A Water Stress–Tolerant Pepper Rootstock Improves the Behavior of Pepper Plants under Deficit Irrigation through Root Biomass Distribution and Physiological Adaptation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Conditions
2.2. Plant Material
2.3. Irrigation Management and Control
2.4. Physiological Parameters
2.5. Production Parameters
2.6. Biomass Parameters
2.7. Experimental Design and Statistical Analysis
3. Results
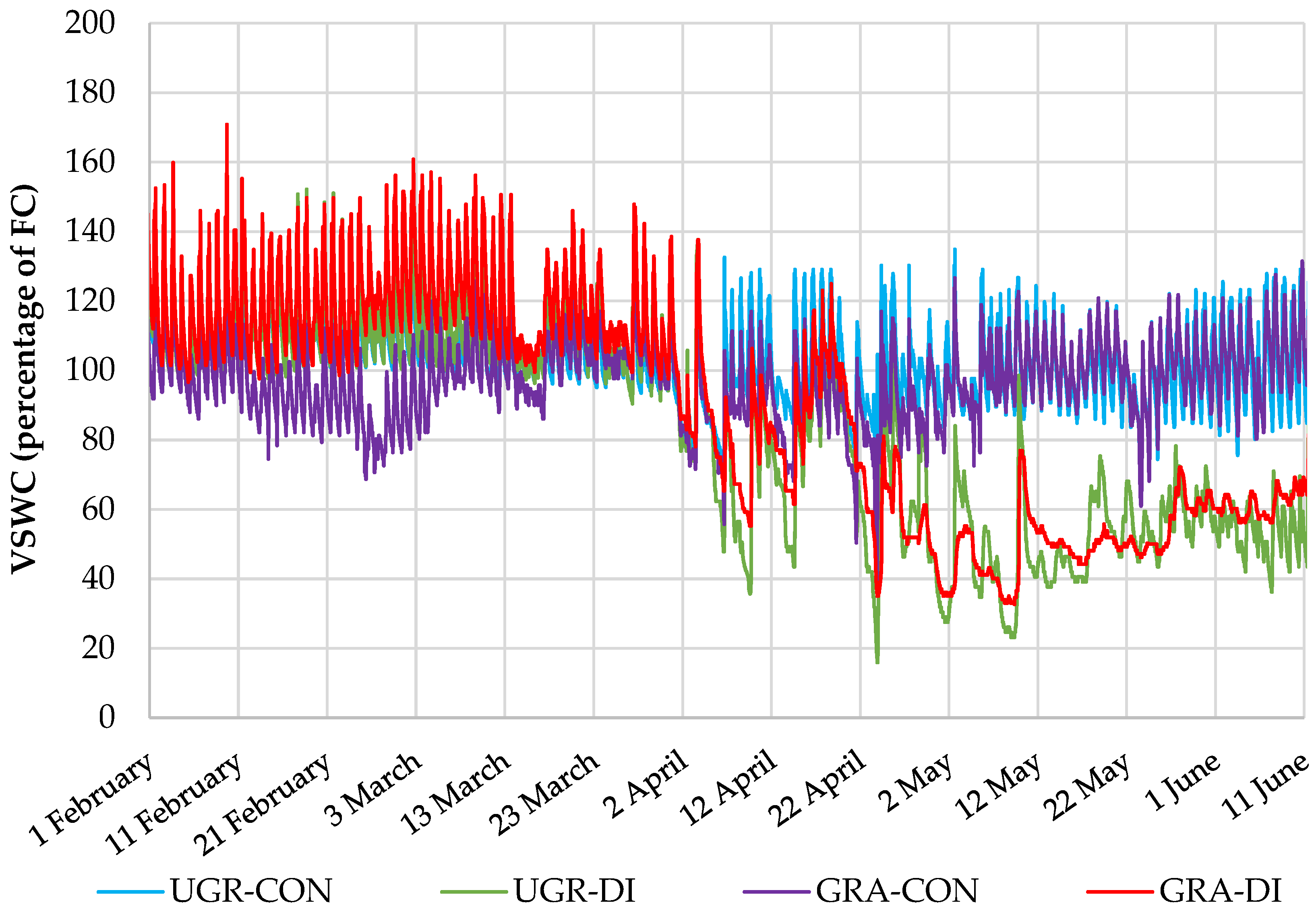
3.1. Irrigation Managment
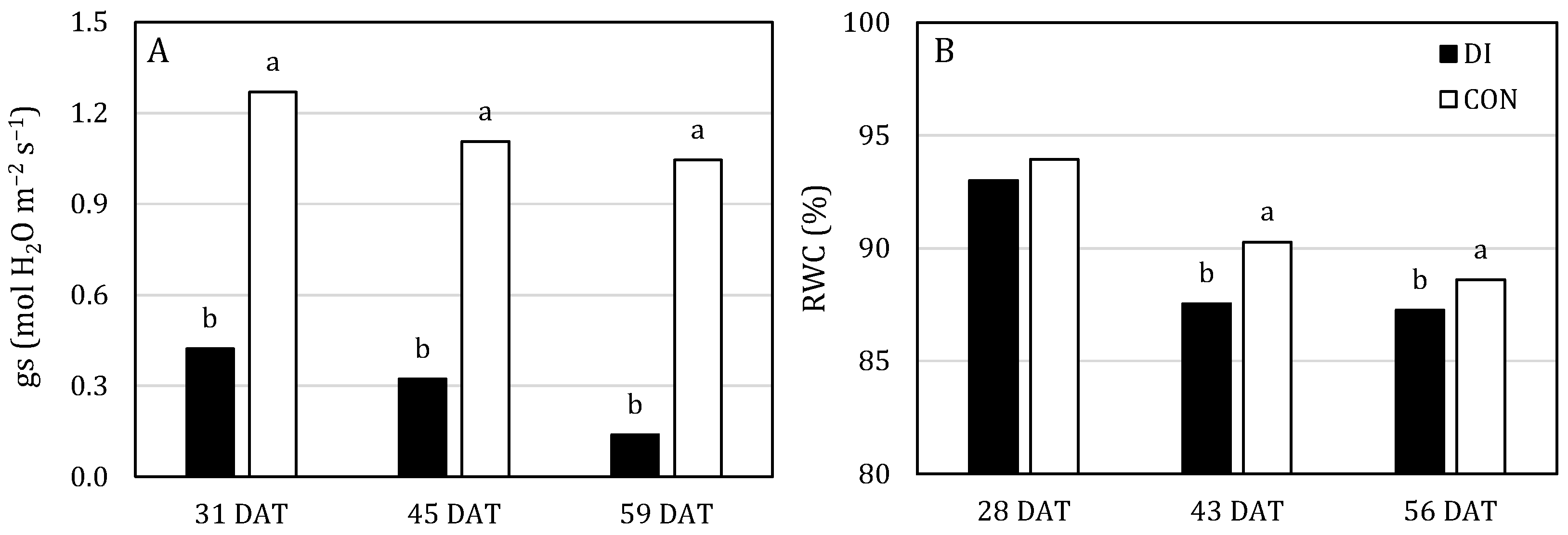
3.2. Physiological Parameters
3.3. Production Parameters
3.4. Biomass Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AQUASTAT Sistema Mundial de Información de la FAO Sobre el Agua en la Agricultura. Available online: https://www.fao.org/aquastat/es/ (accessed on 9 November 2022).
- Fereres, E.; Soriano, M.A. Deficit irrigation for reducing agricultural water use. J. Exp. Bot. 2007, 58, 147–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Saini, R.K.; Singh, S.; Sharma, S.P. Potential of Integrating Biochar and Deficit Irrigation Strategies for Sustaining Vegetable Production in Water-limited Regions: A Review. HortScience 2019, 54, 1872–1878. [Google Scholar] [CrossRef] [Green Version]
- Sezen, S.M.; Yazar, A.; Tekin, S. Physiological response of red pepper to different irrigation regimes under drip irrigation in the Mediterranean region of Turkey. Sci. Hortic. 2019, 245, 280–288. [Google Scholar] [CrossRef]
- Abdelkhalik, A.; Pascual, B.; Nájera, I.; Domene, M.A.; Baixauli, C.; Pascual-Seva, N. Effects of deficit irrigation on the yield and irrigation water use efficiency of drip-irrigated sweet pepper (Capsicum annuum L.) under Mediterranean conditions. Irrig. Sci. 2020, 38, 89–104. [Google Scholar] [CrossRef]
- González-Dugo, V.; Orgaz, F.; Fereres, E. Responses of pepper to deficit irrigation for paprika production. Sci. Hortic. 2007, 114, 77–82. [Google Scholar] [CrossRef]
- Yasuor, H.; Wien, H.C. Peppers. In The Physiology of Vegetable Crops; CABI: Wallingford, UK, 2020; pp. 179–208. [Google Scholar]
- Lee, J.M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Yao, X.; Yang, R.; Zhao, F.; Wang, S.; Li, C.; Zhao, W. An analysis of physiological index of differences in drought tolerance of tomato rootstock seedlings. J. Plant Biol. 2016, 59, 311–321. [Google Scholar] [CrossRef]
- Bikdeloo, M.; Colla, G.; Rouphael, Y.; Hassandokht, M.R.; Soltani, F.; Salehi, R.; Kumar, P.; Cardarelli, M. Morphological and Physio-Biochemical Responses of Watermelon Grafted onto Rootstocks of Wild Watermelon [Citrullus colocynthis (L.) Schrad] and Commercial Interspecific Cucurbita Hybrid to Drought Stress. Horticulturae 2021, 7, 359. [Google Scholar] [CrossRef]
- Penella, C.; Nebauer, S.G.; López-Galarza, S.; Quiñones, A.; San Bautista, A.; Calatayud, Á. Grafting pepper onto tolerant rootstocks: An environmental-friendly technique overcome water and salt stress. Sci. Hortic. 2017, 226, 33–41. [Google Scholar] [CrossRef]
- López-Serrano, L.; Canet-Sanchis, G.; Selak, G.V.; Penella, C.; Bautista, A.S.; López-Galarza, S.; Calatayud, Á. Pepper rootstock and scion physiological responses under drought stress. Front. Plant Sci. 2019, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Gisbert-Mullor, R.; Pascual-Seva, N.; Martínez-Gimeno, M.A.; López-Serrano, L.; Marín, E.B.; Pérez-Pérez, J.G.; Bonet, L.; Padilla, Y.G.; Calatayud, Á.; Pascual, B.; et al. Grafting onto an Appropriate Rootstock Reduces the Impact on Yield and Quality of Controlled Deficit Irrigated Pepper Crops. Agronomy 2020, 10, 1529. [Google Scholar] [CrossRef]
- Penella, C.; Nebauer, S.G.; Bautista, A.S.; López-Galarza, S.; Calatayud, Á. Rootstock alleviates PEG-induced water stress in grafted pepper seedlings: Physiological responses. J. Plant Physiol. 2014, 171, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Ghadirnezhad Shiade, S.R.; Fathi, A.; Taghavi Ghasemkheili, F.; Amiri, E.; Pessarakli, M. Plants’ responses under drought stress conditions: Effects of strategic management approaches—A review. J. Plant Nutr. 2022, 1–33. [Google Scholar] [CrossRef]
- Hsiao, T.C. Plant Responses to Water Stress. Annu. Rev. Plant Physiol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Zapata-Sierra, A.J.; Moreno-Pérez, M.F.; Reyes-Requena, R.; Manzano-Agugliaro, F. Root distribution with the use of drip irrigation on layered soils at greenhouses crops. Sci. Total Environ. 2021, 768, 144944. [Google Scholar] [CrossRef]
- López-Serrano, L.; Penella, C.; Bautista, A.S.; López-Galarza, S.; Calatayud, A. Physiological changes of pepper accessions in response to salinity and water stress. Span. J. Agric. Res. 2017, 15, 15. [Google Scholar] [CrossRef] [Green Version]
- Penella, C.; Landi, M.; Guidi, L.; Nebauer, S.G.; Pellegrini, E.; Bautista, A.S.; Remorini, D.; Nali, C.; López-Galarza, S.; Calatayud, A. Salt-tolerant rootstock increases yield of pepper under salinity through maintenance of photosynthetic performance and sinks strength. J. Plant Physiol. 2016, 193, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Saure, M.C. Why calcium deficiency is not the cause of blossom-end rot in tomato and pepper fruit—A reappraisal. Sci. Hortic. 2014, 174, 151–154. [Google Scholar] [CrossRef]
- De Freitas, S.T.; Shackel, K.A.; Mitcham, E.J. Abscisic acid triggers whole-plant and fruit-specific mechanisms to increase fruit calcium uptake and prevent blossom end rot development in tomato fruit. J. Exp. Bot. 2011, 62, 2645–2656. [Google Scholar] [CrossRef] [Green Version]
- López-Serrano, L.; Canet-Sanchis, G.; Selak, G.V.; Penella, C.; San Bautista, A.; López-Galarza, S.; Calatayud, Á. Physiological characterization of a pepper hybrid rootstock designed to cope with salinity stress. Plant Physiol. Biochem. 2020, 148, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Cardarelli, M.; Colla, G.; Rea, E. Yield, Mineral Composition, Water Relations, and Water Use Efficiency of Grafted Mini-watermelon Plants Under Deficit Irrigation. HortScience 2008, 43, 730–736. [Google Scholar] [CrossRef] [Green Version]
- Kyriacou, M.C.; Rouphael, Y.; Colla, G.; Zrenner, R.; Schwarz, D. Vegetable grafting: The implications of a growing agronomic imperative for vegetable fruit quality and nutritive value. Front. Plant Sci. 2017, 8, 741. [Google Scholar] [CrossRef] [PubMed]
- Trifil, P.; Raimondo, F.; Lo Gullo, M.A.; Nardini, A.; Salleo, S. Hydraulic connections of leaves and fruit to the parent plant in Capsicum frutescens (hot pepper) during fruit ripening. Ann. Bot. 2010, 106, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Guichard, S.; Gary, C.; Leonardi, C.; Bertin, N. Analysis of growth and water relations of tomato fruits in relation to air vapor pressure deficit and plant fruit load. J. Plant Growth Regul. 2005, 24, 201–213. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2015. [Google Scholar]
| Leaf Water Potential (MPa) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 36 DAT | 50 DAT | 63 DAT | ||||||||||
| Ψpredawn | Ψleaf | Ψpredawn | Ψleaf | Ψpredawn | Ψleaf | |||||||
| Grafting (G) | ||||||||||||
| UGR | −0.426 | a | −1.618 | a | −0.398 | a | −1.410 | −0.531 | −1.139 | |||
| GRA | −0.348 | b | −1.463 | b | −0.333 | b | −1.317 | −0.493 | −1.205 | |||
| Water stress (WS) | ||||||||||||
| DI | −0.405 | −1.639 | a | −0.406 | a | −1.444 | a | −0.696 | a | −1.276 | a | |
| CON | −0.369 | −1.441 | b | −0.324 | b | −1.283 | b | −0.328 | b | −1.068 | b | |
| G × WS | ||||||||||||
| UGR-DI | −0.453 | −1.698 | −0.438 | −1.479 | −0.710 | −1.233 | ||||||
| UGR-CON | −0.400 | −1.538 | −0.358 | −1.342 | −0.353 | −1.045 | ||||||
| GRA-DI | −0.358 | −1.580 | −0.375 | −1.408 | −0.683 | −1.320 | ||||||
| GRA-CON | −0.338 | −1.345 | −0.290 | −1.225 | −0.303 | −1.090 | ||||||
| ANOVA (df) | % Sum of squares | |||||||||||
| G (1) | 46.50 | ** | 24.34 | ** | 18.99 | * | 10.69 | n.s | 0.89 | n.s. | 5.95 | n.s |
| WS (1) | 9.85 | n.s | 39.52 | ** | 30.59 | * | 31.29 | * | 80.96 | ** | 59.12 | ** |
| G × WS (1) | 1.98 | n.s | 1.42 | n.s | 0.03 | n.s | 0.64 | n.s | 0.08 | n.s | 0.61 | n.s |
| Residuals (12) | 41.66 | 34.71 | 50.39 | 57.39 | 18.07 | 34.31 | ||||||
| Std. Dev. (+) | 0.043 | 0.107 | 0.061 | 0.125 | 0.101 | 0.092 | ||||||
| Marketable Yield | BER | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (Fruit/Plant) | (kg/Plant) | (g/Fruit) | (Fruit/Plant) | (%) | ||||||
| Grafting (G) | ||||||||||
| UGR | 21.00 | b | 1.550 | b | 71.87 | 21.50 | a | 54.59 | a | |
| GRA | 29.00 | a | 2.152 | a | 72.83 | 13.00 | b | 34.81 | b | |
| Water stress (WS) | ||||||||||
| DI | 11.25 | b | 0.779 | b | 69.26 | b | 19.75 | 62.09 | a | |
| CON | 38.75 | a | 2.923 | a | 75.44 | a | 14.75 | 27.31 | b | |
| G × WS | ||||||||||
| UGR-DI | 8.25 | 0.555 | 68.28 | 25.25 | 74.93 | |||||
| UGR-CON | 33.75 | 2.544 | 75.47 | 17.75 | 34.25 | |||||
| GRA-DI | 14.25 | 1.003 | 70.24 | 14.25 | 49.26 | |||||
| GRA-CON | 43.75 | 3.301 | 75.41 | 11.75 | 20.36 | |||||
| ANOVA (df) | % Sum of squares | |||||||||
| G (1) | 7.10 | ** | 6.65 | ** | 1.14 | n.s | 29.64 | * | 17.96 | ** |
| WS (1) | 83.89 | ** | 84.18 | ** | 47.75 | ** | 10.26 | n.s | 55.57 | ** |
| G × WS (1) | 0.44 | n.s | 0.44 | n.s | 1.29 | n.s | 2.56 | n.s | 1.59 | n.s |
| Residuals (12) | 8.57 | 8.73 | 49.83 | 57.54 | 24.88 | |||||
| Std. Dev. (+) | 5.07 | 0.398 | 3.65 | 6.84 | 13.44 | |||||
| Leaves | Stems | Roots | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FW (g) | DW (g) | FW (g) | DW (g) | FW (g) | DW (g) | Volume (mL) | ||||||||
| Grafting (G) | ||||||||||||||
| UGR | 766.5 | 112.3 | b | 859.9 | 173.9 | 305.0 | b | 33.26 | b | 394.5 | b | |||
| GRA | 715.9 | 119.7 | a | 786.8 | 181.6 | 393.2 | a | 40.58 | a | 517.8 | a | |||
| Water stress (WS) | ||||||||||||||
| DI | 588.5 | b | 91.5 | b | 663.8 | b | 146.8 | b | 296.2 | b | 32.15 | b | 389.3 | b |
| CON | 893.9 | a | 140.4 | a | 983.0 | a | 208.7 | a | 402.0 | a | 41.69 | a | 523.1 | a |
| G × WS | ||||||||||||||
| UGR-DI | 660.9 | 88.4 | 658.9 | 147.3 | 264.4 | c | 30.96 | b | 334.0 | |||||
| UGR-CON | 872.2 | 136.2 | 1061 | 200.6 | 345.6 | b | 35.56 | b | 455.0 | |||||
| GRA-DI | 516.2 | 94.7 | 668.7 | 146.4 | 328.0 | b | 33.34 | b | 444.5 | |||||
| GRA-CON | 915.6 | 144.6 | 905.0 | 216.9 | 458.4 | a | 47.83 | a | 591.1 | |||||
| ANOVA (df) | % Sum of squares | |||||||||||||
| G (1) | 1.22 | n.s | 2.17 | ** | 1.56 | n.s | 1.33 | n.s | 36.72 | ** | 25.56 | ** | 37.69 | ** |
| WS (1) | 44.48 | ** | 95.82 | ** | 29.69 | * | 85.83 | ** | 52.92 | ** | 43.41 | ** | 44.37 | ** |
| G × WS (1) | 4.22 | n.s | 0.04 | n.s | 2.00 | n.s | 1.68 | n.s | 2.85 | * | 11.64 | * | 0.41 | n.s. |
| Residuals (12) | 50.07 | 1.96 | 66.75 | 11.16 | 7.51 | 19.39 | 17.53 | |||||||
| Std. Dev. (+) | 187.06 | 4.04 | 276.35 | 12.89 | 23.02 | 3.68 | 48.56 | |||||||
| DW (g) | Volume (mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–10 cm | 10–20 cm | 20–30 cm | 0–10 cm | 10–20 cm | 20–30 cm | ||||||||
| Grafting (G) | |||||||||||||
| UGR | 21.81 | b | 5.565 | 5.892 | a | 226.1 | b | 69.19 | 99.23 | a | |||
| GRA | 31.70 | a | 4.820 | 4.067 | b | 373.8 | a | 72.31 | 71.71 | b | |||
| Water stress (WS) | |||||||||||||
| DI | 23.41 | b | 4.615 | b | 4.123 | b | 265.4 | b | 57.68 | b | 66.18 | b | |
| CON | 30.09 | a | 5.769 | a | 5.835 | a | 334.5 | a | 83.83 | a | 104.76 | a | |
| G × WS | |||||||||||||
| UGR-DI | 21.22 | b | 5.132 | 4.609 | 211.2 | 55.85 | 66.95 | b | |||||
| UGR-CON | 22.39 | b | 5.997 | 7.175 | 240.9 | 82.53 | 131.50 | a | |||||
| GRA-DI | 25.61 | b | 4.099 | 3.637 | 319.6 | 59.50 | 65.40 | b | |||||
| GRA-CON | 37.79 | a | 5.541 | 4.496 | 428.0 | 85.13 | 78.03 | b | |||||
| ANOVA (df) | % Sum of squares | ||||||||||||
| G (1) | 44.12 | ** | 11.80 | n.s. | 33.10 | ** | 62.64 | ** | 0.74 | n.s. | 20.11 | ** | |
| WS (1) | 20.10 | ** | 28.28 | * | 29.14 | ** | 13.68 | * | 52.09 | ** | 39.56 | ** | |
| G × WS (1) | 13.67 | * | 1.77 | n.s | 7.24 | n.s | 4.44 | n.s | 0.02 | n.s. | 17.91 | ** | |
| Residuals (12) | 22.11 | 58.15 | 30.52 | 19.24 | 47.14 | 22.42 | |||||||
| Std. Dev. (+) | 4.04 | 0.955 | 1.012 | 47.3 | 14.36 | 16.77 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gisbert-Mullor, R.; Martín-García, R.; Bažon Zidarić, I.; Pascual-Seva, N.; Pascual, B.; Padilla, Y.G.; Calatayud, Á.; López-Galarza, S. A Water Stress–Tolerant Pepper Rootstock Improves the Behavior of Pepper Plants under Deficit Irrigation through Root Biomass Distribution and Physiological Adaptation. Horticulturae 2023, 9, 362. https://doi.org/10.3390/horticulturae9030362
Gisbert-Mullor R, Martín-García R, Bažon Zidarić I, Pascual-Seva N, Pascual B, Padilla YG, Calatayud Á, López-Galarza S. A Water Stress–Tolerant Pepper Rootstock Improves the Behavior of Pepper Plants under Deficit Irrigation through Root Biomass Distribution and Physiological Adaptation. Horticulturae. 2023; 9(3):362. https://doi.org/10.3390/horticulturae9030362
Chicago/Turabian StyleGisbert-Mullor, Ramón, Rodrigo Martín-García, Iva Bažon Zidarić, Nuria Pascual-Seva, Bernardo Pascual, Yaiza Gara Padilla, Ángeles Calatayud, and Salvador López-Galarza. 2023. "A Water Stress–Tolerant Pepper Rootstock Improves the Behavior of Pepper Plants under Deficit Irrigation through Root Biomass Distribution and Physiological Adaptation" Horticulturae 9, no. 3: 362. https://doi.org/10.3390/horticulturae9030362
APA StyleGisbert-Mullor, R., Martín-García, R., Bažon Zidarić, I., Pascual-Seva, N., Pascual, B., Padilla, Y. G., Calatayud, Á., & López-Galarza, S. (2023). A Water Stress–Tolerant Pepper Rootstock Improves the Behavior of Pepper Plants under Deficit Irrigation through Root Biomass Distribution and Physiological Adaptation. Horticulturae, 9(3), 362. https://doi.org/10.3390/horticulturae9030362







