Abstract
Browning and contamination are regarded as the main constraints in the plant tissue culture of Musa spp. that can hinder the success of plant propagation in vitro. Browning is caused by enzymatic reactions due to explant injury, while microbial contamination is caused by phyllospheric, rhizospheric, and endophytic microorganisms that reside on, in, and inside the plants. When not properly addressed, they can cause decreased regenerative ability, decreased callus growth, inhibited adventitious shoot growth, and even tissue death. To overcome the browning problem, various attempts have been made in vitro, e.g., immersing the explants in an anti-browning solution, incorporating anti-browning compounds into the medium, and manipulating cultural practices. Correspondingly, to control the problem of contamination, efforts have been made, for example, using various methods, such as thermotherapy, chemotherapy, and cryotherapy, and chemical agents, such as disinfectants, antiseptics, and nanoparticles. This review aims to investigate and provide a comprehensive understanding of the causes of browning and contamination as well as the many approaches used to control browning and contamination problems in Musa spp. tissue cultures.
1. Introduction
Bananas and plantains (Musa spp.) are among the main food commodities in developing countries [1,2]. Bananas are predominantly produced in Asia, Latin America, and Africa. The biggest banana producer countries are India, which produced 29 million tons per year on average between 2010 and 2017, followed by China which can produce up to 11 million tons per annum. Production in both countries mostly serves the domestic market. Other large producers are the Philippines, with an annual average of 7.5 million tons produced between 2010 and 2017, and Ecuador and Brazil, both producing an average of 7 million tons [3].
Currently, the best ways to produce banana and plantain explants are using plant tissue culture micropropagation techniques [4,5]. This method is able to produce disease-free plants, high-quality banana plants, and high-quality planting material, and the rapid production of many uniform plants in a short time, which can support strong plant growth during the subsequent growth cycle [6,7]. However, explant browning and contamination are the main obstacles that often occur in the micropropagation of banana and plantain explants using tissue culture techniques [8,9,10].
Browning is usually caused by a variety of chemical reactions, such as caramelization, phenylalanine deamination, carbonyl amine reaction, o-quinone secondary reactions, lipid oxidation, and enzymatic oxidation [8,11,12]. Enzymatic browning of plant tissues causes unappealing changes in the explants and can cause explant death and failure of regeneration in explant culture for propagation [13], thus affecting both the survival and competitive ability of the explants.
Meanwhile, contamination is caused by phyllospheric, rhizospheric, and endophytic microorganisms that reside on, in, and inside the plants [14,15]. When the contamination of explants is not properly treated, it can cause a decrease in the regenerative ability, reduction in callus growth, and inhibition of adventitious shoot growth [15,16,17]. The presence of microbial contaminants also leads to an increase in plant mortality, variation in growth (reduction in shooting proliferation and rooting), tissue necrosis, and even explant death [18].
Many studies have reported the approaches used in managing lethal browning and contamination in the tissue culture of bananas and plantains [17,19,20,21,22,23,24]. In general, the browning treatment was carried out by removing phenolic compounds, modifying the redox potential, inhibiting the activation of phenol oxidase enzymes, and reducing phenolase activity and substrate availability by immersing the explants in anti-browning solutions, incorporating anti-browning compounds into the medium, and manipulating the cultural practices [9,17,21]. Meanwhile, the treatment of microbial contamination is generally carried out using the methods of chemotherapy, thermotherapy, and cryotherapy. In addition, chemical agents such as antiseptics, disinfectants, and nanoparticles are also incorporated into plant tissue culture media to control microbial contaminants [24,25,26,27].
This article reviews and compiles information on the lethal browning and contamination in the tissue culture of bananas and plantains, as well as the causes of each, in one document. The key species of bacteria and fungi that cause the contamination are also covered in this review, along with the genes responsible for explant browning and several techniques for preventing browning and contamination in different tissue cultures of bananas and plantains. This review will therefore increase our understanding of the mechanisms and occurrence of explant browning and contamination in banana micropropagations and may be helpful for designing methods to effectively control browning and contamination in banana explants.
2. The Causal Agents of Browning in Musa spp. Propagation
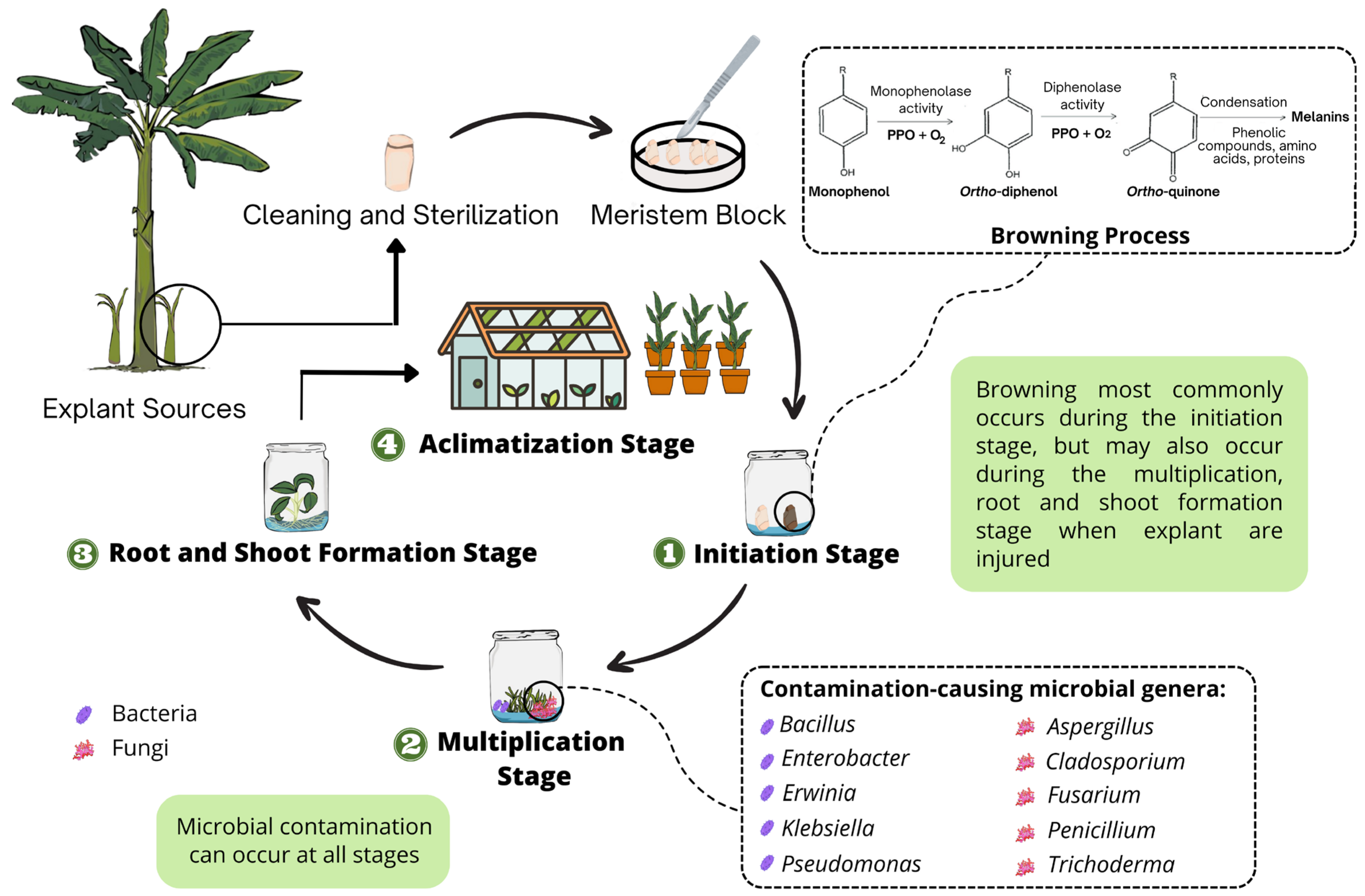
Browning in plant tissue culture refers to the discharge of brown material from the surface to the media during the early differentiation or subdivision of the plant explants, causing the medium to gradually turn brown and the explants to diminish [8]. Tissue browning can be characterized as enzymatic or non-enzymatic, depending on whether enzymes are involved or not in the browning processes [28]. Enzymatic browning is the principal cause of explant browning in plant tissue culture [29]. When explants are sliced, phenols are released and oxidized to quinones by enzymes, resulting in enzymatic browning. Furthermore, quinones formed by enzymatic reactions can form cross-links with proteins or polymerize in tissues via a series of complex biochemical reactions such as dehydration and polymerization, resulting in dark-colored melanin compounds, disrupting tissue metabolism, inhibiting growth, and thereby causing browning and death of the explants [29,30,31]. The schematization of browning process is shown in Figure 1.
Figure 1.
The lethal browning and microbial contamination processes in Musa spp. tissue culture.
The main enzymes involved in browning are polyphenol oxidases (PPOs), peroxidase (POD), and phenylalanine ammonia-lyase (PAL) [32,33,34]. PPOs and POD oxidize phenolic substances to quinone derivatives, which are further oxidized to generate melanin pigmentation, which is present in organisms and is responsible for browning processes [35,36]. PPOs are a group of copper-containing enzymes that can catalyze the o-hydroxylation of monophenols to o-diphenols (tyrosinase activity) as well as the oxidation of o-diphenols to quinones (catecholase activity) in the presence of oxygen [37,38]. PAL converts phenylalanine into trans-cinnamic acid, which acts as a precursor for the further synthesis of phenolic compounds [34]. Other enzymes also play a role in inducing or inhibiting the browning of explants, such as ascorbate peroxidase (APX), catalase (CAT), glutathione reductase (GR), and superoxide dismutase (SOD) [39].
In Musa spp., the PPO gene family has been extensively studied [40,41,42,43]. PPO proteins include two highly conserved copper-binding domains (CuA and CuB), each with three histidine residues, which create a Cu2+ binding site when combined [44]. Typically, PPO proteins also encompass an N-terminal transit peptide, determining the import of PPOs into the thylakoid lumen, a dicopper center, and a C-terminal region [41]. High levels of PPO activity were detected in banana flesh throughout fruit growth and ripening. BP01, BP011, BP034, and BP035 PPO cDNA were recovered from the banana. The BP01 genomic sequence was discovered to have an 85 bp intron, whereas the others did not. Expression analysis revealed a different expression pattern in the genes throughout the different stages of fruit development. BP01 was present in banana flesh early in development [42].
3. Microbial Contamination in Musa spp. Tissue Culture
Tissue culture technology is useful for providing disease-free and consistent planting materials that can be made available throughout the year [45]. Thus, the technique is crucial for banana and plantain micropropagation, ensuring the availability of genetically identical and disease-free plantlets for commercial production of the crop, which would significantly improve food security and economic benefits for farmers and consumers around the world [10]. Despite the multiple benefits of tissue culture technologies to banana and plantain production and agricultural growth, contamination has been a serious impediment to their use in crop micropropagation [46].
Filamentous fungi, bacteria, yeasts, viruses, and micro-arthropods including mites and thrips have been identified as contaminant agents in plant tissue culture [14], breaking through sterilizing barriers to produce contamination. Poorly prepared culture media, insufficient sterilization of explants, and contaminated working instruments are also major contributors to the high occurrence of microbial contaminations [47]. Microbial contamination can cost time, labor, and materials, resulting in significant economic losses [48,49].
3.1. Bacterial Contamination
Endophytic and phyllospheric bacteria, which are colonizing plants in their growing environment, can be considered the single most important damaging factor in plant tissue cultures. Surface disinfection cannot remove internal bacterial contamination (intra- or inter-cellular). These bacteria are not always pathogenic or detrimental to the plants in the natural environment; however, they can cause major contamination during in vitro propagation of the plants. Endophytic bacteria are useful to host plants because they improve disease resistance in plants as well as increase plant fitness [50]; however, they create serious problems in tissue culture micropropagation [51].
Endophytic bacteria are typically difficult to eliminate since systemic sterilizers, such as mercuric chloride and systemic fungicides, might harm the explants [15]. Contaminants are typically not visible at the time of culture establishment, but they can emerge after numerous subcultures. Some explants survive and flourish in the presence of bacteria; however, bacterial infection can cause significant harm to plant material due to bacterial proliferation. Several bacteria genera have been identified as banana colonizers, including Bacillus, Enterobacter, Erwinia, Klebsiella, and Pseudomonas [52,53,54,55,56,57]. Table 1 summarizes bacterial contamination in Musa spp. tissue culture propagation.

Table 1.
Bacterial species that cause contamination in Musa spp. tissue culture.
3.2. Fungal Contamination
Fungal contaminants continue to be a substantial barrier to the successful in vitro micropropagation of plants. They become a serious challenge at every stage of the in vitro plant culture process, and their formation and expansion are faster than the plant culture’s growth [58], resulting in time, resources, and, eventually, huge economic loss [48,51,59]. Accurate identification of common contaminants is required to effectively combat the threat of fungal contamination. Animasaun et al. [10] isolated and identified eleven fungi strains of five genera, Aspergillus, Cladosporium, Fusarium, Penicillium, and Trichoderma as the significant contaminants of in vitro banana culture. In early studies, Odutayo et al. [60] and Rahman et al. [61] identified four and five genera of fungi contaminants in tissue culture materials, respectively. Table 2 summarizes the fungal contamination in Musa spp. tissue culture.

Table 2.
Fungi species that cause contamination in Musa spp. tissue culture.
Fungi have also been reported to form capsules around their spores in order to avoid stressful situations such as hostile host immunological reactions [25,62]. To defend themselves, fungi can produce extracellular enzymes or exhibit an antibiosis mechanism [63]. For example, Fusarium spp. secrete lipases, which allow the fungi to infect the host plants [64], whereas Aspergillus spp., for example, A. flavus, produce various enzymes such as amylases, lipases, and proteases, which allow the fungi to colonize and infect the host [65].
Extracellular enzymes such as esterases, lignases, glutathione 5-transferases, and cytochrome are also involved in fungicide breakdown [66], which explains why fungi are resistant to most preferred sterilants. Most fungal species can produce secondary metabolites and release them into the surrounding environment [67]. These bioactive compounds can negatively influence the plant explants through nutrient and space competition, lysing plant cells, or blocking specific functions related to the host’s growth and metabolism, such as shutting down the host’s defense mechanisms, thereby allowing the pathogen to attack without resistance [22,23,49,67].
4. Managing Browning in Musa spp. Tissue Culture
Potential problems with banana and plantain in tissue culture include browning of the medium and excised faces of explants during the initiation and subculture phases [6], browning of young leaves, shoot necrosis, and plantlets death during the proliferation and rooting phases [68]. These phenomena are mostly caused by phenolic compound oxidation [21], but they are also likely to be caused by a depletion in the medium of mineral nutrients, growth regulators, or both [69].
4.1. Pre-Soaking Explants in Anti-Browning Solutions
Several studies reported the successful use of anti-browning agents during explant preparation to prevent browning in Musa spp. tissue culture (Table 3). For example, Titov et al. [70] reported that an antioxidant wash of 0.125% potassium citrate: citrate (K-C: C in a 4:1 w/w) solution was useful for preparing Musa spp. cv. Kanthali explants. Similarly, Ngomuo et al. [21] reported that dosing explants with 1.2 g/L ascorbic acid during explant preparation reduced the extent of deadly browning in Musa spp. cv. Mzuzu. Onuoha et al. [71] reported that soaking plantain (M. paradisiaca) aux bud in 0.1–0.5 mg/mL potassium citrate and citrate (K-C: C) for 2 h can protect the plants from browning.

Table 3.
Anti-browning agents used in the pre-soaking of explants.
4.2. Incorporation of Anti-Browning into Growing Media
The successful use of anti-browning compounds which were added into the growing media can prevent Musa spp. explants from browning during in vitro tissue culture propagation as reported in several studies (Table 4). For instance, Ko et al. [20] reported on the addition of ascorbic acid to the tissue culture medium during the micropropagation of Cavendish banana cv. Formosana. This chemical compound is involved in cell division, cell differentiation, and cell elongation of apical meristems during plant morphogenesis. Ascorbic acid contains ascorbate, which inhibits PPO directly [72]. Furthermore, AA transforms colorless o-quinones produced by PPO action back to diphenols, preventing browning [73]. According to Titvo et al. [70], AA scavenges oxygen radicals to prevent phenolic component oxidation in injured tissues, minimizing tissue browning. The addition of 15 mL/mL ascorbic acid to the Murashige and Skoog (MS) culture medium effectively prevented oxidation in Musa spp. banana tree explants [73]. Moreover, Nathan et al. [74] added cysteine to the growth medium, which significantly reduced the explant browning in banana tissue culture.
Ascorbic acid has been used by many researchers to manage tissue browning during in vitro propagation. The addition of ascorbic acid to the medium at dosages ranging from 10 mg/L to 150 mg/L has been shown to minimize the occurrence of tissue darkening [75]. This wide range of needed concentrations could be attributed to the genotype [19]. High quantities of ascorbic acid (100 and 200 mg/L) given to the culture media for the banana cultivar Mzuzu are helpful in decreasing tissue browning throughout 4 weeks of culture [21]. However, at low concentrations (50 mg/L), the efficacy of ascorbic acid is limited to the first week of culture.
The addition of ascorbic acid to the culture media during the proliferating or rooting phase prevents the deadly browning of plantlets, reduces the oxidation process, and recovers plantlets that have already been harmed [20]. It is thought that the ascorbic acid taken up by the plantlet migrates to the leaves to avoid phenolic compound oxidation, making its presence in the culture media favorable to plantlet proliferation [20,76]. Because ascorbic acid can be easily damaged by heat, it is best to add it to the surface of the culture media after sterilization. The addition of ascorbic acid to the culture media prior to sterilizing may explain some of the product’s ineffectiveness in decreasing browning as reported in some circumstances [19,20].
Citric acid can also be utilized to efficiently control tissue browning, darkening, and death. Citric acid, like ascorbic acid, can be added to the culture medium at concentrations ranging from 10 to 150 mg/L [75]. A 0.125% solution of citric acid and potassium citrate (4:1 m/m ratio) was successfully used to treat explants such as the banana flower apex [70] and plantain shoot apices [71]. The combination of citrate and citric acid acts as a chelating agent for the ions responsible for polyphenol oxidase activation. A concentration of 50 mg/L cysteine has been proposed to treat the explant for a few minutes before its implantation in vitro [74].
The primary adsorbent which has been widely utilized for in vitro banana and plantain culture is activated charcoal. Activated charcoal is made up of carbon particles that are organized in a quasi-graphitic pattern [77]. It has a fine network of pores with an extremely wide surface area that can be used to absorb several chemical compounds [78]. In plant tissue culture, activated charcoal is frequently used to promote growth and cell development [79,80]. Its primary benefits include the adsorption of inhibitory substances in the culture medium, a significant reduction in phenolic oxidation or brown exudate accumulation, adjustment of the medium pH to an optimum level for morphogenesis, and the establishment of a darkened environment in the medium, which simulates soil conditions [81].
Activated charcoal is commonly used to improve roots in banana and plantain micropropagation at concentrations ranging from 1.0 to 2.5 g/L [74,82,83]. Activated charcoal alone at 1.5 g/L or in combination with 150 mg/L of ascorbic acid or citric acid inhibited phenolic component oxidation and increased plantlet growth in the Grande Naine cultivar [17]. Thus, the adsorbent can aid in browning control, particularly during the initiation phase, when phenolic chemicals are released into the culture media by the excised faces of the explant.

Table 4.
Anti-browning added to the medium of explants.
Table 4.
Anti-browning added to the medium of explants.
| Anti-Browning | Concentration | Plant Species | Explant Types | References |
|---|---|---|---|---|
| Ascorbic Acid | 50 mg/L | Cavendish cv. Formosa | Shoot tip | [20] |
| Ascorbic Acid | 15 mL/mL | Musa spp. | Aux bud | [73] |
| Citric Acid | 150 mg/L | Musa spp. | Shoot tip | [75] |
| Ascorbic Acid | 100–200 mg/L | Musa spp. cv. Mzuzu | Shoot tip | [21] |
| Activated Charcoal | 1.5 g/L | Musa spp. cv. Grand Naine | Shoot tip | [17] |
| Lime Peel Extract | 300 mg/L | M. paradisiaca cv. Kepok Tanjung | Shoot tip | [84] |
4.3. Manipulating Cultural Practices
Another approach for protecting explants from the detrimental effects of browning is to quickly shift them to a fresh medium two or three times during the culture period. Frequent transfer of explants within the same medium or into fresh medium fairly prevents in vitro browning of explants [85]. During this time, the cut end of the explant seals up and phenolic leaching ceases [86]. This also allows for the avoidance of tissue infiltration by harmful by-products of phenol oxidation. These subcultures can be conducted once or twice a week [74].
Light and high temperatures accelerate browning by increasing enzyme activity [85]. Tissues produced in the dark, for example, frequently exhibit lower levels of browning than those grown in the light [87,88]. The most acceptable medium for shoot regeneration was MS media supplemented with 1.6 mg/L IAA and 4.0 mg/L BAP without ascorbic acid and activated charcoal under darkness for 4 weeks [73,89]. Keeping the cultures completely in the dark for a week can also help reduce browning by blocking or lowering the activity of enzymes involved in both phenol production and oxidation [74,86].
5. Control of Contamination in Musa spp. Tissue Culture
Another problem with the culture of Musa spp. in addition to browning is microbial contamination. Even though this contamination does not have a direct impact on the cultured explants, the bacteria and fungi can grow very quickly on the culture medium, which in turn can inhibit the growth of the explants. Thus, microbial contamination must be treated during the culture period.
5.1. Chemotherapy
Bacteria genera such as Pseudomonas, Staphylococcus, Corynebacterium, Bacillus, Agrobacterium, Propionibacterium, and Proteus are the most destructive bacterial contaminants as they can compete with the plant’s explant for nutrients in the growing medium [90,91]. To inhibit the growth of these bacterial contaminants, antibiotics such as tetracycline, streptomycin, vancomycin, rifampicin, gentamycin, cefotaxime, and others can be added to the culture medium [92]. To obtain better outcomes, these antibiotics can be administered singly or in combination. The agar embedding system, in which antibiotics were added to the agar and embedded onto protonema, has been reported as a novel method for eliminating bacterial contamination during the in vitro propagation of Moss protonema, thereby reducing microbial growth due to continuous contact between the tissues and antibiotics [93]. As another example, contamination in Gauda angustifolia Kunth was treated with kanamycin and streptomycin sulfate with kanamycin at a dosage of 10 g/mL, which exhibited the greatest results with no phytotoxicity [94].
Endophytic fungal species such as Acremonium, Alternari, Aspergillus, Cladosporium, Curvularia, Fusarium, Penicillium, Rhizopus, and Trichoderma have been found in meristematic tissue culture [14,47]. These endophytic microorganisms are identified and exploited to produce phytochemicals with anti-cancer, anti-depressant, anti-neoplastic, and other medicinally essential substances such as taxol [95]. They may, on the other hand, constitute a hazard to plants in vitro. Systemic fungicides can also be used to control the growth of most fungal endophytes.
Bavistin (50% carbendazim) is the most used fungicide in culture medium. Bavistin at concentrations ranging from 150 to 300 mg/L added to medium reduced fungal contamination significantly [96]. In fungal cells, it exhibits antimitotic and antineoplastic properties. Its structure is similar to that of cytokinin (adenine derivatives). Bavistin has been shown to promote shoot growth in Stevia rebaudiana cultures [97]. Other fungicides, such as ProClin®300, mancozeb, and thiabendazoles, have been used to prevent yeast contamination in apple cultures [98].
Anti-viral compounds are useful in the control of plant viral diseases. Chemical compounds, such as azidothymidine, ribavirin (RBV) (virazole), and 2-thiouracil [99], and some antiviral drugs, such as S-adenosylhomocysteine hydrolase inhibitors, neuraminidase (NA) inhibitors, and inosine monophosphate dehydrogenase (IMPDH) inhibitors, are generally used in plant chemotherapy [100,101]. Chemotherapy was used prior to meristem tip culture to totally remove the Lily symptomless virus [102].
5.2. Thermotherapy
Plant thermotherapy creates a cellular environment that is less suitable for viral survival [90]. The effects of heat treatment, for example, on the functioning of viral movement proteins result in decreased limitation of infected tissues [103]. The plants are cultivated at high temperatures (38–42 °C) for 4–6 weeks prior to thermotherapy [99]. This is easily performed in tropical or subtropical circumstances by erecting a tiny compartment of a glasshouse fitted with a roof vent on one end and an exhaust fan on the opposite end, both being temperature-regulated [100]. This method eliminates extra heat and offers a steady high-temperature treatment during the day.
In temperate climates, the same effect can be obtained using fluorescent lights, including ballasts, and/or heat-generating incandescent lamps, positioned at the required minimum distance from the plants to be treated in a dark box just large enough to accommodate the plants [103]. A similar approach has been used to eliminate viruses in sweet potatoes [104]. After thermotherapy, 0.2–0.4 mm explants are cultivated separately in test tubes. If the explant is excessively large, it is likely to have a vascular system that contains microbiological pollutants such as viruses. The resulting plants are multiplied and reindexed. A preferable technique would be to cultivate 2–5 mm long explants for 4–5 weeks, then keep the in vitro-grown plant at high temperature for 4–5 weeks before excising 0.2–0.4 mm or even longer explants to commence subcultures [100]. This method eliminates in vivo contamination issues while providing a high rate of survival and multiplication.
After in vitro thermotherapy to remove all contaminating viruses from potatoes, in vitro cultures were grown from several millimeter-long shoot tips and axillary buds [104]. It was feasible to remove viruses A, Y, and X from potato cultivars using this approach in a single step [90]. A method known as the multiple lateral shoot approach for in vitro eradication of three prevalent potato viruses, X, Y, and S, has also been published. A stem with at least five nodes is treated with ribavirin in a liquid medium and cultivated for 5 days at room temperature in this procedure. The stems are then treated with thermotherapy for 25 days at 32–35 °C, following which apical buds are plucked from the lateral shoots and cultivated on a solid medium [105]. ELISA is then used to test the developing plantlets for viral infection.
The capacity of virus particles to travel differently in plant tissues frequently determines the choice of elimination treatment [99]. When compared to meristem culture, which is more suitable for phloematic viruses that are limited to vascular tissues and rarely found in parts of the plant where differentiated tissues are absent, thermotherapy is the more effective control procedure against viruses that are characterized by parenchymatic localization [100]. However, discrepancies in phloem and parenchymatic viral localization in the host tissue have not entirely explained their differing susceptibilities to thermotherapy elimination [105].
Over the last 20 years, advancements in research aimed at investigating the metabolic processes involved in plant defense mechanisms have suggested an interpretation of heat treatment effects based on new metabolic “pathways” triggered by the infected plant’s natural antiviral response, with particular reference to Virus-Induced Gene Silencing (VIGS) induced by the presence of viral RNA in infected plants [100].
RNA silencing has been regarded as such a powerful defense that it constitutes a genetic immunity mechanism [106]. Studies using temperatures lower than those used in typical thermotherapy protocols (36 °C) demonstrated that gene silencing and heat treatments promote recovery in infected plants [107]. Furthermore, in a study performed by Szittya et al. [108], Nicotiana benthamiana plants infected with Cymbidium ringspot virus were subjected to a variety of heat regimes ranging from 15 °C to 27 °C. The amount of short interfering RNA (SiRNA) was measured for each heat treatment, with significant concentrations of SiRNA detected at 27 °C but undetected at 15 °C. In addition, in comparison to the treatments, a growing SiRNA concentration gradient was detected beginning at 21 °C.
Some researchers discovered hyperactivity in the system of temperature-dependent gene silencing as a mechanism of plant antiviral defense in connection to the differential distribution of virus particles seen in the temperature range examined [103]. Virus-induced gene silencing is characterized as a defensive system that acts ineffectively at low temperatures, increasing the plant’s susceptibility to viral infections that do not face virus-blocking gene systems. Increased heat stress, on the other hand, increases the capability of the host defense system by forming a barrier to infection [107]. Chellapan et al. [109] used heat treatment (25–30 °C) on cassava (Manihot esculenta) and tobacco (Nicotiana benthamiana) plants infected with Cassava mosaic disease to assess the impact of temperature on viral silencing in Geminivirus (ssDNA).
5.3. Cryotherapy
Pathogens such as viruses, phytoplasma, and bacteria are subjected to low temperatures (−196 °C) for an extended period of time in plant cryotherapy, which efficiently eradicates viral complexes, resulting in virus-free plants with a high frequency when compared to meristem tip culture [110]. For example, three Closteroviridae viruses that cause leafroll disease in grapevine were reported to be eliminated with vitrification utilizing dehydrating material-based cryotherapy of buds from contaminated clones [111]. Cryotherapy has the advantage of treating a large number of plantlets at the same time, and the approach is appropriate regardless of shoot tip size. However, one significant downside is the high consumption of some gases such as Argon and Nitrogen [90].
5.4. Use of Disinfectants, Antiseptics, and Nanoparticles
In addition to the use of thermotherapy, chemotherapy, and cryotherapy to manage pollutants in culture medium, disinfectants, antiseptics, and nanoparticles are also added into plant tissue culture media. Weber et al. [112], for example, demonstrated the use of 5–10 ppm NaOCl during in vitro potato growth to suppress pathogenic contamination. Disinfection of contaminated culture medium with active chlorine at 0.001% and 0.005% yielded comparable results to traditional techniques [113]. The addition of active chlorine to the medium preserves the stability of heat-sensitive substances such as Vitamin B and growth regulators [114]. Plant Preservative MixturesTM, which comprise a mixture of methylisothiazolinone (MIT) and chloromethylisothiazolone, have also been added into the culture media (CMIT) [115]. Major enzymes generated in the microbial metabolic and energy production pathways are inhibited by these chemical substances [116].
Nanoparticles have been created in order to eliminate the presence of microbial contaminants in plant tissue cultures. At a concentration of 200 mg/L, zinc nanoparticles and zinc oxide nanoparticles have been found to exhibit antibacterial capabilities and no antagonistic action in plant tissue cultures, such as banana cultures [117]. Similarly, silver nanoparticles (AgNPs) have been found to have the capacity to minimize bacterial contamination in Valeriana officinalis tissue cultures at concentrations ranging from 20 to 100 mg/L [118]. The mechanism of action of nanoparticles, particularly silver nanoparticles, for the control of microbial contaminants in meristematic tissue culture includes adhesion to microbial cell membranes [119], penetration inside the cells [120], reduction in oxygen species and free radical generation, induced cellular toxicity and oxidative stress, and modulation of microbial signal transduction pathways [121]. However, silver nanoparticles cause cytotoxicity, genotoxicity, and an inflammatory response in cell-type dependent explants. This has generated concerns about the use of AgNPs in the control of microbial contaminants in the culture of meristematic tissues [122].
6. Challenges and Limitations
Controlling browning by immersing explants in an anti-browning solution during preparation was reported to be capable of inhibiting browning in Musa spp. tissue culture, but this method was not optimal due to the limited ability of the explants to absorb anti-browning compounds, causing oxidation of phenolic compounds to continue during the culture period [20,70]. Other methods such as adding anti-browning compounds to the culture media prior to sterilization were also reported to be capable of effectively inhibiting the browning process [17,75]. Some synthetic anti-browning compounds which have melting points above the autoclave temperature are very likely to be added to the media prior to the sterilization process, but this is not possible for natural anti-browning compounds such as plant extracts [84].
Another method that can be used as an alternative to prevent browning in explants is by increasing the frequency of subcultures or storing the explants in the dark [85]. Increasing the sub-culture frequency has the potential to cause contamination by rhizosphere microbes, making it less effective. Likewise, storing explants in the dark period can only be performed at shoot initiation [73], while for other phases of micropropagation, this is not possible. The combination of using anti-browning compounds in prepared explants with the addition of these compounds to the media may inhibit browning in explants much more effectively in each culture phase by adding to the treatment of storing explants in the dark period during the shoot initiation stage [21,84].
Currently, the most widely used method of handling contamination with chemotherapy is by adding antibiotics to the media, but on the other hand, the continuous use of antibiotics can cause microbial resistance [92]. For thermotherapy and cryotherapy methods, the existence of high-temperature treatment on explants can cause faster oxidation reactions which cause browning in explants; however, cryotherapy requires a lot of argon and nitrogen gasses. Other alternative methods that can be used include adding disinfectants, antiseptics, extracts and plant essential oils, and nanoparticles and genome editing such as clustered regularly interspaced short palindromic repeats (CRISPR) and transcription activator-like effector nucleases (TALENs) [117].
The use of plants’ extracts and essential oils was reported to be capable of inhibiting microbial contamination effectively in Aloe vera, Fragaria ananassa, Phoenix dactylifera, Pennisetum purpureum, Manihot utilissima, and Zea mays cultures [13,123,124,125]. In addition, the application of the CRISPR and TALENs methods was successful in producing disease-free plants in Oryza sativa, Solanum lycopersicum, and Malus domestica towards pathogenic microbes such as Blumeria graminis, Oidium neolycopersici, and Magnaporthe oryzae [126,127,128,129]. In the future, the use of extracts and plant essential oils as well as CRISPR and TALENs has the potential to be applied in handling plant tissue culture browning and contamination, especially for Musa spp.
7. Conclusions
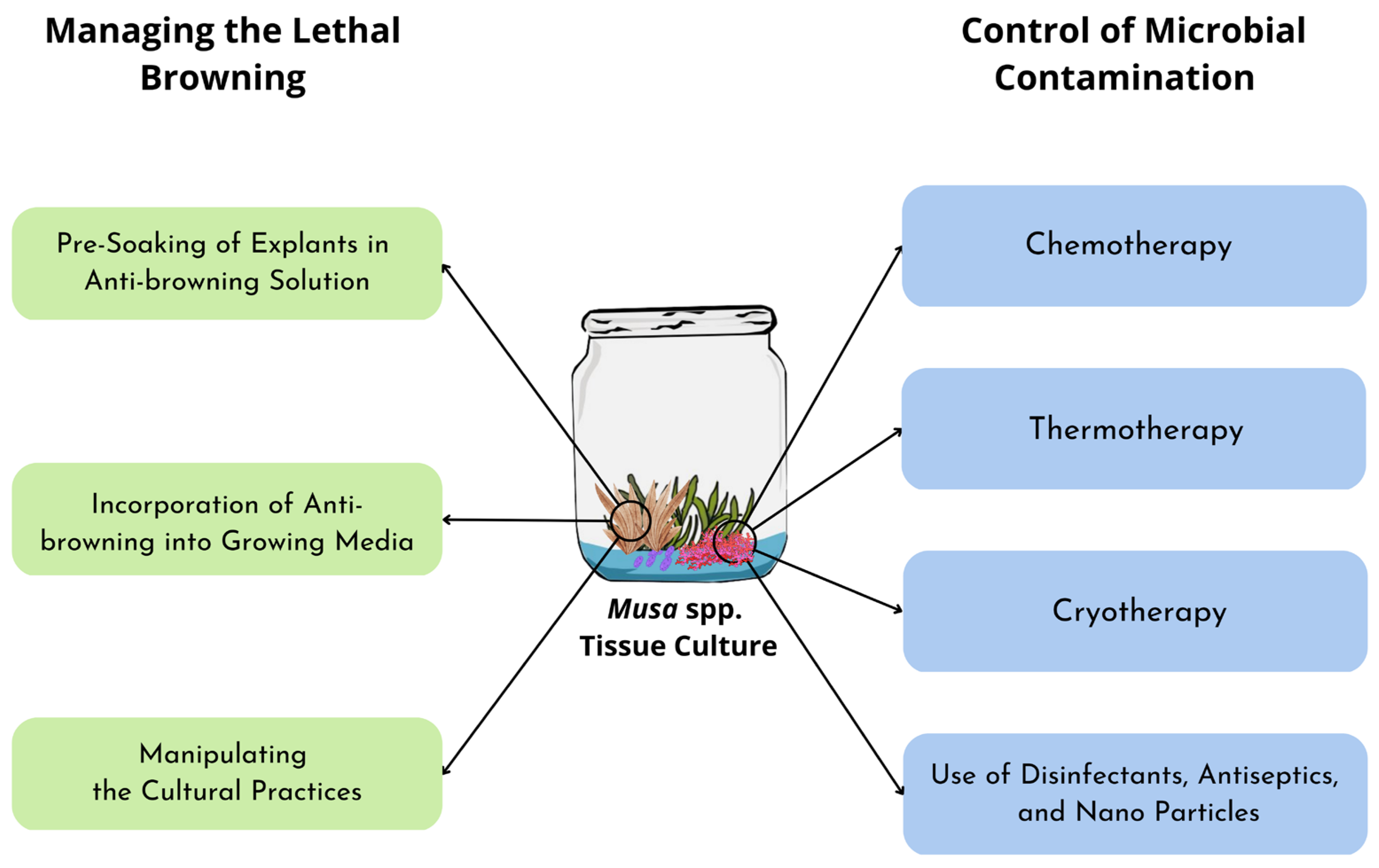
Browning and contamination are significant challenges in Musa spp. plant tissue culture that, if not properly addressed, will eventually decrease banana production. Based on the described protocols, it is possible to control enzymatic browning during the in vitro propagation of Musa spp. by soaking the explants in an anti-browning solution, incorporating the anti-browning compounds into the media, and other methods such as frequent sub-culturing or culture incubation in a dark environment. Similarly, attempts have been made to reduce contamination issues, such as using thermotherapy, chemotherapy, and cryotherapy, as well as the use of chemical agents such as disinfectants, antiseptics, and nanoparticles as summarized in Figure 2. Future research using CRISPR and TALENs to overcome browning and microbial contamination in tissue cultures of Musa spp. must be accelerated in order to increase the productivity and vigor of banana and plantain explants.
Figure 2.
Different strategies used to manage lethal browning and microbial contamination in Musa spp. tissue culture.
Author Contributions
Conceptualization, N.P. and E.J.; writing—original draft preparation, N.P. and F.D.; writing—review and editing, A.N.A., N.P., F.D. and E.J.; visualization, F.D.; supervision, E.J., M.N. and F.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universitas Padjadjaran through the Academic Leadership Grant (ALG) awarded to E.J., grant number: 2203/UN6.3.1/PT.00/2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This study did not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Soorianathasundaram, K.; Narayana, C.K.; Paliyath, G. Bananas and plantains. Encycl. Food Health 2015, 320–327. [Google Scholar] [CrossRef]
- Israeli, Y.; Lahav, E. Banana. Encycl. App. Plant Sci. 2016, 3, 363–381. [Google Scholar]
- FAO. Banana Facts and Figures. Available online: https://www.fao.org/economic/est/est-commodities/oilcrops/bananas/banana-facts/en/#.ZBbqy-xBw1K (accessed on 19 March 2023).
- El-Sayed, I.M.; Salama, W.H.; Salim, R.G.; Taha, L.S. Relevance of nanoparticles on micropropagation, antioxidant activity and molecular characterization of Sequoia sempervirens L. Plant. Jordan J. Biol. Sci. 2021, 14, 374–382. [Google Scholar]
- Mose, W.; Daryono, B.S.; Indrianto, A.; Purwantoro, A.; Semiarti, E. Direct somatic embryogenesis and regeneration of an indonesian orchid Phalaenopsis amabilis (L.) Blume under a variety of plant growth regulators, light regime, and organic substances. Jordan J. Biol. Sci. 2020, 13, 509–518. [Google Scholar]
- Agbadje, E.T.A.E.; Agbidinoukoun, A.; Zandjanakou-Tachin, M.; Cacaï, G.T.H.; Ahanhanzo, C. Mass production of bananas and plantains (Musa spp.) plantlets through in vitro tissue culture partway: A review. Eur. J. Biol. Biotech. 2021, 2, 4. [Google Scholar] [CrossRef]
- Ferdous, M.H.; Masum Billah, A.A.; Mehraj, H.; Taufique, T.; Jamal Uddin, A.F.M. BAP and IBA pulsing for in vitro multiplication of banana cultivars through shoot-tip culture. J. Biosci. Agric. Res. 2015, 3, 87–95. [Google Scholar] [CrossRef]
- Yang, J.; Bao, J.; Lu, X.; Zhang, X.; Tian, P.; Shi, X.; Li, S.; Ma, S. Transcriptomic analysis of the effects of melatonin on genes potentially related to the browning of broccoli (Brassica oleracea L. var. Italica Planch) hairy roots. Plant Growth Regul. 2022, 98, 557–567. [Google Scholar] [CrossRef]
- Chen, G.; Chen, D.; Wang, T.; Xu, C.; Li, L. Analysis of the proteins related to browning in leaf culture of Phalaenopsis. Sci. Hortic. 2012, 141, 17–22. [Google Scholar] [CrossRef]
- Animasaun, D.A.; Nnamdi, C.D.; Ipinmoroti, O.I.; Oyedeji, S.; Olonya, E.A.; Krishnamurthy, R.; Morakinyo, J.A. Molecular identification and phylogenetic analysis of fungi contaminants associated with in vitro cultured banana based on ITS region sequence. Hayati 2022, 29, 288–300. [Google Scholar] [CrossRef]
- Hrynets, Y.; Bhattacherjee, A.; Betti, M. Nonenzymatic browning reactions: Overview. In Reference Module in Food Science; Elsevier: Edmonton, AB, Canada, 2018; Volume 2, pp. 233–244. [Google Scholar]
- Wu, T.; Li, J.; Zhang, J.; Lin, M.; Wu, Z.; Cai, X.; Xiang, W.; Tan, S.; Zhang, Z. Graphene oxide inhibits the lethal browning of Cymbidium sinense by reducing activities of enzymes. J. Plant Biotechnol. Microbiol. 2018, 1, 11–20. [Google Scholar]
- Hamdeni, I.; Slim, S.; Sanaa, A.; Louhaichi, M.; Boulila, A.; Bettaieb, T. Rosemary essential oil enhances culture establishment and inhibits contamination and enzymatic browning: Applications for in vitro propagation of Aloe vera L. S. Afr. J. Bot. 2022, 147, 1199–1205. [Google Scholar] [CrossRef]
- Cobrado, J.; Fernandez, A. Common fungi contamination affecting tissue-cultured abaca (Musa textiles Nee) during initial stage of micropropagation. Asian Res. J. Agric. 2016, 1, 1–7. [Google Scholar] [CrossRef]
- El-Banna, A.N.; El-Mahrouk, M.E.; Dewir, Y.H.; Farid, M.A.; Elyazid, D.M.A.; Schumacher, H.M. Endophytic bacteria in banana in vitro cultures: Molecular identification, antibiotic susceptibility, and plant survival. Horticulturae 2021, 7, 526. [Google Scholar] [CrossRef]
- Xu, C.; Ru, Z.; Li, L.; Zeng, B.; Huang, J.; Huang, W.; Hu, O. The effects of polyphenol oxidase and cycloheximide on the early stage of browning in Phalaenopsis explants. Hortic. Plant. J. 2015, 1, 172–180. [Google Scholar]
- Safwat, G.; Abdul-Rahman, F.; Sharbasy, A.S. The effect of some antioxidants on blackening and growth of in vitro culture of banana (Musa spp. cv. Grand Naine). J. Genet. Cytol 2015, 44, 47–59. [Google Scholar]
- Ray, S.S.; Ali, N. Biotic contamination and possible ways of sterilization: A review with reference to bamboo micropropagation. Braz. Arch. Biol. Technol. 2017, 60, 1–17. [Google Scholar] [CrossRef]
- Chikezie, U.N.Y. Effect of ascorbic acid on blackening and sprouting of Musa spp. shoot tips. J. Biotechnol. Bioinform. 2012, 2, 11–17. [Google Scholar]
- Ko, W.H.; Su, C.C.; Chen, C.L.; Chao, C.P. Control of lethal browning of tissue culture plantlets of cavendish banana cv. Formosana with ascorbic acid. Plant Cell Tissue Organ Cult. 2009, 96, 137–141. [Google Scholar] [CrossRef]
- Ngomuo, M.; Mneney, E.; Ndakidemi, P. Control of lethal browning by using ascorbic acid on shoot tip cultures of a local Musa spp. (Banana) cv. Mzuzu in Tanzania. Afr. J. Biotechnol. 2014, 13, 1721–1725. [Google Scholar]
- Habiba, U.; Reza, S.; Lal Saha, M.; Khan, M.R.; Hadiuzzaman, S. Endogenous bacterial contamination during in vitro culture of table banana: Identification and prevention. Plant Tissue Cult. Biotechnol. 2002, 12, 117–124. [Google Scholar]
- Msogoya, T.; Kanyagha, H.; Mutigitu, J.; Kulebelwa, M.; Mamiro, D. Identification and management of microbial contaminants of banana in vitro cultures. J. Appl. Biosci. 2012, 55, 3987–3994. [Google Scholar]
- Hamill, S.D.; Rames, E. An effective indexing method for banana tissue culture provides long-term freedom from bacterial contamination. Acta. Hortic. 2018, 1205, 741–747. [Google Scholar] [CrossRef]
- Kithaku, E.; Muigai, A.T.; Neondo, J.; Mweu, C. Screening of fungal contaminants in banana tissue cultures in Jkuat, Kenya. Afr. J. Microbiol. Res. 2019, 13, 675–688. [Google Scholar]
- Mokbel, S.A.; Khalil, A.A.; El-Shazly, M.A. Efficiency of eugenol oil nanoemulsion against banana bunchy top virus and contamination with fungi in plant tissue culture. Arab J. Biotech. 2017, 20, 33–50. [Google Scholar]
- Kapadia, C.; Patel, N. Sequential sterilization of banana (Musa spp.) sucker tip reducing microbial contamination with highest establishment percentage. Bangladesh J. Bot. 2021, 50, 1151–1158. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Gao, Y.; Han, Y.; Wu, X. Path analysis of non-enzymatic browning in Dongbei suancai during storage caused by different fermentation conditions. Food Chem. 2021, 335, 127620. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, H.; Liu, K.; Li, L.; Yang, J.; An, X.; Li, P.; Yun, L.; Zhang, Z. The role of JrPPOs in the browning of walnut explants. BMC Plant Biol. 2021, 21, 9. [Google Scholar] [CrossRef]
- He, Y.; Guo, X.; Lu, R.; Niu, B.; Pasapula, V.; Hou, P.; Cai, F.; Xu, Y.; Chen, F. Changes in morphology and biochemical indices in browning callus derived from Jatropha curcas hypocotyls. Plant Cell Tissue Organ Cult. 2009, 98, 11–17. [Google Scholar] [CrossRef]
- Tang, W.; Newton, R.J. Increase of polyphenol oxidase and decrease of polyamines correlate with tissue browning in virginia pine (Pinus virginiana Mill.). Plant Sci. 2004, 167, 621–628. [Google Scholar] [CrossRef]
- Nadafzadeh, M.; Abdanan Mehdizadeh, S.; Soltanikazemi, M. Development of computer vision system to predict peroxidase and polyphenol oxidase enzymes to evaluate the process of banana peel browning using genetic programming modeling. Sci. Hortic. 2018, 231, 201–209. [Google Scholar] [CrossRef]
- Ru, Z.; Lai, Y.; Xu, C.; Li, L. Polyphenol oxidase (PPO) in early stage of browning of Phalaenopsis leaf explants. J. Agric. Sci. 2013, 5, 57–64. [Google Scholar] [CrossRef]
- Jones, A.M.P.; Saxena, P.K. Inhibition of phenylpropanoid biosynthesis in Artemisia annua L.: A novel approach to reduce oxidative browning in plant tissue culture. PLoS ONE 2013, 8, e76802. [Google Scholar] [CrossRef] [PubMed]
- Selvarajan, E.; Veena, R.; Manoj, K.N. Polyphenol oxidase, beyond enzyme browning. In Microbial Bioprospecting for Sustainable Development; Singh, J., Sharma, D., Kumar, G., Sharma, N.R., Eds.; Springer: Singapore, 2018; pp. 203–222. [Google Scholar]
- Rayan, A.; Morsy, N. Thermal inactivation kinetics of peroxidase and polyphenol oxidase from pomegranate arils (Punica granatum L. cv. Wonderful). J. Food Biochem. 2020, 44, e13428. [Google Scholar] [CrossRef] [PubMed]
- Araji, S.; Grammer, T.A.; Gertzen, R.; Anderson, S.D.; Mikulic-Petkovsek, M.; Veberic, R.; Phu, M.L.; Solar, A.; Leslie, C.A.; Dandekar, A.M. Novel roles for the polyphenol oxidase enzyme in secondary metabolism and the regulation of cell death in walnut. Plant Physiol. 2014, 164, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Jiang, Y.; Wang, C.; Song, M.; Liu, Y.; Liu, J.; Jiang, Z.; Yang, Y.; Ren, X.; Ding, Y. Metabolomic and transcriptomic analysis reveal high solar irradiance inhibited the melanin formation in persimmon fruit peel. Environ. Exp. Bot. 2023, 207, 105218. [Google Scholar] [CrossRef]
- Sharafi, Y.; Aghdam, M.S.; Luo, Z.; Jannatizadeh, A.; Razavi, F.; Fard, J.R.; Farmani, B. Melatonin treatment promotes endogenous melatonin accumulation and triggers GABA shunt pathway activity in tomato fruits during cold storage. Sci. Hortic. 2019, 254, 222–227. [Google Scholar] [CrossRef]
- Shekhawat, U.K.S.; Ganapathi, T.R. Transgenic banana plants overexpressing musabZIP53 display severe growth retardation with enhanced sucrose and polyphenol oxidase activity. Plant Cell Tissue Organ Cult. 2014, 116, 387–402. [Google Scholar] [CrossRef]
- Tran, L.T.; Taylor, J.S.; Constabel, C.P. The polyphenol oxidase gene family in land plants: Lineage-specific duplication and expansion. BMC Genom. 2012, 13, 395. [Google Scholar] [CrossRef]
- Gooding, P.S.; Bird, C.; Robinson, S.P. Molecular cloning and characterisation of banana fruit polyphenol oxidase. Planta 2001, 213, 748–757. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Zeng, J.; Yun, Z.; Liu, Y.; Qu, H.; Jiang, Y.; Duan, X.; Xia, R. MicroRNA528, a hub regulator modulating ROS homeostasis via targeting of a diverse set of genes encoding copper-containing proteins in monocots. New Phytol. 2020, 225, 385–399. [Google Scholar] [CrossRef]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.; Pavan, S.; Montemurro, C. Polyphenol oxidases in crops: Biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 2017, 377, 377. [Google Scholar] [CrossRef]
- Eziashi, E.I.; Asemota, O.; Okwuagwu, C.O.; Eke, C.R.; Chidi, N.I.; Oruade-Dimaro, E.A. Screening sterilizing agents and antibiotics for the elimination of bacterial contaminants from oil palm explants for plant tissue culture. Eur. J. Exp. Biol. 2014, 4, 111–115. [Google Scholar]
- Rawal, K.; Keharia, H. Prevention of fungal contamination in plant tissue culture using cyclic lipopeptides secreted by Bacillus amyloliquefaciens AB30a. Plant Tissue Cult. Biotechnol. 2019, 29, 111–119. [Google Scholar] [CrossRef]
- Omamor, I.B.; Asemota, A.O.; Eke, C.R.; Eziashi, E.I. Fungal contaminants of the oil palm tissue culture in nigerian institute for oil palm research (NIFOR). Afr. J. Agric. Res. 2007, 2, 534–537. [Google Scholar]
- Abass, M.H. Microbial contaminants of date palm (Phoenix dactylifera L.) in iraqi tissue culture laboratories. Emir. J. Food Agric. 2013, 25, 875–882. [Google Scholar] [CrossRef]
- Altan, F.; Bürün, B.; Şahin, N. Fungal contaminants observed during micropropagation of Lilium candidum L. and the effect of chemotherapeutic substances applied after sterilization. Afr. J. Biotechnol. 2010, 9, 991–995. [Google Scholar]
- Doni, F.; Miranti, M.; Mispan, M.S.; Mohamed, Z.; Uphoff, N. Multi-omics approaches for deciphering the microbial modulation of plants’ genetic potentials: What’s known and what’s next? Rhizosphere 2022, 24, 100613. [Google Scholar] [CrossRef]
- Guan, S.H.; Sattler, I.; Lin, W.H.; Guo, D.A.; Grabley, S. p-Aminoacetophenonic acids produced by a mangrove endophyte: Streptomyces griseus subsp. J. Nat. Prod. 2005, 68, 1198–1200. [Google Scholar] [CrossRef]
- Ting, A.S.Y.; Meon, S.; Kadir, J.; Radu, S.; Singh, G. Endophytic microorganisms as potential growth promoters of banana. BioControl 2008, 53, 541–553. [Google Scholar] [CrossRef]
- Thomas, P.; Swarna, G.K.; Patil, P.; Rawal, R.D. Ubiquitous presence of normally non-culturable endophytic bacteria in field shoot-tips of banana and their gradual activation to quiescent cultivable form in tissue cultures. Plant Cell Tissue Organ Cult. 2008, 93, 39–54. [Google Scholar] [CrossRef]
- Souza, S.A.; Xavier, A.A.; Costa, M.R.; Cardoso, A.M.S.; Pereira, M.C.T.; Nietsche, S. Endophytic bacterial diversity in banana “Prata Anã” (Musa spp.) toots. Genet. Mol. Biol. 2013, 36, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Caballero-Mellado, J.; Orozco, J.; Martínez-Romero, E. Diazotrophic bacteria associated with banana (Musa spp.). Plant Soil 2003, 257, 35–47. [Google Scholar] [CrossRef]
- van den Houwe, I.; Guns, J.; Swennen, R. Bacterial contamination in musa shoot tip cultures. Acta Hortic. 1998, 490, 485–492. [Google Scholar] [CrossRef]
- Ganen, S.T.S.; Nietsche, S.; Pereira, M.C.T.; Reis, S.T.; Xavier, A.A.; Santos, T.M.; Fernandes, T.P. Microbial contamination in explants of banana cultivars “Galil 18” and “Tropical”. Acta Hortic. 2010, 829, 341–344. [Google Scholar] [CrossRef]
- Mng’omba, S.A.; Sileshi, G.; du Toit, E.S.; Akinnifesi, F.K. Efficacy and utilization of fungicides and other antibiotics for aseptic plant cultures. In Fungicides for Plant and Animal Diseases, 1st ed.; Dhanasekaran, D., Thajuddin, N., Panneerselvam, A., Eds.; InTech: Shanghai, China, 2012; pp. 245–254. [Google Scholar]
- Herman, E.B. Plant tissue culture contamination: Challenges and opportunities. Acta Hortic. 2017, 1155, 231–238. [Google Scholar] [CrossRef]
- Odutayo, O.I.; Amusa, N.A.; Okutade, O.O.; Ogunsanwo, Y.R. Determination of the sources of microbial contaminants of cultured plant tissues. Plant Pathol. J. 2007, 6, 77–81. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Shahinul Islam, S.M.; Chowdhury, A.N.; Subramaniam, S. Identification and prevention of microbial contaminants of potato culture in temporary immersion bioreactor (TIB) system. Malays. J. Microbiol. 2017, 13, 289–297. [Google Scholar]
- Zaragoza, O.; Rodrigues, M.L.; de Jesus, M.; Frases, S.; Dadachova, E.; Casadevall, A. The capsule of the fungal pathogen Cryptococcus neoformans. Adv. Appl. Microbiol. 2009, 68, 133–216. [Google Scholar]
- Compant, S.; Brader, G.; Muzammil, S.; Sessitsch, A.; Lebrihi, A.; Mathieu, F. Use of beneficial bacteria and their secondary metabolites to control grapevine pathogen diseases. BioControl 2013, 58, 435–455. [Google Scholar] [CrossRef]
- Feng, J.; Liu, G.; Selvaraj, G.; Hughes, G.R.; Wei, Y. A secreted lipase encoded by LIP1 ss necessary for efficient use of saturated triglyceride lipids in Fusarium graminearum. Microbiology 2005, 151, 3911–3921. [Google Scholar] [CrossRef]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Hernández, M.L.; Snchez-Salinas, E.; Dantn Gonzlez, E.; Luisa, M. Pesticide biodegradation: Mechanisms, genetics and strategies to enhance the process. In Biodegradation—Life of Science, 1st ed.; Chamy, R., Ed.; InTechOpen: London, UK, 2013; pp. 251–287. [Google Scholar]
- Zhao, P.; Quan, C.; Wang, Y.; Wang, J.; Fan, S. Bacillus amyloliquefaciens Q-426 as a potential biocontrol agent against Fusarium oxysporum f. sp. Spinaciae. J. Basic Microbiol. 2014, 54, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.P.; Zhang, C.L.; Slater, A.; Madassery, J. Control of shoot necrosis and plant death during micropropagation of banana and plantains (Musa spp.). Plant Cell Tissue Organ Cult. 2007, 88, 51–59. [Google Scholar] [CrossRef]
- Bairu, M.W.; Stirk, W.A.; van Staden, J. Factors contributing to in vitro shoot-tip necrosis and their physiological interactions. Plant Cell Tissue Organ Cult. 2009, 98, 239–248. [Google Scholar] [CrossRef]
- Titov, S.; Bhowmik, S.K.; Mandal, A.; Alam, M.S.; Uddin, S.N. Control of phenolic compound secretion and effect of growth regulators for organ formation from Musa spp. cv. Kanthali floral bud explants. Am. J. Biochem. Biotechnol. 2006, 2, 97–104. [Google Scholar] [CrossRef]
- Onuoha, I.C.; Eze, C.J.; Unamba, C.I.N. In vitro prevention of browning in plantain culture. Online J. Biol. Sci. 2011, 11, 13–17. [Google Scholar] [CrossRef]
- Ndakidemi, C.F.; Mneney, E.; Ndakidemi, P.A. Effects of ascorbic acid in controlling lethal browning in vitro culture of Brahylaena using nodal segments. Am. J. Plant Sci. 2014, 05, 187–191. [Google Scholar] [CrossRef]
- Amente, G.; Chimdessa, E. Control of browning in plant tissue culture: A review. J. Sci. Agric. 2021, 5, 67–71. [Google Scholar] [CrossRef]
- Nathan, A.J.; Scobell, A. Banana cell and tissue culture—Review. Foreign Aff. 2012, 91, 1–12. [Google Scholar]
- Strosse, H.; van den Houwe, I.; Panis, B. Banana Cell and Tissue Culture—Review. In Banana Improvement: Cellular, Molecular Biology, and Induced Mutations, 1st ed.; Jain, S.M., Swennen, R., Eds.; Science Publisher Inc.: Enfield, CT, USA, 2004; Volume 49, pp. 1–12. [Google Scholar]
- Ssekiwoko, F.; Talengera, D.; Kiggundu, A.; Namutebi, M.K.; Karamura, E.; Kunert, K. In-vitro proliferation of Musa balbisiana improves with increased vitamin concentration and dark culturing. J. Appl. Biol. Biotechnol. 2014, 2, 1–7. [Google Scholar]
- Utley, W.S. Charcoal. In Encyclopedia of Toxicology, 2nd ed.; Wexler, P., Ed.; Elsevier: New York, NY, USA, 2005; pp. 505–519. [Google Scholar]
- Roy, S.; Das, P.; Sengupta, S.; Manna, S. Calcium impregnated activated charcoal: Optimization and efficiency for the treatment of fluoride containing solution in batch and fixed bed reactor. Process Saf. Environ. Prot. 2017, 109, 18–29. [Google Scholar] [CrossRef]
- Kassahun Bantte, D.S.; Feyissa, T. Effects of polyvinyl pyrrolidone and activated charcoal to control effect of phenolic oxidation on in vitro culture establishment stage of micropropagation of sugarcane (Saccharum officinarum L.). Adv. Crop Sci. Techn. 2015, 3, 4. [Google Scholar] [CrossRef]
- Barbosa, D.A.; Barbosa, E.G.G.; Molinari, M.D.C.; Fuganti Pagliarini, R.; Marin, S.R.R.; Marin, D.R.; Mertz-Henning, L.M.; Nepomuceno, A.L. Activated charcoal added to tissue culture media increases genotype-dependent biomass production in soybean. Agr. Sci. Biotechnol. 2022, 8, 1–11. [Google Scholar] [CrossRef]
- North, J.J.; Ndakidemi, P.A.; Laubscher, C.P. Effects of antioxidants, plant growth regulators and wounding on phenolic compound excretion during micropropagation of Strelitzia reginae. Int. J. Phys. Sci. 2012, 7, 638–646. [Google Scholar] [CrossRef]
- Wirakarnain, S.; Hossain, A.B.M.S.; Chandran, S. Plantlet production through development of competent multiple meristem cultures from male inflorescence of banana, Musa acuminta cv. “Pisang Mas” (AA). Am. J. Biochem. Biotechnol. 2008, 4, 325–328. [Google Scholar]
- Husin, N.; Jalil, M.; Othman, R.Y.; Khalid, N. Enhancement of regeneration efficiency in banana (Musa acuminata cv. Berangan) by using proline and glutamine. Sci. Hortic. 2014, 168, 33–37. [Google Scholar] [CrossRef]
- Nurzaman, M.; Permadi, N.; Setiawati, T.; Hasan, R.; Irawati, Y.; Julaeha, E.; Herlina, T. DPPH free radical scavenging activity of Citrus aurantifolia Swingle peel extracts and their impact in inhibiting the browning of Musa paradisiaca L. var. Kepok Tanjung explants. Jordan J. Biol. Sci. 2022, 15, 771–777. [Google Scholar]
- Dobránszki, J.; Teixeira da Silva, J.A. Micropropagation of apple—A review. Biotechnol. Adv. 2010, 28, 462–488. [Google Scholar] [CrossRef]
- Ahmad, I.; Hussain, T.; Ashraf, I.; Nafees, M.; Rafay, M.; Iqbal, M. Lethal effects of secondary metabolites on plant tissue culture. Environ. Sci. 2013, 13, 539–547. [Google Scholar]
- Ochoa-Alejo, N.; Ramirez-Malagon, R. In vitro chili pepper biotechnology. Vitr. Cell. Dev. Biol. 2001, 37, 701–729. [Google Scholar] [CrossRef]
- Krishna, H.; Sairam, R.K.; Singh, S.K.; Patel, V.B.; Sharma, R.R.; Grover, M.; Nain, L.; Sachdev, A. Mango explant browning: Effect of ontogenic age, mycorrhization and pre-treatments. Sci. Hortic. 2008, 118, 132–138. [Google Scholar] [CrossRef]
- Buah, J.N.; Danso, E.; Taah, K.J.; Abole, E.A.; Bediako, E.A.; Asiedu, J.; Baidoo, R. The effects of different concentrations cytokinins on the in vitro multiplication of plantain (Musa sp.). Biotechnology 2010, 9, 343–347. [Google Scholar] [CrossRef]
- Nsofor, G.C. Conventional methods of controlling microbial contaminants in meristematic tissue cultures: A review. Niger. Agric. J. 2021, 52, 181–186. [Google Scholar]
- Izarra, M.L.; Panta, A.L.; Maza, C.R.; Zea, B.C.; Cruzado, J.; Gutarra, L.R.; Rivera, C.R.; Ellis, D.; Kreuze, J.F. Identification and control of latent bacteria in in vitro cultures of sweetpotato [Ipomoea batatas (L.) Lam]. Front. Plant Sci. 2020, 11, 903. [Google Scholar] [CrossRef]
- Wakil, M.; Mbah, S.I. Screening antibiotics for the elimination of bacteria from in vitro yam plantlets. Au. J. Technol. 2012, 16, 7–18. [Google Scholar]
- Carey, S.B.; Payton, A.C.; McDaniel, S.F. A method for eliminating bacterial contamination from in vitro moss cultures. Appl. Plant Sci. 2015, 3, 1400086. [Google Scholar] [CrossRef]
- Nadha, H.K.; Salwan, R.; Kasana, R.C.; Anand, M.; Sood, A. Identification and elimination of bacterial contamination during in vitro propagation of Guadua angustifolia Kunth. Pharmacogn. Mag. 2012, 8, 93–97. [Google Scholar]
- Shweta, S.; Zuehlke, S.; Ramesha, B.T.; Priti, V.; Mohana Kumar, P.; Ravikanth, G.; Spiteller, M.; Vasudeva, R.; Uma Shaanker, R. Endophytic fungal strains of Fusarium solani, from Apodytes dimidiata E. Mey. ex Arn (Icacinaceae) produce camptothecin, 10-hydroxycamptothecin and 9-methoxycamptothecin. Phytochemistry 2010, 71, 117–122. [Google Scholar] [CrossRef]
- Panathula, C.S.; Mahadev, M.D.N.; Naidu, C.V. The stimulatory effects of the antimicrobial agents bavistin, cefotaxime and kanamycin on in vitro plant regeneration of Centella asiatica (L.)—An important antijaundice medicinal plant. Am. J. Plant Sci. 2014, 05, 279–285. [Google Scholar] [CrossRef]
- Preethi, D.; Sridhar, T.M.; Naidu, C.V. Effect of bavistin and silver thiosulphate on in vitro plant regeneration of Stevia rebaudiana. J. Phytol. 2011, 3, 74–77. [Google Scholar]
- Nagy, K.J.; Sule, S.; Sampaio, J.P. Apple tissue culture contamination by Rhodotorula spp.: Identification and prevention. Vitr. Cell. Dev. Biol. 2005, 41, 520–524. [Google Scholar] [CrossRef]
- Chauhan, P.; Singla, K.; Rajbhar, M.; Singh, A.; Das, N.; Kumar, K. A systematic review of conventional and advanced approaches for the control of plant viruses. J. Appl. Biol. Biotechnol. 2019, 7, 89–98. [Google Scholar]
- Panattoni, A.; Luvisi, A.; Triolo, E. Review. Elimination of viruses in plants: Twenty years of progress. Span. J. Agric. Res. 2013, 11, 173–188. [Google Scholar] [CrossRef]
- De Clercq, E. Antiviral drug discovery and development: Where chemistry meets with biomedicine. Antivir. Res. 2005, 67, 56–75. [Google Scholar] [CrossRef]
- Chinestra, S.C.; Curvetto, N.R.; Marinangeli, P.A. Production of virus-free plants of Lilium spp. from bulbs obtained in vitro and ex vitro. Sci. Hortic. 2015, 194, 304–312. [Google Scholar] [CrossRef]
- Jones, R.A.C. Control of plant virus diseases. Adv. Virus Res. 2006, 67, 205–244. [Google Scholar]
- O’Brien, P.A. Biological control of plant diseases. Australas. Plant Pathol. 2017, 46, 293–304. [Google Scholar] [CrossRef]
- Panattoni, A.; Triolo, E. Susceptibility of grapevine viruses to thermotherapy on in vitro collection of kober 5BB. Sci. Hortic. 2010, 125, 63–67. [Google Scholar] [CrossRef]
- Voinnet, O. RNA silencing as a plant immune system against viruses. Trends Genet. 2001, 17, 449–459. [Google Scholar] [CrossRef]
- Qu, F.; Ye, X.; Hou, G.; Sato, S.; Clemente, T.E.; Morris, T.J. RDR6 has a broad-spectrum but temperature-dependent antiviral defense role in Nicotiana benthamiana. J. Virol. 2005, 79, 15209–15217. [Google Scholar] [CrossRef]
- Szittya, G.; Silhavy, D.; Molnár, A.; Havelda, Z.; Lovas, Á.; Lakatos, L.; Bánfalvi, Z.; Burgyán, J. Low temperature inhibits RNA silencing-mediated defence by the control of SiRNA generation. EMBO J. 2003, 22, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Chellappan, P.; Vanitharani, R.; Ogbe, F.; Fauquet, C.M. Effect of temperature on geminivirus-induced RNA silencing in plants. Plant Physiol. 2005, 138, 1828–1841. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.; Pant, M.; Rani, A. The who’s who of plant viruses: A cognitive approach. Asian J. Pharm. Clin. Res. 2015, 8, 60–68. [Google Scholar]
- Pathirana, R.; McLachlan, A.; Hedderley, D.; Carra, A.; Carimi, F.; Panis, B. Removal of leafroll viruses from infected grapevine plants by droplet vitrification. Acta Hortic. 2015, 1083, 491–498. [Google Scholar] [CrossRef]
- Weber, B.N.; Witherell, R.A.; Charkowski, A.O. Low-cost potato tissue culture with microwave and bleach media preparation and sterilization. Am. J. Potato Res. 2015, 92, 128–137. [Google Scholar] [CrossRef]
- Brondani, G.E.; de Oliveira, L.S.; Bergonci, T.; Brondani, A.E.; França, F.A.M.; da Silva, A.L.L.; Gonçalves, A.N. Chemical sterilization of culture medium: A low cost alternative to in vitro establishment of plants. Sci. For. 2013, 41, 257–264. [Google Scholar]
- Peiris, S.E.; de Silva, E.D.U.D.; Edussuriya, M.; Attanayake, A.M.U.R.K.; Peiris, B.C.N. CSUP technique: A low cost sterilization method using sodium hypochlorite to replace the use of expensive equipment in micropropagation. J. Natl. Sci. Found. 2012, 40, 49–54. [Google Scholar] [CrossRef]
- Luna, C.; Acevedo, R.; Collavino, M.; González, A.; Mroginski, L.; Sansberro, P. Endophytic bacteria from Ilex paraguariensis shoot cultures: Localization, characterization, and response to isothiazolone biocides. Vitr. Cell. Dev. Biol. 2013, 49, 727–738. [Google Scholar] [CrossRef]
- Williams, T.M. The mechanism of action of isothiazolone biocides. Power Plant Chem. 2006, 9, 14–22. [Google Scholar]
- Helaly, M.N.; El-Metwally, M.A.; El-Hoseiny, H.; Omar, S.A.; El-Sheery, N.I. Effect of nanoparticles on biological contamination of in vitro cultures and organogenic regeneration of banana. Aust. J. Crop Sci. 2014, 8, 612–624. [Google Scholar]
- Abdi, G.; Salehi, H.; Khosh-Khui, M. Nano silver: A novel nanomaterial for removal of bacterial contaminants in valerian (Valeriana officinalis L.) tissue culture. Acta Physiol. Plant 2008, 30, 709–714. [Google Scholar] [CrossRef]
- Habash, M.B.; Park, A.J.; Vis, E.C.; Harris, R.J.; Khursigara, C.M. Synergy of silver nanoparticles and aztreonam against Pseudomonas aeruginosa PAO1 biofilms. Antimicrob Agents Chemother 2014, 58, 18–30. [Google Scholar] [CrossRef]
- Singh, B.R.; Singh, B.N.; Singh, A.; Khan, W.; Naqvi, A.H.; Singh, H.B. Mycofabricated biosilver nanoparticles interrupt Pseudomonas aeruginosa quorum sensing systems. Sci. Rep. 2015, 5, 13719. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.S.; Ryu, D.S.; Choi, S.J.; Lee, D.S. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Solgi, M.; Shahrjerdi, I. Essential oil as an alternative to chemical antimicrobial agent for the culture of strawberry in vitro. J. Hortic. 2016, 20, 99–106. [Google Scholar]
- Sharma, Y.; Bhardwaj, M.; Nagar, A.; Bhagat, N. Isolation and characterization of mdr bacteria from in vitro culture of Bacopa monniera and supplementation of natural extracts to control bacterial contamination. Int. J. Pharm. Sci. 2016, 8, 102–107. [Google Scholar] [CrossRef]
- Jasim, N.S.; Salih, A.M.; Ati, M.A. Evaluating the efficiency of plants essential oils against common fungal contamination affecting tissue culture of date palms (Phoenix dactylifera L.) by in vitro culture. Res. J. Chem. Environ. 2021, 25, 40–45. [Google Scholar]
- Min, T.; Hwarari, D.; Li, D.; Movahedi, A.; Yang, L. CRISPR-based genome editing and its applications in woody plants. Int. J. Mol. Sci. 2022, 23, 10175. [Google Scholar] [CrossRef]
- Zaynab, M.; Sharif, Y.; Fatima, M.; Afzal, M.Z.; Aslam, M.M.; Raza, M.F.; Anwar, M.; Raza, M.A.; Sajjad, N.; Yang, X. CRISPR/Cas9 to generate plant immunity against pathogen. Microb. Pathog. 2020, 141, 103996. [Google Scholar] [CrossRef]
- Boubakri, H. Recent progress in CRISPR/Cas9-based genome editing for enhancing plant disease resistance. Gene 2023, 866, 147334. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Pooja; Yadav, S.K. CRISPR-cas for genome editing: Classification, mechanism, designing and applications. Int. J. Biol. Macromol. 2023, 238, 124054. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).