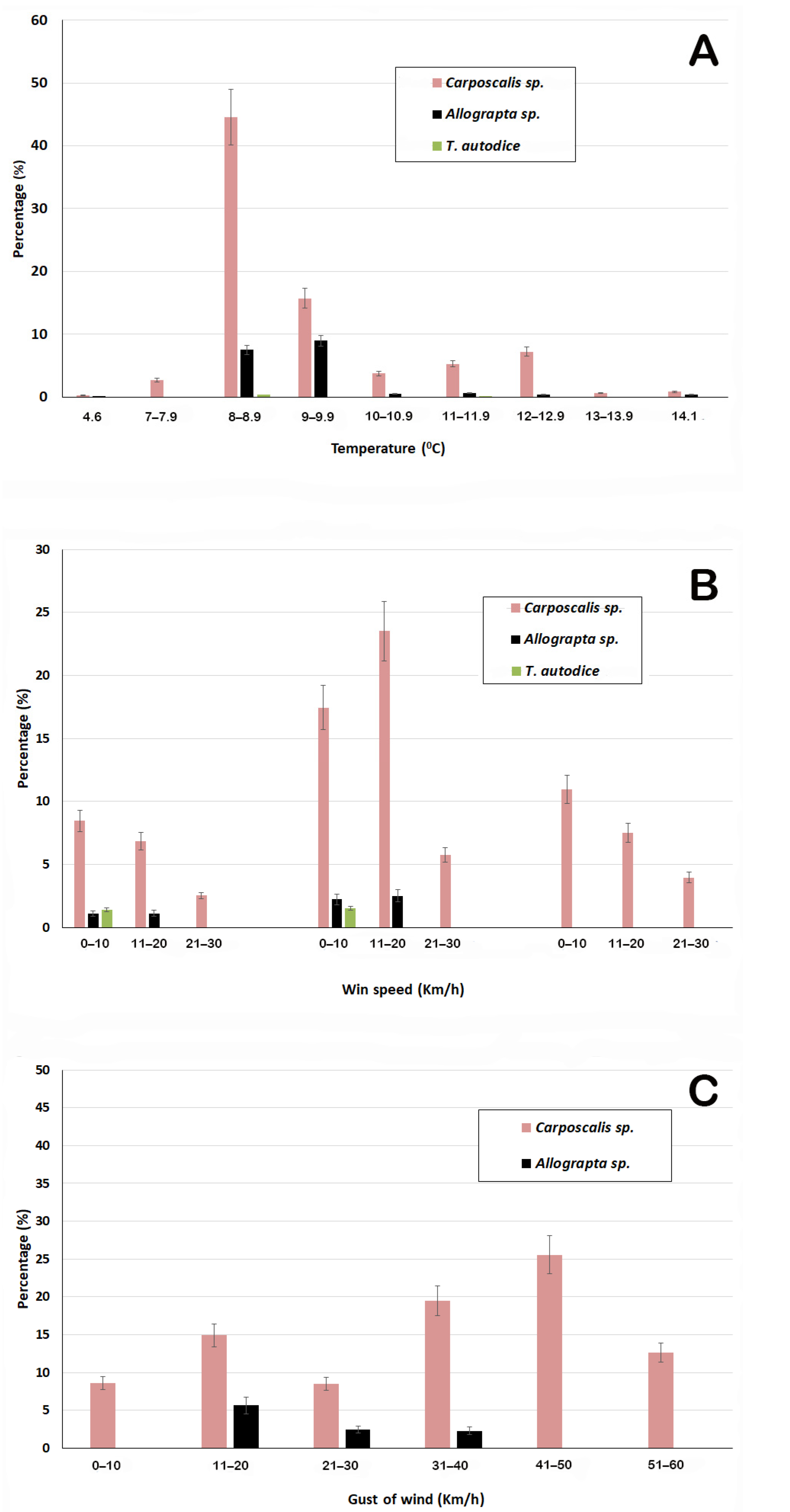

| Variable | Year | October | November | ||||
|---|---|---|---|---|---|---|---|
| Mean | Max | Min | Mean | Max | Min | ||
| Temperature °C | 2010 | 7.4b | 18.0 | −1.8 | 8.3a | 19.7 | −0.8 |
| 2014 | 6.1c | 17.4 | −2.9 | 6.9b | 17.3 | −1.1 | |
| 2015 | 6.4c | 19.9 | −0.6 | 8.0a | 17.9 | 0.1 | |
| 2016 | 8.5a | 17.8 | 1.8 | 8.2a | 19.7 | 0.7 | |
| RH % | 2010 | 62c | 90 | 32 | 64c | 91 | 29 |
| 2014 | 69a | 100 | 26 | 69b | 92 | 40 | |
| 2015 | 66b | 96 | 22 | 68b | 95 | 34 | |
| 2016 | 70a | 96 | 32 | 73a | 95 | 37 | |
| Mean Wind Speed Km/h | 2010 | 8.8b | 35.4 | 1.6 | 10.3b | 32.2 | 1.6 |
| 2014 | 9.0a | 37.0 | 1.6 | 11.0ab | 37 | 1.6 | |
| 2015 | 8.9ab | 35.4 | 1.6 | 11.6a | 35.4 | 1.6 | |
| 2016 | 10.1a | 35.4 | 1.6 | 10.0b | 38.6 | 1.6 | |
| Wind Gust Km/h | 2010 | 24.6a | 83.7 | 1.6 | 26.0ab | 74 | 1.6 |
| 2014 | 24.4a | 72.4 | 1.6 | 27.1a | 66 | 1.6 | |
| 2015 | 23.3a | 69.2 | 1.6 | 28.3a | 70.8 | 1.6 | |
| 2016 | 24.0a | 66.0 | 1.6 | 25.0b | 74 | 1.6 | |
| Year | October (Day) | November (Day) | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 11 | 12 | 13 | 20 | 30 | 31 | 2 | 3 | 4 | 6 | ||
| 2010 | - | - | - | - | - | - | - | - | - | - | 0 |
| 2014 | 2.0 | 1.5 | 12.6 | - | 2.0 | 3.0 | - | - | - | - | 21.1 |
| 2015 | - | - | - | 2.4 | - | - | 2.3 | 10.4 | - | - | 15.1 |
| 2016 | - | - | - | - | - | - | - | - | 15.4 | 1.0 | 16.4 |
| Date | Year | |||
|---|---|---|---|---|
| 2010 | 2014 | 2015 | 2016 | |
| 26 October | 320b | 278bc | 225c | 387a |
| 2 November | 486b | 423bc | 332c | 612a |
| Month | Year | |||
|---|---|---|---|---|
| 2010 | 2014 | 2015 | 2016 | |
| June | 2.6b | 2.9b | 1.8b | 5.3a |
| July | 2.6a | 2.8a | 0.9b | 2.9a |
| August | 3.8a | 3.3ab | 2.3b | 2.8ab |
| September | 4.9b | 4.1bc | 2.6c | 6.6a |
| Year | DJF | JFM | FMA | MAM | AMJ | MJJ | JJA | JAS | ASO | SON | OND | NDJ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 1.5 | 1.2 | 0.8 | 0.4 | −0.2 | −0.7 | −1.0 | −1.3 | −1.6 | −1.6 | −1.6 | −1.6 |
| 2014 | −0.4 | −0.5 | −0.3 | 0.0 | 0.2 | 0.2 | 0.0 | 0.1 | 0.2 | 0.5 | 0.6 | 0.7 |
| 2015 | 0.5 | 0.5 | 0.5 | 0.7 | 0.9 | 1.2 | 1.5 | 1.9 | 2.2 | 2.4 | 2.6 | 2.6 |
| 2016 | 2.5 | 2.1 | 1.6 | 0.9 | 0.4 | −0.1 | −0.4 | −0.5 | −0.6 | −0.7 | −0.7 | −0.6 |
| Year | Percentage (%) | ||
|---|---|---|---|
| Carposcalis | Allograpta | BHF | |
| 2010 | 75a | ||
| 2014 | 85a | 15b | 25c |
| 2015 | 39b | 61a | 41b |
| 2016 | 21b | 79a | 11c |
| Insect | AirTemp | RH | Wind Speed | Wind Gust |
|---|---|---|---|---|
| Carposcalis sp. | −0.076 ** | −0.059 ** | 0.042 ** | 0.070 ** |
| Allograpta sp. | −0.100 * | −0.095 * | 0.154 ** | 0.126 ** |
| Tatochila autodice | −0.594 ** | −0.594 ** | −0.594 ** | −0.594 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radice, S.; Giordani, E.; Arena, M.E. Effect of Climatic Variations in the Floral Phenology of Berberis microphylla and Its Pollinator Insects. Horticulturae 2023, 9, 1254. https://doi.org/10.3390/horticulturae9121254
Radice S, Giordani E, Arena ME. Effect of Climatic Variations in the Floral Phenology of Berberis microphylla and Its Pollinator Insects. Horticulturae. 2023; 9(12):1254. https://doi.org/10.3390/horticulturae9121254
Chicago/Turabian StyleRadice, Silvia, Edgardo Giordani, and Miriam E. Arena. 2023. "Effect of Climatic Variations in the Floral Phenology of Berberis microphylla and Its Pollinator Insects" Horticulturae 9, no. 12: 1254. https://doi.org/10.3390/horticulturae9121254
APA StyleRadice, S., Giordani, E., & Arena, M. E. (2023). Effect of Climatic Variations in the Floral Phenology of Berberis microphylla and Its Pollinator Insects. Horticulturae, 9(12), 1254. https://doi.org/10.3390/horticulturae9121254







