Comparison of Growth Patterns and Metabolite Composition of Different Ginseng Cultivars (Yunpoong and K-1) Grown in a Vertical Farm
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Growth Characteristics
2.3. Analysis
2.3.1. Biological Compounds and Antioxidant Properties
2.3.2. Ginsenoside Analysis
2.4. Analysis of Metabolites
2.4.1. Quantification Methods and Gas Chromatography–Mass Spectrometry (GC/MS) Conditions for Primary Metabolite
2.4.2. Quantification Methods and Ultra-Performance Liquid Chromatography/Time-of-Flight Mass Spectrometry (UPLC/Q-TOF-MS) Conditions for Secondary Metabolites
2.4.3. Metabolite Data Processing
2.5. Statistical Analysis
3. Results and Discussion
3.1. Growth Characteristics
3.2. Biological Compounds and Antioxidant Properties
3.2.1. Biological Compounds (Total Phenolic and Flavonoid Contents)
3.2.2. Antioxidant Properties (DPPH, FRAP, and ABTS)
3.3. Ginsenoside
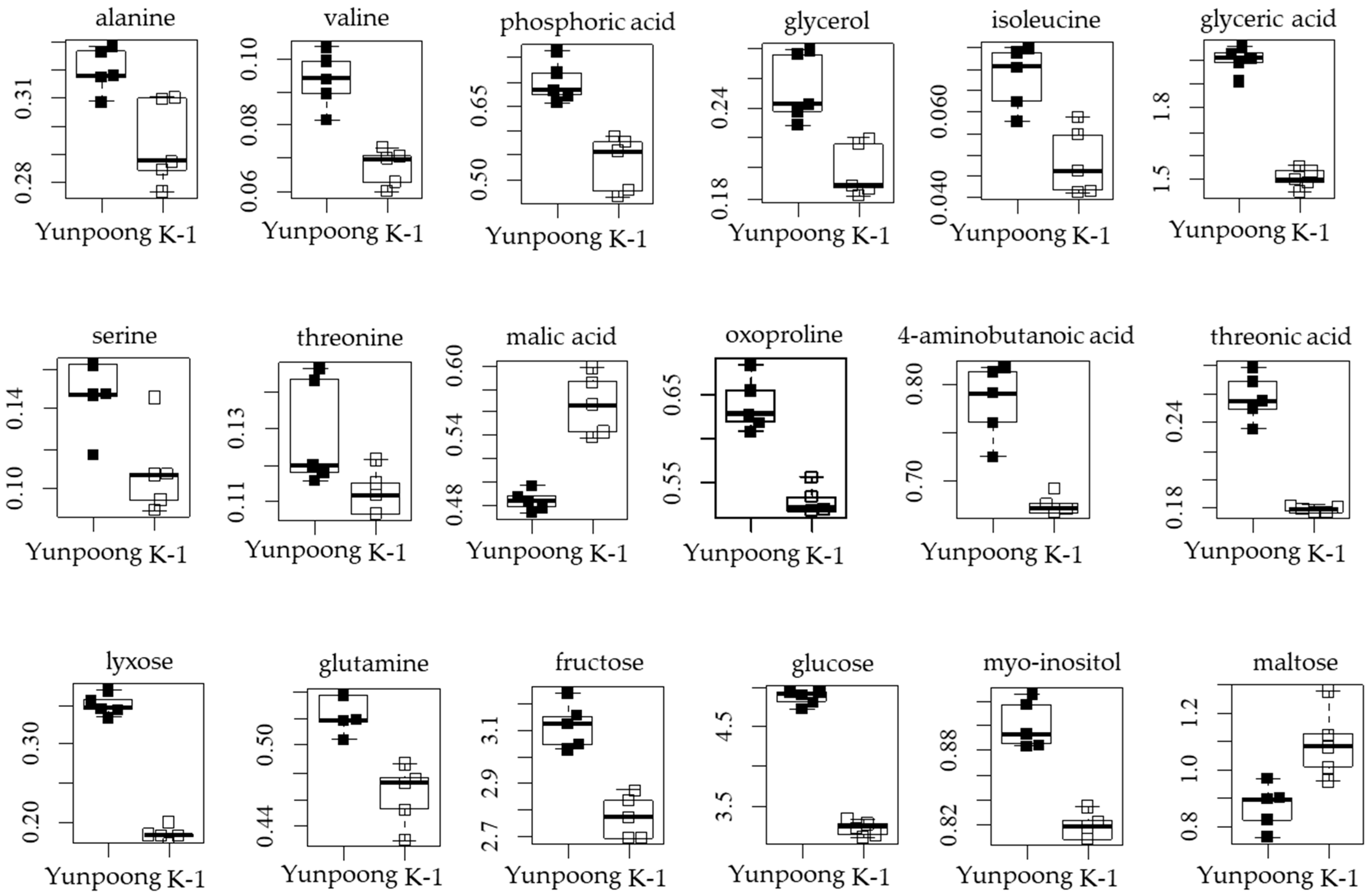
3.4. Metabolites
3.5. Annual Net Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Agriculture, Food and Rural Affairs (MAFRA). 2021 Ginseng Statistics; Ministry of Agriculture, Food and Rural Affairs: Sejong, Republic of Korea, 2022; pp. 1–36.
- Kim, Y.C.; Kim, Y.B.; Park, H.W.; Bang, K.Y.; Kim, J.U.; Jo, I.H.; Kim, K.H.; Song, B.H.; Kim, D.H. Optimal harvesting time of ginseng seeds and effect of gibberellic acid (GA3) treatment for improving stratification rate of ginseng (Panax ginseng C. A. Meyer) seeds. Korean J. Med. Crop Sci. 2014, 22, 423–428. [Google Scholar] [CrossRef]
- Ahn, I.O.; Lee, S.S.; Lee, J.H.; Lee, M.J.; Jo, B.G. Comparison of ginsenoside contents and pattern similarity between root parts of new cultivars in Panax ginseng C. A. Meyer. J. Ginseng Res. 2008, 32, 15–18. [Google Scholar] [CrossRef]
- Kwon, W.S.; Lee, M.G.; Lee, J.H. Characteristics of flowering and fruiting in new varieties and lines of Panax ginseng C. A. Meyer. J. Ginseng Res. 2001, 25, 41–44. [Google Scholar]
- Lee, S.O.; Kim, M.J.; Kim, D.G.; Choi, H.J. Antioxidative activities of temperature-stepwise water extracts from Inonotus obliquus. J. Korean Soc. Food Sci. Nutr. 2005, 34, 139–147. [Google Scholar] [CrossRef]
- Kwon, W.S.; Lee, M.G.; Choi, K.T. Breeding process and characteristics of Yunpoong; a new variety of Panax ginseng C. A. Meyer. J. Ginseng Res. 2000, 24, 1–7. [Google Scholar]
- Wang, H.; Xu, F.; Wang, X.; Kwon, W.S.; Yang, D.C. Molecular discrimination of Panax ginseng cultivar K-1 using pathogenesis-related protein 5 gene. J. Ginseng Res. 2019, 43, 482–487. [Google Scholar] [CrossRef]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides; the main active components of Panax ginseng; in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef]
- Kim, D.H.; Moon, Y.S.; Lee, T.H.; Jung, J.S.; Suh, H.W.; Song, D.K. The inhibitory effect of ginseng saponins on the stress-induced plasma interleukin-6 level in mice. Neurosci. Lett. 2003, 353, 13–16. [Google Scholar] [CrossRef]
- López, M.V.N.; Cuadrado, M.P.G.-S.; RuizPoveda, O.M.P.; Del Fresno, A.M.V.; Accame, M.E.C. Neuroprotective effect of individual ginsenosides on astrocytes primary culture. Biochim. Biophys. Acta 2007, 1770, 1308–1316. [Google Scholar] [CrossRef]
- Vuksan, V.; Sung, M.K.; Sievenpiper, J.L.; Stavro, P.M.; Jenkins, A.L.; Di Buono, M.; Lee, K.S.; Leiter, L.A.; Nam, K.Y.; Arnason, J.T. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled; type 2 diabetes, Results of a randomized; double-blind; placebo-controlled study of efficacy and safety. Nutr. Metabol. Cardiovasc. Dis. 2008, 18, 46–56. [Google Scholar] [CrossRef]
- Choi, J.E.; Li, X.; Han, Y.H.; Lee, K.T. Changes of saponin contents of leaves; stems and flower buds of Panax ginseng C. A. Meyer by harvesting days. Korean J. Med. Crop Sci. 2009, 17, 251–256. [Google Scholar]
- Lim, W.S. Effects of interactions among age; cultivation method (location) and population on ginsenoside content of wild Panax quinquefolium L. one year after transplanting from wild. Korean J. Med. Crop Sci. 2005, 13, 254–261. [Google Scholar]
- Li, X.G.; Nam, K.Y.; Choi, J.E. Difference of the ginsenosides contents according to the planting location in Panax ginseng C. A. Meyer. Korean J. Crop Sci. 2009, 54, 159–164. [Google Scholar]
- Yahara, S.; Tanaka, O.; Komori, T. Saponins of the leaves of Panax ginseng C. A. Meyer. Chem. Pharm. Bull. 1976, 24, 2204–2208. [Google Scholar] [CrossRef]
- Xie, J.T.; Lin, E.; Wang, C.Z.; Yuan, C.S. Constituents and effects of ginseng leaf. Orient. Pharm. Exp. Med. 2004, 4, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, J.Y.; Xiao, X.Y.; Lin, R.C.; Cheng, Y.Y. Simultaneous determination of ginsenosides in Panax ginseng with different growth ages using high-performance liquid chromatography-mass spectrometry. Phytochem. Anal. 2006, 17, 424–430. [Google Scholar] [CrossRef]
- Kim, G.S.; Hyun, D.Y.; Kim, Y.O.; Lee, S.E.; Kwon, H.; Cha, S.W.; Park, C.B.; Kim, Y.B. Investigation of ginsenosides in different parts of Panax ginseng cultured by hydroponics. Korean J. Hortic. Sci. Technol. 2010, 28, 216–226. [Google Scholar]
- Kozai, T. Why LED Lighting for Urban Agriculture? In LED Lighting for Urban Agriculture, 1st ed.; Kosai, T., Fujiwara, K., Runkle, E., Eds.; Springer: Singapore, 2016; pp. 3–18. [Google Scholar]
- Kim, J.H.; Chang, S.D. Industrialization condition and possibility of plant factory. Korean J. Agric. Manag. Policy 2009, 36, 918–948. [Google Scholar]
- Goto, E. Chapter 15—Production of Pharmaceuticals in a Specially Designed Plant Factory A2—Kozai, Toyoki. In Plant Factory; Niu, G., Takagaki, M., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 193–200. [Google Scholar] [CrossRef]
- Cheon, S.K.; Mok, S.K.; Lee, S.S.; Shin, D.Y. Effects of light intensity and quality on the growth and quality of Korean ginseng (Panax ginseng C. A. Meyer) I. Effects of light intensity on the growth and yield of ginseng plants. J. Ginseng Res. 1991, 15, 21–30. [Google Scholar]
- Kim, M.J.; Li, X.; Han, J.S.; Lee, S.E.; Choi, J.E. Effect of blue and red LED irradiation on growth characteristics and saponin contents in Panax ginseng C. A. Meyer. Korean J. Med. Crop Sci. 2009, 17, 187–191. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hahida, S.; Yoshihara, T. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ. Exp. Bot. 2012, 75, 128–133. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Nájera, C.; del Mar Gea, M. Effect of the spectral quality and intensity of light-emitting diodes on several horticultural crops. HortScience 2016, 51, 268–271. [Google Scholar] [CrossRef]
- Lee, D.U.; Ku, H.B.; Lee, Y.J.; Kim, G.N.; Lee, S.C. Antioxidant and antimelanogenic activities of Panax ginseng sprout extract. J. Korean Soc. Food Sci. Nutr. 2019, 48, 699–703. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Lee, J.-H.; Lee, G.O.; Cho, K.M.; Cho, D.Y.; Son, K.-H. Optimization of Cultivation Type and Temperature for the Production of Balloon Flower (Platycodongrandiflorum A. DC) Sprouts in a Plant Factory with Artificial Lighting. Horticulturae 2022, 8, 315. [Google Scholar] [CrossRef]
- Cho, K.M.; Lee, H.Y.; Lee, Y.M.; Seo, E.Y.; Kim, D.H.; Son, K.-H.; Lee, J.; Cho, D.Y.; Lee, J.H. Comparative assessment of compositional constituents and antioxidant effects in ginseng sprouts (Panax ginseng) through aging and fermentation processes. LWT 2022, 164, 113644. [Google Scholar] [CrossRef]
- Kim, S.C.; Kang, Y.M.; Seong, J.A.; Lee, H.Y.; Cho, D.Y.; Joo, O.S.; Lee, J.H.; Cho, K.M. Comprehensive changes of nutritional constituents and antioxidant activities of ginseng sprouts according to the roasting process. Korean J. Food Preserv. 2021, 28, 72–87. [Google Scholar] [CrossRef]
- Cho, K.M.; Hwang, C.E.; Joo, O.S. Change of physicochemical properties; phytochemical contents and biological activities during the vinegar fermentation of Elaeagnus multiflora fruit. Korean J. Food Preserv. 2017, 24, 125–133. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, D.H.; Kim, S.C.; Cho, D.Y.; Cho, K.M. Changes in nutritional components and antioxidant activities from soybean leaves containing high isoflavone contents according to different storage temperatures and periods. J. Appl. Biol. Chem. 2020, 63, 305–317. [Google Scholar] [CrossRef]
- Jang, S.-N.; Lee, G.O.; Sim, H.-S.; Bae, J.-S.; Lee, A.-R.; Cho, D.-Y.; Cho, K.-M.; Son, K.-H. Effect of Pre-Harvest Irradiation of UV-A and UV-B LED in Ginsenosides Content of Ginseng Sprouts. J. Bio-Environ. Control. 2022, 31, 28–34. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.C.; Lee, H.Y.; Cho, D.Y.; Jung, J.G.; Kang, D.; Kang, S.S.; Cho, K.M. Changes in nutritional compositions of processed mountain-cultivated ginseng sprouts (Panax ginseng) and screening for their antioxidant and anti-inflammatory properties. J. Funct. Foods 2021, 86, 104668. [Google Scholar] [CrossRef]
- Jang, I.B.; Yu, J.; Suh, S.J.; Jang, I.B.; Kwon, K.B. Growth and Ginsenoside Content in Different Parts of Ginseng Sprouts Depending on Harvest Time. Korean J. Med. Crop Sci. 2018, 26, 205–213. [Google Scholar] [CrossRef]
- Proctor, J.T.A.; Dorais, M.; Bleiholder, H.; Willis, A.; Hack, H.; Meier, V. Phenological growth stages of North American ginseng (Panax quinquefolius). Ann. Appl. Biol. 2003, 143, 311–317. [Google Scholar] [CrossRef]
- Minghui, J.; Xiaotong, J.; Yunlong, Z. Effects of long-term nitrogen addition on community aboveground and belowground biomass and their ratio in a typical steppe of Inner Mongolia. Chin. J. Ecol. 2020, 39, 3185–3193. [Google Scholar] [CrossRef]
- Kim, Y.J.; Nguyen, T.K.L.; Oh, M.M. Growth and Ginsenosides Content of Ginseng Sprouts According to LED-Based Light Quality Changes. Agronomy 2020, 10, 1979. [Google Scholar] [CrossRef]
- Song, B.H.; Chang, Y.G.; Lee, K.A.; Lee, S.W.; Kang, S.W.; Cha, S.W. Studies on Analysis of growth characteristics; ability of dry matter production; and yield of Panax ginseng C. A. Meyer at different growth stages with different cultivars and shading nets in paddy field. Korean J. Med. Crop Sci. 2011, 19, 90–96. [Google Scholar] [CrossRef]
- Haralampidis, K.; Trojanowska, M.; Osbourn, A.E. Biosynthesis of triterpenoid saponin in plants. Adv. Biochem. Eng. Biotechnol. 2002, 75, 31–49. [Google Scholar] [CrossRef]
- Eom, S.J.; Hwang, J.E.; Kim, H.S.; Kim, K.T.; Paik, H.D. Anti-inflammatory and cytotoxic effects of ginseng extract bioconverted by Leuconostoc mesenteroides KCCM 12010P isolated from kimchi. Int. J. Food Sci. Technol. 2018, 53, 1331–1337. [Google Scholar] [CrossRef]
- Lee, K.S.; Seong, B.J.; Kim, G.H.; Kim, S.I.; Han, S.H.; Kim, H.H.; Baik, N.D. Ginsenoside; phenolic acid composition and physiological significances of fermented ginseng leaf. J. Korean Soc. Food Sci. Nutr. 2010, 39, 1194–1200. [Google Scholar] [CrossRef]
- Chung, I.M.; Lim, J.J.; Ahn, M.S.; Jeong, H.N.; An, T.J.; Kim, S.H. Comparative phenolic compound profiles and antioxidant activity of the fruit; leaves; and roots of Korean ginseng (Panax ginseng Meyer) according to cultivation years. J. Ginseng Res. 2016, 40, 68–75. [Google Scholar] [CrossRef]
- Tian, M.; Xu, X.; Liu, Y.; Xie, L.; Pan, S. Effect of Se treatment on glucosinolate metabolism and health-promoting compounds in the broccoli sprouts of three cultivars. Food Chem. 2016, 190, 374–380. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Khaneghah, A.M.; Barba, F.J.; Nemati, Z.; Shokofti, S.S.; Alizadeh, F. Fermented sweet lemon juice (Citrus limetta) using Lactobacillus plantarum LS5, Chemical composition; antioxidant and antibacterial activities. J. Funct. Foods 2017, 38, 409–414. [Google Scholar] [CrossRef]
- Cho, K.M.; Ha, T.J.; Lee, Y.B.; Seo, W.D.; Kim, J.Y.; Ryu, H.W.; Jeong, S.H.; Kang, Y.M.; Lee, J.H. Soluble phenolics and antioxidant properties of soybean (Glycine max L.) cultivars with varying seed coat colours. J. Funct. Foods 2013, 5, 1065–1076. [Google Scholar] [CrossRef]
- Lee, S.S.; Lee, J.H.; Ahn, I.O. Characteristics of new cultivars in Panax ginseng C.A. Meyer. In Proceedings of the Ginseng Society Conference; Korean Society Ginseng: Seoul, Republic of Korea, 2005; Volume 18, pp. 3–18. [Google Scholar]
- Park, S.J. Antioxidant activities and whitening effects of ethanol extract from Panax ginseng sprout powder. J. Korean Soc. Food Sci. Nutr. 2019, 48, 276–281. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Kim, H.Y.; Yamabe, N.; Yokozawa, T. Stereospecificity in hydroxyl radical scavenging activities of four ginsenosides produced by heat processing. Bioorg. Med. Chem. Lett. 2006, 16, 5028–5031. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits; vegetables; and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Yoshida, T.; Mori, K.; Hatano, T.; Okumura, T.; Uehara, I.; Komagoe, K.; Fujita, Y.; Okuda, T. Studies on inhibition mechanism of autoxidation by tannins and flavonoids. V. Radical-scavenging effects of tannins and related polyphenols on 1;1-diphenyl-2-picrylhydrazyl radical. Chem. Pharm. Bull. 1989, 37, 1919–1921. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, D.; Lee, H.L.; Kim, C.E.; Jung, K.; Kang, K.S. Beneficial effects of Panax ginseng for the treatment and prevention of neurodegenerative diseases, Past findings and future directions. J. Ginseng Res. 2018, 42, 239–247. [Google Scholar] [CrossRef]
- Hwang, S.I.; Joo, J.M.; Joo, S.Y. ICT-based smart farm factory systems through the case of hydroponic ginseng plant factory. J. Korean Inst. Commun. Inf. Sci. 2015, 40, 780–790. [Google Scholar] [CrossRef]
- Seong, B.J.; Kim, S.I.; Jee, M.G.; Lee, H.C.; Kwon, A.R.; Kim, H.H.; Won, J.Y.; Lee, K.S. Changes in Growth; Active Ingredients; and Rheological Properties of Greenhouse-Cultivated Ginseng Sprout during Its Growth Period. Korean J. Med. Crop Sci. 2019, 27, 126–135. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, S.C.; Seong, J.A.; Lee, H.Y.; Cho, D.Y.; Kim, M.J.; Jung, J.G.; Jeong, E.H.; Son, K.-H.; Cho, K.M. Comparison of ginsenoside contents and antioxidant activity according to the size of ginseng sprout has produced in a plant factory. J. Appl. Biol. Chem. 2021, 64, 253–261. [Google Scholar] [CrossRef]
- Yang, L.; Yu, Q.T.; Ge, Y.Z.; Zhang, W.S.; Fan, Y.; Ma, C.W.; Liu, Q.; Qi, L.W. Distinct urine metabolome after Asian ginseng and American ginseng intervention based on GC-MS metabolomics approach. Sci. Rep. 2016, 6, 39045. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-E.; Kim, H.; Kim, J.; Choi, S.-J.; Ham, J.; Nho, C.W.; Yoo, G. A comparative study of ginseng berry production in a vertical farm and an open field. Ind. Crops Prod. 2019, 140, 111612. [Google Scholar] [CrossRef]

| Cultivar | WAT | Shoot (g) | Root (g) | Top/Root Ratio | Total Yield | ||
|---|---|---|---|---|---|---|---|
| Fresh Weight | Dry Weight | Fresh Weight | Dry Weight | ||||
| Yunpoong | 2 | 0.70 ± 0.03 b | 0.09 ± 0.00 b | 0.71 ± 0.03 a | 0.10 ± 0.00 c | 0.99 ± 0.04 b | 1.42 ± 0.05 b |
| 3 | 1.04 ± 0.04 a | 0.17 ± 0.01 a | 0.79 ± 0.04 a | 0.13 ± 0.01 ab | 1.34 ± 0.08 a | 1.84 ± 0.07 a | |
| 4 | 1.01 ± 0.07 a | 0.16 ± 0.01 a | 0.72 ± 0.05 a | 0.10 ± 0.01 bc | 1.41 ± 0.06 a | 1.73 ± 0.11 a | |
| 5 | 0.95 ± 0.05 a | 0.17 ± 0.01 a | 0.74 ± 0.04 a | 0.14 ± 0.01 a | 1.29 ± 0.04 a | 1.70 ± 0.09 a | |
| K-1 | 2 | 0.40 ± 0.02 c | 0.04 ± 0.00 c | 0.42 ± 0.02 b | 0.05 ± 0.00 b | 0.96 ± 0.03 b | 0.83 ± 0.03 c |
| 3 | 0.55 ± 0.03 b | 0.08 ± 0.01 b | 0.55 ± 0.05 a | 0.07 ± 0.01 a | 1.03 ± 0.06 b | 1.10 ± 0.07 b | |
| 4 | 0.81 ± 0.04 a | 0.13 ± 0.01 a | 0.52 ± 0.04 a | 0.08 ± 0.01 a | 1.58 ± 0.09 a | 1.33 ± 0.07 a | |
| 5 | 0.74 ± 0.03 a | 0.12 ± 0.01 a | 0.53 ± 0.04 a | 0.08 ± 0.01 a | 1.43 ± 0.08 a | 1.26 ± 0.06 a | |
| Cultivar | WAT | Total Phenolic Contents (GAE mg g−1) | Total Flavonoid Contents (RE mg g−1) | ||
|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | ||
| Yunpoong | 3 | 2.72 ± 0.04 b | 1.43 ± 0.02 b | 1.17 ± 0.01 a | 0.25 ± 0.00 a |
| 4 | 3.19 ± 0.02 a | 1.86 ± 0.04 a | 1.08 ± 0.01 b | 0.26 ± 0.01 b | |
| 5 | 3.06 ± 0.04 a | 2.10 ± 0.09 a | 1.06 ± 0.01 b | 0.26 ± 0.00 b | |
| K-1 | 3 | 2.57 ± 0.01 a | 1.60 ± 0.02 a | 0.90 ± 0.01 b | 0.18 ± 0.00 b |
| 4 | 2.46 ± 0.01 b | 1.49 ± 0.04 a | 0.98 ± 0.02 a | 0.21 ± 0.00 a | |
| 5 | 2.32 ± 0.02 c | 1.51 ± 0.01 a | 0.79 ± 0.01 c | 0.24 ± 0.00 c | |
| Cultivar | WAT | DPPH Radical Scavenging Activity (%) | Ferric Reducing/Antioxidant Power (OD593nm) | ABTS Radical Scavenging Activity (%) | |||
|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | Shoot | Root | ||
| Yunpoong | 3 | 33.59 ± 0.22 c | 10.01 ± 0.38 b | 1.30 ± 0.01 c | 0.34 ± 0.00 b | 75.22 ± 0.32 b | 25.02 ± 0.72 c |
| 4 | 37.70 ± 0.53 b | 11.43 ± 0.11 ab | 1.40 ± 0.01 b | 0.41 ± 0.01 a | 73.95 ± 0.56 c | 35.02 ± 0.47 b | |
| 5 | 43.59 ± 0.05 a | 12.21 ± 0.23 a | 1.47 ± 0.02 a | 0.40 ± 0.01 a | 78.01 ± 0.42 a | 39.17 ± 1.00 a | |
| K-1 | 3 | 32.73 ± 0.31 b | 11.16 ± 0.23 c | 1.12 ± 0.01 b | 0.42 ± 0.00 c | 71.03 ± 0.30 a | 36.13 ± 0.28 b |
| 4 | 35.93 ± 0.76 a | 12.99 ± 0.16 b | 1.25 ± 0.01 a | 0.48 ± 0.00 b | 61.35 ± 0.40 b | 35.44 ± 0.35 b | |
| 5 | 33.35 ± 0.29 ab | 14.26 ± 0.42 a | 1.26 ± 0.01 a | 0.53 ± 0.00 a | 62.09 ± 0.10 b | 39.74 ± 0.61 a | |
| Cultivar | WAT | Protopanaxatriol Types (mg g−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rg1 | Re | Rf | F5 | F3 | Rg2 | Rh1 | F1 | PPT | Total PPT-Type | |||
| Shoot | Yunpoong | 3 | 2.71 ± 0.03 c | 12.62 ± 0.02 b | 0.19 ± 0.00 a | 1.10 ± 0.01 b | 1.61 ± 0.01 b | 0.59 ± 0.01 b | 0.05 ± 0.03 a | 1.38 ± 0.00 c | 0.13 ± 0.00 b | 20.38 ± 0.01 c |
| 4 | 2.85 ± 0.01 b | 15.25 ± 0.08 a | 0.54 ± 0.08 a | 1.26 ± 0.04 ab | 1.88 ± 0.02 a | 0.69 ± 0.01 a | ND | 1.52 ± 0.01 a | 0.16 ± 0.00 a | 24.16 ± 0.23 b | ||
| 5 | 3.67 ± 0.02 a | 15.31 ± 0.09 a | 0.58 ± 0.20 a | 1.45 ± 0.09 a | 1.91 ± 0.00 a | 0.70 ± 0.01 a | 0.05 ± 0.02 a | 1.47 ± 0.00 b | 0.12 ± 0.00 b | 25.26 ± 0.27 a | ||
| K-1 | 3 | 4.50 ± 0.01 c | 14.87 ± 0.02 c | 1.08 ± 0.02 a | 1.77 ± 0.03 a | 1.83 ± 0.01 c | 0.75 ± 0.02 b | 0.08 ± 0.00 b | 1.69 ± 0.01 b | 0.15 ± 0.01 b | 26.73 ± 0.02 c | |
| 4 | 5.28 ± 0.01 b | 17.79 ± 0.03 b | 0.31 ± 0.21 b | 1.72 ± 0.07 a | 2.12 ± 0.02 b | 0.90 ± 0.01 a | 0.13 ± 0.01 a | 1.72 ± 0.02 b | 0.14 ± 0.01 b | 30.10 ± 0.20 b | ||
| 5 | 6.67 ± 0.31 a | 19.67 ± 0.29 a | 0.40 ± 0.15 b | 1.95 ± 0.10 a | 2.41 ± 0.02 a | 0.93 ± 0.04 a | 0.13 ± 0.00 a | 1.94 ± 0.04 a | 0.19 ± 0.00 a | 34.28 ± 0.19 a | ||
| Root | Yunpoong | 3 | 1.09 ± 0.01 b | 4.95 ± 0.01 b | 0.92 ± 0.10 a | 0.47 ± 0.03 b | 2.75 ± 0.04 a | 0.82 ± 0.08 a | ND | 0.05 ± 0.02 a | 0.06 ± 0.00 a | 11.12 ± 0.21 b |
| 4 | 1.08 ± 0.06 b | 4.68 ± 0.06 c | 0.89 ± 0.03 a | 0.51 ± 0.02 b | 0.19 ± 0.10 c | 0.56 ± 0.03 b | ND | 0.05 ± 0.00 a | 0.03 ± 0.00 b | 7.99 ± 0.13 c | ||
| 5 | 1.52 ± 0.05 a | 5.71 ± 0.03 a | 0.94 ± 0.03 a | 4.45 ± 0.06 a | 0.66 ± 0.07 b | ND | ND | 0.09 ± 0.01 a | 0.06 ± 0.00 a | 13.44 ± 0.13 a | ||
| K-1 | 3 | 0.99 ± 0.01 a | 3.78 ± 0.01 b | 0.61 ± 0.03 a | 0.43 ± 0.00 b | 0.27 ± 0.01 a | 0.44 ± 0.00 b | ND | 0.05 ± 0.00 a | 0.03 ± 0.00 b | 6.61 ± 0.04 b | |
| 4 | 0.86 ± 0.01 b | 4.32 ± 0.05 a | 0.59 ± 0.01 a | 0.53 ± 0.01 a | 0.19 ± 0.02 b | 0.55 ± 0.01 a | ND | 0.04 ± 0.00 b | 0.07 ± 0.00 a | 7.14 ± 0.09 a | ||
| 5 | 0.77 ± 0.02 c | 3.33 ± 0.01 c | 0.47 ± 0.02 b | 0.35 ± 0.00 c | ND | 0.31 ± 0.00 c | ND | 0.04 ± 0.00 b | 0.03 ± 0.00 b | 5.30 ± 0.05 c | ||
| Cultivar | WAT | Protopanaxadiol Type (mg g−1) | Oleanane Type | Total Ginsenoside | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rb1 | Rc | Rb2 | Rb3 | Rd | Rd2 | F2 | Rg3 | C.K | Rh2 | PPD | Total PPD-Type | Ro | ||||
| Shoot | Yunpoong | 3 | 0.55 ± 0.01 c | 2.48 ± 0.08 b | 3.49 ± 0.03 b | 0.40 ± 0.04 a | 11.17 ± 0.22 b | 0.22 ± 0.02 a | 0.51 ± 0.09 a | 0.18 ± 0.03 a | ND | 0.10 ± 0.00 a | 0.92 ± 0.01 b | 20.03 ± 0.44 b | 0.38 ± 0.01 a | 40.80 ± 0.45 b |
| 4 | 0.63 ± 0.02 b | 2.84 ± 0.01 a | 3.95 ± 0.03 a | 0.45 ± 0.02 a | 12.50 ± 0.09 a | 0.28 ± 0.01 a | 0.58 ± 0.01 a | 0.17 ± 0.01 a | 0.01 ± 0.00 a | 0.07 ± 0.00 b | 1.10 ± 0.01 a | 22.59 ± 0.15 a | 0.37 ± 0.00 a | 47.12 ± 0.38 a | ||
| 5 | 0.68 ± 0.01 a | 2.71 ± 0.05 a | 3.99 ± 0.04 a | 0.49 ± 0.05 a | 12.20 ± 0.28 a | 0.27 ± 0.08 a | 0.70 ± 0.01 a | 0.15 ± 0.00 a | 0.01 ± 0.01 a | 0.06 ± 0.00 b | 0.99 ± 0.04 b | 22.25 ± 0.39 a | 0.24 ± 0.01 b | 47.75 ± 0.50 a | ||
| K-1 | 3 | 0.95 ± 0.02 a | 3.23 ± 0.04 c | 4.98 ± 0.03 c | 0.52 ± 0.01 b | 13.46 ± 0.08 c | 0.19 ± 0.02 a | 0.54 ± 0.01 b | 0.28 ± 0.11 a | 0.01 ± 0.00 a | 0.13 ± 0.00 a | 0.84 ± 0.01 b | 25.14 ± 0.17 c | 0.32 ± 0.01 b | 52.19 ± 0.18 c | |
| 4 | 0.94 ± 0.03 a | 4.20 ± 0.03 b | 5.89 ± 0.01 b | 0.68 ± 0.06 a | 14.63 ± 0.40 b | 0.26 ± 0.01 a | 0.69 ± 0.06 b | 0.19 ± 0.02 a | 0.01 ± 0.01 a | 0.12 ± 0.00 b | 1.38 ± 0.05 a | 28.99 ± 0.49 b | 0.46 ± 0.02 a | 59.55 ± 0.47 b | ||
| 5 | 1.04 ± 0.04 a | 4.77 ± 0.10 a | 6.54 ± 0.03 a | 0.75 ± 0.01 a | 18.62 ± 0.22 a | 0.40 ± 0.08 a | 0.87 ± 0.03 a | 0.26 ± 0.01 a | 0.01 ± 0.00 a | 0.11 ± 0.00 b | 1.30 ± 0.03 a | 34.68 ± 0.07 a | 0.49 ± 0.06 a | 69.44 ± 0.20 a | ||
| Root | Yunpoong | 3 | 0.01 ± 0.00 b | 2.82 ± 0.02 b | 1.73 ± 0.02 b | 0.27 ± 0.00 ab | 0.80 ± 0.01 b | 0.04 ± 0.00 a | 0.01 ± 0.00 b | 0.06 ± 0.01 a | 0.03 ± 0.00 c | 0.25 ± 0.00 b | 0.45 ± 0.01 a | 6.47 ± 0.03 b | 0.92 ± 0.01 a | 18.51 ± 0.25 b |
| 4 | 3.92 ± 0.17 a | 2.61 ± 0.03 c | 1.36 ± 0.01 c | 0.17 ± 0.08 b | 0.77 ± 0.01 b | 0.05 ± 0.02 a | ND | 0.04 ± 0.01 b | 0.05 ± 0.00 b | 0.23 ± 0.00 c | 0.48 ± 0.16 a | 9.66 ± 0.38 a | 1.24 ± 0.15 a | 18.90 ± 0.34 b | ||
| 5 | 0.37 ± 0.01 b | 4.37 ± 0.03 a | 2.72 ± 0.03 a | 0.40 ± 0.00 a | 1.60 ± 0.01 a | 0.07 ± 0.01 a | 0.04 ± 0.01 a | 0.05 ± 0.01 ab | 0.11 ± 0.00 a | 0.67 ± 0.00 a | 0.22 ± 0.00 a | 10.61 ± 0.08 a | 1.48 ± 0.39 a | 25.52 ± 0.18 a | ||
| K-1 | 3 | 3.59 ± 0.01 a | 2.85 ± 0.01 a | 1.63 ± 0.02 a | 0.27 ± 0.01 a | 0.74 ± 0.02 a | 0.04 ± 0.00 b | 0.01 ± 0.00 a | 0.03 ± 0.00 a | 0.06 ± 0.00 b | 0.36 ± 0.00 a | 0.31 ± 0.01 b | 9.89 ± 0.02 a | 0.39 ± 0.05 b | 16.89 ± 0.05 a | |
| 4 | 3.42 ± 0.05 b | 2.51 ± 0.04 b | 1.16 ± 0.02 c | 0.23 ± 0.01 b | 0.57 ± 0.01 b | 0.04 ± 0.00 ab | 0.02 ± 0.01 a | 0.03 ± 0.00 a | 0.03 ± 0.00 c | 0.31 ± 0.01 b | 0.42 ± 0.01 a | 8.74 ± 0.15 c | 0.43 ± 0.03 ab | 16.31 ± 0.27 a | ||
| 5 | 3.59 ± 0.01 a | 2.51 ± 0.01 b | 1.51 ± 0.01 b | 0.27 ± 0.01 a | 0.70 ± 0.01 a | 0.06 ± 0.01 a | 0.03 ± 0.00 a | 0.04 ± 0.00 a | 0.09 ± 0.00 a | 0.31 ± 0.01 b | 0.30 ± 0.00 b | 9.41 ± 0.03 b | 0.58 ± 0.01 a | 15.29 ± 0.07 b | ||
| Cultivar | WAT | Annual Net Production | |
|---|---|---|---|
| Yield (g m2 Year) | Ginsenoside (mg m2 Year) | ||
| Yunpoong | 3 | 1.72 ± 0.06 a | 102.12 ± 1.01 a |
| 4 | 1.22 ± 0.08 b | 80.28 ± 0.22 b | |
| 5 | 0.96 ± 0.05 c | 69.99 ± 0.64 c | |
| K-1 | 3 | 1.03 ± 0.06 a | 71.26 ± 0.16 a |
| 4 | 0.94 ± 0.05 a | 71.02 ± 0.68 a | |
| 5 | 0.71 ± 0.03 b | 60.18 ± 0.12 b | |
| Significance | |||
| Cultivar (C) | <0.0001 | <0.0001 | |
| Week (W) | <0.0001 | <0.0001 | |
| C × W | 0.0005 | <0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.O.; Jang, S.-N.; Kim, M.J.; Cho, D.Y.; Cho, K.M.; Lee, J.H.; Son, K.-H. Comparison of Growth Patterns and Metabolite Composition of Different Ginseng Cultivars (Yunpoong and K-1) Grown in a Vertical Farm. Horticulturae 2023, 9, 583. https://doi.org/10.3390/horticulturae9050583
Lee GO, Jang S-N, Kim MJ, Cho DY, Cho KM, Lee JH, Son K-H. Comparison of Growth Patterns and Metabolite Composition of Different Ginseng Cultivars (Yunpoong and K-1) Grown in a Vertical Farm. Horticulturae. 2023; 9(5):583. https://doi.org/10.3390/horticulturae9050583
Chicago/Turabian StyleLee, Ga Oun, Seong-Nam Jang, Min Ju Kim, Du Yong Cho, Kye Man Cho, Ji Hyun Lee, and Ki-Ho Son. 2023. "Comparison of Growth Patterns and Metabolite Composition of Different Ginseng Cultivars (Yunpoong and K-1) Grown in a Vertical Farm" Horticulturae 9, no. 5: 583. https://doi.org/10.3390/horticulturae9050583
APA StyleLee, G. O., Jang, S.-N., Kim, M. J., Cho, D. Y., Cho, K. M., Lee, J. H., & Son, K.-H. (2023). Comparison of Growth Patterns and Metabolite Composition of Different Ginseng Cultivars (Yunpoong and K-1) Grown in a Vertical Farm. Horticulturae, 9(5), 583. https://doi.org/10.3390/horticulturae9050583







