Analysis of Floral Fragrance Components in Different Parts of Iris typhifolia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Research Methodology
2.2.1. Electronic Nose
2.2.2. SPME-GC-MS Analysis
2.2.3. Identification and Analysis Methods
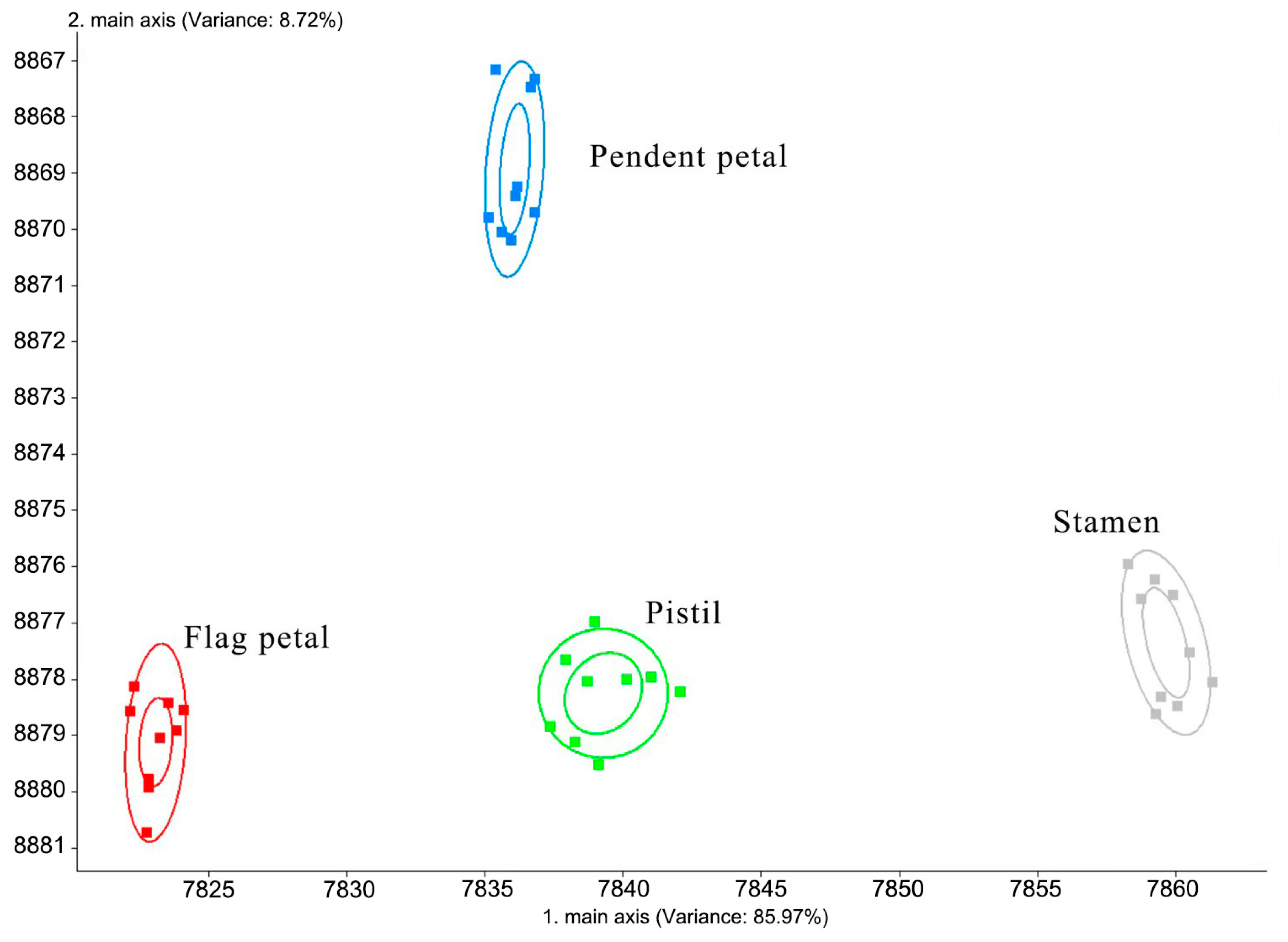
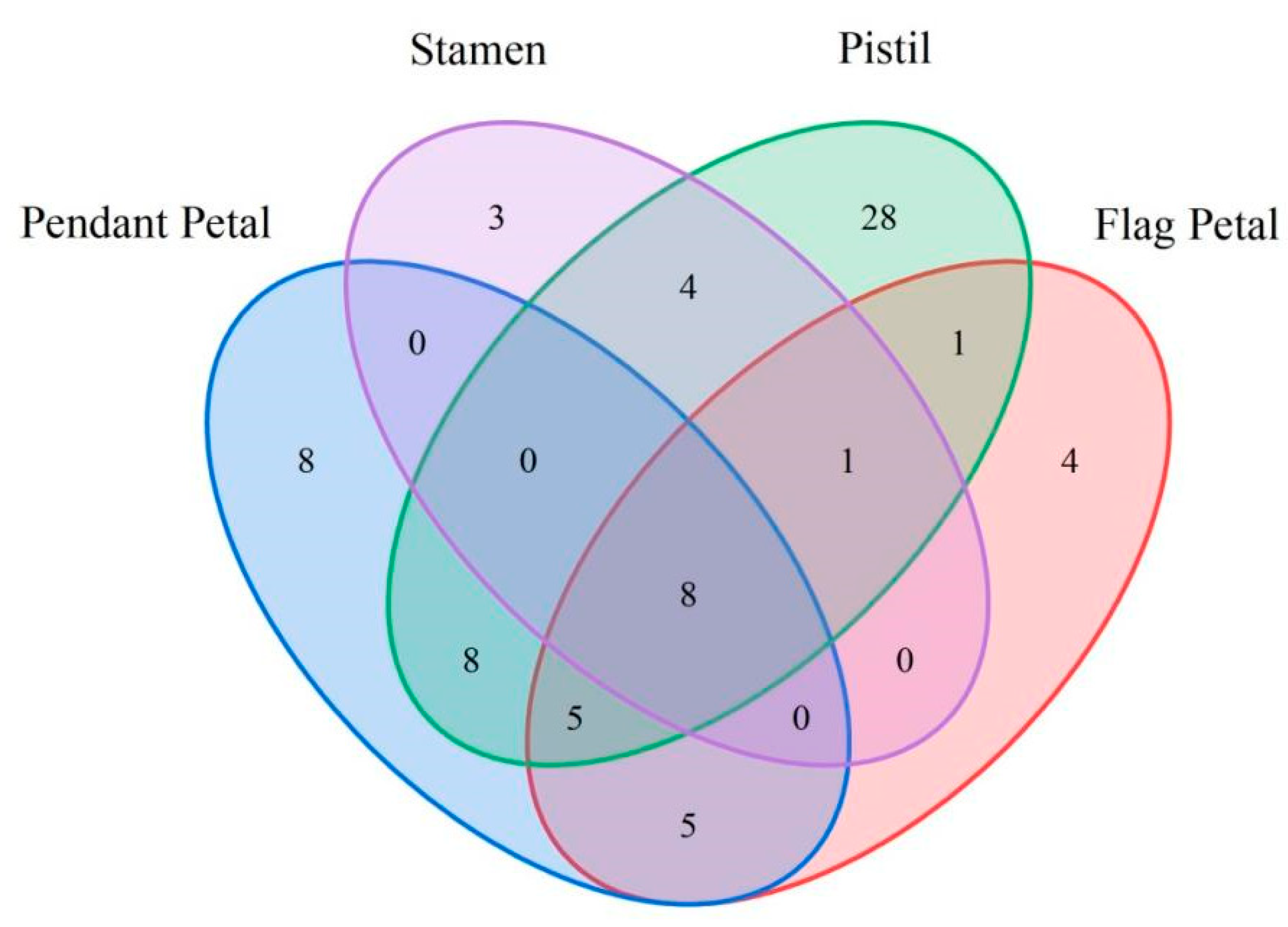
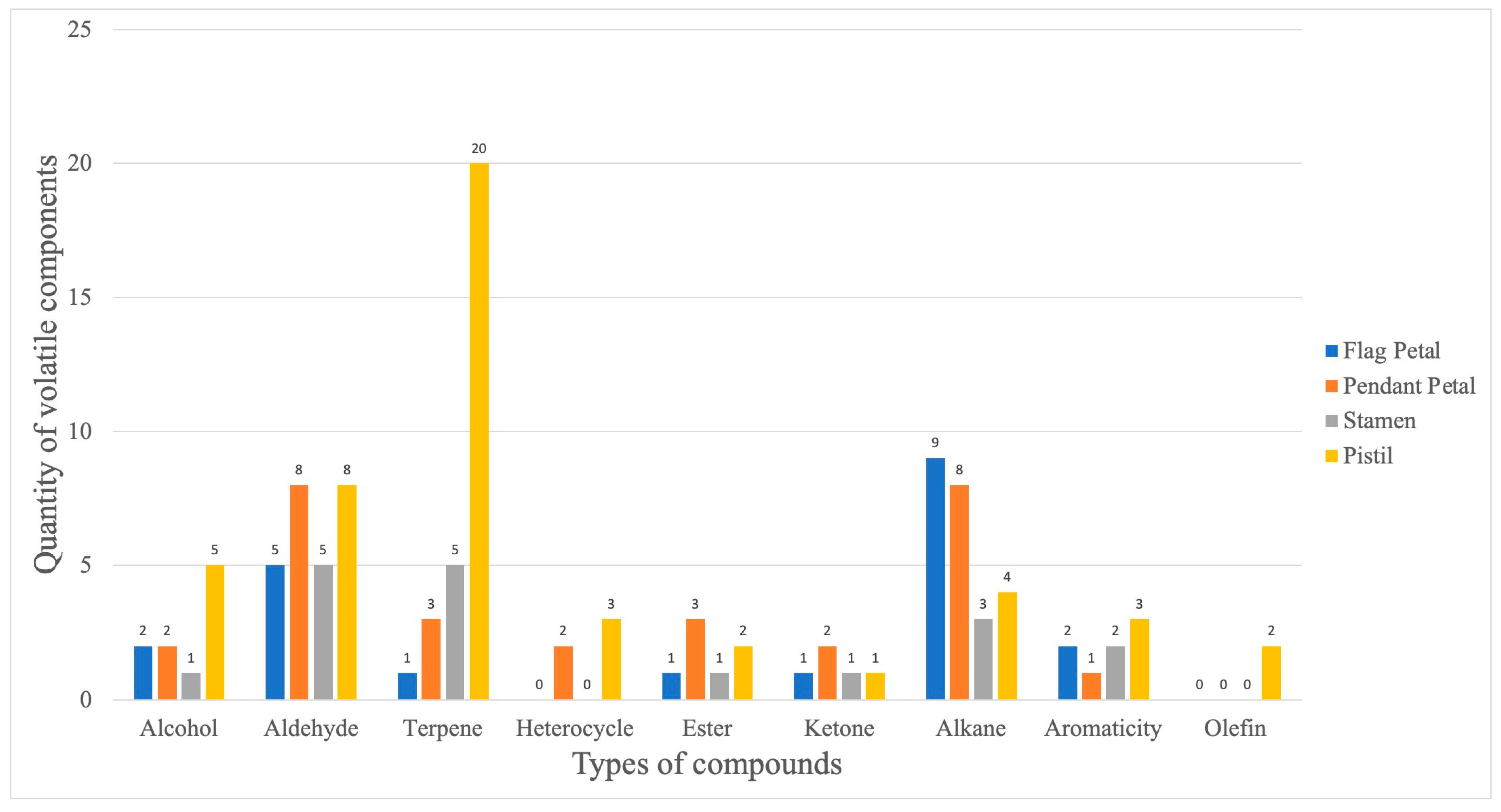
3. Results
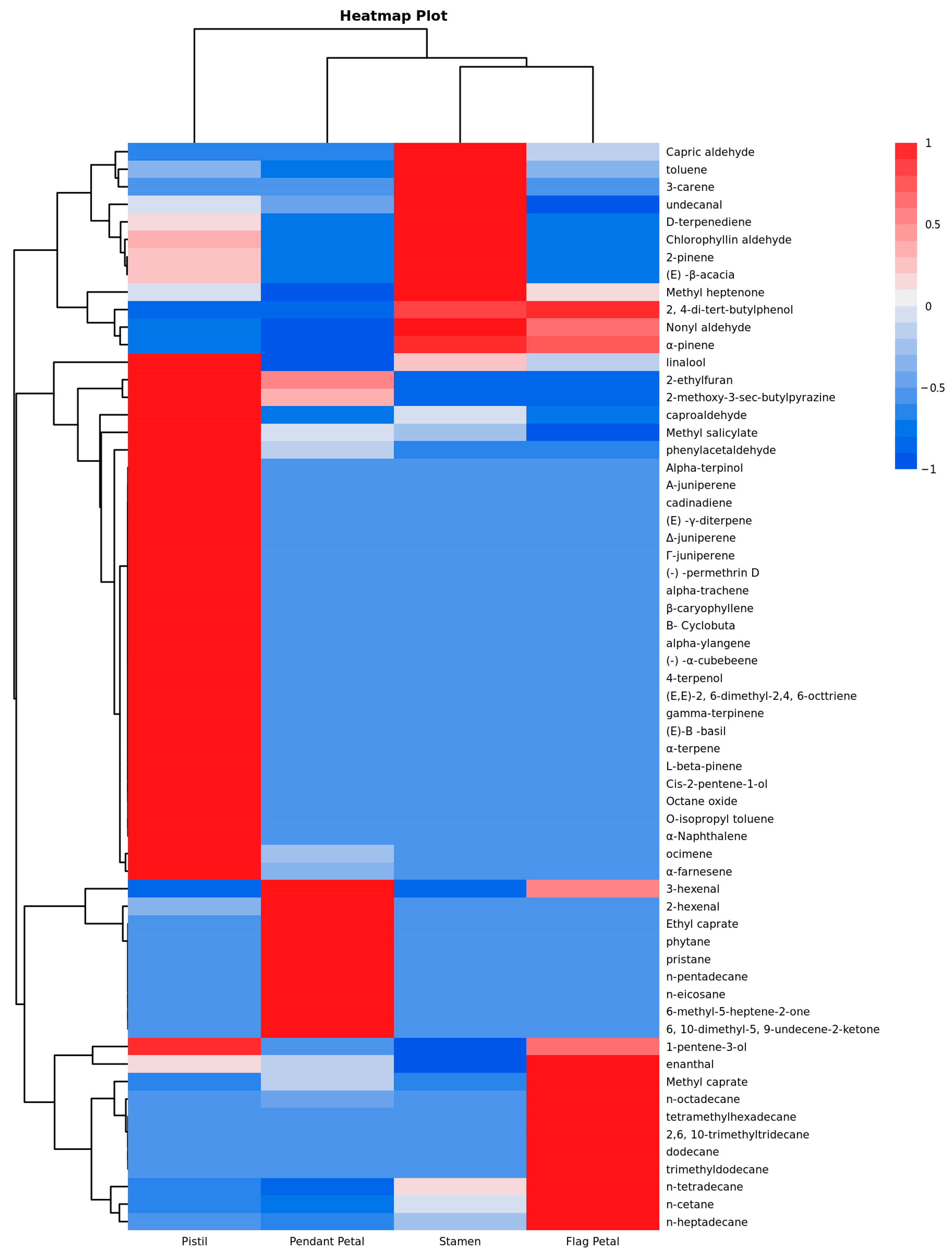
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brunoa, M.; Maggioa, A.; Sajeva, M. Floral scent in Iris planifolia (Iridaceae) suggests food reward. Phytochemistry 2019, 158, 86–90. [Google Scholar] [CrossRef]
- Krick, W.; Marner, F.J.; Jaenicke, L. Isolation and Structure Determination of the Precursors of a-and y-Irone and Homologous Compounds from Iris pallida and Iris florentina. Z. Für Naturforschung C 1983, 38, 179–184. [Google Scholar] [CrossRef]
- Marner, F.J.; Hanisch, B. Hoogianal, a β-Irone Precursor from Iris hoogiana Dykes (Iridaceae). Helv. Chim. Acta 2001, 84, 933–938. [Google Scholar] [CrossRef]
- Cai, K.; Feng, C.; Xu, S.; Sun, Y.; Lou, Q.; Sun, J.; Chen, H. The volatile components in different flowering stages of Iris uniflora. J. Northeast. For. Univ. 2023, 51, 53–58. [Google Scholar]
- Xiang, Q.; Duan, F.; Zou, Y.; Zhang, H. Research progress of Iris plants in China. Rural. Sci. Technol. 2020, 11, 2. [Google Scholar]
- Nan, H. Isolation and Identification of Aroma-Producing Strains from Iris Rhizomes and Biosynthesis of Irone. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2019; pp. 78–81. [Google Scholar]
- Başer, K.H.; Demirci, B.; Orhan, I.E.; Kartal, M.; Sekeroglu, N.; Sener, B. Composition of Volatiles from Three Iris Species of Turkey. J. Essent. Oil Res. 2011, 23, 66–71. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Flamini, G. Iris lutescens on serpentine soil: Volatile emission profiles in different organs of its two colour morph. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2021, 155, 406–414. [Google Scholar] [CrossRef]
- Wang, H.; Conchou, L.; Bessiere, J.-M.; Cazals, G.; Schatz, B.; Imbert, E. Flower color polymorphism in Iris lutescens (Iridaceae): Biochemical analyses in light of plant-insect interactions. Phytochemistry 2013, 94, 123–134. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, Y.; Zhao, Y.; Liu, C.; Chen, X.; Li, F.; Bao, J. Identification of Floral Scent Profiles in Bearded Irises. Molecules 2019, 24, 1773. [Google Scholar] [CrossRef]
- Sheng, X.; Lin, Y.; Cao, J.; Ning, Y.; Pang, X.; Wu, J. Comparative evaluation of key aroma-active compounds in sweet osmanthus (Osmanthus fragrans Lour.) with different enzymatic treatments. J. Agric. Food Chem. 2021, 69, 332–344. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Wang, C.; Li, C.W.; Liu, S.H.; Zhang, C.X.; Li, L.W.; Jiang, D.H. Characterization of Aroma-Active Compounds of Pu-erh Tea by Headspace Solid-Phase Microextraction (HS-SPME) and Simultaneous Distillation-Extraction (SDE) Coupled with GC-Olfactometry and GC-MS. FAM 2016, 9, 1188–1198. [Google Scholar] [CrossRef]
- Feng, Y.; Cai, Y.; Fu, X.; Zheng, L.; Xiao, Z.; Zhao, M. Comparison of aroma-active compounds in broiler broth and native chicken broth by aroma extract dilution analysis (AEDA), odor activity value (OAV) and omission experiment. Food Chem. 2018, 265, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Niu, Y.; Xiao, Z. Characterization of the key aroma compounds in Laoshan green teas by application of odour activity value (OAV), gas chromatography-mass spectrometry-olfactometry (GC-MS-O) and comprehensive two-dimensional gas chromatography-mass spectrometry (GC × GC-qMS). Food Chem. 2021, 339, 128136. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, W.; Zhou, T.; Zhang, D.; Zhang, D.; Zhang, L.; Cao, F. Discrimination of Malus taxa with different scent intensities using electronic nose and gas chromatography–mass spectrometry. Sensors 2018, 18, 3429. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ren, J.; Wang, Y.; Hu, Q. Diurnal fluctuation of volatile compounds emitted from four seasons rose (Rosa damascena Mill.) cultivated in Beijing. J. Appl. Bot. Food Qual. 2014, 87, 9–15. [Google Scholar]
- Awano, K.; Honda, T.; Ogawa, T.; Suzuki, S.; Matsunaga, Y. Volatile components of Phalaenopsis schilleriana Rehb. f. Flavour Fragr. J. 1997, 12, 341–344. [Google Scholar] [CrossRef]
- Kong, Y.; Bai, J.; Lang, L.; Bao, F.; Dou, X.; Wang, H.; Shang, H. Variation in floral scent compositions of different lily hybrid groups. J. Am. Soc. Hortic. Sci. 2017, 142, 175–183. [Google Scholar] [CrossRef]
- Schiestl, F.P. The evolution of floral scent and insect chemical communication. Ecol. Lett. 2010, 13, 643–656. [Google Scholar] [CrossRef]
- Dong, X.; Wei, C.; Wang, W. Comprehensive profiling and natural variation of flavonoids in rice. J. Integr. Plant Biol. 2014, 56, 876–886. [Google Scholar] [CrossRef]
- Wang, X.; Xie, K.; Zhuang, H.; Ye, R.; Fang, Z.; Feng, T. Volatile flavor compounds, total polyphenolic contents and antioxidant activities of a China gingko wine. Food Chem. 2015, 182, 41–46. [Google Scholar] [CrossRef]
- Hyun, J.; Lee, J.G.; Yang, K.-Y.; Lim, S.; Lee, E.J. Postharvest Fumigation of (E)-2-Hexenal on Kiwifruit (Actinidia chinensis cv. ‘Haegeum’) Enhances Resistance to Botrytis cinerea. Postharvest Biol. Technol. 2022, 187, 111854. [Google Scholar] [CrossRef]
- Shimizu, Y.; Imayoshi, Y.; Kato, M.; Maeda, K.; Iwabuchi, H.; Shimomura, K. New eudesmane-type sesquiterpenoids and other volatile constituents from the roots of Gynura bicolor DC. Flavour Fragr. J. 2011, 26, 55–64. [Google Scholar] [CrossRef]
- Gutbrod, P.; Yang, W.; Grujicic, G.V.; Peisker, H.; Gutbrod, K.; Du, L.F.; Dörmann, P. Phytol derived from chlorophyll hydrolysis in plants is metabolized via phytenal. J. Biol. Chem. 2021, 296, 100530. [Google Scholar] [CrossRef] [PubMed]
- León, D.C.S.; Ortíz, D.K.R.; González, D.F.J. Sensory approach and chiral analysis for determination of odour active compounds from feijoa (Acca sellowiana). Food Chem. 2020, 317, 126383. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Kusumoto, N.; Matsui, N.; Makino, R.; Hashida, K.; Arai, D.; Iiduka, Y.; Ashitani, T. Antitermitic and antifungal properties of enantiopure linalool and furanoid linalool oxide confirmed in Lindera umbellata var. membranacea. J. Wood Chem. Technol. 2021, 42, 37–45. [Google Scholar] [CrossRef]
- Castellar, A.; Oliveira, D.R.; Leitão, S.G.; Bizzo, H.R.; Soares, M.d.L.C.; Kinupp, V.F.; Veiga-Junior, V.F. Essential oil from Philodendron fragrantissimum, an aromatic Araceae from Amazonia, Brazil. J. Essent. Oil Res. 2013, 25, 194–197. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, W.; Zhang, D.; Wang, G.; Cao, F. Flowering Stage and Daytime Affect Scent Emission of Malus ioensis “Prairie Rose”. Molecules 2019, 24, 2356. [Google Scholar] [CrossRef] [PubMed]
- Lesage, P.; Candy, J.P.; Hirigoyen, C.; Humblot, F.; Basset, J.M. Selective dehydrogenation of dipentene (R-(+)-limonene) into paracymene on silica supported palladium assisted by α-olefins as hydrogen acceptor. J. Mol. Catal. A Chem. 1996, 112, 431–435. [Google Scholar] [CrossRef]
- Al-Onazi, W.; Al-Mohaimeed, A.M.; Amina, M.; El-Tohamy, M.F. Identification of Chemical Composition and Metal Determination of Retama raetam (Forssk) Stem Constituents Using ICP-MS, GC-MS-MS, and DART-MS. J. Anal. Methods Chem. 2021, 2021, 6667238. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Wang, F.; Huang, A. Identification and analysis of the aroma components of different species of Matcha by electronic nose. Food Ferment. Ind. 2019, 45, 270–276. [Google Scholar]
- Sowmyal, R.S.; Sugrivl, G.; Annapure, U.S. Effect of basil herb on cookies development and its effect on the nutritive, elemental, phytochemical, textural and sensory quality. Food Sci. Technol. 2022, 59, 3482–3491. [Google Scholar] [CrossRef]
- Rudnicki, K.; Sobczak, K.; Kaliszczak, M.; Sipa, K.; Powałka, E.; Skrzypek, S.; Poltorak, L.; Herzog, G. Voltammetric study of cefotaxime at the macroscopic and miniaturized interface between two immiscible electrolyte solutions. Microchim. Acta 2021, 188, 413. [Google Scholar] [CrossRef] [PubMed]
- Chaia, K.; Wang, X.; She, G. Biotransformation and metabolism of three methyl salicylate glycosides by gut microbiota in vitro. J. Pharm. Biomed. Anal. 2023, 233, 115474. [Google Scholar] [CrossRef]
- Li, X.; Tieman, D.; Alseekh, S.; Fernie, A.R.; Klee, H.J. Natural variations in the Sl-AKR9 aldo/keto reductase gene impact fruit flavor volatile and sugar contents. Plant J. 2023, 115, 1134–1150. [Google Scholar] [CrossRef] [PubMed]
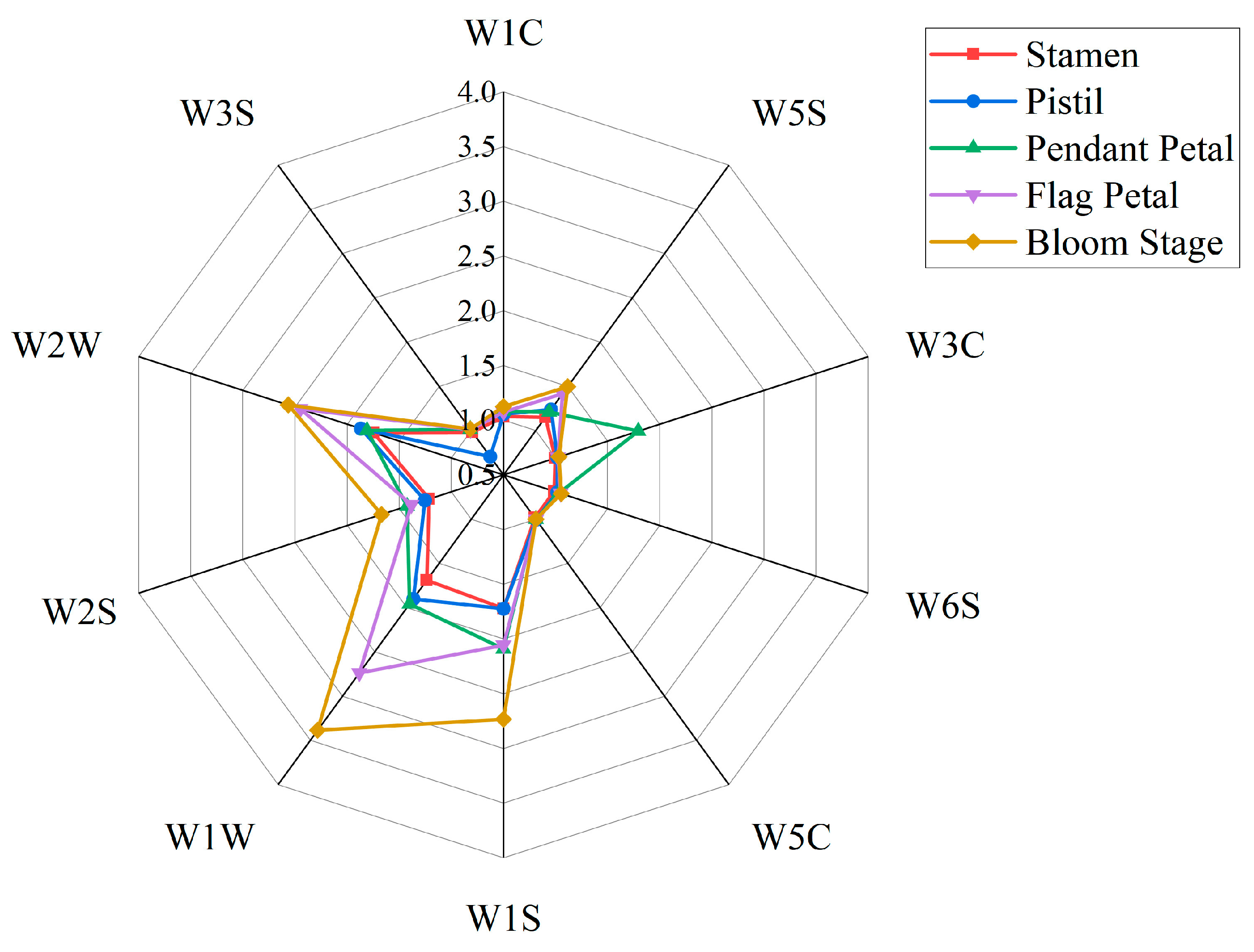



| Array Serial No. | Sensor Name | Performance Description |
|---|---|---|
| 1 | W1C | aromatic |
| 2 | W5S | broad range |
| 3 | W3C | aromatic |
| 4 | W6S | hydrogen |
| 5 | W5C | arom–aliph |
| 6 | W1S | broad–methane |
| 7 | W1W | sulphur–organic |
| 8 | W2S | broad–alcohol |
| 9 | W2W | sulph–chlor |
| 10 | W3S | methane–aliph |
| No. | Case | RT | Compound | Stamen | Pistil | Flag Petal | Pendant Petal |
|---|---|---|---|---|---|---|---|
| Alcohol | |||||||
| 1 | 616-25-1 | 3.25 | 1-pentene-3-ol | 1.71 0.8 d | 1.46 0.42 d | 0.4 0.11 c | |
| 2 | 1576-95-0 | 5.12 | Cis-2-pentene-1-ol | 0.77 0.38 d | |||
| 3 | 562-74-3 | 17.72 | 4-terpenol | 0.31 0.15 d | |||
| 4 | 78-70-6 | 15.37 | linalool | 8.21 3.15 bcd | 12.41 7.27 b | 6.38 7.18 cd | |
| 5 | 10482-56-1 | 18.11 | alpha-terpinol | 0.1 0.07 d | |||
| Heterocycle | |||||||
| 6 | 3208-16-0 | 3.57 | 2-ethylfuran | 0.35 0.14 d | 0.24 0.03 c | ||
| 7 | 24168-70-5 | 17.55 | 2-methoxy-3-sec-butylpyrazine | 0.24 0.09 d | 0.14 0.04 c | ||
| 8 | 29837-12-5 | 26.93 | cadinadiene | 0.09 0.06 d | |||
| Aromaticity | |||||||
| 9 | 108-88-3 | 4.99 | toluene | 3.66 1.41 d | 0.61 0.52 d | 0.56 0.4 d | |
| 10 | 527-84-4 | 13.01 | O-isopropyl toluene | 0.17 0.09 d | |||
| 11 | 96-76-4 | 26.42 | 2, 4-di-tert-butylphenol | 17.06 5.82 a | 1.09 0.64 d | 17.73 6.69 b | 1.23 1.85 c |
| Aldehyde | |||||||
| 12 | 4440-65-7 | 5.8 | 3-hexenal | 20.07 4.12 a | 28.53 2.19 a | ||
| 13 | 66-25-1 | 5.86 | caproaldehyde | 14.1 4.8 b | 43.36 16.81 a | ||
| 14 | 6728-26-3 | 7.49 | chlorophyllin aldehyde | 5.02 4.9 cd | 2.66 1.06 d | 19.35 7.18 a | 28.53 2.19 a |
| 15 | 111-71-7 | 9.02 | enanthal | 0.39 0.13 d | 0.68 0.26 d | 0.29 0.13 c | |
| 16 | 505-57-7 | 7.43 | 2-hexenal | 2.66 1.06 d | 28.53 2.19 a | ||
| 17 | 124-19-6 | 15.48 | nonyl aldehyde | 8.44 0.93 bcd | 1.53 1.05 d | 6.68 2.57 cd | 1 0.87 c |
| 18 | 122-78-1 | 13.56 | phenylacetaldehyde | 0.65 0.28 d | 0.15 0.06 c | ||
| 19 | 112-31-2 | 18.49 | capric aldehyde | 4.83 2.28 d | 0.58 0.44 d | 1.44 0.82 d | 0.41 0.22 c |
| 20 | 112-44-7 | 21.34 | undecanal | 0.65 0.46 d | 0.24 0.17 d | 0.15 0.05 c | |
| Terpene | |||||||
| 21 | 13466-78-9 | 10.09 | 3-carene | 1.98 1.51 d | |||
| 22 | 80-56-8 | 10.09 | 2-pinene | 3.96 1.6 d | |||
| 23 | 5989-27-5 | 13.16 | D-terpenediene | 9.26 4.07 bcd | 3.75 1.72 cd | ||
| 24 | 13877-91-3 | 13.78 | ocimene | 2.17 0.62 d | 0.31 0.25 c | ||
| 25 | 18172-67-3 | 11.48 | L-beta-pinene | 0.41 0.21 d | |||
| 26 | 99-86-5 | 12.77 | α-terpene | 0.13 0.06 d | |||
| 27 | 3779-61-1 | 13.46 | (E)-Β -basil | 0.34 0.05 d | |||
| 28 | 18794-84-8 | 25.25 | (E) -β-acacia | 0.96 0.38 d | 0.46 0.31 d | ||
| 29 | 99-85-4 | 14.11 | gamma-terpinene | 0.26 0.13 d | |||
| 30 | 586-62-9 | 15.04 | terpinolene | 0.13 0.06 d | |||
| 31 | 17699-14-8 | 22.58 | (-) -α-cubebeene | 0.38 0.14 d | |||
| 32 | 3856-25-5 | 23.31 | α-pinene | 2.2 0.85 d | 0.38 0.14 d | 2.03 0.64 d | 0.12 0.09 c |
| 33 | 5208-59-3 | 23.55 | Β- Cyclobuta | 0.3 0.29 d | |||
| 34 | 87-44-5 | 24.45 | β-caryophyllene | 1 0.77 d | |||
| 35 | 6753-98-6 | 25.3 | alpha-trachene | 0.75 0.57 d | |||
| 36 | 10208-80-7 | 26.28 | α-Naphthalene | 0.21 0.17 d | |||
| 37 | 502-61-4 | 26.33 | α-farnesene | 1.44 1.32 d | 0.14 0.07 c | ||
| 38 | 39029-41-9 | 26.58 | Γ-juniperene | 0.48 0.44 d | |||
| 39 | 483-76-1 | 26.74 | Δ-juniperene | 1.28 1.14 d | |||
| 40 | 53585-13-0 | 26.88 | (E) -γ-diterpene | 0.25 0.11 d | |||
| 41 | 24406-05-1 | 27.03 | A-juniperene | 0.38 0.27 d | |||
| Ketone | |||||||
| 42 | 110-93-0 | 11.79 | methyl heptenone | 11.48 1.3 bc | 6.05 2.1 c | 7.1 0.93 cd | 5.23 0.85 b |
| 43 | 689-67-8 | 25.07 | 6, 10-dimethyl-5, 9-undecene-2-ketone | 0.21 0.23 c | |||
| Alkane | |||||||
| 44 | 2984-50-1 | 9.14 | octane oxide | 0.27 0.1 d | |||
| 45 | 112-40-3 | 18.36 | dodecane | 0.58 0.3 d | |||
| 46 | 31295-56-4 | 20.64 | trimethyldodecane | 0.46 0.47 d | |||
| 47 | 629-59-4 | 23.8 | n-tetradecane | 0.58 0.06 d | 0.19 0.08 d | 1.09 0.23 d | 0.11 0.06 c |
| 48 | 544-76-3 | 27.99 | n-cetane | 0.88 0.39 d | 0.22 0.07 d | 2.4 0.88 d | 0.15 0.05 c |
| 49 | 3891-99-4 | 25.35 | 2,6,10-trimethyltridecane | 0.37 0.45 d | |||
| 50 | 593-45-3 | 26.11 | n-octadecane | 3.89 1.54 d | 0.15 0.11 c | ||
| 51 | 504-44-9 | 26.12 | tetramethylhexadecane | 0.56 0.69 d | |||
| 52 | 629-78-7 | 29.48 | n-heptadecane | 0.82 0.29 d | 0.26 0.1 d | 3.78 1.61 d | 0.15 0.12 c |
| 53 | 629-94-7 | 26.08 | n-Heneicosane | 0.09 0.11 c | |||
| 54 | 1921-70-6 | 29.53 | pristane | 0.1 0.12 c | |||
| 55 | 638-36-8 | 30.85 | phytane | 0.13 0.12 c | |||
| 56 | 629-62-9 | 26.14 | n-pentadecane | 1.4 0.3 d | 0.11 0.06 c | ||
| Ester | |||||||
| 57 | 119-36-8 | 18.2 | methyl salicylate | 0.81 0.76 d | 2.35 1.46 d | 1.03 0.22 c | |
| 58 | 110-42-9 | 21.76 | methyl caprate | 1.54 0.42 d | 0.37 0.21 c | ||
| 59 | 110-38-3 | 23.62 | ethyl caprate | 0.23 0.17 c | |||
| 60 | 23986-74-5 | 25.92 | (-) -permethrin D | 0.29 0.13 d | |||
| Olefin | |||||||
| 61 | 3016-19-1 | 16.65 | (E,E)-2, 6-dimethyl-2,4, 6-octtriene | 0.22 0.1 d | |||
| 62 | 14912-44-8 | 23.19 | alpha-ylangene | 0.93 0.59 d | |||
| No. | Compound | Odor Descriptions | OAV | |||
|---|---|---|---|---|---|---|
| Stamen | Pistil | Flag Petal | Pendant Petal | |||
| 1 | caproaldehyde | apple, fat, fresh, green, oil | 18.99 | 214.73 | ||
| 2 | trans-2-hexenal | fresh, fruit | 1.94 | 0.66 | 13.73 | 64.2 |
| 3 | 2-pinene | pine, resin | 1.91 | 0.64 | ||
| 4 | methyl heptenone | fresh, fruit | 6.66 | 2.25 | 2.16 | 4.61 |
| 5 | D-terpenediene | pine, resin | 2.44 | 0.63 | ||
| 6 | 2-hexenal | sweet, fruit, apple | 0.66 | 17.12 | ||
| 8 | linalool | coriander, floral, lavender, lemon, rose | 317.34 | 37.34 | 12.66 | 43 |
| 9 | nonyl aldehyde | bitter almond, burnt matches, fat, floral | 139.86 | 16.22 | 54.16 | 25.65 |
| 10 | phenylacetaldehyde | berry, geranium, honey, nut, pungent | 2.69 | 1.54 | ||
| 11 | α-pinene | pine, resin | 1.64 | 0.12 | 0.49 | 0.94 |
| 12 | methyl salicylate | almond, caramel, peppermint, sharp | 0.79 | 1.46 | 1.54 | |
| 13 | capric aldehyde | citrus, fat, green, oil, pungent | 56.35 | 4.28 | 8.16 | 7.43 |
| 14 | undecanal | rose | 2.71 | 0.65 | 0.95 | |
| 15 | 2-methoxy-3-sec-butylpyrazine | fresh legumes | 222.55 | 316.88 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, K.; Tian, K.; Ban, Z.; Xu, H.; Jia, W.; Zhu, Y.; Chen, H. Analysis of Floral Fragrance Components in Different Parts of Iris typhifolia. Horticulturae 2023, 9, 1268. https://doi.org/10.3390/horticulturae9121268
Cai K, Tian K, Ban Z, Xu H, Jia W, Zhu Y, Chen H. Analysis of Floral Fragrance Components in Different Parts of Iris typhifolia. Horticulturae. 2023; 9(12):1268. https://doi.org/10.3390/horticulturae9121268
Chicago/Turabian StyleCai, Keyu, Kexin Tian, Zhengjie Ban, Haowen Xu, Wenxu Jia, Ying Zhu, and Hongwu Chen. 2023. "Analysis of Floral Fragrance Components in Different Parts of Iris typhifolia" Horticulturae 9, no. 12: 1268. https://doi.org/10.3390/horticulturae9121268
APA StyleCai, K., Tian, K., Ban, Z., Xu, H., Jia, W., Zhu, Y., & Chen, H. (2023). Analysis of Floral Fragrance Components in Different Parts of Iris typhifolia. Horticulturae, 9(12), 1268. https://doi.org/10.3390/horticulturae9121268





