Managing Lethal Browning and Microbial Contamination in Musa spp. Tissue Culture: Synthesis and Perspectives
Abstract
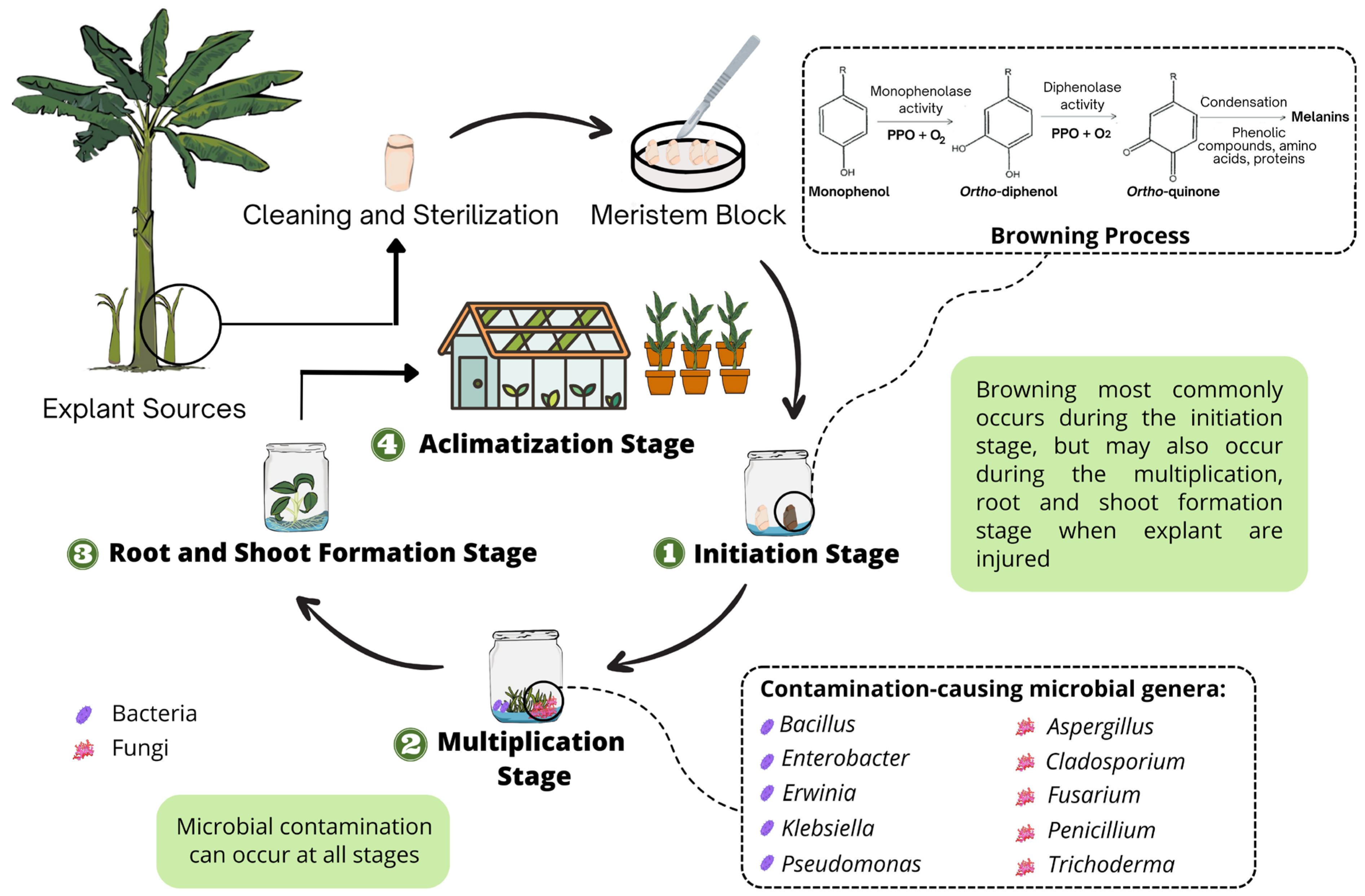
:1. Introduction
2. The Causal Agents of Browning in Musa spp. Propagation
3. Microbial Contamination in Musa spp. Tissue Culture
3.1. Bacterial Contamination
3.2. Fungal Contamination
4. Managing Browning in Musa spp. Tissue Culture
4.1. Pre-Soaking Explants in Anti-Browning Solutions
4.2. Incorporation of Anti-Browning into Growing Media
| Anti-Browning | Concentration | Plant Species | Explant Types | References |
|---|---|---|---|---|
| Ascorbic Acid | 50 mg/L | Cavendish cv. Formosa | Shoot tip | [20] |
| Ascorbic Acid | 15 mL/mL | Musa spp. | Aux bud | [73] |
| Citric Acid | 150 mg/L | Musa spp. | Shoot tip | [75] |
| Ascorbic Acid | 100–200 mg/L | Musa spp. cv. Mzuzu | Shoot tip | [21] |
| Activated Charcoal | 1.5 g/L | Musa spp. cv. Grand Naine | Shoot tip | [17] |
| Lime Peel Extract | 300 mg/L | M. paradisiaca cv. Kepok Tanjung | Shoot tip | [84] |
4.3. Manipulating Cultural Practices
5. Control of Contamination in Musa spp. Tissue Culture
5.1. Chemotherapy
5.2. Thermotherapy
5.3. Cryotherapy
5.4. Use of Disinfectants, Antiseptics, and Nanoparticles
6. Challenges and Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soorianathasundaram, K.; Narayana, C.K.; Paliyath, G. Bananas and plantains. Encycl. Food Health 2015, 320–327. [Google Scholar] [CrossRef]
- Israeli, Y.; Lahav, E. Banana. Encycl. App. Plant Sci. 2016, 3, 363–381. [Google Scholar]
- FAO. Banana Facts and Figures. Available online: https://www.fao.org/economic/est/est-commodities/oilcrops/bananas/banana-facts/en/#.ZBbqy-xBw1K (accessed on 19 March 2023).
- El-Sayed, I.M.; Salama, W.H.; Salim, R.G.; Taha, L.S. Relevance of nanoparticles on micropropagation, antioxidant activity and molecular characterization of Sequoia sempervirens L. Plant. Jordan J. Biol. Sci. 2021, 14, 374–382. [Google Scholar]
- Mose, W.; Daryono, B.S.; Indrianto, A.; Purwantoro, A.; Semiarti, E. Direct somatic embryogenesis and regeneration of an indonesian orchid Phalaenopsis amabilis (L.) Blume under a variety of plant growth regulators, light regime, and organic substances. Jordan J. Biol. Sci. 2020, 13, 509–518. [Google Scholar]
- Agbadje, E.T.A.E.; Agbidinoukoun, A.; Zandjanakou-Tachin, M.; Cacaï, G.T.H.; Ahanhanzo, C. Mass production of bananas and plantains (Musa spp.) plantlets through in vitro tissue culture partway: A review. Eur. J. Biol. Biotech. 2021, 2, 4. [Google Scholar] [CrossRef]
- Ferdous, M.H.; Masum Billah, A.A.; Mehraj, H.; Taufique, T.; Jamal Uddin, A.F.M. BAP and IBA pulsing for in vitro multiplication of banana cultivars through shoot-tip culture. J. Biosci. Agric. Res. 2015, 3, 87–95. [Google Scholar] [CrossRef]
- Yang, J.; Bao, J.; Lu, X.; Zhang, X.; Tian, P.; Shi, X.; Li, S.; Ma, S. Transcriptomic analysis of the effects of melatonin on genes potentially related to the browning of broccoli (Brassica oleracea L. var. Italica Planch) hairy roots. Plant Growth Regul. 2022, 98, 557–567. [Google Scholar] [CrossRef]
- Chen, G.; Chen, D.; Wang, T.; Xu, C.; Li, L. Analysis of the proteins related to browning in leaf culture of Phalaenopsis. Sci. Hortic. 2012, 141, 17–22. [Google Scholar] [CrossRef]
- Animasaun, D.A.; Nnamdi, C.D.; Ipinmoroti, O.I.; Oyedeji, S.; Olonya, E.A.; Krishnamurthy, R.; Morakinyo, J.A. Molecular identification and phylogenetic analysis of fungi contaminants associated with in vitro cultured banana based on ITS region sequence. Hayati 2022, 29, 288–300. [Google Scholar] [CrossRef]
- Hrynets, Y.; Bhattacherjee, A.; Betti, M. Nonenzymatic browning reactions: Overview. In Reference Module in Food Science; Elsevier: Edmonton, AB, Canada, 2018; Volume 2, pp. 233–244. [Google Scholar]
- Wu, T.; Li, J.; Zhang, J.; Lin, M.; Wu, Z.; Cai, X.; Xiang, W.; Tan, S.; Zhang, Z. Graphene oxide inhibits the lethal browning of Cymbidium sinense by reducing activities of enzymes. J. Plant Biotechnol. Microbiol. 2018, 1, 11–20. [Google Scholar]
- Hamdeni, I.; Slim, S.; Sanaa, A.; Louhaichi, M.; Boulila, A.; Bettaieb, T. Rosemary essential oil enhances culture establishment and inhibits contamination and enzymatic browning: Applications for in vitro propagation of Aloe vera L. S. Afr. J. Bot. 2022, 147, 1199–1205. [Google Scholar] [CrossRef]
- Cobrado, J.; Fernandez, A. Common fungi contamination affecting tissue-cultured abaca (Musa textiles Nee) during initial stage of micropropagation. Asian Res. J. Agric. 2016, 1, 1–7. [Google Scholar] [CrossRef]
- El-Banna, A.N.; El-Mahrouk, M.E.; Dewir, Y.H.; Farid, M.A.; Elyazid, D.M.A.; Schumacher, H.M. Endophytic bacteria in banana in vitro cultures: Molecular identification, antibiotic susceptibility, and plant survival. Horticulturae 2021, 7, 526. [Google Scholar] [CrossRef]
- Xu, C.; Ru, Z.; Li, L.; Zeng, B.; Huang, J.; Huang, W.; Hu, O. The effects of polyphenol oxidase and cycloheximide on the early stage of browning in Phalaenopsis explants. Hortic. Plant. J. 2015, 1, 172–180. [Google Scholar]
- Safwat, G.; Abdul-Rahman, F.; Sharbasy, A.S. The effect of some antioxidants on blackening and growth of in vitro culture of banana (Musa spp. cv. Grand Naine). J. Genet. Cytol 2015, 44, 47–59. [Google Scholar]
- Ray, S.S.; Ali, N. Biotic contamination and possible ways of sterilization: A review with reference to bamboo micropropagation. Braz. Arch. Biol. Technol. 2017, 60, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Chikezie, U.N.Y. Effect of ascorbic acid on blackening and sprouting of Musa spp. shoot tips. J. Biotechnol. Bioinform. 2012, 2, 11–17. [Google Scholar]
- Ko, W.H.; Su, C.C.; Chen, C.L.; Chao, C.P. Control of lethal browning of tissue culture plantlets of cavendish banana cv. Formosana with ascorbic acid. Plant Cell Tissue Organ Cult. 2009, 96, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Ngomuo, M.; Mneney, E.; Ndakidemi, P. Control of lethal browning by using ascorbic acid on shoot tip cultures of a local Musa spp. (Banana) cv. Mzuzu in Tanzania. Afr. J. Biotechnol. 2014, 13, 1721–1725. [Google Scholar]
- Habiba, U.; Reza, S.; Lal Saha, M.; Khan, M.R.; Hadiuzzaman, S. Endogenous bacterial contamination during in vitro culture of table banana: Identification and prevention. Plant Tissue Cult. Biotechnol. 2002, 12, 117–124. [Google Scholar]
- Msogoya, T.; Kanyagha, H.; Mutigitu, J.; Kulebelwa, M.; Mamiro, D. Identification and management of microbial contaminants of banana in vitro cultures. J. Appl. Biosci. 2012, 55, 3987–3994. [Google Scholar]
- Hamill, S.D.; Rames, E. An effective indexing method for banana tissue culture provides long-term freedom from bacterial contamination. Acta. Hortic. 2018, 1205, 741–747. [Google Scholar] [CrossRef]
- Kithaku, E.; Muigai, A.T.; Neondo, J.; Mweu, C. Screening of fungal contaminants in banana tissue cultures in Jkuat, Kenya. Afr. J. Microbiol. Res. 2019, 13, 675–688. [Google Scholar]
- Mokbel, S.A.; Khalil, A.A.; El-Shazly, M.A. Efficiency of eugenol oil nanoemulsion against banana bunchy top virus and contamination with fungi in plant tissue culture. Arab J. Biotech. 2017, 20, 33–50. [Google Scholar]
- Kapadia, C.; Patel, N. Sequential sterilization of banana (Musa spp.) sucker tip reducing microbial contamination with highest establishment percentage. Bangladesh J. Bot. 2021, 50, 1151–1158. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Gao, Y.; Han, Y.; Wu, X. Path analysis of non-enzymatic browning in Dongbei suancai during storage caused by different fermentation conditions. Food Chem. 2021, 335, 127620. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, H.; Liu, K.; Li, L.; Yang, J.; An, X.; Li, P.; Yun, L.; Zhang, Z. The role of JrPPOs in the browning of walnut explants. BMC Plant Biol. 2021, 21, 9. [Google Scholar] [CrossRef]
- He, Y.; Guo, X.; Lu, R.; Niu, B.; Pasapula, V.; Hou, P.; Cai, F.; Xu, Y.; Chen, F. Changes in morphology and biochemical indices in browning callus derived from Jatropha curcas hypocotyls. Plant Cell Tissue Organ Cult. 2009, 98, 11–17. [Google Scholar] [CrossRef]
- Tang, W.; Newton, R.J. Increase of polyphenol oxidase and decrease of polyamines correlate with tissue browning in virginia pine (Pinus virginiana Mill.). Plant Sci. 2004, 167, 621–628. [Google Scholar] [CrossRef]
- Nadafzadeh, M.; Abdanan Mehdizadeh, S.; Soltanikazemi, M. Development of computer vision system to predict peroxidase and polyphenol oxidase enzymes to evaluate the process of banana peel browning using genetic programming modeling. Sci. Hortic. 2018, 231, 201–209. [Google Scholar] [CrossRef]
- Ru, Z.; Lai, Y.; Xu, C.; Li, L. Polyphenol oxidase (PPO) in early stage of browning of Phalaenopsis leaf explants. J. Agric. Sci. 2013, 5, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.M.P.; Saxena, P.K. Inhibition of phenylpropanoid biosynthesis in Artemisia annua L.: A novel approach to reduce oxidative browning in plant tissue culture. PLoS ONE 2013, 8, e76802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvarajan, E.; Veena, R.; Manoj, K.N. Polyphenol oxidase, beyond enzyme browning. In Microbial Bioprospecting for Sustainable Development; Singh, J., Sharma, D., Kumar, G., Sharma, N.R., Eds.; Springer: Singapore, 2018; pp. 203–222. [Google Scholar]
- Rayan, A.; Morsy, N. Thermal inactivation kinetics of peroxidase and polyphenol oxidase from pomegranate arils (Punica granatum L. cv. Wonderful). J. Food Biochem. 2020, 44, e13428. [Google Scholar] [CrossRef] [PubMed]
- Araji, S.; Grammer, T.A.; Gertzen, R.; Anderson, S.D.; Mikulic-Petkovsek, M.; Veberic, R.; Phu, M.L.; Solar, A.; Leslie, C.A.; Dandekar, A.M. Novel roles for the polyphenol oxidase enzyme in secondary metabolism and the regulation of cell death in walnut. Plant Physiol. 2014, 164, 1191–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Y.; Jiang, Y.; Wang, C.; Song, M.; Liu, Y.; Liu, J.; Jiang, Z.; Yang, Y.; Ren, X.; Ding, Y. Metabolomic and transcriptomic analysis reveal high solar irradiance inhibited the melanin formation in persimmon fruit peel. Environ. Exp. Bot. 2023, 207, 105218. [Google Scholar] [CrossRef]
- Sharafi, Y.; Aghdam, M.S.; Luo, Z.; Jannatizadeh, A.; Razavi, F.; Fard, J.R.; Farmani, B. Melatonin treatment promotes endogenous melatonin accumulation and triggers GABA shunt pathway activity in tomato fruits during cold storage. Sci. Hortic. 2019, 254, 222–227. [Google Scholar] [CrossRef]
- Shekhawat, U.K.S.; Ganapathi, T.R. Transgenic banana plants overexpressing musabZIP53 display severe growth retardation with enhanced sucrose and polyphenol oxidase activity. Plant Cell Tissue Organ Cult. 2014, 116, 387–402. [Google Scholar] [CrossRef]
- Tran, L.T.; Taylor, J.S.; Constabel, C.P. The polyphenol oxidase gene family in land plants: Lineage-specific duplication and expansion. BMC Genom. 2012, 13, 395. [Google Scholar] [CrossRef] [Green Version]
- Gooding, P.S.; Bird, C.; Robinson, S.P. Molecular cloning and characterisation of banana fruit polyphenol oxidase. Planta 2001, 213, 748–757. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Zeng, J.; Yun, Z.; Liu, Y.; Qu, H.; Jiang, Y.; Duan, X.; Xia, R. MicroRNA528, a hub regulator modulating ROS homeostasis via targeting of a diverse set of genes encoding copper-containing proteins in monocots. New Phytol. 2020, 225, 385–399. [Google Scholar] [CrossRef]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.; Pavan, S.; Montemurro, C. Polyphenol oxidases in crops: Biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 2017, 377, 377. [Google Scholar] [CrossRef] [Green Version]
- Eziashi, E.I.; Asemota, O.; Okwuagwu, C.O.; Eke, C.R.; Chidi, N.I.; Oruade-Dimaro, E.A. Screening sterilizing agents and antibiotics for the elimination of bacterial contaminants from oil palm explants for plant tissue culture. Eur. J. Exp. Biol. 2014, 4, 111–115. [Google Scholar]
- Rawal, K.; Keharia, H. Prevention of fungal contamination in plant tissue culture using cyclic lipopeptides secreted by Bacillus amyloliquefaciens AB30a. Plant Tissue Cult. Biotechnol. 2019, 29, 111–119. [Google Scholar] [CrossRef]
- Omamor, I.B.; Asemota, A.O.; Eke, C.R.; Eziashi, E.I. Fungal contaminants of the oil palm tissue culture in nigerian institute for oil palm research (NIFOR). Afr. J. Agric. Res. 2007, 2, 534–537. [Google Scholar]
- Abass, M.H. Microbial contaminants of date palm (Phoenix dactylifera L.) in iraqi tissue culture laboratories. Emir. J. Food Agric. 2013, 25, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Altan, F.; Bürün, B.; Şahin, N. Fungal contaminants observed during micropropagation of Lilium candidum L. and the effect of chemotherapeutic substances applied after sterilization. Afr. J. Biotechnol. 2010, 9, 991–995. [Google Scholar]
- Doni, F.; Miranti, M.; Mispan, M.S.; Mohamed, Z.; Uphoff, N. Multi-omics approaches for deciphering the microbial modulation of plants’ genetic potentials: What’s known and what’s next? Rhizosphere 2022, 24, 100613. [Google Scholar] [CrossRef]
- Guan, S.H.; Sattler, I.; Lin, W.H.; Guo, D.A.; Grabley, S. p-Aminoacetophenonic acids produced by a mangrove endophyte: Streptomyces griseus subsp. J. Nat. Prod. 2005, 68, 1198–1200. [Google Scholar] [CrossRef]
- Ting, A.S.Y.; Meon, S.; Kadir, J.; Radu, S.; Singh, G. Endophytic microorganisms as potential growth promoters of banana. BioControl 2008, 53, 541–553. [Google Scholar] [CrossRef]
- Thomas, P.; Swarna, G.K.; Patil, P.; Rawal, R.D. Ubiquitous presence of normally non-culturable endophytic bacteria in field shoot-tips of banana and their gradual activation to quiescent cultivable form in tissue cultures. Plant Cell Tissue Organ Cult. 2008, 93, 39–54. [Google Scholar] [CrossRef]
- Souza, S.A.; Xavier, A.A.; Costa, M.R.; Cardoso, A.M.S.; Pereira, M.C.T.; Nietsche, S. Endophytic bacterial diversity in banana “Prata Anã” (Musa spp.) toots. Genet. Mol. Biol. 2013, 36, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Caballero-Mellado, J.; Orozco, J.; Martínez-Romero, E. Diazotrophic bacteria associated with banana (Musa spp.). Plant Soil 2003, 257, 35–47. [Google Scholar] [CrossRef]
- van den Houwe, I.; Guns, J.; Swennen, R. Bacterial contamination in musa shoot tip cultures. Acta Hortic. 1998, 490, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Ganen, S.T.S.; Nietsche, S.; Pereira, M.C.T.; Reis, S.T.; Xavier, A.A.; Santos, T.M.; Fernandes, T.P. Microbial contamination in explants of banana cultivars “Galil 18” and “Tropical”. Acta Hortic. 2010, 829, 341–344. [Google Scholar] [CrossRef]
- Mng’omba, S.A.; Sileshi, G.; du Toit, E.S.; Akinnifesi, F.K. Efficacy and utilization of fungicides and other antibiotics for aseptic plant cultures. In Fungicides for Plant and Animal Diseases, 1st ed.; Dhanasekaran, D., Thajuddin, N., Panneerselvam, A., Eds.; InTech: Shanghai, China, 2012; pp. 245–254. [Google Scholar]
- Herman, E.B. Plant tissue culture contamination: Challenges and opportunities. Acta Hortic. 2017, 1155, 231–238. [Google Scholar] [CrossRef]
- Odutayo, O.I.; Amusa, N.A.; Okutade, O.O.; Ogunsanwo, Y.R. Determination of the sources of microbial contaminants of cultured plant tissues. Plant Pathol. J. 2007, 6, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.Z.; Shahinul Islam, S.M.; Chowdhury, A.N.; Subramaniam, S. Identification and prevention of microbial contaminants of potato culture in temporary immersion bioreactor (TIB) system. Malays. J. Microbiol. 2017, 13, 289–297. [Google Scholar]
- Zaragoza, O.; Rodrigues, M.L.; de Jesus, M.; Frases, S.; Dadachova, E.; Casadevall, A. The capsule of the fungal pathogen Cryptococcus neoformans. Adv. Appl. Microbiol. 2009, 68, 133–216. [Google Scholar]
- Compant, S.; Brader, G.; Muzammil, S.; Sessitsch, A.; Lebrihi, A.; Mathieu, F. Use of beneficial bacteria and their secondary metabolites to control grapevine pathogen diseases. BioControl 2013, 58, 435–455. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Liu, G.; Selvaraj, G.; Hughes, G.R.; Wei, Y. A secreted lipase encoded by LIP1 ss necessary for efficient use of saturated triglyceride lipids in Fusarium graminearum. Microbiology 2005, 151, 3911–3921. [Google Scholar] [CrossRef] [Green Version]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Hernández, M.L.; Snchez-Salinas, E.; Dantn Gonzlez, E.; Luisa, M. Pesticide biodegradation: Mechanisms, genetics and strategies to enhance the process. In Biodegradation—Life of Science, 1st ed.; Chamy, R., Ed.; InTechOpen: London, UK, 2013; pp. 251–287. [Google Scholar]
- Zhao, P.; Quan, C.; Wang, Y.; Wang, J.; Fan, S. Bacillus amyloliquefaciens Q-426 as a potential biocontrol agent against Fusarium oxysporum f. sp. Spinaciae. J. Basic Microbiol. 2014, 54, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.P.; Zhang, C.L.; Slater, A.; Madassery, J. Control of shoot necrosis and plant death during micropropagation of banana and plantains (Musa spp.). Plant Cell Tissue Organ Cult. 2007, 88, 51–59. [Google Scholar] [CrossRef]
- Bairu, M.W.; Stirk, W.A.; van Staden, J. Factors contributing to in vitro shoot-tip necrosis and their physiological interactions. Plant Cell Tissue Organ Cult. 2009, 98, 239–248. [Google Scholar] [CrossRef]
- Titov, S.; Bhowmik, S.K.; Mandal, A.; Alam, M.S.; Uddin, S.N. Control of phenolic compound secretion and effect of growth regulators for organ formation from Musa spp. cv. Kanthali floral bud explants. Am. J. Biochem. Biotechnol. 2006, 2, 97–104. [Google Scholar] [CrossRef]
- Onuoha, I.C.; Eze, C.J.; Unamba, C.I.N. In vitro prevention of browning in plantain culture. Online J. Biol. Sci. 2011, 11, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Ndakidemi, C.F.; Mneney, E.; Ndakidemi, P.A. Effects of ascorbic acid in controlling lethal browning in vitro culture of Brahylaena using nodal segments. Am. J. Plant Sci. 2014, 05, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Amente, G.; Chimdessa, E. Control of browning in plant tissue culture: A review. J. Sci. Agric. 2021, 5, 67–71. [Google Scholar] [CrossRef]
- Nathan, A.J.; Scobell, A. Banana cell and tissue culture—Review. Foreign Aff. 2012, 91, 1–12. [Google Scholar]
- Strosse, H.; van den Houwe, I.; Panis, B. Banana Cell and Tissue Culture—Review. In Banana Improvement: Cellular, Molecular Biology, and Induced Mutations, 1st ed.; Jain, S.M., Swennen, R., Eds.; Science Publisher Inc.: Enfield, CT, USA, 2004; Volume 49, pp. 1–12. [Google Scholar]
- Ssekiwoko, F.; Talengera, D.; Kiggundu, A.; Namutebi, M.K.; Karamura, E.; Kunert, K. In-vitro proliferation of Musa balbisiana improves with increased vitamin concentration and dark culturing. J. Appl. Biol. Biotechnol. 2014, 2, 1–7. [Google Scholar]
- Utley, W.S. Charcoal. In Encyclopedia of Toxicology, 2nd ed.; Wexler, P., Ed.; Elsevier: New York, NY, USA, 2005; pp. 505–519. [Google Scholar]
- Roy, S.; Das, P.; Sengupta, S.; Manna, S. Calcium impregnated activated charcoal: Optimization and efficiency for the treatment of fluoride containing solution in batch and fixed bed reactor. Process Saf. Environ. Prot. 2017, 109, 18–29. [Google Scholar] [CrossRef]
- Kassahun Bantte, D.S.; Feyissa, T. Effects of polyvinyl pyrrolidone and activated charcoal to control effect of phenolic oxidation on in vitro culture establishment stage of micropropagation of sugarcane (Saccharum officinarum L.). Adv. Crop Sci. Techn. 2015, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, D.A.; Barbosa, E.G.G.; Molinari, M.D.C.; Fuganti Pagliarini, R.; Marin, S.R.R.; Marin, D.R.; Mertz-Henning, L.M.; Nepomuceno, A.L. Activated charcoal added to tissue culture media increases genotype-dependent biomass production in soybean. Agr. Sci. Biotechnol. 2022, 8, 1–11. [Google Scholar] [CrossRef]
- North, J.J.; Ndakidemi, P.A.; Laubscher, C.P. Effects of antioxidants, plant growth regulators and wounding on phenolic compound excretion during micropropagation of Strelitzia reginae. Int. J. Phys. Sci. 2012, 7, 638–646. [Google Scholar] [CrossRef]
- Wirakarnain, S.; Hossain, A.B.M.S.; Chandran, S. Plantlet production through development of competent multiple meristem cultures from male inflorescence of banana, Musa acuminta cv. “Pisang Mas” (AA). Am. J. Biochem. Biotechnol. 2008, 4, 325–328. [Google Scholar]
- Husin, N.; Jalil, M.; Othman, R.Y.; Khalid, N. Enhancement of regeneration efficiency in banana (Musa acuminata cv. Berangan) by using proline and glutamine. Sci. Hortic. 2014, 168, 33–37. [Google Scholar] [CrossRef]
- Nurzaman, M.; Permadi, N.; Setiawati, T.; Hasan, R.; Irawati, Y.; Julaeha, E.; Herlina, T. DPPH free radical scavenging activity of Citrus aurantifolia Swingle peel extracts and their impact in inhibiting the browning of Musa paradisiaca L. var. Kepok Tanjung explants. Jordan J. Biol. Sci. 2022, 15, 771–777. [Google Scholar]
- Dobránszki, J.; Teixeira da Silva, J.A. Micropropagation of apple—A review. Biotechnol. Adv. 2010, 28, 462–488. [Google Scholar] [CrossRef]
- Ahmad, I.; Hussain, T.; Ashraf, I.; Nafees, M.; Rafay, M.; Iqbal, M. Lethal effects of secondary metabolites on plant tissue culture. Environ. Sci. 2013, 13, 539–547. [Google Scholar]
- Ochoa-Alejo, N.; Ramirez-Malagon, R. In vitro chili pepper biotechnology. Vitr. Cell. Dev. Biol. 2001, 37, 701–729. [Google Scholar] [CrossRef]
- Krishna, H.; Sairam, R.K.; Singh, S.K.; Patel, V.B.; Sharma, R.R.; Grover, M.; Nain, L.; Sachdev, A. Mango explant browning: Effect of ontogenic age, mycorrhization and pre-treatments. Sci. Hortic. 2008, 118, 132–138. [Google Scholar] [CrossRef]
- Buah, J.N.; Danso, E.; Taah, K.J.; Abole, E.A.; Bediako, E.A.; Asiedu, J.; Baidoo, R. The effects of different concentrations cytokinins on the in vitro multiplication of plantain (Musa sp.). Biotechnology 2010, 9, 343–347. [Google Scholar] [CrossRef] [Green Version]
- Nsofor, G.C. Conventional methods of controlling microbial contaminants in meristematic tissue cultures: A review. Niger. Agric. J. 2021, 52, 181–186. [Google Scholar]
- Izarra, M.L.; Panta, A.L.; Maza, C.R.; Zea, B.C.; Cruzado, J.; Gutarra, L.R.; Rivera, C.R.; Ellis, D.; Kreuze, J.F. Identification and control of latent bacteria in in vitro cultures of sweetpotato [Ipomoea batatas (L.) Lam]. Front. Plant Sci. 2020, 11, 903. [Google Scholar] [CrossRef]
- Wakil, M.; Mbah, S.I. Screening antibiotics for the elimination of bacteria from in vitro yam plantlets. Au. J. Technol. 2012, 16, 7–18. [Google Scholar]
- Carey, S.B.; Payton, A.C.; McDaniel, S.F. A method for eliminating bacterial contamination from in vitro moss cultures. Appl. Plant Sci. 2015, 3, 1400086. [Google Scholar] [CrossRef]
- Nadha, H.K.; Salwan, R.; Kasana, R.C.; Anand, M.; Sood, A. Identification and elimination of bacterial contamination during in vitro propagation of Guadua angustifolia Kunth. Pharmacogn. Mag. 2012, 8, 93–97. [Google Scholar]
- Shweta, S.; Zuehlke, S.; Ramesha, B.T.; Priti, V.; Mohana Kumar, P.; Ravikanth, G.; Spiteller, M.; Vasudeva, R.; Uma Shaanker, R. Endophytic fungal strains of Fusarium solani, from Apodytes dimidiata E. Mey. ex Arn (Icacinaceae) produce camptothecin, 10-hydroxycamptothecin and 9-methoxycamptothecin. Phytochemistry 2010, 71, 117–122. [Google Scholar] [CrossRef]
- Panathula, C.S.; Mahadev, M.D.N.; Naidu, C.V. The stimulatory effects of the antimicrobial agents bavistin, cefotaxime and kanamycin on in vitro plant regeneration of Centella asiatica (L.)—An important antijaundice medicinal plant. Am. J. Plant Sci. 2014, 05, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Preethi, D.; Sridhar, T.M.; Naidu, C.V. Effect of bavistin and silver thiosulphate on in vitro plant regeneration of Stevia rebaudiana. J. Phytol. 2011, 3, 74–77. [Google Scholar]
- Nagy, K.J.; Sule, S.; Sampaio, J.P. Apple tissue culture contamination by Rhodotorula spp.: Identification and prevention. Vitr. Cell. Dev. Biol. 2005, 41, 520–524. [Google Scholar] [CrossRef]
- Chauhan, P.; Singla, K.; Rajbhar, M.; Singh, A.; Das, N.; Kumar, K. A systematic review of conventional and advanced approaches for the control of plant viruses. J. Appl. Biol. Biotechnol. 2019, 7, 89–98. [Google Scholar]
- Panattoni, A.; Luvisi, A.; Triolo, E. Review. Elimination of viruses in plants: Twenty years of progress. Span. J. Agric. Res. 2013, 11, 173–188. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, E. Antiviral drug discovery and development: Where chemistry meets with biomedicine. Antivir. Res. 2005, 67, 56–75. [Google Scholar] [CrossRef]
- Chinestra, S.C.; Curvetto, N.R.; Marinangeli, P.A. Production of virus-free plants of Lilium spp. from bulbs obtained in vitro and ex vitro. Sci. Hortic. 2015, 194, 304–312. [Google Scholar] [CrossRef]
- Jones, R.A.C. Control of plant virus diseases. Adv. Virus Res. 2006, 67, 205–244. [Google Scholar]
- O’Brien, P.A. Biological control of plant diseases. Australas. Plant Pathol. 2017, 46, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Panattoni, A.; Triolo, E. Susceptibility of grapevine viruses to thermotherapy on in vitro collection of kober 5BB. Sci. Hortic. 2010, 125, 63–67. [Google Scholar] [CrossRef]
- Voinnet, O. RNA silencing as a plant immune system against viruses. Trends Genet. 2001, 17, 449–459. [Google Scholar] [CrossRef]
- Qu, F.; Ye, X.; Hou, G.; Sato, S.; Clemente, T.E.; Morris, T.J. RDR6 has a broad-spectrum but temperature-dependent antiviral defense role in Nicotiana benthamiana. J. Virol. 2005, 79, 15209–15217. [Google Scholar] [CrossRef] [Green Version]
- Szittya, G.; Silhavy, D.; Molnár, A.; Havelda, Z.; Lovas, Á.; Lakatos, L.; Bánfalvi, Z.; Burgyán, J. Low temperature inhibits RNA silencing-mediated defence by the control of SiRNA generation. EMBO J. 2003, 22, 633–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chellappan, P.; Vanitharani, R.; Ogbe, F.; Fauquet, C.M. Effect of temperature on geminivirus-induced RNA silencing in plants. Plant Physiol. 2005, 138, 1828–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal, A.; Pant, M.; Rani, A. The who’s who of plant viruses: A cognitive approach. Asian J. Pharm. Clin. Res. 2015, 8, 60–68. [Google Scholar]
- Pathirana, R.; McLachlan, A.; Hedderley, D.; Carra, A.; Carimi, F.; Panis, B. Removal of leafroll viruses from infected grapevine plants by droplet vitrification. Acta Hortic. 2015, 1083, 491–498. [Google Scholar] [CrossRef]
- Weber, B.N.; Witherell, R.A.; Charkowski, A.O. Low-cost potato tissue culture with microwave and bleach media preparation and sterilization. Am. J. Potato Res. 2015, 92, 128–137. [Google Scholar] [CrossRef]
- Brondani, G.E.; de Oliveira, L.S.; Bergonci, T.; Brondani, A.E.; França, F.A.M.; da Silva, A.L.L.; Gonçalves, A.N. Chemical sterilization of culture medium: A low cost alternative to in vitro establishment of plants. Sci. For. 2013, 41, 257–264. [Google Scholar]
- Peiris, S.E.; de Silva, E.D.U.D.; Edussuriya, M.; Attanayake, A.M.U.R.K.; Peiris, B.C.N. CSUP technique: A low cost sterilization method using sodium hypochlorite to replace the use of expensive equipment in micropropagation. J. Natl. Sci. Found. 2012, 40, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Luna, C.; Acevedo, R.; Collavino, M.; González, A.; Mroginski, L.; Sansberro, P. Endophytic bacteria from Ilex paraguariensis shoot cultures: Localization, characterization, and response to isothiazolone biocides. Vitr. Cell. Dev. Biol. 2013, 49, 727–738. [Google Scholar] [CrossRef]
- Williams, T.M. The mechanism of action of isothiazolone biocides. Power Plant Chem. 2006, 9, 14–22. [Google Scholar]
- Helaly, M.N.; El-Metwally, M.A.; El-Hoseiny, H.; Omar, S.A.; El-Sheery, N.I. Effect of nanoparticles on biological contamination of in vitro cultures and organogenic regeneration of banana. Aust. J. Crop Sci. 2014, 8, 612–624. [Google Scholar]
- Abdi, G.; Salehi, H.; Khosh-Khui, M. Nano silver: A novel nanomaterial for removal of bacterial contaminants in valerian (Valeriana officinalis L.) tissue culture. Acta Physiol. Plant 2008, 30, 709–714. [Google Scholar] [CrossRef]
- Habash, M.B.; Park, A.J.; Vis, E.C.; Harris, R.J.; Khursigara, C.M. Synergy of silver nanoparticles and aztreonam against Pseudomonas aeruginosa PAO1 biofilms. Antimicrob Agents Chemother 2014, 58, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.R.; Singh, B.N.; Singh, A.; Khan, W.; Naqvi, A.H.; Singh, H.B. Mycofabricated biosilver nanoparticles interrupt Pseudomonas aeruginosa quorum sensing systems. Sci. Rep. 2015, 5, 13719. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Lee, H.S.; Ryu, D.S.; Choi, S.J.; Lee, D.S. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [Green Version]
- Taghizadeh, M.; Solgi, M.; Shahrjerdi, I. Essential oil as an alternative to chemical antimicrobial agent for the culture of strawberry in vitro. J. Hortic. 2016, 20, 99–106. [Google Scholar]
- Sharma, Y.; Bhardwaj, M.; Nagar, A.; Bhagat, N. Isolation and characterization of mdr bacteria from in vitro culture of Bacopa monniera and supplementation of natural extracts to control bacterial contamination. Int. J. Pharm. Sci. 2016, 8, 102–107. [Google Scholar] [CrossRef]
- Jasim, N.S.; Salih, A.M.; Ati, M.A. Evaluating the efficiency of plants essential oils against common fungal contamination affecting tissue culture of date palms (Phoenix dactylifera L.) by in vitro culture. Res. J. Chem. Environ. 2021, 25, 40–45. [Google Scholar]
- Min, T.; Hwarari, D.; Li, D.; Movahedi, A.; Yang, L. CRISPR-based genome editing and its applications in woody plants. Int. J. Mol. Sci. 2022, 23, 10175. [Google Scholar] [CrossRef]
- Zaynab, M.; Sharif, Y.; Fatima, M.; Afzal, M.Z.; Aslam, M.M.; Raza, M.F.; Anwar, M.; Raza, M.A.; Sajjad, N.; Yang, X. CRISPR/Cas9 to generate plant immunity against pathogen. Microb. Pathog. 2020, 141, 103996. [Google Scholar] [CrossRef]
- Boubakri, H. Recent progress in CRISPR/Cas9-based genome editing for enhancing plant disease resistance. Gene 2023, 866, 147334. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Pooja; Yadav, S.K. CRISPR-cas for genome editing: Classification, mechanism, designing and applications. Int. J. Biol. Macromol. 2023, 238, 124054. [Google Scholar] [CrossRef] [PubMed]

| No. | Bacteria Species | Explants | Refs. |
|---|---|---|---|
| 1 | Acinetobacter baylyi | Cavendish AAA | [24] |
| 2 | Bacillus flexus | Cavendish AAA | [24] |
| 3 | Bacillus licheniformis | Musa spp. | [6] |
| 4 | Bacillus megaterium | Cavendish AAA | [24] |
| 5 | Bacillus pumilus | Musa paradisiaca L. Grand Naine; Cavendish AAA | [15,24] |
| 6 | Bacillus subtilis | Musa paradisiaca L. Grand Naine; Musa spp. | [6,15] |
| 7 | Corynebacterium spp. | Musa spp. | [6] |
| 8 | Enterobacter cloacae | Galil 18 and Tropical | [57] |
| 9 | Enterobacter gergoviae | Galil 18 and Tropical | [57] |
| 10 | Erwinia spp. | Musa spp. | [6] |
| 11 | Erwinia chrysanthemi | Galil 18 and Tropical | [57] |
| 12 | Geobacillus stearothermophilus | Musa paradisiaca L. Grand Naine | [15] |
| 13 | Herbaspirillum seropedicae | Cavendish AAA | [24] |
| 14 | Herbaspirillum rubrisubalbicans | Cavendish AAA | [24] |
| 15 | Klebsiella pneumoniae | Galil 18 and Tropical | [57] |
| 16 | Paenibacillus spp. | Musa paradisiaca L. Grand Naine | [15] |
| 17 | Paenibacillus polymyxa | Galil 18 and Tropical | [57] |
| 18 | Pseudomonas fulva | Cavendish AAA | [24] |
| 19 | Pseudomonas huttiensi | Galil 18 and Tropical | [57] |
| 20 | Pseudomonas putida | Cavendish AAA | [24] |
| 21 | Pseudomonas syringae | Musa spp. | [6] |
| 22 | Rhizobium radiobacter | Cavendish AAA | [24] |
| 23 | Rhizobium etli | Cavendish AAA | [24] |
| 24 | Salmonella spp. | Galil 18 and Tropical | [57] |
| No. | Fungi Species | Explant | Refs. |
|---|---|---|---|
| 1 | Alterneria tenius | Musa spp. | [6] |
| 2 | Aspergillus spp. | Musa textiles Nee; Musa spp. | [14,25] |
| 3 | Aspergillus awamori | Banana FIA 9 | [10] |
| 4 | Aspergillus flavus | Banana FIA 9; Musa spp. cv. Grand Nain | [10,26] |
| 5 | Aspergillus fumigatus | Musa spp. | [6] |
| 6 | Aspergillus niger | Musa spp.; Banana FIA 9; Musa spp. cv. Grand Nain | [6,10,26] |
| 7 | Aspergillus parvissclerotigenus | Banana FIA 9 | [10] |
| 8 | Chrysosporium spp. | Musa textiles Nee | [14] |
| 9 | Cladosporium spp. | Musa spp. | [25] |
| 10 | Cladosporium tenuissimum | Banana FIA 9 | [10] |
| 11 | Cunninghamella spp. | Musa spp. | [25] |
| 12 | Fusarium spp. | Musa spp. | [25] |
| 13 | Fusarium chlamydosporum | Banana FIA 9 | [10] |
| 14 | Fusarium culmorum | Musa spp. | [6] |
| 15 | Penincillium spp. | Musa spp. | [25] |
| 16 | Penincillium citrinum | Banana FIA 9 | [10] |
| 17 | Penincillium expansum | Musa spp. cv. Grand Nain | [26] |
| 18 | Trichoderma viride | Banana FIA 9 | [10] |
| Anti-Browning | Concentration | Plant Species | Explant Type | References |
|---|---|---|---|---|
| Potassium Citrate and Citrate | 0.125% | Musa spp. cv. Kanthali | Shoot tip | [70] |
| Potassium Citrate and Citrate | 0.1–0.5 mg/mL | M. paradisiaca | Aux bud | [71] |
| Ascorbic Acid | 1.2 g/L | Musa spp. cv. Mzuzu | Shoot tip | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Permadi, N.; Nurzaman, M.; Alhasnawi, A.N.; Doni, F.; Julaeha, E. Managing Lethal Browning and Microbial Contamination in Musa spp. Tissue Culture: Synthesis and Perspectives. Horticulturae 2023, 9, 453. https://doi.org/10.3390/horticulturae9040453
Permadi N, Nurzaman M, Alhasnawi AN, Doni F, Julaeha E. Managing Lethal Browning and Microbial Contamination in Musa spp. Tissue Culture: Synthesis and Perspectives. Horticulturae. 2023; 9(4):453. https://doi.org/10.3390/horticulturae9040453
Chicago/Turabian StylePermadi, Nandang, Mohamad Nurzaman, Arshad Naji Alhasnawi, Febri Doni, and Euis Julaeha. 2023. "Managing Lethal Browning and Microbial Contamination in Musa spp. Tissue Culture: Synthesis and Perspectives" Horticulturae 9, no. 4: 453. https://doi.org/10.3390/horticulturae9040453
APA StylePermadi, N., Nurzaman, M., Alhasnawi, A. N., Doni, F., & Julaeha, E. (2023). Managing Lethal Browning and Microbial Contamination in Musa spp. Tissue Culture: Synthesis and Perspectives. Horticulturae, 9(4), 453. https://doi.org/10.3390/horticulturae9040453










