Foliar Spraying of ZnO Nanoparticals on Curcuma longa Had Increased Growth, Yield, Expression of Curcuminoid Synthesis Genes, and Curcuminoid Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Chemical Evaluation
2.2.1. Photosynthetic Pigment Measure
2.2.2. Mineral Composition
2.2.3. Analysis of C. longa Rhizomes Powder Compositions
2.3. GC/MS Analysis of Dried C. longa Rhizomes Ethanolic Extract
2.4. Determination of Curcumin, Bisdemethoxycurcumin, and Demethoxycurcumin Contents in Ethanolic Extracts of Dried C. longa Rhizome by High-Performance Liquid Chromatography (HPLC)
2.5. Determined CURS1, -2, -3, and DCS Gene Expression Using Real-Time Reverse Transcriptase Polymerase Chain Reaction (Real-Time RT–PCR)
2.6. Statistical Analysis
3. Results
3.1. The Impact of ZnO NPs on the Growth and Yield of Plants
3.2. Photosynthetic Pigments, Mineral Contents, and Rhizomes Powder Compositions
3.3. GC-MS Analysis
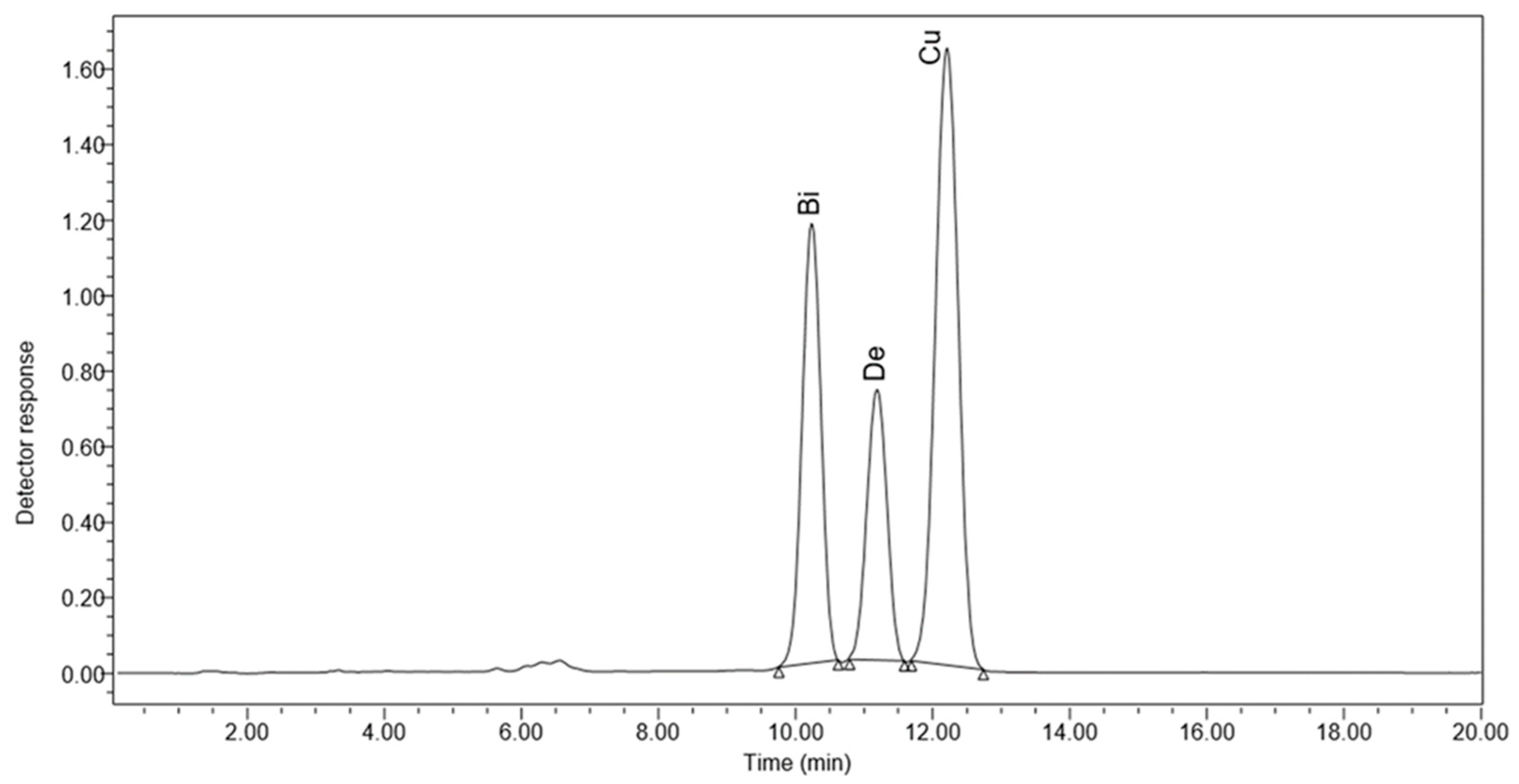
3.4. HPLC Results
3.5. The Influence of ZnO Nanoparticles on the Expression of Curcuminoid Biosynthesis Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, M.; Song, Y.; Kanwar, M.K.; Ahammed, G.J.; Shao, S.; Zhou, J. Phytonanotechnology Applications in Modern Agriculture. J. Nanobiotechnol. 2021, 19, 430. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Kamrul Hasan, M.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [PubMed]
- Kadi, V.P.; Vishwavidyalaya, S.; Rudani, K.; Patel, V.; Prajapati, K. The Importance of Zinc in Plant Growth—A Review. Int. Res. J. Nat. Appl. Sci. ISSN 2018, 46, 2349–4077. [Google Scholar]
- Sharma, A.; Patni, B.; Shankhdhar, D.; Shankhdhar, S.C. Zinc—An Indispensable Micronutrient. Physiol. Mol. Biol. Plants 2013, 19, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Hacisalihoglu, G. Zinc (Zn): The Last Nutrient in the Alphabet and Shedding Light on Zn Efficiency for the Future of Crop Production under Suboptimal Zn. Plants 2020, 9, 1471. [Google Scholar] [CrossRef]
- Al Jabri, H.; Saleem, M.H.; Rizwan, M.; Hussain, I.; Usman, K.; Alsafran, M. Zinc Oxide Nanoparticles and Their Biosynthesis: Overview. Life 2022, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Bhat, J.A.; Noureldeen, A.; Ahmad, P.; Yu, F. Zinc Oxide Nanoparticles and 24-Epibrassinolide Alleviates Cu Toxicity in Tomato by Regulating ROS Scavenging, Stomatal Movement and Photosynthesis. Ecotoxicol. Environ. Saf. 2021, 218, 112293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, R.; Chen, Z.; Cui, P.; Lu, H.; Yang, Y.; Zhang, H. The Effect of Zinc Oxide Nanoparticles for Enhancing Rice (Oryza sativa L.) Yield and Quality. Agriculture 2021, 11, 1247. [Google Scholar] [CrossRef]
- Awan, S.; Shahzadi, K.; Javad, S.; Tariq, A.; Ahmad, A.; Ilyas, S. A Preliminary Study of Influence of Zinc Oxide Nanoparticles on Growth Parameters of Brassica Oleracea Var Italic. J. Saudi Soc. Agric. Sci. 2021, 20, 18–24. [Google Scholar] [CrossRef]
- Bautista-Diaz, J.; Cruz-Alvarez, O.; Hernández-Rodríguez, O.A.; Sánchez-Chávez, E.; Jacobo-Cuellar, J.L.; Preciado-Rangel, P.; Avila-Quezada, G.D.; Ojeda-Barrios, D.L. Zinc Sulphate or Zinc Nanoparticle Applications to Leaves of Green Beans. Folia Hortic. 2021, 33, 365–375. [Google Scholar] [CrossRef]
- Adil, M.; Bashir, S.; Bashir, S.; Aslam, Z.; Ahmad, N.; Younas, T.; Asghar, R.M.A.; Alkahtani, J.; Dwiningsih, Y.; Elshikh, M.S. Zinc Oxide Nanoparticles Improved Chlorophyll Contents, Physical Parameters, and Wheat Yield under Salt Stress. Front. Plant Sci. 2022, 13, 932861. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; El Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharm. 2020, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Ajanaku, C.O.; Ademosun, O.T.; Atohengbe, P.O.; Ajayi, S.O.; Obafemi, Y.D.; Owolabi, O.A.; Akinduti, P.A.; Ajanaku, K.O. Functional Bioactive Compounds in Ginger, Turmeric, and Garlic. Front. Nutr. 2022, 9, 1012023. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological Activities of Curcuminoids, Other Biomolecules from Turmeric and Their Derivatives—A Review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Filho, J.G.; de Almeida, M.J.; Sousa, T.L.; dos Santos, D.C.; Egea, M.B. Bioactive Compounds of Turmeric (Curcuma longa L.). In Bioactive Compounds in Underutilized Vegetables and Legumes; Springer: Cham, Switzerland, 2021; pp. 297–318. [Google Scholar] [CrossRef]
- Sabir, S.M.; Zeb, A.; Mahmood, M.; Abbas, S.R.; Ahmad, Z.; Iqbal, N. Phytochemical Analysis and Biological Activities of Ethanolic Extract of Curcuma longa Rhizome. Braz. J. Biol. 2021, 81, 737–740. [Google Scholar] [CrossRef]
- Katsuyama, Y.; Kita, T.; Funa, N.; Horinouchi, S. Curcuminoid Biosynthesis by Two Type III Polyketide Synthases in the Herb Curcuma longa. J. Biol. Chem. 2009, 284, 11160–11170. [Google Scholar] [CrossRef]
- Katsuyama, Y.; Kita, T.; Horinouchi, S. Identification and Characterization of Multiple Curcumin Synthases from the Herb Curcuma longa. FEBS Lett. 2009, 583, 2799–2803. [Google Scholar] [CrossRef]
- El Sherif, F.; Alkuwayti, M.A.; Khattab, S. Foliar Spraying of Salicylic Acid Enhances Growth, Yield, and Curcuminoid Biosynthesis Gene Expression as Well as Curcuminoid Accumulation in Curcuma longa. Horticulturae 2022, 8, 417. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ishimine, Y.; Akamine, H.; Motomura, K. Effects of Seed Rhizome Size on Growth and Yield of Turmeric (Curcuma longa L.). Plant Prod. Sci. 2005, 8, 86–94. [Google Scholar] [CrossRef]
- Buurman, P.; Van Lagen, B.; Velthorst, E.J. Manual for Soil and Water Analysis; Backhuys Publishers: Leiden, The Netherlands, 1996; ISBN 9073348587. [Google Scholar]
- AOAC. Official Methods of Analysis of the AOAC, 14th ed.; Howitz, Ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1984. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; Hans Publishers: Cambridge, MA, USA, 1942. [Google Scholar]
- Jackson, M.L. Soil Chemica Analysis; Prentice Hall: New Delhi, India, 1967. [Google Scholar]
- Murphy, J.; Riley, J.P. A Modified Single-Solution Method for the Determination of Phosphorus in Natural Water. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Mazumdar, B.; Majumder, K.; Mazumdar, B.C.; Majumder, K. Methods of Physiochemical Analysis of Fruits; Daya Publishing House: Delhi, India, 2003. [Google Scholar]
- Page, A.L. Methods of Soil Analysis-Part 2: Chemical and Microbiological Properties, 2nd ed.; ASA and SSSA: Madison, WI, USA, 1982; Volume 9, ISBN 0891180729. [Google Scholar]
- Noel, S.J.; Jørgensen, H.J.H.; Knudsen, K.E.B. The Use of Near-Infrared Spectroscopy (NIRS) to Determine the Energy Value of Individual Feedstuffs and Mixed Diets for Pigs. Anim. Feed Sci. Technol. 2022, 283, 115156. [Google Scholar] [CrossRef]
- González-Martín, I.; Álvarez-García, N.; González-Cabrera, J.M. Near-Infrared Spectroscopy (NIRS) with a Fibre-Optic Probe for the Prediction of the Amino Acid Composition in Animal Feeds. Talanta 2006, 69, 706–710. [Google Scholar] [CrossRef] [PubMed]
- StatSoft STATISTICA for Windows, Version 6; 2300; StatSoft Inc.: Tulsa, OK, USA, 2001.
- Ellison, E.; Blaylock, A.D.; Sanchez, C.; Smith, R. Exploring Controlled Release Nitrogen Fertilizers for Vegetable and Melon Crop Production in California and Arizona. In Proceedings of the 2013 Western Nutrient Management Conference, Reno, NV, USA, 5–8 March 2013; Volume 10, pp. 17–22. [Google Scholar]
- Derosa, M.C.; Monreal, C.; Schnitzer, M.; Walsh, R.; Sultan, Y. Nanotechnology in Fertilizers. Nat. Nanotechnol. 2010, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Akanbi-gada, M.A.; Ogunkunle, C.O.; Ilesanmi, A.O.; Femi-adepoju, A.G.; Sidiq, L.O.; Fatoba, P.O. Effects of Zinc Oxide Nanoparticles on Chlorophyll Content, Growth Attributes, Antioxidant Enzyme Activities And Bioaccumulation of Common Bean (Phaseolus vulgaris L.) Grown In Soil Medium. IOSR J. Environ. Sci. Toxicol. Food Technol. 2019, 13, 9–15. [Google Scholar] [CrossRef]
- Miliauskienė, J.; Brazaitytė, A.; Sutulienė, R.; Urbutis, M.; Tučkutė, S. ZnO Nanoparticle Size-Dependent Effects on Swiss Chard Growth and Nutritional Quality. Agriculture 2022, 12, 1905. [Google Scholar] [CrossRef]
- Mogazy, A.M.; Hanafy, R.S. Foliar Spray of Biosynthesized Zinc Oxide Nanoparticles Alleviate Salinity Stress Effect on Vicia Faba Plants. J. Soil Sci. Plant Nutr. 2022, 22, 2647–2662. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Hafeez, M.; Rizwan, M.; Hussain, K.; Asrar, M.; Alyemeni, M.N.; Wijaya, L.; Ali, S. Foliar Exposure of Zinc Oxide Nanoparticles Improved the Growth of Wheat (Triticum aestivum L.) and Decreased Cadmium Concentration in Grains under Simultaneous Cd and Water Deficient Stress. Ecotoxicol. Environ. Saf. 2021, 208, 111627. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Van Der Ent, A.; Cheng, M.; Jiang, H.; Read, T.L.; Lombi, E.; Tang, C.; De Jonge, M.D.; Menzies, N.W.; et al. Absorption of Foliar-Applied Zn in Sunflower (Helianthus annuus): Importance of the Cuticle, Stomata and Trichomes. Ann. Bot. 2019, 123, 57–68. [Google Scholar] [CrossRef]
- Kisan, B.; Shruthi, H.; Sharanagouda, H.; Revanappa, S.B.; Pramod, N.K. Effect of Nano-Zinc Oxide on the Leaf Physical and Nutritional Quality of Spinach. Agrotechnology 2015, 5, 135. [Google Scholar] [CrossRef]
- Rahman, M.H.; Hasan, M.N.; Khan, M.Z.H. Study on Different Nano Fertilizers Influencing the Growth, Proximate Composition and Antioxidant Properties of Strawberry Fruits. J. Agric. Food Res. 2021, 6, 100246. [Google Scholar] [CrossRef]
- Fan, Y.; Jiang, T.; Chun, Z.; Wang, G.; Yang, K.; Tan, X.; Zhao, J.; Pu, S.; Luo, A. Zinc Affects the Physiology and Medicinal Components of Dendrobium Nobile Lindl. Plant Physiol. Biochem. 2021, 162, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Shaghaleh, H.; Hamoud, Y.A.; Holford, P.; Shao, H.; Qi, W.; Hashmi, M.Z.; Wu, T. Zinc Oxide Nanoparticles: Potential Effects on Soil Properties, Crop Production, Food Processing, and Food Quality. Environ. Sci. Pollut. Res. 2021, 28, 36942–36966. [Google Scholar] [CrossRef]
- Liu, R.; Bao, Z.X.; Zhao, P.J.; Li, G.H. Advances in the Study of Metabolomics and Metabolites in Some Species Interactions. Molecules 2021, 26, 3311. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.K.; Prasad, S.; Yuan, W.; Li, S.; Aggarwal, B.B. Identification of a Novel Compound (β-Sesquiphellandrene) from Turmeric (Curcuma longa) with Anticancer Potential: Comparison with Curcumin. Investig. New Drugs 2015, 33, 1175–1186. [Google Scholar] [CrossRef]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef]
- Ghosh, M.; Jana, A.; Sinha, S.; Jothiramajayam, M.; Nag, A.; Chakraborty, A.; Mukherjee, A.; Mukherjee, A. Effects of ZnO Nanoparticles in Plants: Cytotoxicity, Genotoxicity, Deregulation of Antioxidant Defenses, and Cell-Cycle Arrest. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2016, 807, 25–32. [Google Scholar] [CrossRef]
- Mahendra, S.; Zhu, H.; Colvin, V.L.; Alvarez, P.J. Quantum Dot Weathering Results in Microbial Toxicity. Environ. Sci. Technol. 2008, 42, 9424–9430. [Google Scholar] [CrossRef]
- Zafar, H.; Ali, A.; Ali, J.S.; Haq, I.U.; Zia, M. Effect of ZnO Nanoparticles on Brassica Nigra Seedlings and Stem Explants: Growth Dynamics and Antioxidative Response. Front. Plant Sci. 2016, 7, 535. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Khan, T.; Khan, M.A.; Nadhman, A.; Aasim, M.; Khan, N.Z.; Ali, W.; Nazir, N.; Zahoor, M. Iron-Doped Zinc Oxide Nanoparticles-Triggered Elicitation of Important Phenolic Compounds in Cell Cultures of Fagonia Indica. Plant Cell Tissue Organ Cult. 2021, 147, 287–296. [Google Scholar] [CrossRef]
- Hezaveh, T.A.; Pourakbar, L.; Rahmani, F.; Alipour, H. Effects of ZnO NPs on Phenolic Compounds of Rapeseed Seeds under Salinity Stress. J. Plant Process Funct. 2020, 8, 11–18. [Google Scholar]
- Al Dayel, M.F.; El Sherif, F. Spirulina Platensis Foliar Spraying Curcuma longa Has Improved Growth, Yield, and Curcuminoid Biosynthesis Gene Expression, as Well as Curcuminoid Accumulation. Horticulturae 2022, 8, 469. [Google Scholar] [CrossRef]
- Park, W.T.; Arasu, M.V.; Al-Dhabi, N.A.; Yeo, S.K.; Jeon, J.; Park, J.S.; Lee, S.Y.; Park, S.U. Yeast Extract and Silver Nitrate Induce the Expression of Phenylpropanoid Biosynthetic Genes and Induce the Accumulation of Rosmarinic Acid in Agastache Rugosa Cell Culture. Molecules 2016, 21, 426. [Google Scholar] [CrossRef] [PubMed]

| ZnO NPs (mgL−1) | Plant Height (cm) | Number of Leaves (n) | Number of Roots (n) | Root Length (cm) | Weight of the Dried Root (g) | Weight of Dried Leaves (g) |
|---|---|---|---|---|---|---|
| Control | 120.0 d ± 0.605551 | 10.0 d ± 0.0516611 | 20.25 c ± 0.429101 | 12.25 b ± 0.00331 | 2.90 b ± 0.22073 | 23.53 d ± 0.208167 |
| 5 | 159.7 b ± 0.50287 | 15.5 b ± 0.081666 | 36.7 b ± 0.507571 | 13.3 b ± 1.00525 | 2.33 b ± 0.52758 | 49.7 bc ± 0.568624 |
| 10 | 177 ab ± 0.888194 | 13.3 c ± 0.886751 | 46.3 a ± 1.527525 | 22.7 a ± 1.527525 | 3.6 a ± 0.688878 | 88.73 a ± 1.113553 |
| 20 | 148.7 c ± 0.74223 | 14.17 bc ± 0.57735 | 33.7 b ± 0.767453 | 11.7 b ± 0.763763 | 2.4 b ± 0.616276 | 34.2 c ± 0.556776 |
| 40 | 187.3 a ± 00098 | 16 a ± 0.033223 | 39 b ± 0557439 | 16.7 ab ± 0.732051 | 2.93 b ± 0.960109 | 62.77 b ± 0.85049 |
| ZnO NPs (mgL−1) | Number. of Rhizomes (n) | Weight of Dried Rhizome (g) | Diameters of Rhizomes (mm) |
|---|---|---|---|
| Control | 16.67 c ± 0.50925 | 9.7 c ± 1.93132 | 8.0 d ± 1.113553 |
| 5 | 19.7 b ± 0.131601 | 16 b ± 1.255398 | 14.3 b ± 1.123892 |
| 10 | 26.14 a ± 0.618802 | 27.1 a ± 1.05183 | 22.7 a ± 0.923398 |
| 20 | 18.37 b ± 0.645751 | 11.07 b ± 1.87460 | 9.0 c ± 1.042874 |
| 40 | 22.25 a ± 1.527525 | 15.27 b ± 1.41109 | 13.7 b ± 1.562861 |
| ZnO NPs (mgL−1) | Chl a (mg/100 g F.W.) | Chl b (mg/100 g F.W.) | Carotenoids (mg/100 g F.W.) |
|---|---|---|---|
| Control | 64.126 c ± 0.422089 | 29.003 c ± 0.02369 | 70.0825 c ± 0.94639 |
| 5 | 84.483 b ± 1.15505 | 38.860 b ± 0.12018 | 72.8609 d ± 0.05155 |
| 10 | 77.577 b ± 0.12125 | 30.287 c ± 0.4654 | 88.733 c ± 0.28711 |
| 20 | 83.129 b ± 0.86419 | 36.4314 b ± 0.35553 | 102.918 b ± 1.7836 |
| 40 | 105.934 a ± 1.05027 | 49.509 a ± 0.63638 | 133.499 a ± 0.48326 |
| ZnO NPs (mgL−1) | N (g kg−1) | P (mg kg−1) | K (mg kg−1) | Zn (mg kg−1) |
|---|---|---|---|---|
| Control | 61.525 a ± 0.00264575 | 0.0375 a ± 0.002646 | 11.01538 a ± 0.189180152 | 0.03215 b ± 0.004709 |
| 5 | 39.75 a ± 0.002828427 | 0.021 b ± 0.002828 | 9.32245 b ± 1.506632419 | 0.0417 b ± 0.009051 |
| 10 | 40.35 a ± 0.000707107 | 0.0235 b ± 0.000707 | 9.9004 ab ± 0.297550534 | 0.033375 b ± 0.003323 |
| 20 | 51.3 a ± 0.0234507 | 0.0245 b ± 0.000707 | 10.2673 ab ± 0.137461558 | 0.0502 b ± 0.006223 |
| 40 | 50.75 a ± 0.00212132 | 0.0255 b ± 0.002121 | 9.45515 b ± 0.304975155 | 0.0773 a ± 0.024466 |
| ZnO NPs (mgL−1) | Fat (%) | Fiber (%) | Moisture (%) | Protein (%) | Starch (%) | Ash (%) |
|---|---|---|---|---|---|---|
| Control | 3.74 b ± 0.113137 | 10.02 a ± 1.569777 | 14.43 c ± 0.289914 | 4.02 e ± 0.685894 | 24.21 cd ± 2.001112 | 2.02 ab ± 1.19501 |
| 5 | 3.9 a ± 0.021213 | 5.41 c ± 0.007071 | 16.81 a ± 0.007071 | 5.95 c ± 0.070711 | 29.95 a ± 0.070711 | 0.29 c ± 0.014142 |
| 10 | 3.48 b ± 0.028284 | 7.32 b ± 0.021213 | 14.88 b ± 0.028284 | 5.05 d ± 0.070711 | 26.39 b ± 0.007071 | 1.61 bc ± 0.007071 |
| 20 | 3.05 c ± 0.070711 | 8.42 ab ± 0.028284 | 14.02 d ± 0.028284 | 11.08 a ± 0.035355 | 27.29 b ± 0.021213 | 1.96 ab ± 0.007071 |
| 40 | 3.88 a ± 0.028284 | 9.52 a ± 0.028284 | 14.08 d ± 0.035355 | 8.42 b ± 0.028284 | 22.81 d ± 0.007071 | 3.19 a ± 0.021213 |
| Phytochemical | Molecular Formula | Composition (Area %) | ||||
|---|---|---|---|---|---|---|
| Control | ZnO NPs (5 mgL−1) | ZnO NPs (10 mgL−1) | ZnO NPs (20 mgL−1) | ZnO NPs (40 mgL−1) | ||
| Coumaran | C9H6O2 | 0.77 d | 4.67 a | 2.35 b | 1.76 c | 1.26 c |
| 4-Hydroxy-3-methylacetophenone | C9H10O2 | 1.31 d | 6.15 a | 3.44 b | 3.24 b | 2.33 c |
| alpha.-Curcumene | C15H22 | 2.84 b | 4.53 a | 1.41 c | 0.8 d | 1.08 c |
| Ar-tumerone | C15H20O | 56.45 a | 36.11 c | 53.75 a | 51.36 ab | 46.65 b |
| beta.-Sesquiphellandrene | C15H24 | 3.02 b | 4.25 a | 1.33 c | 1.12 c | 1.19 c |
| Curlone | C15H22O | 19.85 b | 9.28 c | 21.83 a | 22.60 a | 20.59 a |
| Vanillin | C8H8O3 | 0.25 c | 1.88 a | 1.15 a | 0.66 b | 0.81 b |
| Phytochemical | Molecular Formula | Composition (Area %) | ||||
|---|---|---|---|---|---|---|
| Control | ZnO NPs (5 mgL−1) | ZnO NPs (10 mgL−1) | ZnO NPs (20 mgL−1) | ZnO NPs (40 mgL−1) | ||
| (-)-Zingiberene | C15H24 | ND | 0.60 a | 0.32 a | ND | ND |
| P-hydroxybenzaldehyde | C7H6O2 | ND | 0.93 a | 0.43 b | 0.16 d | 0.29 c |
| Caryophyllene | C15H24 | 0.09 c | 0.38 a | 0.16 b | ND | ND |
| cis oleic acid | C18H34O2 | ND | 1.02 a | 0.52 b | 0.61 b | ND |
| cis,cis-Linoleic acid | C17H30O2 | ND | 1.83 a | 0.79 c | 0.95 b | 0.90 b |
| Humulane-1,6-dien-3-ol | C15H22O | ND | 3.57 a | 1.67 b | 1.58 b | 1.13 b |
| Oleic acid, ethyl ester | C20H38O2 | ND | 0.17 b | 0.19 b | 0.28 a | ND |
| Pentadecanoic acid | C15H30O2 | ND | ND | 2.56 b | 3.49 a | 2.06 b |
| Thymol | C10H14O | ND | ND | ND | 0.16 b | 0.35 a |
| Tumerone | C15H22O | 6.2 a | ND | ND | ND | 5.63 a |
| Phytochemical | Molecular Formula | Composition (Area %) | |||
|---|---|---|---|---|---|
| Control | ZnO NPs (10 mgL−1) | ZnO NPs (20 mgL−1) | ZnO NPs (40 mgL−1) | ||
| 4-propylguaiacol | C10H14O2 | ND | 0.11 | ND | ND |
| Benzene, (1,1-dimethylnonyl)- | C17H28 | ND | 2.45 | ND | ND |
| Benzene, 1,4-dimethyl-2-(2-methylpropyl)- | C18H22 | ND | ND | 1.54 | ND |
| Germacron | C15H22O | 2.56 | ND | ND | ND |
| Palmitic acid methyl ester | C17H34O2 | ND | ND | ND | 1.26 |
| Camphor | C10H16O | ND | ND | 1.34 | ND |
| (+)-.alpha.-Bisabolol | C15H26O | ND | ND | 1.26 | ND |
| Dicumene | C18H24Cr | ND | ND | ND | 1.25 |
| Palmitic acid, ethyl ester | C18H36O2 | ND | ND | 1.42 | ND |
| Palmitic acid | C16H32O2 | ND | ND | ND | 3.57 |
| ZnO NPs (mgL−1) | Bisdemethoxycurcumin (µg/mL) | Demethoxycurcumin (µg/mL) | Curcumin (µg/mL) |
|---|---|---|---|
| Control | 140.02985935 e ± 0.000199 | 72.339401155 e ± 0.227121 | 225.70146805 e ± 0.002076 |
| 5 | 390.9309998 b ± 0.001414 | 191.9427214 b ± 0.010293 | 628.0079489 b ± 0.002901 |
| 10 | 297.7843364 c ± 0.022152 | 155.2782524 c ± 0.313598 | 502.7190404 c ± 0.001357 |
| 20 | 235.8495247 d ± 0.043099 | 129.44198 d ± 0.011342 | 469.839536 d ± 0.000656 |
| 40 | 398.4860058 a ± 0.019791 | 212.6428497 a ± 0.000213 | 715.05218605 a ± 0.003092 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khattab, S.; Alkuwayti, M.A.; Yap, Y.-K.; Meligy, A.M.A.; Bani Ismail, M.; El Sherif, F. Foliar Spraying of ZnO Nanoparticals on Curcuma longa Had Increased Growth, Yield, Expression of Curcuminoid Synthesis Genes, and Curcuminoid Accumulation. Horticulturae 2023, 9, 355. https://doi.org/10.3390/horticulturae9030355
Khattab S, Alkuwayti MA, Yap Y-K, Meligy AMA, Bani Ismail M, El Sherif F. Foliar Spraying of ZnO Nanoparticals on Curcuma longa Had Increased Growth, Yield, Expression of Curcuminoid Synthesis Genes, and Curcuminoid Accumulation. Horticulturae. 2023; 9(3):355. https://doi.org/10.3390/horticulturae9030355
Chicago/Turabian StyleKhattab, Salah, Mayyadah Abdullah Alkuwayti, Yun-Kiam Yap, Ahmed M. A. Meligy, Mohammad Bani Ismail, and Fadia El Sherif. 2023. "Foliar Spraying of ZnO Nanoparticals on Curcuma longa Had Increased Growth, Yield, Expression of Curcuminoid Synthesis Genes, and Curcuminoid Accumulation" Horticulturae 9, no. 3: 355. https://doi.org/10.3390/horticulturae9030355
APA StyleKhattab, S., Alkuwayti, M. A., Yap, Y.-K., Meligy, A. M. A., Bani Ismail, M., & El Sherif, F. (2023). Foliar Spraying of ZnO Nanoparticals on Curcuma longa Had Increased Growth, Yield, Expression of Curcuminoid Synthesis Genes, and Curcuminoid Accumulation. Horticulturae, 9(3), 355. https://doi.org/10.3390/horticulturae9030355






