Effect of Nisin on the Quality and Antioxidant Activity of Fresh-Cut Pumpkins (Cucurbita moschata Duch.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Processing and Treatments of Fresh-Cut Pumpkins
2.2. Weight Loss
2.3. Firmness
2.4. Color
2.5. Respiration Intensity
2.6. Superoxide Anion (O2−·) Production Rate
2.7. H2O2 Content
2.8. Superoxide Dismutase Activity (SOD)
2.9. Catalase Activity (CAT)
2.10. Ascorbate Peroxidase Activity (APX)
2.11. Glutathione Reductase Activity (GR)
2.12. Reduced Glutathione Content (GSH)
2.13. Ascorbic Acid Content (AsA)
2.14. DPPH, ABTS Free Radical Scavenging Rate
2.15. Total Antioxidant Capacity (T-AOC)
2.16. Statistical Analysis
3. Results
3.1. Weight Loss and Firmness
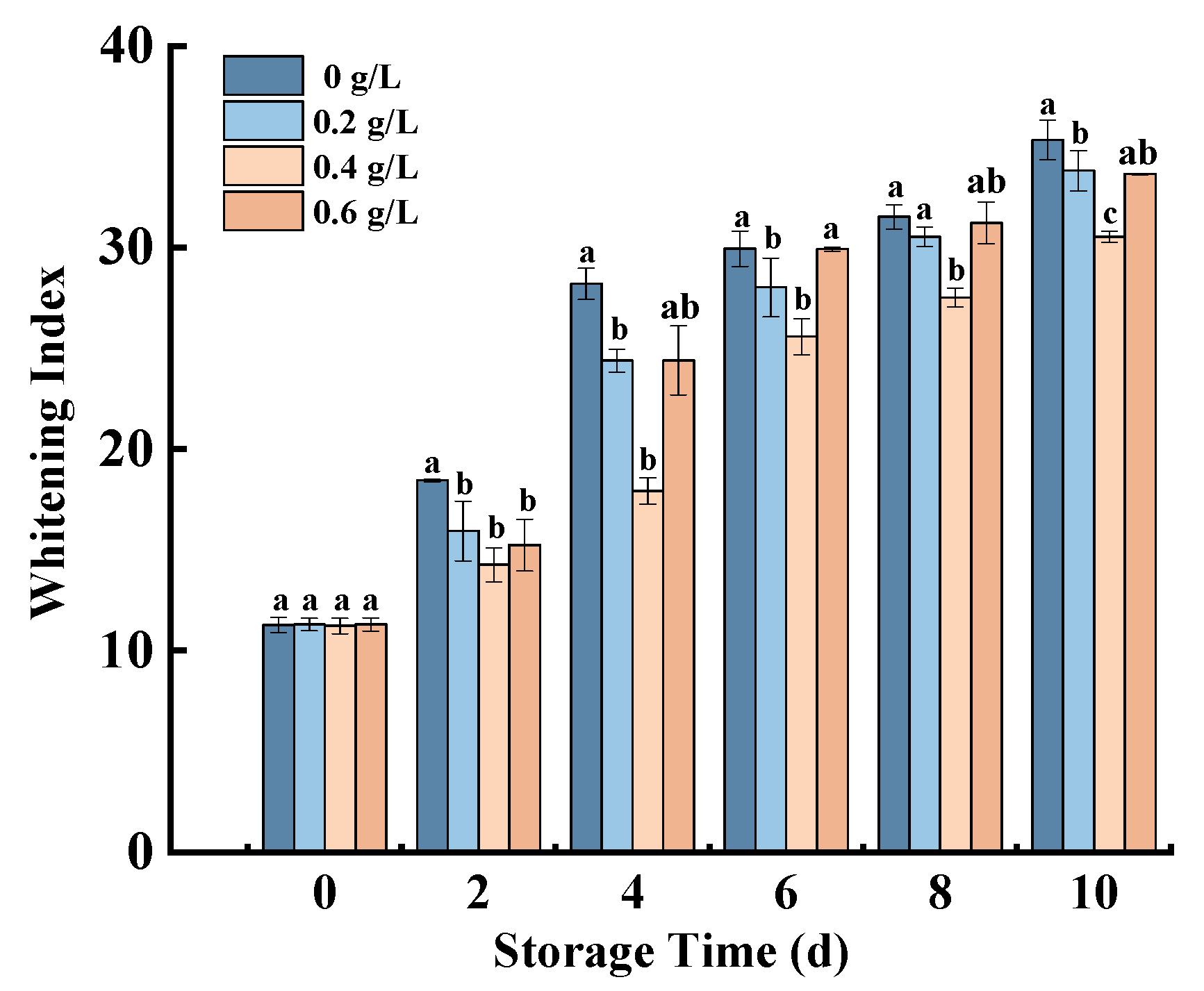
3.2. Whitening Index (WI)
3.3. Respiration Intensity
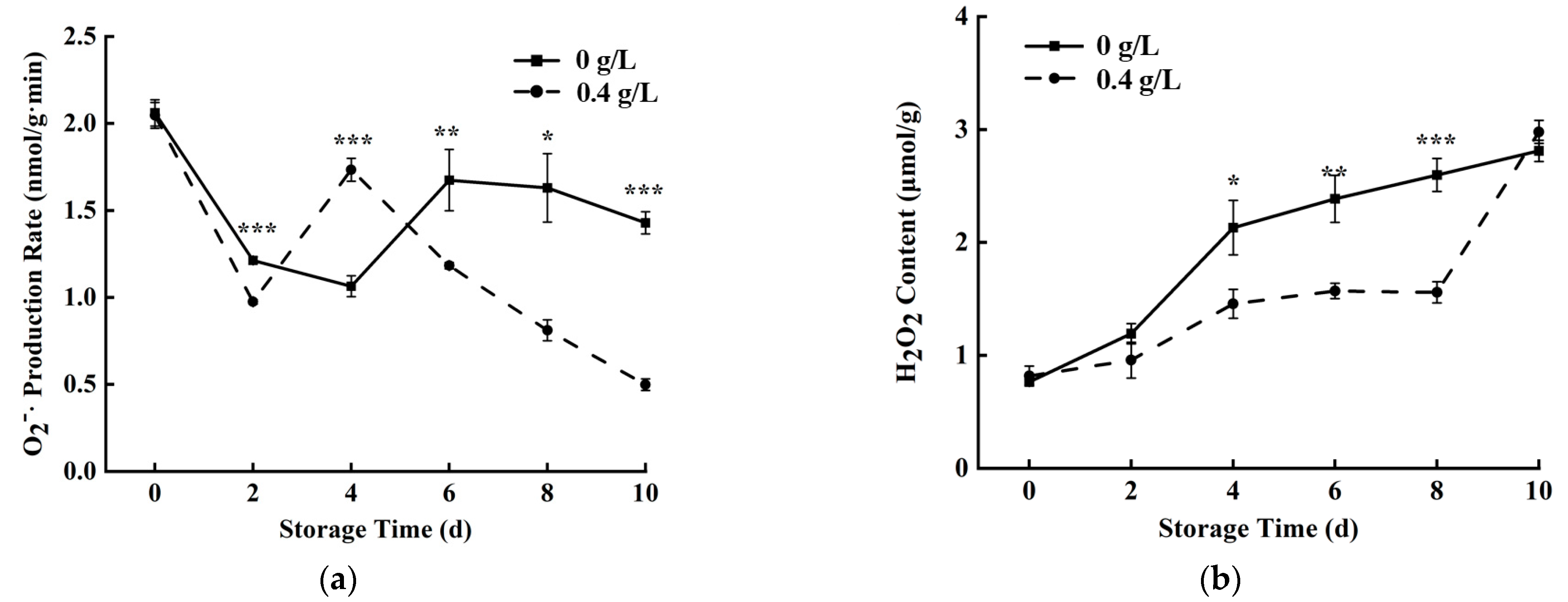
3.4. O2−· Production Rate and H2O2 Contents
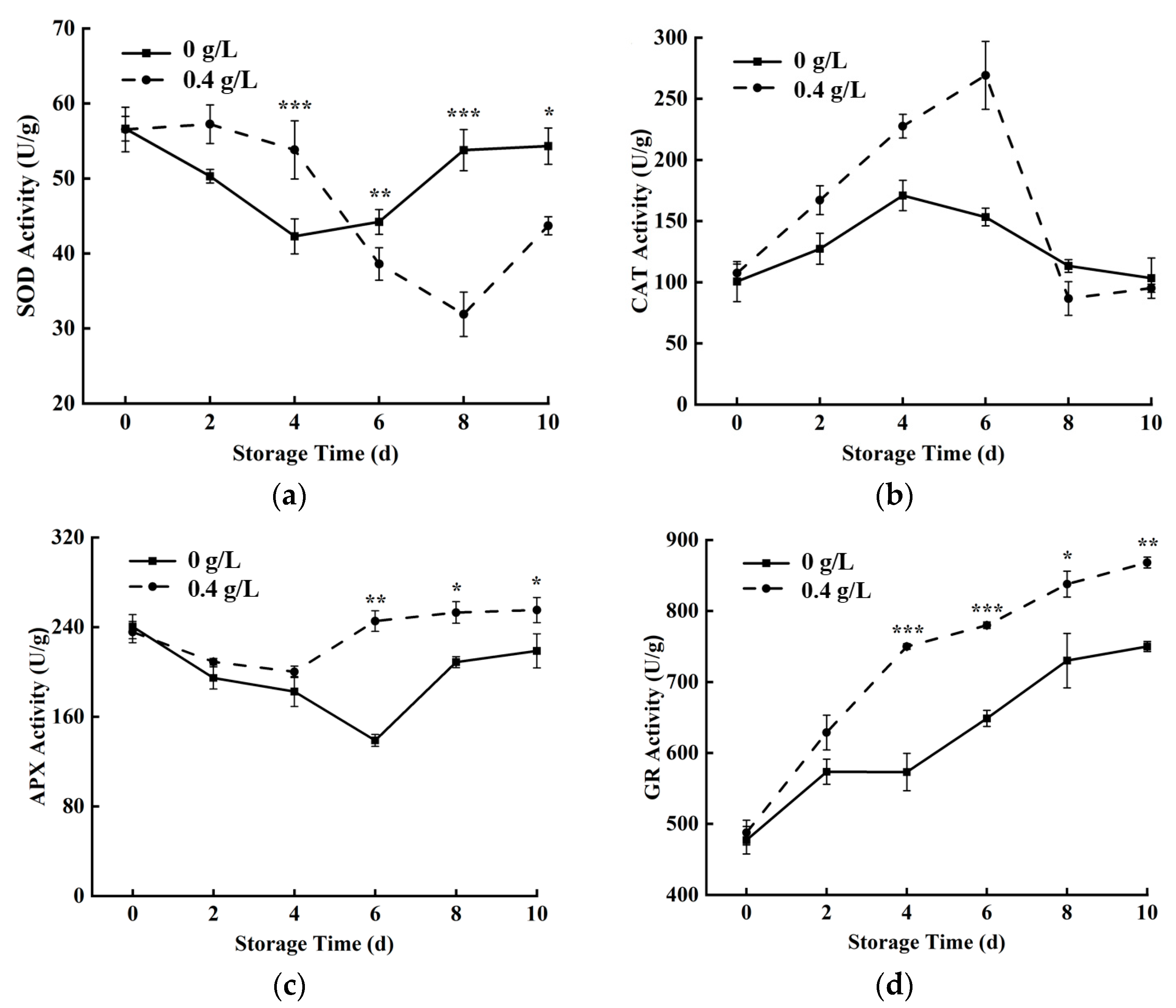
3.5. Antioxidant Metabolism-Related Enzyme Activities
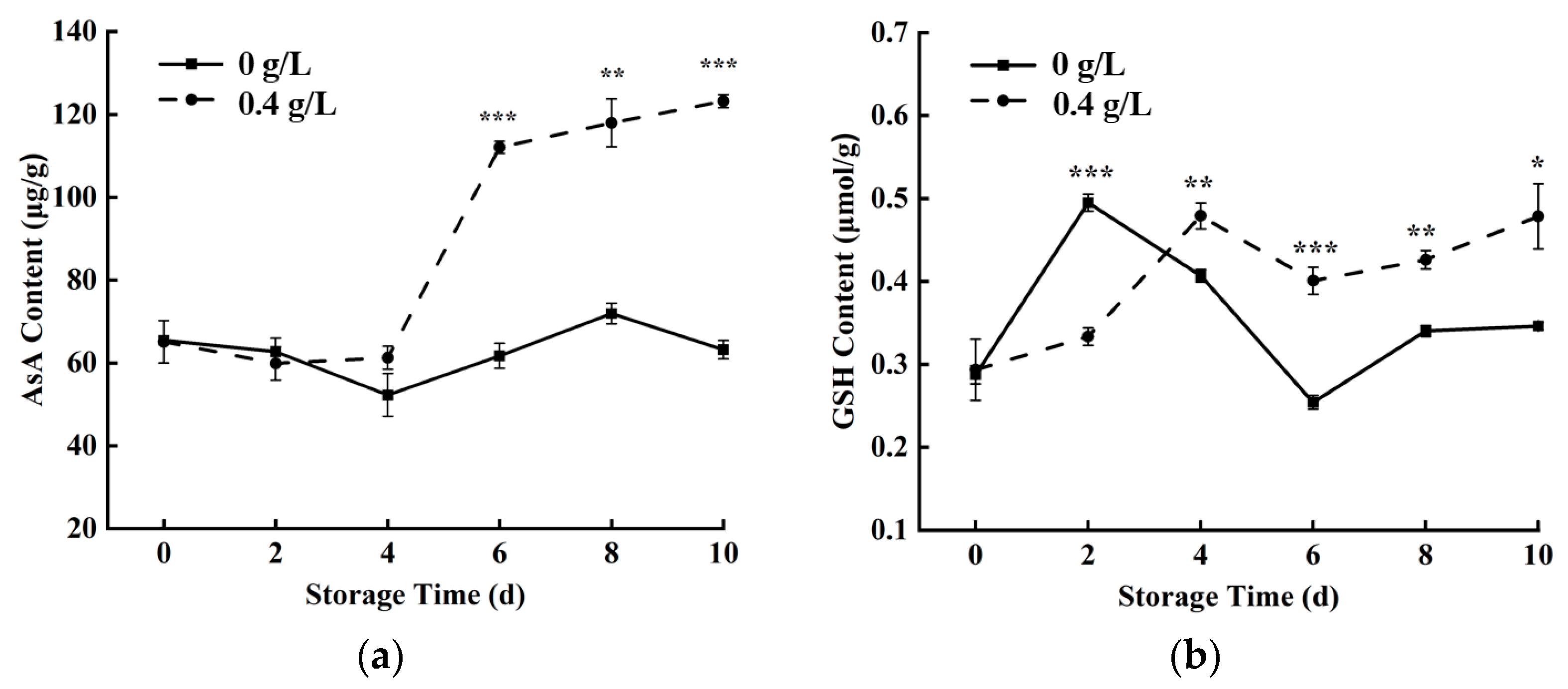
3.6. Antioxidant Metabolism-Related Substances Contents
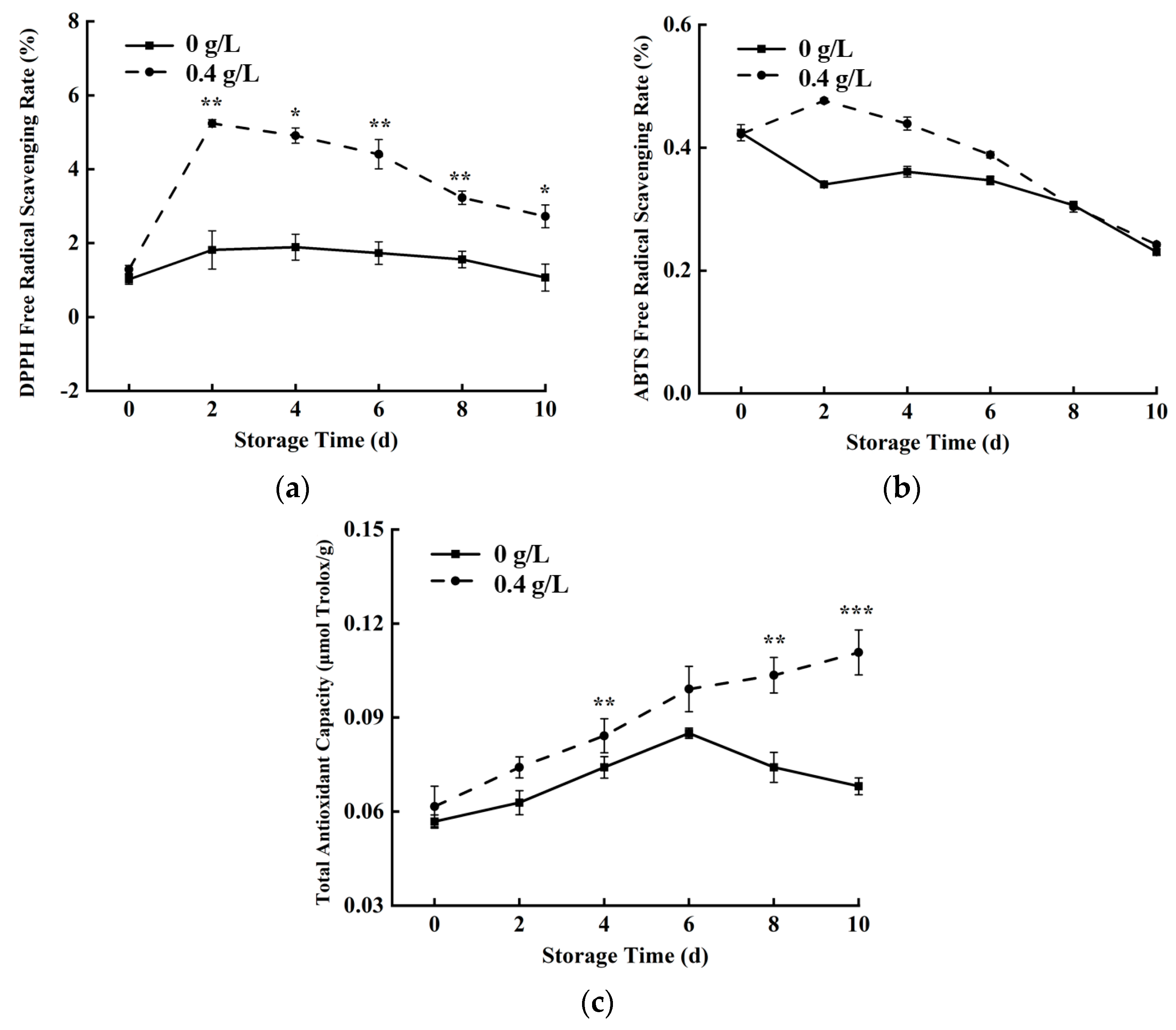
3.7. Antioxidant Capacity
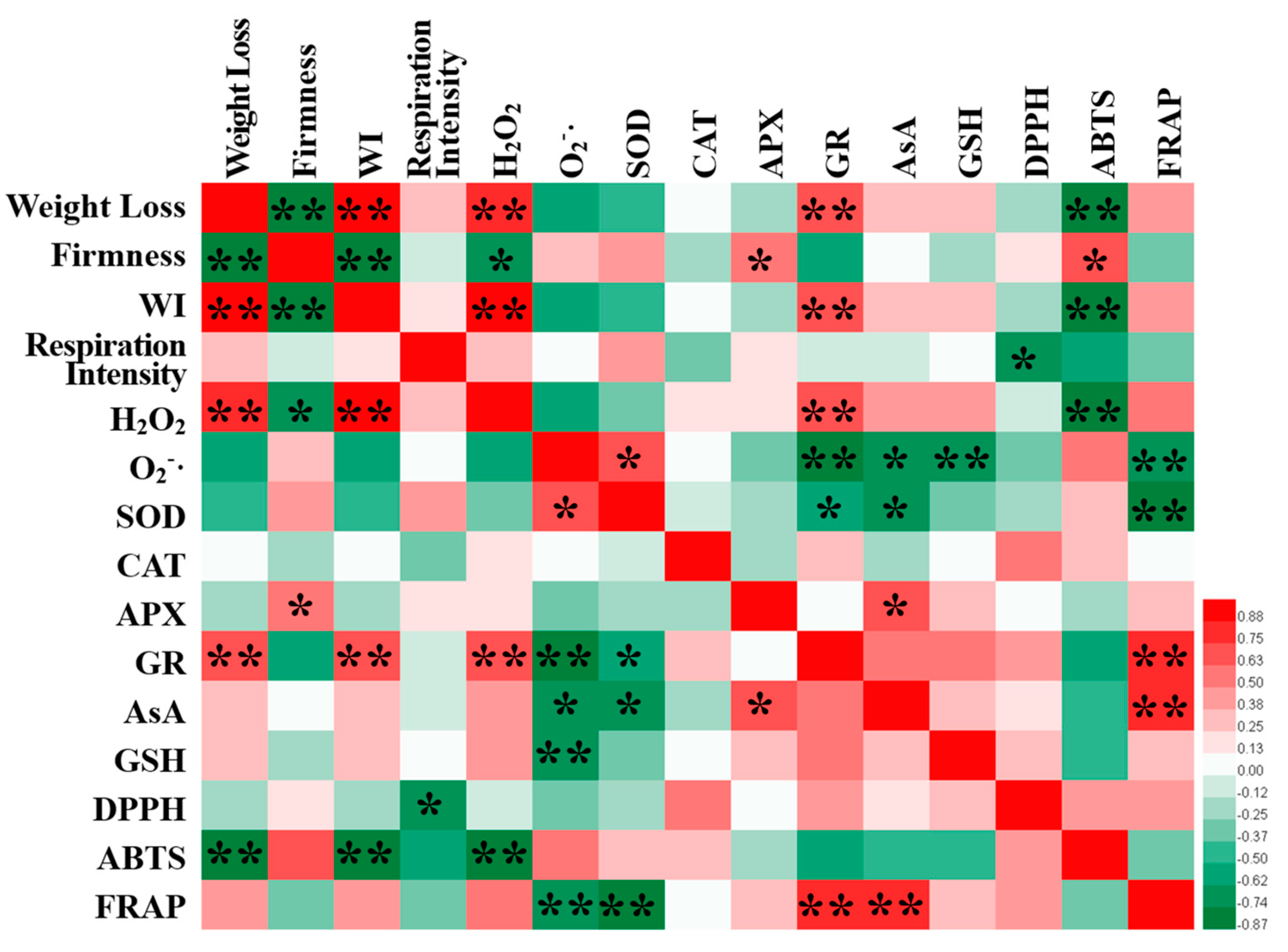
3.8. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ji, X.; Peng, B.; Ding, H.; Cui, B.; Nie, H.; Yan, Y. Purification, Structure and Biological Activity of Pumpkin Polysaccharides: A Review. Food Rev. Int. 2021, 39, 307–319. [Google Scholar] [CrossRef]
- Sharma, S.; Ramana Rao, T.V. Nutritional quality characteristics of pumpkin fruit as revealed by its biochemical analysis. Int. Food Res. J. 2013, 20, 2309–2316. [Google Scholar]
- Oloyede, F.M.; Adebooye, O.C.; Obuotor, E.M. Planting date and fertilizer affect antioxidants in pumpkin fruit. Sci. Hortic. 2014, 168, 46–50. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A. The Profile of Carotenoids and Other Bioactive Molecules in Various Pumpkin Fruits (Cucurbita maxima Duchesne) Cultivars. Molecules 2019, 24, 3212. [Google Scholar] [CrossRef] [PubMed]
- Zdunic, G.M.; Menkovic, N.R.; Jadranin, M.B.; Novakovic, M.M.; Savikin, K.P.; Zivkovic, J.C. Phenolic compounds and carotenoids in pumpkin fruit and related traditional products. Hem. Ind. 2016, 70, 429–433. [Google Scholar] [CrossRef]
- Yadav, M.; Jain, S.; Tomar, R.; Prasad, G.B.K.S.; Yadav, H. Medicinal and biological potential of pumpkin: An updated review. Nutr. Res. Rev. 2010, 23, 184–190. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, E.J.; Kim, Y.N.; Choi, C.; Lee, B.H. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr. Res. Pract. 2012, 6, 21–27. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data (accessed on 1 March 2023).
- Yüksel, Ç.; Atalay, D.; Erge, H.S. The effects of chitosan coating and vacuum packaging on quality of fresh-cut pumpkin slices during storage. J. Food Process. Preserv. 2022, 46, e16365. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017, 64, 23–38. [Google Scholar] [CrossRef]
- Rashid, M.H.; Khan, M.R.; Roobab, U.; Rajoka, M.S.R.; Inam-ur-Raheem, M.; Anwar, R.; Ahmed, W.; Jahan, M.; Ijaz, M.R.A.; Asghar, M.M.; et al. Enhancing the shelf stability of fresh-cut potatoes via chemical and nonthermal treatments. J. Food Process. Preserv. 2021, 45, e15582. [Google Scholar] [CrossRef]
- Khan, I.; Oh, D.-H. Integration of nisin into nanoparticles for application in foods. Innov. Food Sci. Emerg. 2016, 34, 376–384. [Google Scholar] [CrossRef]
- Ali, A.H.; Hale, O.W.; Khasawneh, F.A.; Urban, R.S.; Werner, H.V.; Smalligan, R.D. NISIN and Clostridium difficile: A Potentially Effective Treatment for an Increasingly Problematic Disease. Am. J. Gastroenterol. 2013, 108, 625. [Google Scholar] [CrossRef] [PubMed]
- Fusieger, A.; Perin, L.M.; Teixeira, C.G.; de Carvalho, A.F.; Nero, L.A. The ability of Lactococcus lactis subsp. lactis bv. diacetylactis strains in producing nisin. Antonie Van Leeuwenhoek 2020, 113, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.H.A.; Cutter, C.N. Development and evaluation of pullulan-based composite antimicrobial films (CAF) incorporated with nisin, thymol and lauric arginate to reduce foodborne pathogens associated with muscle foods. Int. J. Food Microbiol. 2020, 320, 108519. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.V.; Jagus, R.J.; Aguero, M.V. Application of a combined treatment using natural antimicrobials and modified atmosphere packaging to enhance safety, quality, and shelf-life of fresh-cut beet leaves. J. Food Saf. 2018, 38, e12556. [Google Scholar] [CrossRef]
- Song, Z.Y.; Li, F.; Guan, H.; Xu, Y.F.; Fu, Q.J.; Li, D.P. Combination of nisin and epsilon-polylysine with chitosan coating inhibits the white blush of fresh-cut carrots. Food Control 2017, 74, 34–44. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Wang, J.Y.; Wu, Z.H.; Xu, Y.P.; Wu, Z.X. Ozone treatment promotes physicochemical properties and antioxidant capacity of fresh-cut red pitaya based on phenolic metabolism. Front. Nutr. 2022, 9, 1–14. [Google Scholar] [CrossRef]
- Sarengaowa; Hu, W.Z.; Jiang, A.L.; Xiu, Z.L.; Feng, K. Effect of thyme oil-alginate-based coating on quality and microbial safety of fresh-cut apples. J. Sci. Food Agric. 2018, 98, 2302–2311. [Google Scholar] [CrossRef]
- BOLIN, H.R.; HUXSOLL, C.C. Control of minimally processed carrot (Daucus carotova) surface discoloration caused by abrasion peeling. J. Food Sci. 1991, 56, 416–418. [Google Scholar]
- Zhou, F.H.; Xu, D.Y.; Liu, C.H.; Chen, C.; Tian, M.X.; Jiang, A.L. Ascorbic acid treatment inhibits wound healing of fresh-cut potato strips by controlling phenylpropanoid metabolism. Postharvest Biol. Technol. 2021, 181, 111644. [Google Scholar] [CrossRef]
- Coming Biotechnology Company Home Page of SOD Activity Kit. Available online: http://www.cominbio.com/index.html (accessed on 2 November 2022).
- Yan, J.; Song, Y.; Li, J.; Jiang, W. Forced-air precooling treatment enhanced antioxidant capacities of apricots. J. Food Process. Pres. 2018, 42, e13320. [Google Scholar] [CrossRef]
- Zhou, F.; Zuo, J.; Xu, D.; Gao, L.; Wang, Q.; Jiang, A. Low intensity white light-emitting diodes (LED) application to delay senescence and maintain quality of postharvest pakchoi (Brassica campestris L. ssp. chinensis (L.) Makino var. communis Tsen et Lee). Sci. Hortic. 2020, 262, 109060. [Google Scholar]
- Guan, Y.; Ji, Y.; Yang, X.; Pang, L.; Cheng, J.; Lu, X.; Zheng, J.; Yin, L.; Hu, W. Antioxidant activity and microbial safety of fresh-cut red cabbage stored in different packaging films. LWT-Food Sci. Technol. 2023, 175, 114478. [Google Scholar] [CrossRef]
- Piagentini, A.M.; Güemes, D.R. Shelf life of fresh-cut spinach as affected by chemical treatment and type of packaging film. Braz. J. Chem. Eng. 2002, 19, 383–389. [Google Scholar] [CrossRef]
- Qiao, H.; Zhang, B.; Chen, X.; Su, L.; Jiao, C.; Chen, S.; Fan, J.; Liu, H. Short peptides secreted by Bacillus subtilis inhibit the growth of mold on fresh-cut pumpkin (Cucurbita pepo). J. Sci. Food Agric. 2020, 100, 936–944. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, A.; Almushhin, A.; Alharbi, M.; Almatrafi, M.; Benajiba, N.; Fikry, M.; Helal, M. Enhancement in physicochemical parameters and microbial populations of mushrooms as influenced by nano-coating treatments. Sci. Rep. 2021, 11, 7915. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, H.; Huang, M.; Zhou, D.; Cao, Q.; Ma, K. Effects of high pressure nitrogen treatments on the quality of fresh-cut pears at cold storage. Innov. Food Sci. Emerg. Technol. 2015, 32, 56–63. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Fikry, M.; Helal, M. The combined effect of coating treatments to nisin, nano-silica, and chitosan on oxidation processes of stored button mushrooms at 4 °C. Sci. Rep. 2021, 11, 6031. [Google Scholar] [CrossRef]
- Li, C.; Tao, J.; Wu, Z. Gaseous ozone regulates reactive oxygen species metabolism and ascorbate-glutathione cycle to delay the senescence of fresh-cut red pitaya (Selenicereus undatus) fruit. Sci. Hortic. 2023, 312, 111839. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Y.; Wu, X.; Liu, W.; Jing, X.; Liu, Y.; Yan, J.; Liu, S.; Qin, W. Combination of calcium lactate impregnation with UV-C irradiation maintains quality and improves antioxidant capacity of fresh-cut kiwifruit slices. Food Chem. X 2022, 14, 100329. [Google Scholar] [CrossRef]
- Li, B.; Li, M.; Liu, J.; Sun, W.; Min, D.; Li, F.; Li, X. Methyl salicylate pretreatment maintains quality and antioxidant capacity of fresh-cut pitaya fruit by modulating phenylpropanoid metabolism and antioxidant system. Sci. Hortic. 2023, 309, 111705. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Li, M.; Fu, X.; Zhao, X.; Min, D.; Li, F.; Li, X.; Zhang, X. Hot air pretreatment alleviates browning of fresh-cut pitaya fruit by regulating phenylpropanoid pathway and ascorbate-glutathione cycle. Postharvest Biol. Technol. 2022, 190, 111954. [Google Scholar] [CrossRef]
- Chen, C.; Hu, W.Z.; Zhang, R.D.; Jiang, A.L.; Liu, C.H. Effects of hydrogen sulfide on the surface whitening and physiological responses of fresh-cut carrots. J. Sci. Food Agric. 2018, 98, 4726–4732. [Google Scholar] [CrossRef]
- Zhou, C.-L.; Liu, W.; Zhao, J.; Yuan, C.; Song, Y.; Chen, D.; Ni, Y.-Y.; Li, Q.-H. The effect of high hydrostatic pressure on the microbiological quality and physical–chemical characteristics of Pumpkin (Cucurbita maxima Duch.) during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2014, 21, 24–34. [Google Scholar] [CrossRef]
- Nicola, S.; Tibaldi, G.; Gaino, W.; Pignata, G. Cutting shape, film and storage temperature affect the shelf-life of fresh-cut pumpkin. Acta Hortic. 2018, 1209, 399–408. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef]
- McManamon, O.; Kaupper, T.; Scollard, J.; Schmalenberger, A. Nisin application delays growth of Listeria monocytogenes on fresh-cut iceberg lettuce in modified atmosphere packaging, while the bacterial community structure changes within one week of storage. Postharvest Biol. Technol. 2019, 147, 185–195. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.; Liu, F.; Dong, P.; Huang, W.; Xiong, L.; Liao, X. Comparing the effects of high hydrostatic pressure and thermal pasteurization combined with nisin on the quality of cucumber juice drinks. Innov. Food Sci. Emerg. Technol. 2013, 17, 27–36. [Google Scholar] [CrossRef]
- Mok, J.H.; Pyatkovskyy, T.; Yousef, A.; Sastry, S.K. Effects of combination shear stress, moderate electric field (MEF), and nisin on kinetics and mechanisms of inactivation of Escherichia coli K12 and Listeria innocua in fresh apple-kale blend juice. J. Food Eng. 2021, 292, 110262. [Google Scholar] [CrossRef]
- Ma, T.; Wang, J.; Wang, L.; Yang, Y.; Yang, W.; Wang, H.; Lan, T.; Zhang, Q.; Sun, X. Ultrasound-Combined Sterilization Technology: An Effective Sterilization Technique Ensuring the Microbial Safety of Grape Juice and Significantly Improving Its Quality. Foods 2020, 9, 1512. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hu, W.; Zhang, R.; Jiang, A.; Zou, Y. Levels of phenolic compounds, antioxidant capacity, and microbial counts of fresh-cut onions after treatment with a combination of nisin and citric acid. Hortic. Environ. Biotechnol. 2016, 57, 266–273. [Google Scholar] [CrossRef]
- Nieva, S.G.; Jagus, R.J.; Agüero, M.V.; Fernandez, M.V. Fruit and vegetable smoothies preservation with natural antimicrobials for the assurance of safety and quality. LWT-Food Sci. Technol. 2022, 154, 112663. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, W.; Xu, Y.; Yang, X.; Ji, Y.; Feng, K.; Sarengaowa. Metabolomics and physiological analyses validates previous findings on the mechanism of response to wounding stress of different intensities in broccoli. Food Res. Int. 2021, 140, 110058. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.-J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sharma, P.; Gill, S.S.; Hasanuzzaman, M.; Khan, E.A.; Kachhap, K.; Mohamed, A.A.; Thangavel, P.; Devi, G.D.; Vasudhevan, P.; et al. Catalase and ascorbate peroxidase-representative H2O2-detoxifying heme enzymes in plants. Environ. Sci. Pollut. Res. 2016, 23, 19002–19029. [Google Scholar] [CrossRef]
- Jian, W. Effect of Chitosan and Nisin on the Antioxidant Quality of Feijoa during Storage. Master’s Thesis, Southwest University of Science and Technology, Mianyang, China, 2020. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, N.; Wang, Y.; Guan, Y.; Chen, C.; Hu, W. Effect of Nisin on the Quality and Antioxidant Activity of Fresh-Cut Pumpkins (Cucurbita moschata Duch.). Horticulturae 2023, 9, 529. https://doi.org/10.3390/horticulturae9050529
Yuan N, Wang Y, Guan Y, Chen C, Hu W. Effect of Nisin on the Quality and Antioxidant Activity of Fresh-Cut Pumpkins (Cucurbita moschata Duch.). Horticulturae. 2023; 9(5):529. https://doi.org/10.3390/horticulturae9050529
Chicago/Turabian StyleYuan, Ning, Yi Wang, Yuge Guan, Chen Chen, and Wenzhong Hu. 2023. "Effect of Nisin on the Quality and Antioxidant Activity of Fresh-Cut Pumpkins (Cucurbita moschata Duch.)" Horticulturae 9, no. 5: 529. https://doi.org/10.3390/horticulturae9050529
APA StyleYuan, N., Wang, Y., Guan, Y., Chen, C., & Hu, W. (2023). Effect of Nisin on the Quality and Antioxidant Activity of Fresh-Cut Pumpkins (Cucurbita moschata Duch.). Horticulturae, 9(5), 529. https://doi.org/10.3390/horticulturae9050529






