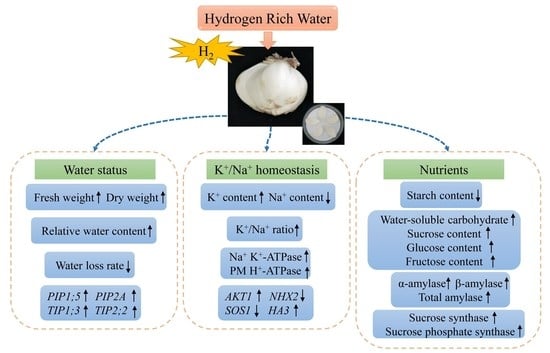
Effects of Hydrogen-Rich Water on Postharvest Physiology in Scales of Lanzhou Lily during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Hydrogen-Rich Water and Treatment
2.3. Measurement of Fresh Weight, Dry Weight, and Relative Water Content
2.4. Measurement of Water Loss Rate
2.5. Measurement of K+ and Na+ Contents
2.6. Measurement of Starch, Water-Soluble Carbohydrate, Sucrose, Glucose, and Fructose Content
2.7. Measurement of Enzyme Activities
2.8. Extraction of RNA and Real-Time Reverse Transcription-PCR Assay
2.9. Statistical Analysis
3. Results
3.1. Effects of Different Concentrations of HRW on Water Content of Lanzhou Lily Scales
3.2. Effects of HRW on K+ Content, Na+ Content, and ATP Enzyme Activity
3.3. Effect of HRW on the Expression of Genes Related to K+/Na+ Homeostasis
3.4. Effect of HRW on the Expression of Aquaporin Genes
3.5. Effects of HRW on Starch, Water-Soluble Carbohydrate, Sucrose, Glucose, and Fructose Content
3.6. Effects of HRW on the Activity of Total Amylase, α-Amylase, β-Amylase, Sucrose Synthase, and Sucrose Phosphate Synthase
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fang, H.; Wang, C.L.; Wang, S.Y.; Liao, W.B. Hydrogen gas increases the vase life of cut rose ‘Movie star’ by regulating bacterial community in the stem ends. Postharvest Biol. Technol. 2021, 181, 111685. [Google Scholar] [CrossRef]
- Mizuno, K.; Sasaki, A.T.; Ebisu, K.; Tajima, K.; Kajimoto, O.; Nojima, J.; Kuratsune, H.; Hori, H.; Watanabe, Y. Hydrogen-rich water for improvements of mood, anxiety, and autonomic nerve function in daily life. Med. Gas Res. 2018, 7, 247–255. [Google Scholar] [PubMed] [Green Version]
- Hancock, J.T.; Russell, G.; Stratakos, A.C. Molecular hydrogen: The postharvest use in fruits, vegetables and the floriculture industry. Appl. Sci. 2022, 12, 10448. [Google Scholar] [CrossRef]
- Dong, B.Y.; Zhu, D.Q.; Yao, Q.P.; Tang, H.M.; Ding, X.C. Hydrogen-rich water treatment maintains the quality of Rosa sterilis fruit by regulating antioxidant capacity and energy metabolism. LWT 2022, 161, 113361. [Google Scholar] [CrossRef]
- Guan, Q.; Ding, X.W.; Jiang, R.; Ouyang, P.L.; Gui, J.; Feng, L.; Yang, L.; Song, L.H. Effects of hydrogen-rich water on the nutrient composition and antioxidative characteristics of sprouted black barley. Food Chem. 2019, 299, 125095. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.P.; Li, C.X.; Liu, H.W.; Zhao, Z.X.; Liao, W.B. Hydrogen gas improves seed germination in cucumber by regulating sugar and starch metabolisms. Horticulturae 2021, 7, 456. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Chen, Q.H.; Wang, Y.M.; Shen, Z.G.; Shen, W.B.; Xu, X.M. Hydrogen-rich water induces aluminum tolerance in maize seedlings by enhancing antioxidant capacities and nutrient homeostasis. Ecotox. Environ. Safe 2017, 144, 369–379. [Google Scholar] [CrossRef]
- Lin, Y.T.; Zhang, W.; Qi, F.; Cui, W.T.; Xie, Y.J.; Shen, W.B. Hydrogen-rich water regulates cucumber adventitious root development in a heme oxygenase-1/carbon monoxide-dependent manner. J. Plant Physiol. 2014, 171, 1–8. [Google Scholar] [CrossRef]
- Wang, C.L.; Fang, H.; Gong, T.Y.; Zhang, J.; Niu, L.J.; Huang, D.J.; Huo, J.Q.; Liao, W.B. Hydrogen gas alleviates postharvest senescence of cut rose ‘Movie star’ by antagonizing ethylene. Plant Mol. Biol. 2020, 102, 271–285. [Google Scholar] [CrossRef]
- Hu, H.L.; Li, P.X.; Wang, Y.N.; Gu, R.X. Hydrogen-rich water delays postharvest ripening and senescence of kiwifruit. Food Chem. 2014, 156, 100–109. [Google Scholar] [CrossRef]
- Fu, X.M.; Ma, L.; Gui, R.F.; Ashraf, U.; Li, Y.Z.; Yang, X.J.; Zhang, J.W.; Imran, M.; Tang, X.R.; Tian, H.; et al. Differential response of fragrant rice cultivars to salinity and hydrogen rich water in relation to growth and antioxidative defense mechanisms. Int. J. Phytoremediat. 2021, 23, 1203–1211. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhao, G.; Cheng, P.F.; Yan, X.Y.; Li, Y.; Cheng, D.; Wang, R.; Chen, J.; Shen, W.B. Nitrite accumulation during storage of tomato fruit as prevented by hydrogen gas. Int. J. Food Prop. 2019, 22, 1425–1438. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Huang, L.P.; Su, N.N.; Shabala, L.; Wang, H.Y.; Huang, X.; Wen, R.Y.; Yu, M.; Cui, J.; Shabala, S. Calcium-dependent hydrogen peroxide mediates hydrogen-rich water-reduced cadmium uptake in plant roots. Plant Physiol. 2020, 183, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Yao, Y.D.; Mou, K.P.; Dan, Y.Y.; Li, W.T.; Wang, C.L.; Liao, W.B. The involvement of abscisic acid in hydrogen gas-enhanced drought resistance in tomato seedlings. Sci. Hortic. 2022, 292, 110631. [Google Scholar] [CrossRef]
- Chen, Q.H.; Zhao, X.Q.; Lei, D.K.; Hu, S.B.; Shen, Z.G.; Shen, W.B.; Xu, X.M. Hydrogen-rich water pretreatment alters photosynthetic gas exchange, chlorophyll fluorescence, and antioxidant activities in heat-stressed cucumber leaves. Plant Growth Regul. 2017, 83, 69–82. [Google Scholar] [CrossRef]
- Lawson, T.; Vialet-Chabrand, S. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.M.; Lakso, A.N.; Eissenstat, D.M. Interactive effects of soil temperature and moisture on Concord grape root respiration. J. Exp. Bot. 2005, 56, 2651–2660. [Google Scholar] [CrossRef] [Green Version]
- Gardner, W.R.; Ehlig, C.F. The influence of soil water on transpiration by plants. J. Geophys. Res. 1963, 68, 5719–5724. [Google Scholar] [CrossRef]
- Chen, W.; Yao, X.Q.; Cai, K.Z.; Chen, J.N. Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol. Trace Elem. Res. 2011, 142, 67–76. [Google Scholar] [CrossRef]
- Hu, X.J.; Cui, Y.; Lu, X.M.; Song, W.B.; Lei, L.; Zhu, J.J.; Lai, J.S.; Lizhu, E.; Zhao, H.M. Maize WI5 encodes an endo-1, 4-β-xylanase required for secondary cell wall synthesis and water transport in xylem. J. Integr. Plant Biol. 2020, 62, 1607–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyerman, S.D.; Niemietz, C.M.; Bramley, H. Plant aquaporins: Multifunctional water and solute channels with expanding roles. Plant Cell Environ. 2002, 25, 173–194. [Google Scholar] [CrossRef] [Green Version]
- Sade, N.; Gebretsadik, M.; Seligmann, R.; Schwartz, A.; Wallach, R.; Moshelion, M. The role of tobacco Aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiol. 2010, 152, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Maurel, C.; Verdoucq, L.; Luu, D.T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Ann. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogiers, S.Y.; Coetzee, Z.A.; Walker, R.R.; Deloire, A.; Tyerman, S.D. Potassium in the Grape (Vitis vinifera L.) berry: Transport and function. Front. Plant Sci. 2017, 8, 1629. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S. Salinity and programmed cell death: Unravelling mechanisms for ion specific signalling. J. Exp. Bot. 2009, 60, 709–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Shi, J.Y.; Wang, Z.P.; Zhang, W.W.; Yang, H.Q. H2S pretreatment mitigates the alkaline salt stress on Malus hupehensis roots by regulating Na+/K+ homeostasis and oxidative stress. Plant Physiol. Biochem. 2020, 156, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Li, C.X.; Yan, M.; Zhao, Z.X.; Huang, P.P.; Wei, L.J.; Wu, X.T.; Wang, C.L.; Liao, W.B. Strigolactone is involved in nitric oxide-enhanced the salt resistance in tomato seedlings. J. Plant Res. 2022, 135, 337–350. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B.R. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.Q.; Wang, T.; Li, W.J.; Tang, W.; Zhang, D.M.; Dong, H.Z. Exogenous nitric oxide delays salt-induced leaf senescence in Cotton (Gossypium hirsutum L.). Acta Physiol. Plant. 2016, 38, 1–9. [Google Scholar] [CrossRef]
- Allu, A.D.; Soja, A.M.; Wu, A.; Szymanski, J.; Balazadeh, S. Salt stress and senescence: Identification of cross-talk regulatory components. J. Exp. Bot. 2014, 65, 3993–4008. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zheng, Q.S.; Shen, Q.R.; Guo, S.W. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.H.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbani, A.; Omran, V.O.G.; Razavi, S.M.; Pirdashti, H.; Ranjbar, M. Piriformospora indica confers salinity tolerance on tomato (Lycopersicon esculentum Mill.) through amelioration of nutrient accumulation, K+/Na+ homeostasis and water status. Plant Cell Rep. 2019, 38, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Wu, H.H.; Chen, L.M.; Liu, L.L.; Wan, X.C. Maintenance of mesophyll potassium and regulation of plasma membrane H+-ATPase are associated with physiological responses of tea plants to drought and subsequent rehydration. Crop J. 2018, 6, 611–620. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Mukherjee, J.; Jacquin, L.; Mukherjee, D.; Mitra, P.; Ray, S.; Chakraborty, S.B. Physiological and behavioural responses to acid and osmotic stress and effects of mucuna extract in guppies. Ecotox. Environ. Safe 2018, 163, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Li, W.T.; Huang, D.J.; Wang, B.; Hou, X.M.; Zhang, R.; Yan, M.; Liao, W.B. Changes of starch and sucrose content and related gene expression during the growth and development of Lanzhou lily bulb. PLoS ONE 2022, 17, e0262506. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Yang, Y.; Liu, X.H.; Huang, J.; Wang, Q.; Gu, J.H.; Lu, Y.M. Transcriptome profiling of the cold response and signaling pathways in Lilium lancifolium. BMC Genom. 2014, 15, 203. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.P.; Li, C.X.; Che, P.P.; Liu, H.W.; Zhao, Z.X.; Feng, L.; Liu, X.J.; Liao, W.B. Hydrogen Gas Enhanced Seed Germination by Increasing Trehalose Biosynthesis in Cucumber. J. Plant Growth Regul. 2022, 11, 1–15. [Google Scholar] [CrossRef]
- Du, F.; Fan, J.; Wang, T.; Wu, Y.; Grierson, D.; Gao, Z.S.; Xia, Y.P. Identification of differentially expressed genes in Flower, Leaf and bulb scale of Lilium oriental hybrid ‘Sorbonne’and putative control network for scent genes. BMC Genom. 2017, 18, 899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.M.; Wang, Y.J.; Zhang, Y.B.; Wang, R.Y.; Guo, Z.H.; Xie, Z.K. Impacts of drought stress on the morphology, physiology, and sugar content of Lanzhou lily (Lilium davidii var. unicolor). Acta Physiol. Plant. 2020, 42, 127. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Li, C.X.; Liu, H.W.; Yang, J.J.; Huang, P.P.; Liao, W.B. The involvement of glucose in Hydrogen gas-medicated adventitious Rooting in Cucumber. Plants 2021, 10, 1937. [Google Scholar] [CrossRef]
- Ling, L.J.; Jiao, Z.L.; Ma, W.X.; Zhao, J.; Feng, J.J.; Zhang, X.; Li, Z.B.; Zhang, J.; Lu, L. Preliminary report on the study of postharvest fruit rot bacteria and yeasts in Lanzhou Lily (Lilium davidii var. unicolor) in China. J. Phytopathol. 2019, 167, 135–145. [Google Scholar] [CrossRef]
- Huang, D.J.; Li, W.T.; Dawuda, M.M.; Huo, J.Q.; Li, C.X.; Wang, C.L.; Liao, W.B. Hydrogen sulfide reduced colour change in Lanzhou lily-bulb scales. Postharvest. Biol. Technol. 2021, 176, 111520. [Google Scholar] [CrossRef]
- Zhao, G.; Cheng, P.F.; Zhang, T.; Abdalmegeed, D.; Xu, S.; Shen, W.B. Hydrogen-rich water prepared by ammonia borane can enhance rapeseed (Brassica napus L.) seedlings tolerance against salinity, drought or cadmium. Ecotox. Environ. Safe 2021, 224, 112640. [Google Scholar] [CrossRef]
- Hou, X.M.; Qi, N.N.; Wang, C.L.; Li, C.X.; Huang, D.J.; Li, Y.H.; Wang, N.; Liao, W.B. Hydrogen-rich water promotes the formation of bulblets in Lilium davidii var. unicolor through regulating sucrose and starch metabolism. Planta 2021, 254, 106. [Google Scholar] [CrossRef]
- Shaheen, R.; Hassan, I.; Hafiz, I.A.; Jilani, G.; Abbasi, N.A. Balanced zinc nutrition enhances the antioxidative activities in Oriental lily cut-flower leading to improved growth and vase quality. Sci. Hortic. 2015, 197, 644–649. [Google Scholar] [CrossRef]
- Díaz–Pérez, J.C.; Muy–Rangel, M.D.; Mascorro, A.G. Fruit size and stage of ripeness affect postharvest water loss in bell pepper fruit (Capsicum annuum L.). J. Sci. Food Agric. 2007, 87, 68–73. [Google Scholar] [CrossRef]
- Wei, L.J.; Wang, C.L.; Liao, W.B. Hydrogen sulfide improves the vase life and quality of cut roses and chrysanthemums. J. Plant Growth Regul. 2021, 40, 2532–2547. [Google Scholar] [CrossRef]
- Ozaki, K.; Uchida, A.; Takabe, T.; Shinagawa, F.; Tanaka, Y.; Takabe, T.; Hayashi, T.; Hattori, T.; Rai, A.K.; Takabe, T. Enrichment of sugar content in melon fruits by hydrogen peroxide treatment. J. Plant Physiol. 2009, 166, 569–578. [Google Scholar] [CrossRef]
- Wu, X.T.; Li, W.T.; Liu, Z.Y.; Liu, H.W.; Gao, R.; Luo, Y.Y.; Liao, W.B. Trehalose promotes the formation of Lanzhou lily bulblets by increasing carbohydrate content. J. Hortic. Sci. Biotechnol. 2022, 97, 503–513. [Google Scholar] [CrossRef]
- Ren, P.J.; Jin, X.; Liao, W.B.; Wang, M.; Niu, L.J.; Li, X.P.; Xu, X.T.; Zhu, Y.C. Effect of hydrogen-rich water on vase life and quality in cut lily and rose flowers. Hortic. Environ. Biotechnol. 2017, 58, 576–584. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, H.N.; Xing, H.Y.; Cui, N.; Liu, X.Y.; Meng, X.N.; Wang, X.Y.; Fan, L.; Fan, H.Y. Regulation of growth and salt resistance in cucumber seedlings by hydrogen-rich water. J. Plant Growth Regul. 2021, 42, 134–153. [Google Scholar] [CrossRef]
- Esmann, M.; Fedosova, N.U.; Marsh, D. Osmotic stress and viscous retardation of the Na, K-ATPase ion pump. Biophys. J. 2008, 94, 2767–2776. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.H.; Pottosin, I.I.; Cuin, T.A.; Fuglsang, A.T.; Tester, M.; Jha, D.; Zepeda-Jazo, I.; Zhou, M.X.; Palmgren, M.G.; Newman, I.A.; et al. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol. 2007, 145, 1714–1725. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Cheng, P.F.; Wang, J.; Zhao, Z.S.; Kong, L.S.; Lou, W.; Zhang, T.; Jing, D.D.; Yu, J.L.; Shu, Z.L.; Huang, L.Q.; et al. Molecular hydrogen increases quantitative and qualitative traits of rice grain in field trials. Plants 2021, 10, 2331. [Google Scholar] [CrossRef]
- Li, C.X.; Huang, D.J.; Wang, C.L.; Wang, N.; Yao, Y.D.; Li, W.F.; Liao, W.B. NO is involved in H2-induced adventitious rooting in cucumber by regulating the expression and interaction of plasma membrane H+-ATPase and 14-3-3. Planta 2020, 252, 9. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Nadira, U.A.; Qiu, C.W.; Cao, F.B.; Zhang, G.P.; Holford, P.; Wu, F.B. Tolerance to drought, low pH and ai combined stress in tibetan wild barley is associated with improvement of ATPase and modulation of antioxidant defense system. Int. J. Mol. Sci. 2018, 19, 3553. [Google Scholar] [CrossRef] [Green Version]
- Li, J.S.; Jia, H.L.; Wang, J.; Cao, Q.H.; Wen, Z.C. Hydrogen sulfide is involved in maintaining ion homeostasis via regulating plasma membrane Na+/H+ antiporter system in the hydrogen peroxide-dependent manner in salt-stress Arabidopsis thaliana root. Protoplasma 2014, 251, 899–912. [Google Scholar] [CrossRef]
- Myo, T.; Wei, F.; Zhang, H.H.; Hao, J.F.; Zhang, B.; Liu, Z.X.; Cao, G.Q.; Tian, B.M.; Shi, G.Y. Genome-wide identification of the BASS gene family in four Gossypium species and functional characterization of GhBASSs against salt stress. Sci. Rep. 2021, 11, 11342. [Google Scholar] [CrossRef]
- Khan, M.S.; Ahmad, D.; Khan, M.A. Trends in genetic engineering of plants with (Na+/H+) antiporters for salt stress tolerance. Biotechnol. Biotecnol. Eq. 2015, 29, 815–825. [Google Scholar] [CrossRef]
- Garriga, M.; Raddatz, N.; Véry, A.A.; Sentenac, H.; Rubio-Meléndez, M.E.; González, W.; Dreyer, I. Cloning and functional characterization of HKT1 and AKT1 genes of Fragaria spp.—Relationship to plant response to salt stress. J. Plant Physiol. 2017, 210, 9–17. [Google Scholar] [CrossRef]
- Wu, W.W.; He, S.S.; An, Y.Y.; Cao, R.X.; Sun, Y.P.; Tang, Q.; Wang, L.J. Hydrogen peroxide as a mediator of 5–aminolevulinic acid–induced Na+ retention in roots for improving salt tolerance of strawberries. Physiol. Plant. 2019, 167, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly regulated channels controlling plant water relations. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.Y.; Kim, D.G.; Kim, Y.O.; Kim, J.S.; Kang, H. An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Mol. Biol. 2004, 54, 713–725. [Google Scholar] [CrossRef]
- He, J.D.; Dong, T.; Wu, H.H.; Zou, Y.N.; Wu, Q.S.; Kuca, K. Mycorrhizas induce diverse responses of root TIP aquaporin gene expression to drought stress in trifoliate orange. Sci. Hortic. 2019, 243, 64–69. [Google Scholar]
- Wang, Z.; Cao, J.K.; Jiang, W.B. Changes in sugar metabolism caused by exogenous oxalic acid related to chilling tolerance of apricot fruit. Postharvest Biol. Technol. 2016, 114, 10–16. [Google Scholar] [CrossRef]









| Gene Name | Gene ID | Annotation | Primer Sequence (Forward/Reverse) |
|---|---|---|---|
| AKT1 | transcript_HQ_Ld3_vu_transcript5092/f3p0/2924 | Inward rectifier potassium channel | F: GATGACAACAAGCAGAGACGGAGAG R: CAACGAACCTGGTAGGAGCACAAG |
| NHX2 | transcript_HQ_Ld3_vu_transcript10872/f16p0/2127 | Sodium/hydrogen exchanger family | F: TCACCACCATTCCAGGCTCTCC R: AATCCTCGCTGAACACCAAGATGC |
| SOS1 | transcript_HQ_Ld3_vu_transcript2231/f3p0/3731 | Sodium/hydrogen exchanger family | F: TGCGACTGGAAGGGATTGAATGC R: TCTTAACTACACGGAGGACCTGAGG |
| HA3 | transcript_HQ_Ld3_vu_transcript27371/f2p0/604 | Plasma membrane H+-transporting ATPase P Inorganic ion transport and metabolism | F: AAGAACTCTGCACGGGCTTCAAC R: CAGACTGTGTAGTGCTGCTGGATC |
| PIP1;5 | transcript_HQ_Ld3_vu_transcript20905/f18p0/1042 | Aquaporin G Carbohydrate transport and metabolism | F: GGAGGGCAAAGAAGAAGATGTGAGG R: ACGGTGAGGATGGTGATGTAGAGG |
| PIP2A | transcript_HQ_Ld3_vu_transcript19036/f4p0/1288 | Aquaporin G Carbohydrate transport and metabolism | F: ACAAGCACCAATCCGACACCAC R: ACAAGCACGAAGGTTCCGATGATC |
| TIP1;3 | transcript_HQ_Ld3_vu_transcript22649/f4p0/950 | Aquaporin G Carbohydrate transport and metabolism | F: TCATCCGTGGCGTCCTCTACTG R: TCCGACTATGAAACCAATGGCGATC |
| TIP2;2 | transcript_HQ_Ld3_vu_transcript19036/f4p0/1288 | Aquaporin G Carbohydrate transport and metabolism | F: TTATTGTTCGTGTTTGCGGGTGTTG R: AGCCGAGAAGTTGTGCAATCCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Fang, H.; Huang, P.; Feng, L.; Ye, F.; Wei, L.; Wu, X.; Zhang, H.; Liao, W. Effects of Hydrogen-Rich Water on Postharvest Physiology in Scales of Lanzhou Lily during Storage. Horticulturae 2023, 9, 156. https://doi.org/10.3390/horticulturae9020156
Liu X, Fang H, Huang P, Feng L, Ye F, Wei L, Wu X, Zhang H, Liao W. Effects of Hydrogen-Rich Water on Postharvest Physiology in Scales of Lanzhou Lily during Storage. Horticulturae. 2023; 9(2):156. https://doi.org/10.3390/horticulturae9020156
Chicago/Turabian StyleLiu, Xingjuan, Hua Fang, Panpan Huang, Li Feng, Fujin Ye, Lijuan Wei, Xuetong Wu, Hongsheng Zhang, and Weibiao Liao. 2023. "Effects of Hydrogen-Rich Water on Postharvest Physiology in Scales of Lanzhou Lily during Storage" Horticulturae 9, no. 2: 156. https://doi.org/10.3390/horticulturae9020156
APA StyleLiu, X., Fang, H., Huang, P., Feng, L., Ye, F., Wei, L., Wu, X., Zhang, H., & Liao, W. (2023). Effects of Hydrogen-Rich Water on Postharvest Physiology in Scales of Lanzhou Lily during Storage. Horticulturae, 9(2), 156. https://doi.org/10.3390/horticulturae9020156