1. Introduction
During plant development, specific changes occur both at the level of cells, tissues, entire organs, and in metabolism and physiological processes. All these changes are controlled at the genetic level and by environmental conditions. Anatomical, morphological, and biochemical characteristics of roots, leaves and flowers during their development are often discussed in the literature, as well as the genes involved in regulation of these processes [
1,
2,
3,
4,
5]. The ontogenetic changes occurring in the stem of herbaceous dicotyledon plants [
3,
4,
5] are studied less frequently compared to monocotyledons [
2,
6,
7,
8,
9]. However, significant changes occur in this plant organ with age, leading to the formation of anatomical and morphological structures that ensure the effective performance of its main functions—the transport of minerals and photosynthates and the mechanical maintenance of the shoot in an upright position [
2,
3,
6]. These processes are associated with the deposition of lignin in the cell walls of xylem vessels and fibers, while it is absent in parenchymal cells of the cortex and the central cylinder [
3,
10,
11]. Lignification limits the growth of cell walls through the formation of the bonds between the residues of hydroxycinnamic acids in lignin and cell wall polysaccharides [
12]. In angiosperms, lignin consists of
p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) lignin monomers, presented in different proportions in different species and organs of the same species [
13].
Lignification in plant cells and tissues is studied on several model plants, including mono- and dicotyledonous species [
2,
3,
6,
7,
8,
9,
10,
11,
12,
13]. One of the popular dicotyledon model plants is zinnia, an annual horticultural crop from the Asteraceae family, which is popular in urban and rural areas. Zinnia is known as an object for studying lignification processes both in vitro and ex vivo [
11,
14,
15]. It was determined that lignin of zinnia hypocotyl and epicotyl [
15] is different: G- and S-units of lignin predominate in the hypocotyl; G-units predominate in the epicotyl; and H- and S- are present in equal proportions. In another classical model,
Arabidopsis thaliana L. xylem and fiber lignin in mature stem of is composed of G- (above 70%) and S-units, respectively [
3,
4,
10]. The biosynthesis of phenylpropanoids as lignin precursors is a multi-step process. Phenylalanine ammonia-lyase (PAL; EC 4.3.1.5) catalyzes the first reaction, in which phenylalanine is converted to
trans-cinnamic acid [
16]. Cinnamate 4-hydroxylase (C4H; EC 1.14.13.11) converts cinnamic acid to
p-coumaric acid, which is a precursor to caffeic, ferulic, and sinapic acids. In next subsequent reactions, 4-coumarate:CoA ligase (4CL; EC 6.2.1.12) [
16] and cinnamoyl-CoA reductase (CCR; EC 1.2.1.44) produce hydroxycinnamaldehydes. Cinnamyl alcohol dehydrogenase (CAD; EC 1.1.1.195) catalyzes the final step in the biosynthesis of G- and S-lignin monomers such as coniferyl and sinapyl alcohol monolignols, respectively [
16]. In the secondary cell wall, monolignols are oxidized to radicals with the participation of class III peroxidases (PRX, EC 1.11.1.7) and laccases (LAC, EC 1.10.3.2). The final polymerization of lignin occurs according to the free radical mechanism [
14,
15,
16,
17].
Lignification is a process controlled by many factors. It is determined by metabolism of phenolic compounds, including the activity and expression of genes of the phenylpropanoid pathway and lignin biosynthesis [
2,
3,
4,
12,
14,
16]. It was shown that the expression of monolignols biosynthesis,
PRX and
LAC genes are dynamic during development of stem [
3,
4,
10,
12].
Despite the study of many aspects of lignification, there are still many questions about the regulation of biosynthesis and deposition of lignin in cell walls at different stages of the development of vascular and mechanical tissues in the plant stem [
2,
4]. If changes in mechanical and vascular tissues during the linear growth of axial organs are widely discussed [
2,
6,
18], then changes in these tissues during radial growth have been studied to a lesser extent. Therefore, investigation of lignification in plant organs of different ontogenetic status is still relevant. In addition, it is not clear why the composition of lignin units is different in different parts of the zinnia stem, and whether there is specificity of lignin synthesis in organs of different ages. If this is so, then in the zinnia plant there is a time- and organ-specific expression of genes responsible for the formation of phenylpropanoids as lignin precursors, which determines their amount and spectrum. The amount and composition of phenolic compounds and the activity of peroxidase are responsible for the biosynthesis of H-, S-, G-units of lignin in different parts of the stem at different stages of their formation.
In this regard, the purpose of this research was to study the deposition of lignin in zinnia stem tissues at different periods of their radial growth and the factors regulating this process, including phenolic metabolism, the activity of enzymes involved in lignification, and the genes that regulate these processes.
2. Materials and Methods
2.1. Plant Material and Growth Condition, Growth Characteristics of Zinnia
Zinnia plants (
Zinnia elegans Jacq. cv.
Rotkappchen) were grown from seeds in a growth chamber under a 16 h (day): 8 h (night) photoperiod; 23 ± 2 °C temperature; and 65 ± 5% humidity. Plants were cultivated in a soil mixture, consisting of 75% neutralized peat (pH 6.5, total nitrogen 1.5, phosphorus 2.5, potassium 3.0 g kg
−1 of dry weight) and 25% coco substrate in 0.2 L vegetative vessels, and watered two times a week. Plants were grown for 40 days until they finished their vegetative growth. From the moment of seed germination to the end of apical vegetative growth, the lengths of stem segments, hypocotyl, epicotyl (the first internode from the cotyledons), and the second internode, were measured (the data are presented in
Supplementary Material, Table S1). The diameter of the internodes was measured on transverse sections.
2.2. Lignin Histochemical Staining and Microscopy
For histochemical analysis of lignin localization in zinnia internodes, fragments of the hypocotyl and internodes at the age of 20 and 40 days were fixed in a mixture of 96% ethanol: glacial acetic acid (3:1,
v/
v) at 4 °C [
19]. After 48 h, the samples were washed and stored in 96% ethanol at 4 °C. The middle part of the internodes was used for making transverse sections. Cross sections 100 µm-thick were made on a freezing microtome MZP-01 (TECHNOM, Ekaterinburg, Russia). Lignin was stained with phloroglucinol-HCl [
20]. The transverse sections were studied using a wide-field microscope Olympus BX51 WI (Olympus Corporation, Tokyo, Japan). Measurements of cell and tissue parameters were performed using SIMAGIS
® Meso-Plant™ software version 2.1 for Windows XP. Five sections from one plant for each organ were analyzed. The total number of viewed sections was at least 30.
2.3. Determination of Total Lignin Content
Extractives from the crushed plant material were removed by treatment with 96% ethanol for 5 h. The extract was separated by centrifugation, the precipitate was dried to constant weight. The plant material after extraction was used to determine the lignin content by two methods.
Cysteine-assisted sulfuric method (CASA-lignin). To 10 ± 0.1 mg of plant material, 1.0 mL of 72% sulfuric acid with L-cysteine (0.1 g mL
–1) was added. The mixture was incubated with continuous stirring at 24 °C until complete dissolution of the plant material (60–70 min). The resulting solution was transferred to a volumetric flask and diluted with deionized water to 100 mL. The optical density of solutions was measured at 283 nm on a Shimadzu UV-1800 spectrophotometer (Shimadzu, Kyoto, Japan). To calculate the lignin content, an extinction coefficient of 11.26 g
−1 L cm
−1 for the hypocotyl (G: S ratios < 1) and 12.50 for the epicotyl (G: S ratios ≥ 2) were used [
21]. The analysis was performed in 3 biological and 3 analytical replicates.
The thioglycolic acid (TGA-lignin) method. To 10 mg of plant material, 1 mL of 2 N HCl, 100 μL of thioglycolic acid were added, and the mixture was incubated at 96 °C for 8 h with periodical stirring. The tubes were then cooled on ice and centrifuged at 14,000×
g, 30 min. The precipitate was washed once with 1 mL of distilled water, centrifuged, and the supernatant was discarded. The precipitate was resuspended in 1 mL of 1 N NaOH and incubated on a shaker (250 rpm) at room temperature for 18 h. Samples were centrifuged (14,000×
g, 30 min), the supernatant containing the lignin thioglycolate was transferred to new tubes, and 200 µL of concentrated sulfuric acid was added. Samples were incubated for 10 h at 4 °C. The precipitate was collected by centrifugation at 14,000×
g for 30 min and dissolved in 200 µL of 1 N NaOH [
22]. The optical density of the solution was measured at 280 nm on a Shimadzu UV-1800 spectrophotometer (Shimadzu, Kyoto, Japan). Calibration curves were performed by subjecting increasing amounts of 1.0–3.0 mg of commercial lignin (Kraft lignin, Sigma Aldrich, Schnelldorf, Germany) to the same procedure (R
2 = 0.97). The analysis was performed in 3 biological and 3 analytical replicates.
2.4. Determination of Phenolic Compounds Content
Easily soluble free phenolic acids were extracted from 100 mg of plant material with 1 mL of 95% ethanol at 45 °C and sonication for 1 h. An aqueous solution of sodium hydroxide was added to the test tube to a final concentration of 2 N NaOH and a volume of 3 mL. Saponification of phenolic glycosides was processed for 3 h. At the end of saponification, the content of the tubes was acidified to pH = 2 with concentrated hydrochloric acid to convert phenolates into free phenolic compounds. Hydrolysis of phenolic ethers lasted overnight in an acidic medium at room temperature.
The hydrolysate was separated by centrifugation (5 min at 10,000× g). The precipitate was washed twice with 3 mL of distilled water. The supernatants were pooled together and amounted to 10 mL.
Purification from impurities and concentration of phenolic compounds was carried out by re-extraction into ethyl acetate by 5 mL for 3 times. The combined ethyl acetate extract was evaporated on a rotary evaporator at 0.2 atm and 45 °C. The dry residue was dissolved in 1 mL of 95% ethanol and used for analysis [
23].
The quantitative determination of the total phenolic compounds was carried out by a semi-micro method using the Folin–Ciocalteu reagent according to the method [
24] with minor modifications [
25]. Each sample was consequently extracted by water 6 times, and 60 µL of the pooled extract was added to the microplate well with 100 µL of 0.1 N Folin–Ciocalteu reagent. After 3 min, 50 µL of 7.5% sodium carbonate solution was added. An hour later, the optical density of solutions was determined in microcuvettes at 760 nm using a Shimadzu UV-1800 spectrophotometer (Shimadzu, Kyoto, Japan). The analysis was carried out in 3 biological and 3 analytical replicates. The concentration was expressed in terms of gallic acid.
To characterize the qualitative composition of phenolic compounds, thin layer chromatography (TLC) was used on Sorbfil plates (Sorbfil, Krasnodar, Russia), 10
× 10 cm in size, with silicon oxide as a sorbent, the layer thickness of which was 0.09–0.12 mm, and the granule size was 5–17 µm. The test solution was applied to the start line five times in portions of 3 μL. As standards, a mixture of ferulic, gallic, salicylic acids (mixture 1) and
p-coumaric and cinnamic acids (mixture 2) were used in an amount of 4 μg of each substance. Chromatography was carried out in a mixture of toluene: ethyl acetate: formic acid (30:18:2). At the end of the separation, the plates were dried and viewed in UV at 312 nm. Fluorescent spots were noted. Then, the plates were treated with 7.5% sodium carbonate solution, heated for 5 min at 100 °C, and viewed in visible light and UV [
26].
Phenolic acids involved in the synthesis of lignin were eluted from undeveloped chromatograms [
27]. The zones corresponding to the Rf of the standard compounds were scraped off the plates and eluted in 1 mL of 95% ethanol. To prove the presence of the desired compound in the eluate, the absorption spectra of the extracts were recorded (in the range from 210 to 440 nm) using a Shimadzu UV-1800 spectrophotometer (Shimadzu, Kyoto, Japan). The absorption maxima and minima of the standard sample were compared with the absorption spectrum of the eluate. Eluates of standard compounds, taken in amounts of 3, 6, and 9 µg from TLC were used for calibration curves. The optical density was determined at the corresponding absorption maxima. A calibration graph and a linear regression equation (R
2 = 0.99 for ferulic acid, 0.99 for
p-coumaric acid, and 0.90 for cinnamic acid) were used for the determination of phenolic acid concentration in the samples.
2.5. Peroxidases Activity and Isoforms Assay
Frozen tissues were ground to a powder with liquid nitrogen and extracted with 100 mM Phosphate buffer (pH 7.0); then, the samples were centrifuged (12,000×
g, 20 min, 4 °C). The obtained supernatant was used for peroxidases III class activity assay and isoform identification. Guaiacol peroxidases (GPOD) activity was measured by the increase in absorbance at 470 nm for 120 s and expressed as µmol tetraguaiacol min
−1 mg
−1 total protein [
28]. Syringaldazine peroxidase (SPOD) activity was measured by the increase in absorbance at 530 nm for 120 s and expressed as µmol syringaldazine oxidized min
−1 mg
−1 total protein [
29]. Benzidine peroxidase (BPOD) activity was measured by the increase oinf absorbance at 590 nm for 120 s and expressed as µmol benzidine oxidized min
−1 mg
−1 total protein [
30]. The amount of total protein was determined according to Bradford method, using bovine serum albumin as a standard [
31]. Optical densities of the samples were measured on a Shimadzu UV-1800 spectrophotometer (Shimadzu, Kyoto, Japan). All analyses were conducted in 3 biological and 3 analytical replicates.
Protein electrophoresis was performed under non-denaturing conditions in a 10% polyacrylamide gel, adding 30 µg of protein to each well. Horizontal electrophoresis in 1% agarose gel and Tris-glycine buffer (pH 8.3) was performed to identify anionic and cationic isoforms of peroxidases [
6]. Peroxidase isoforms were detected by a modified method [
32]. Gels were stained for 10 min with a reaction medium containing of 0.2% benzidine or 0.2% benzidine in 2% acetic acid; to remove excess substrate, the gel was washed in a solution containing 2% acetic acid, then incubated for 3 min in 0.5% hydrogen peroxide until clear bands appeared on a non-stained background. Molecular weight marker Precision Plus Protein
TM Kaleidoscope
TM Standards (Bio-Rad, Hercules, CA, USA) was used as a standard.
2.6. Total RNA Isolation and Real-Time Quantitive PCR
Total RNA was isolated from hypocotyl and internodes, frozen in liquid nitrogen, byTrizol (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions. In total, 100 ng of total RNA from each sample was used for Reverse Transcription PCR with Oligo(dT)23VN primers to obtain the first strand of cDNA in accordance with the instructions of the manufacturer (HiScriptII first standard cDNA synthesis kit, Vasyme, Nanjing, China). Gene expression was assessed by qRT-PCR in a qTOWER 2.0 96-well optical amplifier (Analytikjena, Jena, Germany) using TransStrat® Tip Green qPCR SuperMix (TransGenBiotech, Beijing, China, Cat#AQ141).
The forward and reverse primers for the reaction were selected using the Blast Primer designee online program (
www.ncbi.nlm.nih.gov/tools/primer-blast, accessed on 30 November 2019). Gene-specific primer sequences were performed in a previously published article [
33]. Amplification was carried out under standard conditions (1 cycle: 30 s at 94 °C; 40 cycles: 5 s at 94 °C, 15 s at 60 °C, and 10 s at 72 °C; 5 s at 60 °C). The relative expression level was calculated using the 2
−ΔΔCt method; the resulting value was raised to the power of 6 [
34]. The data were normalized to the gene encoding the 18S rRNA. The analysis was performed in 3 biological and 3 analytical replicates.
2.7. Statistical Analysis
The experiment was repeated 3 times. The data are presented as the arithmetic mean values and the standard error. Statistical data processing was carried out in the STATISTICA 13 program for Windows 10 using Student’s t-test and Mann–Whitney U-test (p < 0.05).
3. Results
During the development of zinnia plants, a hypocotyl appeared on the third day after sowing. Its exponential linear growth occurred within 10 days, then the growth was slowed down, reaching the final values by the 20th day. The epicotyl (the first internode) appeared on the 11th day from the germination. Its growth was also completed in the 20-day-old plants. The second internode appeared on the 17th day after germination, and its linear growth stopped by the 25th day (the data performed in
Supplementary Material Table S1). The end of linear growth does not mean a complete termination of growth—all parts of the zinnia stem continued the radial growth. The diameter of the hypocotyl, epicotyl increased, which led to an increase in the cross-sectional area (
Table 1). The radial growth was well expressed in zinnia on the 20th and 40th days after sowing.
3.1. Anatomical Characteristics of the Hypocotyl and Epicotyl in Zinnia of Different Ages
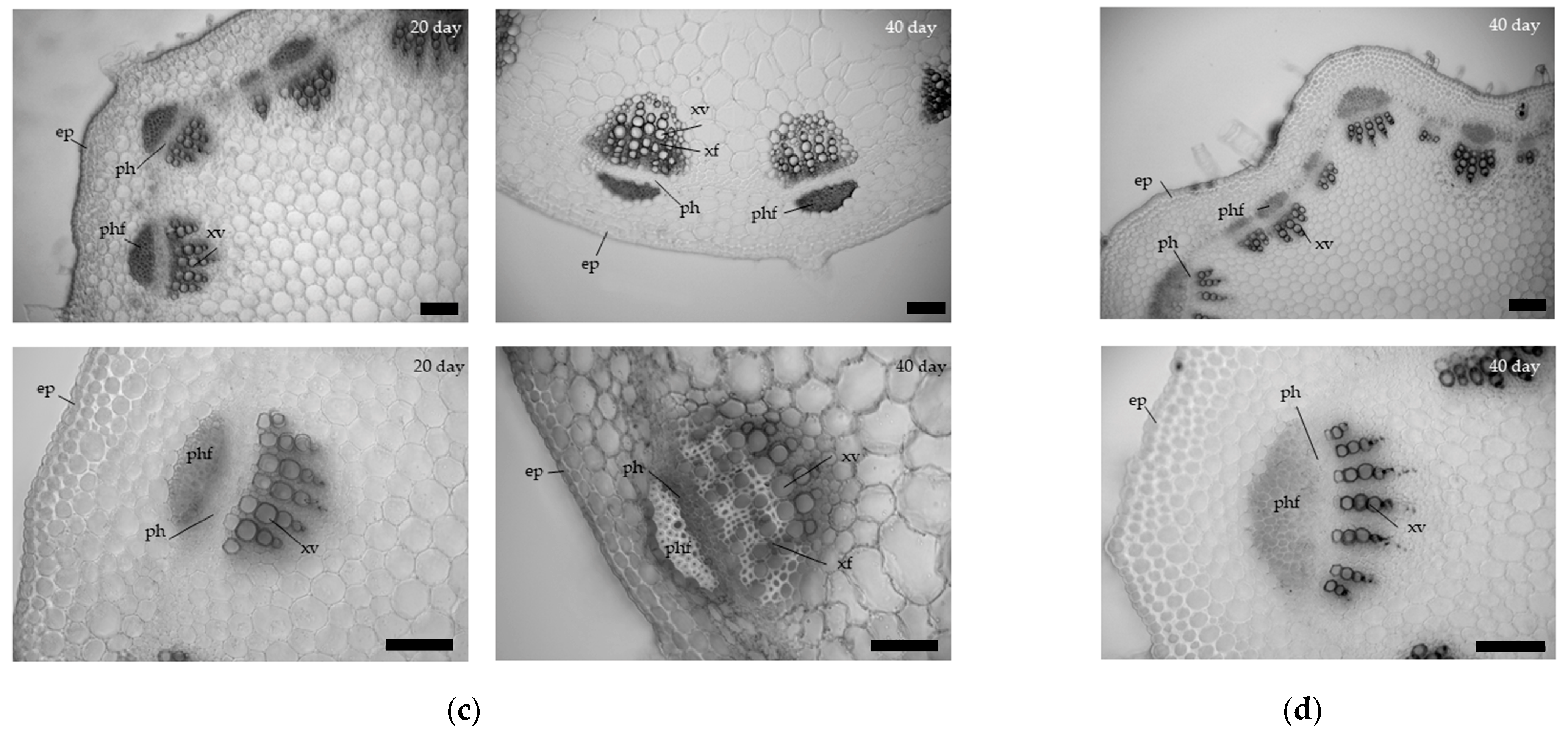
Cross sections of the zinnia hypo- and epicotyl at the age of 20 and 40 days are shown in
Figure 1. In zinnia hypocotyl, the number of lignified cells in the xylem of the vascular bundle increased from day 20th to 40th, and a xylem ring was formed by the development of interfascicular xylem fibers (
Figure 1). The proportion of xylem in the vascular bundle increased by 10% compared to day 20, as the number of fibers in the xylem. The cross-sectional area of the metaxylem vessels also increased by 44% (
Table 1). The xylem area on the cross sections increased by 19% to the 40th day of zinnia growth. At the same time, the thickness of the cell walls of the metaxylem vessels did not change during the radial growth of the hypocotyl.
An increase in the cross-sectional area of the first internode was also due to xylem formation. An increase in the proportion of xylem in vascular bundles and the cross-sectional area of metaxylem vessels by 12% was observed (
Table 1) during radial growth from 20 to 40 day. The number of fibers and vessels in the xylem also increased (
Figure 1c). The thickness of the cell walls of the metaxylem vessels did not change (
Table 1).
In the second internode, no fibers were detected in the secondary bundles. Metaxylem vessels had a smaller cross-sectional area, and the cell wall thickness was compared to the first internode of 20-day-old zinnia plants. The proportion of xylem in transverse sections and the proportion of xylem in the vascular bundles were like the 1st internode on the 20th day of zinnia growth.
3.2. Changes in the Content of Phenolic Compounds and Lignin
In 40-day-old plants, compared with 20-day-old plants, the content of phenolic compounds in the hypocotyl increased by 71%, which positively correlated with an increase in the content of TGA- and CASA-lignin. The percent of
p-coumaric and ferulic acids in the total content of phenolic compounds decreased compared to 20-day-old plants (
Table 2). Cinnamic acid was not detected in any of the samples.
In the epicotyl, the content of phenolic compounds did not change during radial growth between 20th and 40th days. At the same time, the content of CASA-lignin increased, and the proportion of ferulic acid among phenolic compounds increased. In the second internode, the content of phenolic compounds and the proportion of ferulic acid were higher than in the first internode on the 20th day of growth. At the same time, their spectrum, revealed by TLC, was more diverse (
Figure 2).
The spectrum of phenolic compounds revealed by TLC in the hypocotyl and epicotyl at the same age were more similar than in the same part of the stem, but at different ages (
Figure 2). All parts of the stem on the 20th day had a greater variety of phenolic compounds (more than 10 fluorescent spots on TLC) than on the 40th (5–6-fluorescent spots).
During the radial growth of the stem (
Table 2), an upward trend was observed for the TGA- and CASA-lignin amount. The second internode on the 40th day was comparable in the amount of lignin to the first internode on the 20th day of zinnia growth.
3.3. Activity of Class III Peroxidases and Profile of Their Isoforms
A lot of enzymes are involved in the process of lignification, among which peroxidases are of particular importance. Not only the total activity of peroxidases, but also their isozyme spectrum can change during development in different organs and even tissues.
High molecular weight isoforms of anionic peroxidases (>75 kDa) predominated in the hypocotyl and epicotyl (
Figure 3a). Low molecular weight isoforms (<75 kDa) were detected in hypocotyl only on the 20th day, and in epicotyl and the second internode they were detected both on 20th and 40th day. In general, at least five isoforms of peroxidases were detected. At the same time, the activity of the lightest isoform (A
1) was not detected in the epicotyl of 40-day-old plants, and the activity of A
2–A
4 isoforms decreased. In the first internode in 40-day-old plants, the A
5 form was not detected, but the activity of A
1 increased significantly. In the second internode, the light form of the A
5 isozyme was characterized by the highest activity, also as the A
2 isoform. Thus, the spectra of peroxidases were not the same in different parts of the zinnia shoot.
The profile of cationic zinnia peroxidases in different parts of the stem also differed. In the hypocotyl of different age, high activity of C1, C2, and C4 isoforms was detected. By day 40, the activity of the C3 isoform increased. In the epicotyl (1 internode), C1, C2, and C4 isoforms were detected. By the 40th day, the activity of the C4 isoform increased, while the others practically did not differ in activity. In the second internode, isoforms C1 and C4 revealed activity, the latter being more active. Anionic peroxidases were difficult to differentiate when separated on an agarose gel. The AA1 isoform was found in the hypocotyl; enzymatic activity of light isoform AA2 was detected in the second internode.
Class III peroxidases are characterized by a wide range of oxidizing substrates. The substrate specificity could differ in various tissues [
29,
35]. Therefore, three substrates were used: syringaldazine, benzidine, and guaiacol. BPOD activity was measured at pH 5.5, SPOD, and GPOD in a neutral medium (pH 7.4 and 7.0, respectively). The total activity of class III peroxidases was higher in the hypocotyl in comparison with the first and second internodes. The trends in changes in enzyme activity were similar (
Figure 3c).
At earlier stages of vascular tissue development, a high activity of anionic isoforms of peroxidases was revealed, which was visualized using benzidine (
Figure 3a). The decrease in the activity of A
2–A
4 isoforms, detected by differential staining of the gels, coincided with a decrease in the total activity of BPOD in the hypocotyl and epicotyl (first internode) at later stages of plant development (day 40) (
Figure 3c). SPOD and GPOD activity in the hypocotyl and epicotyl (first internode) increased with age, which positively correlated with lignin content and the development of vascular and mechanical elements in these organs (
Figure 3b,c).
3.4. Relative Level of Expression of Genes Involved in the Biosynthesis of Phenylpropanoids and Lignin
Since the hypocotyl and epicotyl of zinnia differed in age and level of lignification, they showed different trends in the number of transcripts of genes encoding enzymes of the phenylpropanoid pathway and lignin synthesis. For genes of the early stages (
PAL,
C4H), a higher level of expression was shown in comparison with the genes of the late stages (
CAD). By the 40th day of growth, the relative number of transcripts of the
PAL,
C4H, and
CAD genes in the hypocotyl increased in comparison with the 20th day. Expression of the
PRX and
LAC genes decreased (
Figure 4).
In the epicotyl (first internode) of zinnia, the level of expression of the early-stage genes (
PAL) and synthesis of lignin (
PRX) was comparable both on 20 and 40 days. The relative level of expression of the
C4H and
LAC genes decreased (
Figure 4). The lower level of expression of these genes could lead to a decrease in the spectrum of phenolic compounds (
Figure 2). A high level of
CAD gene expression was detected in the epicotyl on the 40th day. In the second internode, the level of gene expression was different.
4. Discussion
Ontogenetic changes in cells, tissues and plant organs are expressed both in quantitative and qualitative characteristics. One of the markers of the growth termination and differentiation in xylem vessels and fiber cells is lignification [
3,
5,
11,
12,
34]. Lignin, deposited in the cell walls, limits cell elongation, and marks the transition of the primary cell wall to the secondary one. The development of vascular tissues and fibers of plants and the lignification of their cell walls is a long process [
3,
12]. In plants at the initial stages of stem growth, a low degree of lignification of the cell walls in xylem vessels was shown, as well as the absence of fibers in vascular bundles [
3,
5]. During this period, the internodes grow due to the cell extension.
Stem fragments of different localization and different ages were characterized in terms of tissue lignification, changes in phenolic metabolism, including enzyme activity and expression of genes involved in lignification. It was shown that the limitation of stem linear growth did not mean the end of growth as a whole: the stem continued its radial growth due to the new formation of vessel elements and fibers. Increased deposition of lignin during the radial growth of axial organs is a common mechanism for strengthening the stems of angiosperms [
3,
36]. In the hypocotyl of zinnia, it was shown that during its radial growth, interfascicular xylem develops in addition to the fascicular xylem, which lead to an increase in the lignin content in tissues. An increase in the number of fibers in the vascular fibrous bundles was shown in the epicotyl, which also led to an increase in total lignin in this part of the stem.
Cell wall lignification is an important process of changing the properties and structure of the secondary cell wall [
11,
13,
18]. This process depends on many factors, including the content of phenolic compounds, which are used by peroxidases and laccases to form monolignol radicals, from which lignin is built [
37,
38]. The content of free phenolic compounds in the hypocotyl was increased with plant age, which may be due to an increase in the level of expression of the genes, involved in early stages of the phenylpropanoid pathway—
PAL and
C4H.
PAL catalyzes the conversion of phenylalanine to cinnamic acid at the initial step of phenylpropanoids biosynthesis [
16]. The absence of cinnamic acid may be associated with the highest level of
C4H expression among all the studied genes. In zinnia hypocotyl and epicotyl, the content of phenolic compounds coincided with the level of
PAL gene expression both on 20th and 40th days of plant development. It is likely that an increase in the relative amount of
PAL gene transcripts in the hypocotyl of 40-day-old zinnia led to an increase in the diversity of phenolic compounds and to an increase in stem lignification at the stage of xylem ring formation.
The percent of
p-coumaric acid which is a product of the C4H enzyme in the total pool of phenolic compounds decreased, which may be explained by an increase in the syntheses of other phenylpropanoids and phenolic compounds, the diversity of which was shown by TLC (
Figure 2a,b).
Monolignol radicals, which are the structural units of lignin, are formed due to the work of peroxidases and laccases [
10,
11,
16]. Peroxidases are characterized by broad substrate specificity and differ in isoform profiles in different organs, which makes it difficult to identify the role of individual genes and isoforms in lignification [
3,
6,
14,
17]. The total SPOD activity increased at the stage of radial plant growth and correlated with an increase in lignin content. Histochemical localization of SPOD in the secondary cell walls of vessels and xylem fibers was shown at the stage of cell wall lignification, while the enzyme was not detected in fully mature cells [
29]. Our study has shown an increase in the total GPOD activity at later stages of stem maturation. GPOD is localized in the secondary cell walls of metaxylem and fiber in zinnia [
35]. In the cell walls of protoxylem, peroxidases that use diaminobenzidine or benzidine as substrates are mainly detected at the early stages of growth [
29,
35]. Thus, class III peroxidases differ in their localization in the tissues of the zinnia stem, which changes the process of lignification of the cell walls of xylem vessels and fibers. The change in the total activity of peroxidases is determined by the spectrum of isoforms, including cationic and anionic ones.
Both peroxidases and laccases are represented by multigene families, the expression of which depends on the organ and stage of ontogeny and has a complex spatiotemporal regulation [
3,
6,
16]. This makes it difficult to identify the role of individual genes and isoforms in lignification [
39]. We determined the expression of the
PRX gene, which encodes the cationic peroxidase ZPO-C [
40]. The ZPO-C transcript was accumulated transiently during secondary wall thickening in xylogenic culture of zinnia cells. It was shown by in situ hybridization [
40]. In our study, the expression of the
PRX and
LAC genes was higher in the hypocotyl on the 20th day of growth, which allows us to assume their participation in the lignification during the formation of vascular fibrous bundles, while their expression decreased during the formation of the interfascicular xylem. Lignification of secondary cell walls of xylem vessels and fibers in the epicotyl occurs, most likely, due to increased expression of the
PRX gene.
An increase in the activity of SPOD and GPOD at later stages of the hypocotyl and epicotyl radial growth coincided with a change in the relative number of transcripts of the
CAD gene, which is involved in the formation of cinnamyl alcohols—precursors of lignin [
41].
5. Conclusions
During zinnia stem maturation, it was determined that the formation of a radial xylem ring in the hypocotyl leads to increased lignification of stem tissues due to the formation and thickening of secondary cell walls of fibers and vessels. In the epicotyl, the xylem volume also increased due the formation of fibers and vessels. A marker of xylem development at this growth stage was an increase in the proportion of ferulic acid in the composition of phenolic compounds. In both hypocotyl and epicotyl of zinnia, an increase in SPOD and GPOD activity was accompanied by the development of xylem. A high expression level of the PAL gene provided the synthesis of phenylpropanoid compounds, and an increase in the expression of the CAD gene led to the formation of oxycinnamic alcohols, the direct precursors of lignin, and stimulated the activity of peroxidases. In the zinnia plant, a time- and organ-specific expression of genes responsible for the formation of phenylpropanoids as lignin precursors was detected. This determines the amount and spectrum of phenylpropanoids. The amount and composition of phenolic compounds and the activity of peroxidase were responsible for the biosynthesis of H-, S-, G-units of lignin in different parts of the stem at different stages of their formation.
Thus, during the radial growth of the zinnia stem, both the anatomical and biochemical characteristics of cells and tissues and the expression of the key genes for the biosynthesis of lignin and its precursors changed.