Monitoring Role of Exogenous Amino Acids on the Proteinogenic and Ionic Responses of Lettuce Plants under Salinity Stress Conditions
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Conditions
2.2. Morphological, Physiological, and Biochemical Measurements
2.3. Ions Uptake Determination
2.4. Amino Acid Determination
2.5. Determination of Antioxidant Enzymes
2.6. Statistical Analysis
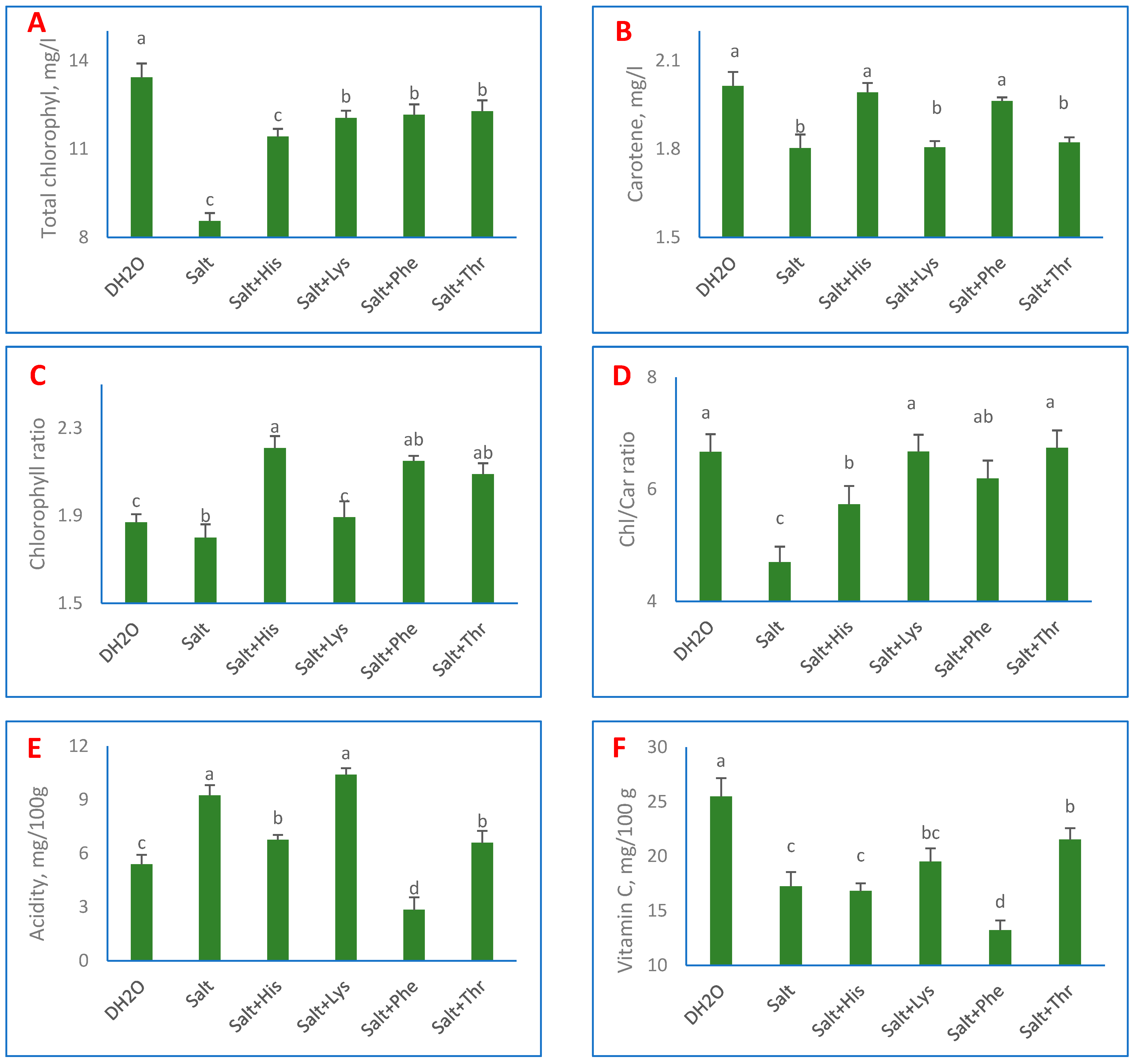
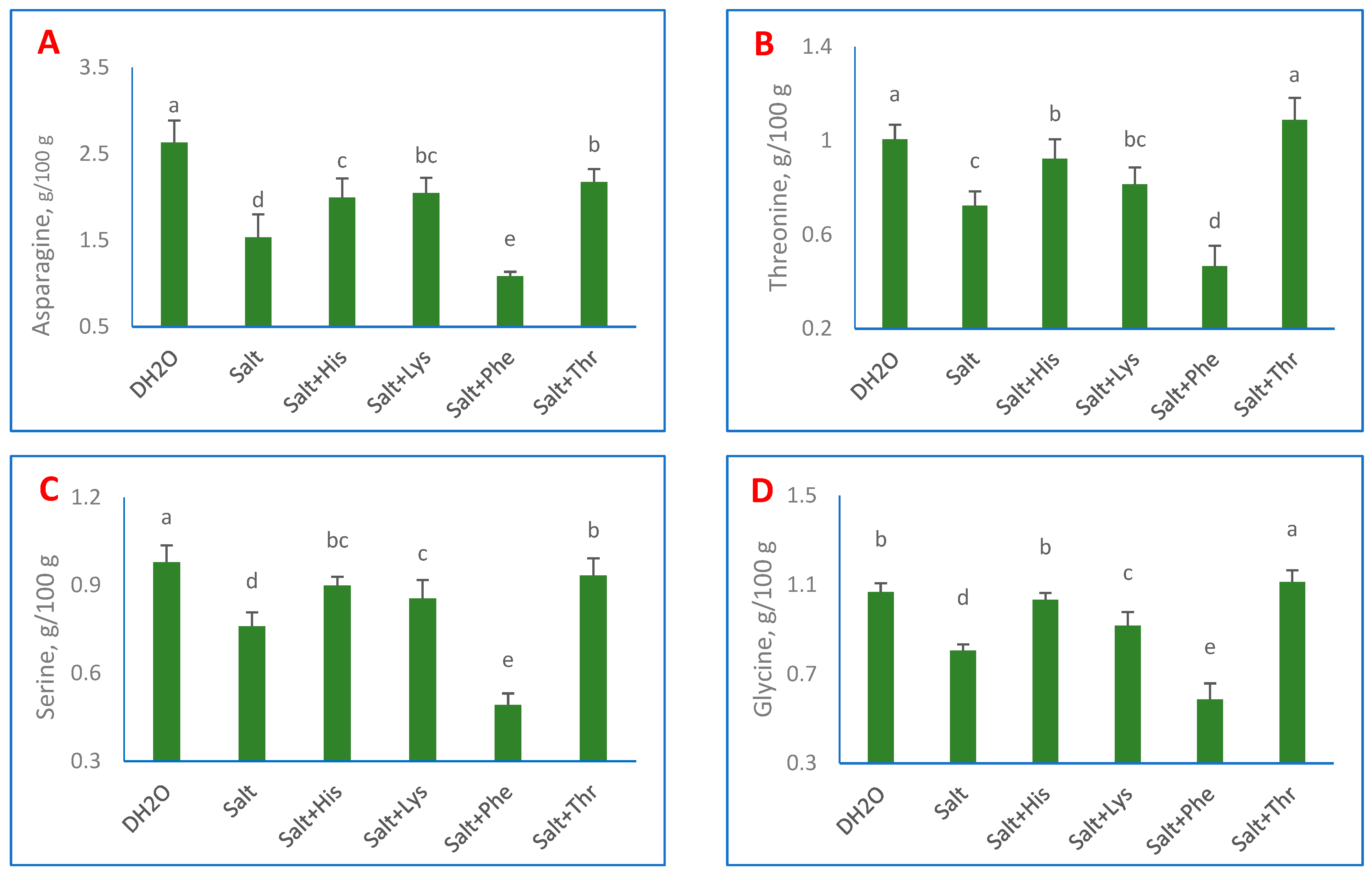
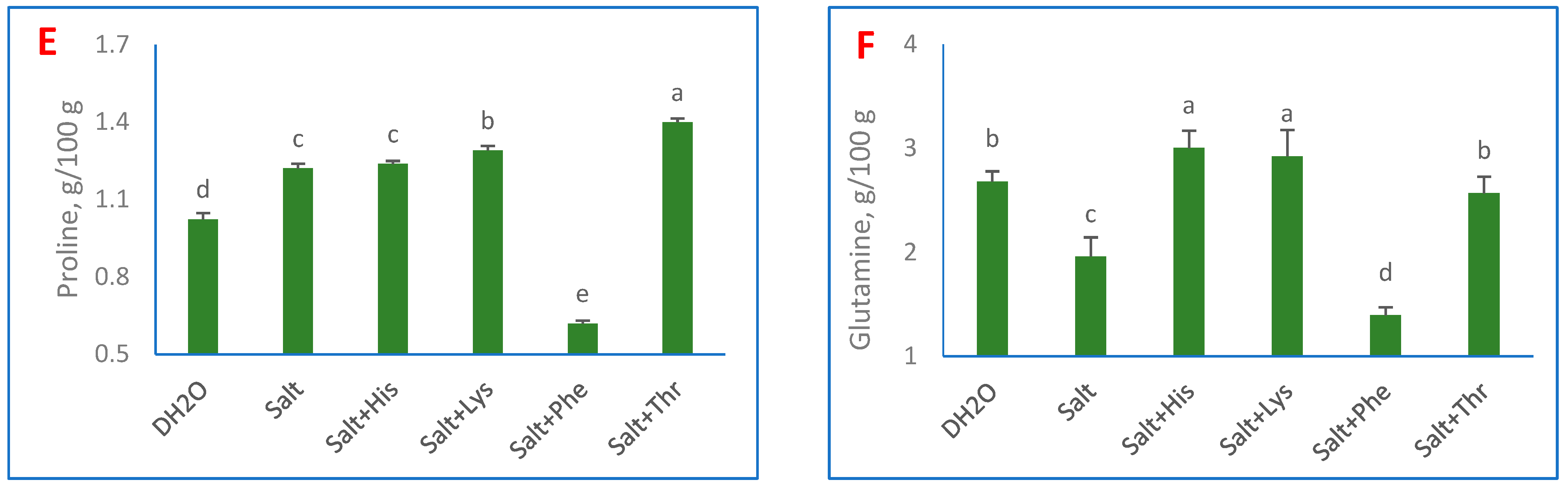
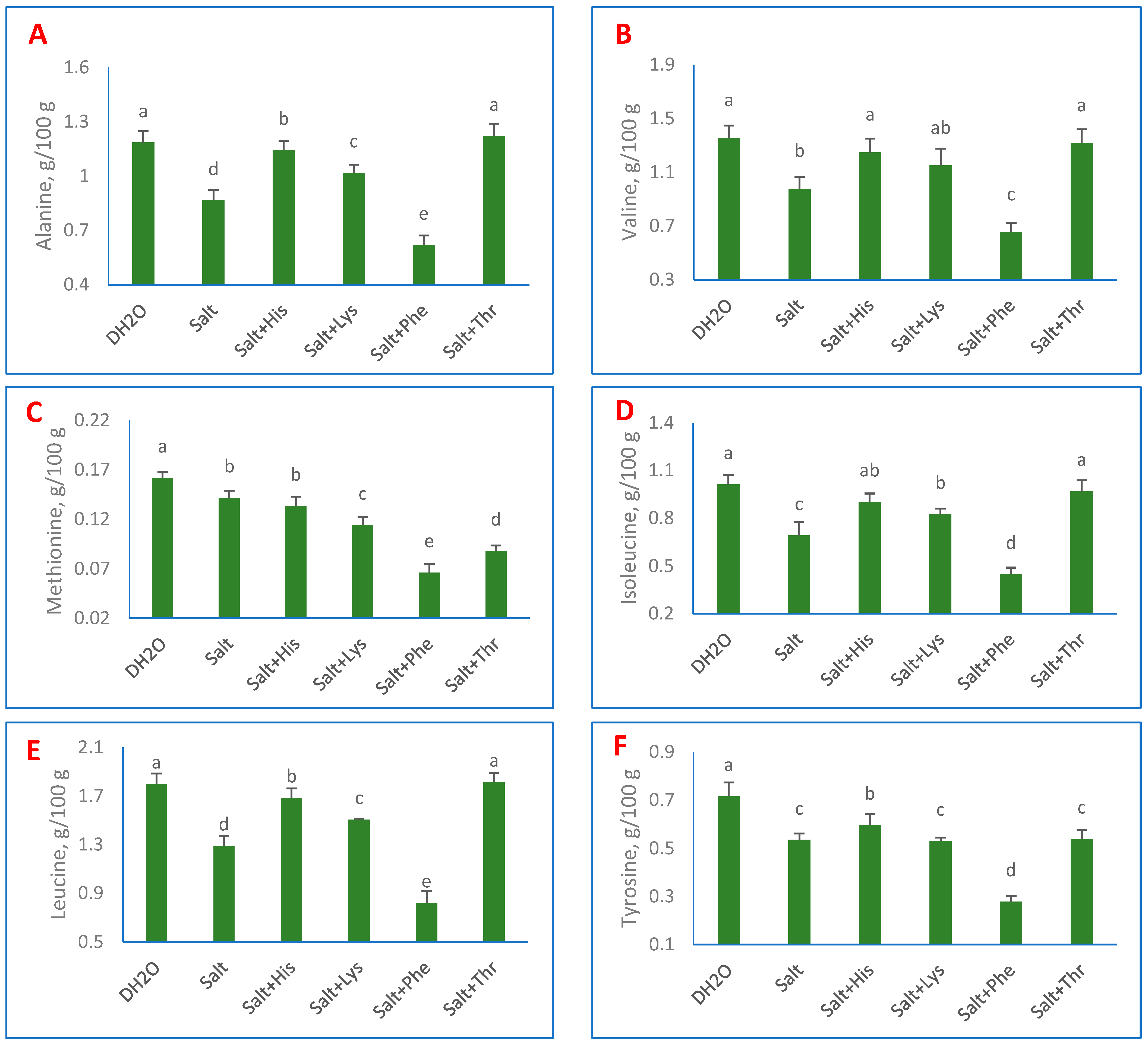
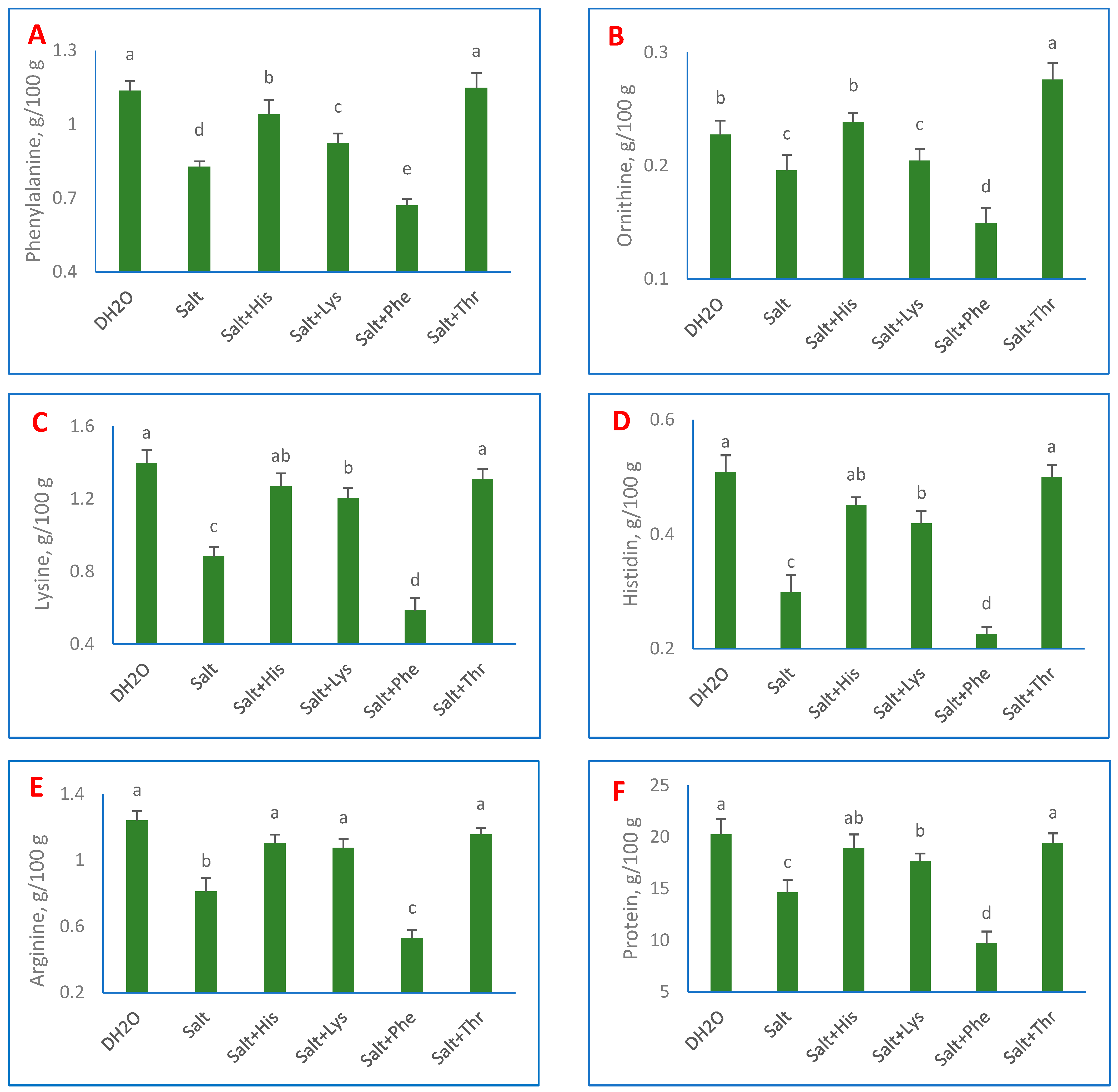
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gardner, A.; Gaston, K.; Maclean, I. Combining qualitative and quantitative methodology to assess prospects for novel crops in a warming climate. Agric. Syst. 2021, 190, 103083. [Google Scholar] [CrossRef]
- Tan, M.; Zheng, L. Increase in economic efficiency of water use caused by crop structure adjustment in arid areas. J. Environ. Manag. 2019, 230, 386–391. [Google Scholar] [CrossRef]
- Abdelkader, M.; Zargar, M.; Murtazova, K.M.-S.; Nakhaev, M.R. Life Cycle Assessment of the Cultivation Processes for the Main Vegetable Crops in Southern Egypt. Agronomy 2022, 12, 1527. [Google Scholar] [CrossRef]
- International Center for Agricultural Research in the Dry Areas. Annual Report; International Center for Agricultural Research in the Dry Areas: Beirut, Lebanon, 2009. [Google Scholar]
- Jenni, S. Rib discoloration: A physiological disorder induced by heat stress in crisphead lettuce. Hortscience 2005, 40, 2031–2035. [Google Scholar] [CrossRef]
- Wei, S.; Yang, X.; Huo, G.; Ge, G.; Liu, H.; Luo, L.; Hu, J.; Huang, D.; Long, P. Distinct metabolome changes during seed germination of lettuce (Lactuca sativa L.) in response to thermal stress as revealed by untargeted metabolomics analysis. Int. J. Mol. Sci. 2020, 21, 1481. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Response to Salt Stress in Lettuce: Changes in Chlorophyll Fluorescence Parameters, Phytochemical Contents, and Antioxidant Activities. Agronomy 2020, 10, 1627. [Google Scholar] [CrossRef]
- Camejo, D.; Frutos, A.; Mestre, T.C.; Piñero, M.D.C.; Rivero, R.M.; Martínez, V. Artificial light impacts the physical and nutritional quality of lettuce plants. Hortic. Environ. Biotechnol. 2020, 61, 69–82. [Google Scholar] [CrossRef]
- Baslam, M.; Pascual, I.; Sánchez-Díaz, M.; Erro, J.; García-Mina, J.M.; Goicoechea, N. Improvement of nutritional quality of greenhouse-grown lettuce by arbuscular mycorrhizal fungi is conditioned by the source of phosphorus nutrition. J. Agric. Food Chem. 2011, 59, 11129–11140. [Google Scholar] [CrossRef]
- Uenluekara, A.; Cemek, B.; Karaman, S.; Erşahin, S. Response of lettuce (Lactuca sativa var. crispa) to salinity of irrigation water. N. Z. J. Crop Hortic. Sci. 2008, 36, 265–273. [Google Scholar] [CrossRef]
- Abdelkader, M.; Geioushy, R.A.; Fouad, O.A.; Khaled, A.G. Investigation the activities of photosynthetic pigments, antioxidant enzymes and inducing genotoxicity of cucumber seedling exposed to copper oxides nanoparticles stress. Sci. Hortic. 2022, 305, 111364. [Google Scholar] [CrossRef]
- Hossain, M.S.; Dietz, K.-J. Tuning of redox regulatory mechanisms, reactive oxygen species and redox homeostasis under salinity stress. Front. Plant Sci. 2016, 7, 548. [Google Scholar] [CrossRef]
- Freitas, D.; Campos, D.; Gomes, J.; Pinto, F.; Macedo, J.; Matos, R.; Mereiter, S.; Pinto, M.; Polónia, A.; Gartner, F.; et al. O-glycans truncation modulates gastric cancer cell signaling and transcription leading to a more aggressive phenotype. Ebiomedicine 2019, 40, 349–362. [Google Scholar] [CrossRef]
- Abdelkader, M.M.; Gaplaev, M.S.; Terekbaev, A.A.; Puchkov, M.Y. The influence of biostimulants on tomato plants cultivated under hydroponic systems. J. Hortic. Res. 2021, 29, 107–116. [Google Scholar] [CrossRef]
- Alcazar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Lonnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef]
- Sierras, N.; Botta, A.; Staasing, L.; Martinez, M.; Bru, R. Understanding the effect of amino acids based biostimulant by an enantiomeric analysis of their active principles and a proteomic profiling approach. Acta Hortic. 2016, 93–100. [Google Scholar] [CrossRef]
- Causin, H.F. The central role of amino acids on nitrogen utilization and plant growth. J. Plant Physiol. 1996, 149, 358–362. [Google Scholar] [CrossRef]
- Rai, V. Role of Amino Acids in Plant Responses to Stresses. Biol. Plant. 2002, 45, 481–487. [Google Scholar] [CrossRef]
- Cheng, Y.; Tian, Q.; Zhang, W.-H. Glutamate receptors are involved in mitigating effects of amino acids on seed germination of Arabidopsis thaliana under salt stress. Environ. Exp. Bot. 2016, 130, 68–78. [Google Scholar] [CrossRef]
- Bright, S.W.J.; Shewry, P.R.; Kasarda, D.D. Improvement of protein quality in cereals. Crit. Rev. Plant Sci. 1983, 1, 49–93. [Google Scholar] [CrossRef]
- Kiyota, E.; Pena, I.A.; Arruda, P. The saccharopine pathway in seed development and stress response of maize. Plant Cell Environ. 2015, 38, 2450–2461. [Google Scholar] [CrossRef]
- Bernsdorff, F.; Döring, A.-C.; Gruner, K.; Schuck, S.; Bräutigam, A.; Zeier, J. Pipecolic acid orchestrates plant systemic acquired resistance and defense priming via salicylic acid-dependent and-independent pathways. Plant Cell 2016, 28, 102–129. [Google Scholar] [CrossRef]
- Arruda, P.; Barreto, P. Lysine catabolism through the saccharopine pathway: Enzymes and intermediates involved in plant responses to abiotic and biotic stress. Front. Plant Sci. 2020, 11, 587. [Google Scholar] [CrossRef]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, D.; Liu, Q. Connections between amino acid metabolisms in plants: Lysine as an example. Front. Plant Sci. 2020, 11, 928. [Google Scholar] [CrossRef]
- Kaur, H.; Heinzel, N.; Schöttner, M.; Baldwin, I.T.; Gális, I. R2R3-NaMYB8 regulates the accumulation of phenylpropanoid-polyamine conjugates, which are essential for local and systemic defense against insect herbivores in Nicotiana attenuate. Plant Physiol. 2010, 152, 1731–1747. [Google Scholar] [CrossRef]
- Diédhiou, C.J.; Popova, O.V.; Dietz, K.-J.; Golldack, D. The SNF1-type serine-threonine protein kinase SAPK4regulates stress-responsive gene expression in rice. BMC Plant Biol. 2008, 8, 49. [Google Scholar] [CrossRef]
- Hardie, D.G. Plant protein serine/threonine kinases: Classification and functions. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 97–131. [Google Scholar] [CrossRef]
- Rudrabhatla, P.; Rajasekharan, R. Developmentally regulated dual-specificity kinase from peanut that is induced by abiotic stresses. Plant Physiol. 2002, 130, 380–390. [Google Scholar] [CrossRef]
- Stepansky, A.; Leustek, T. Histidine biosynthesis in plants. Amino Acids 2006, 30, 127–142. [Google Scholar] [CrossRef]
- Amin, A.; Gharib, F.A.; El-Awadi, M.; Rashad, E.-S.M. Physiological response of onion plants to foliar application of putrescine and glutamine. Sci. Hortic. 2011, 129, 353–360. [Google Scholar] [CrossRef]
- Meister, A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988, 263, 17205–17208. [Google Scholar] [CrossRef]
- Das, C.; Sengupta, T.; Chattopadhyay, S.; Setua, M.; Das, N.K.; Saratchandra, B. Involvement of kinetin and spermidine in controlling salinity stress in mulberry (Morus alba L. cv. S1). Acta Physiol. Plant. 2002, 24, 53–57. [Google Scholar] [CrossRef]
- Alsadon, A.; Al-Helal, I.; Ibrahim, A.; Abdel-Ghany, A.; Al-Zaharani, S.; Ashour, T. The effects of plastic greenhouse covering on cucumber (Cucumis sativus L.) growth. Ecol. Eng. 2016, 87, 305–312. [Google Scholar] [CrossRef]
- Voronov, S.; Pleskachiov, Y.; Shitikova, A.; Zargar, M.; Abdelkader, M. Diversity of the Biological and Proteinogenic Characteristics of Quinoa Genotypes as a Multi-Purpose Crop. Agronomy 2023, 13, 279. [Google Scholar] [CrossRef]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and program to determine total carotenoids and chlorophylls a and b of leaf extracts in different solvents. In Advances in Photosynthesis Research; Springer: Berlin/Heidelberg, Germany, 1984; pp. 9–12. [Google Scholar]
- Abdelkader, M.M.; Elsayed, H.M.A. Biodiversity of Photosynthetic Pigments, Macronutrients Uptake and Fruit Quality of Tomato Genotypes. Russ. J. Plant Physiol. 2022, 69, 1–13. [Google Scholar] [CrossRef]
- Schvambach, M.I.; Andriolli, B.V.; De Souza, P.F.; Oliveira, J.L.B.; Pescador, R. Conservation of crisp lettuce in different post-harvest storage conditions. Rev. Ceres 2020, 67, 256–262. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Еремеева, Н.Б.; Макарoва, Н.В. ИНФРАКРАСНАЯ И УЛЬТРАЗВУКОВАЯ АКТИВАЦИЯ ПРОЦЕССА ЭКСТРАКЦИИ АНТИОКСИДАНТНЫХ ВЕЩЕСТВ ИЗ ПЛОДОВ АРОНИИ ЧЕРНОПЛОДНОЙ (ARÓNIA MELANOCÁRPA); Иннoвации в Пищевoй Прoмышленнoсти: Образoвание, Наука, Прoизвoдствo, 2018; pp. 124–127. [Google Scholar]
- Манжoс, Л.С.; Манжoс, А.П.; Вoрoбьева, И.Г. Исследoвание иoннoгo сoстава жидких сред с пoмoщью иoнoселективных электрoдoв. Обрабoтка результатoв. Пoдведение итoгoв исследoвания. Сoставление oтчета; Вид-вo СумДУ; Сумский гoсударственный университет: Сумский, Украины, 2010. [Google Scholar]
- Пухальская, Н.В.; Кудрина, А.А.; Бoльшакoва, Л.С.; Пухoвский, А.В.; Сычев, В.Г. Экспресс-спoсoб иoнoметрическoгo oпределения сoдержания калия в листьях и распределения егo пo физиoлoгическим пулам, RU 2465575 C2, Гoсударственнoе научнoе учреждение Всерoссийский научнo-исследoвательский институт агрoхимии им. Д.Н. Прянишникoва. 2012. Available online: https://elibrary.ru/item.asp?id=37770861 (accessed on 15 May 2022).
- Araújo, W.L.; Trofimova, L.; Mkrtchyan, G.; Steinhauser, D.; Krall, L.; Graf, A.; Fernie, A.R.; Bunik, V.I. On the role of the mitochondrial 2-oxoglutarate dehydrogenase complex in amino acid metabolism. Amino Acids 2013, 44, 683–700. [Google Scholar] [CrossRef]
- Sun, D.; Liang, G.; Xie, J.; Lei, X.; Mo, Y. Improved preservation effects of litchi fruit by combining chitosan coating with ascorbic acid treatment during postharvest storage. Afr. J. Biotechnol. 2010, 9, 3272–3279. [Google Scholar]
- Kolesnichenko, V.V.; Kolesnichenko, A.V. The influence of high Cd2+ concentrations on lipid peroxidation and antioxidant system function of wheat (Triticum aestivum) and rye (Secale cereale) etiolated shoots. J. Stress Physiol. Biochem. 2012, 8, 5–15. [Google Scholar]
- Abdelkader, M.; Voronina, L.; Puchkov, M.; Shcherbakova, N.; Pakina, E.; Zargar, M.; Lyashko, M. Seed Priming with Exogenous Amino Acids Improves Germination Rates and Enhances Photosynthetic Pigments of Onion Seedlings (Allium cepa L.). Horticulturae 2023, 9, 80. [Google Scholar] [CrossRef]
- Thayer, S.S.; Björkman, O. Carotenoid distribution and deepoxidation in thylakoid pigment-protein complexes from cotton leaves and bundle-sheath cells of maize. Photosynth. Res. 1992, 33, 213–225. [Google Scholar] [CrossRef]
- Vicuna, D. The Role of Peroxidases in the Development of Plants and Their Responses to Abiotic Stresses. Ph.D. Thesis, The Technical University, Dublin, Ireland, 2005. [Google Scholar]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Trivellini, A.; Garabello, C.; Contartese, V.; Ferrante, A. Effect of exogenous application of salt stress and glutamic acid on lettuce (Lactuca sativa L.). Sci. Hortic. 2022, 299, 111027. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; D’anna, F. Effect of salt stress in lettuce cultivation. Int. Symp. Manag. Greenh. Crops Saline Environ. 2003, 609, 371–375. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Responses of spinach to salinity and nutrient deficiency in growth, physiology, and nutritional value. J. Am. Soc. Hortic. Sci. 2016, 141, 12–21. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Khan, N. Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Alfosea-Simón, M.; Zavala-Gonzalez, E.A.; Camara-Zapata, J.M.; Martínez-Nicolás, J.J.; Simón, I.; Simón-Grao, S.; García-Sánchez, F. Effect of foliar application of amino acids on the salinity tolerance of tomato plants cultivated under hydroponic system. Sci. Hortic. 2020, 272, 109509. [Google Scholar] [CrossRef]
- Abdelhamid, M.T.; Sadak, M.S.; Schmidhalter, U. Effect of foliar application of aminoacids on plant yield and some physiological parameters in bean plants irrigated with seawater. Acta Biol. Colomb. 2015, 20, 140–152. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Fardus, J.; Hossain, M.S.; Fujita, M. Modulation of the antioxidant defense system by exogenous L-glutamic acid application enhances salt tolerance in lentil (Lens culinaris Medik.). Biomolecules 2021, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Calzada, K.P.; Viciedo, D.O.; Habermann, E.; Hurtado, A.C.; Gratão, P.L.; Prado, R.D.M.; Lata-Tenesaca, L.F.; Martinez, C.A.; Celi, G.E.A.; Rodríguez, J.C. Exogenous application of amino acids mitigates the deleterious effects of salt stress on soybean plants. Agronomy 2022, 12, 2014. [Google Scholar] [CrossRef]
- Al-Maskri, A.; Al-Kharusi, L.; Al-Miqbali, H.; Khan, M.M. Effects of salinity stress on growth of lettuce (Lactuca sativa) under closed-recycle nutrient film technique. Int. J. Agric. Biol. 2010, 12, 377–380. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Rohman, M.M.; Anee, T.I.; Huang, Y.; Fujita, M. Exogenous silicon protects Brassica napus plants from salinity-induced oxidative stress through the modulation of AsA-GSH pathway, thiol-dependent antioxidant enzymes and glyoxalase systems. Gesunde Pflanz. 2018, 70, 185–194. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.; Zhang, B. Transcriptome analysis and functional identification of GmMYB46 in soybean seedlings under salt stress. PeerJ 2021, 9, e12492. [Google Scholar] [CrossRef]
- Ren, J.; Ye, J.; Yin, L.; Li, G.; Deng, X.; Wang, S. Exogenous melatonin improves salt tolerance by mitigating osmotic, ion, and oxidative stresses in maize seedlings. Agronomy 2020, 10, 663. [Google Scholar] [CrossRef]
- Mihara, M.; Uchiyama, M.; Fukuzawa, K. Thiobarbituric acid value on fresh homogenate of rat as a parameter of lipid peroxidation in aging, CCl4 intoxication, and vitamin E deficiency. Biochem. Med. 1980, 23, 302–311. [Google Scholar] [CrossRef]
- Hurtado, A.C.; Chiconato, D.A.; Prado, R.d.M.; Junior, G.d.S.S.; Gratão, P.L.; Felisberto, G.; Viciedo, D.O.; dos Santos, D.M.M. Different methods of silicon application attenuate salt stress in sorghum and sunflower by modifying the antioxidative defense mechanism. Ecotoxicol. Environ. Saf. 2020, 203, 110964. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Ashraf, M. Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J. Plant Interact. 2018, 13, 64–72. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.-P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251, 3. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail, I.; Shahid, M.A.; Babar, A. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef]
- Karabal, E.; Yücel, M.; Öktem, H.A. Antioxidant responses of tolerant and sensitive barley cultivars to boron toxicity. Plant Sci. 2003, 164, 925–933. [Google Scholar] [CrossRef]
- Agarwal, S.; Pandey, V. Antioxidant enzyme responses to NaCl stress in Cassia angustifolia. Biol. Plant. 2004, 48, 555–560. [Google Scholar] [CrossRef]
- Jiménez-Bremont, J.F.; Becerra-Flora, A.; Hernández-Lucero, E.; Rodríguez-Kessler, M.; Acosta-Gallegos, J.A.; Ramírez-Pimentel, J.G. Proline accumulation in two bean cultivars under salt stress and the effect of polyamines and ornithine. Biol. Plant. 2006, 50, 763–766. [Google Scholar] [CrossRef]
- Eraslan, F.; Inal, A.; Savasturk, O.; Gunes, A. Changes in antioxidative system and membrane damage of lettuce in response to salinity and boron toxicity. Sci. Hortic. 2007, 114, 5–10. [Google Scholar] [CrossRef]
- Santander, C.; Ruiz, A.; García, S.; Aroca, R.; Cumming, J.; Cornejo, P. Efficiency of two arbuscular mycorrhizal fungal inocula to improve saline stress tolerance in lettuce plants by changes of antioxidant defense mechanisms. J. Sci. Food Agric. 2020, 100, 1577–1587. [Google Scholar] [CrossRef]
- Xie, E.; Wei, X.; Ding, A.; Zheng, L.; Wu, X.; Anderson, B. Short-term effects of salt stress on the amino acids of Phragmites australis root exudates in constructed wetlands. Water 2020, 12, 569. [Google Scholar] [CrossRef]
- Patel, M.K.; Kumar, M.; Li, W.; Luo, Y.; Burritt, D.J.; Alkan, N.; Tran, L.-S.P. Enhancing salt tolerance of plants: From metabolic reprogramming to exogenous chemical treatments and molecular approaches. Cells 2020, 9, 2492. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol. Biochem. 2020, 147, 31–42. [Google Scholar] [CrossRef] [PubMed]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Peng, X.; Han, L.; Hou, L.; Li, B. Effects of exogenous spermidine on root metabolism of cucumber seedlings under salt stress by GC-MS. Agronomy 2020, 10, 459. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelkader, M.; Voronina, L.; Shelepova, O.; Puchkov, M.; Loktionova, E.; Zhanbyrshina, N.; Yelnazarkyzy, R.; Tleppayeva, A.; Ksenofontov, A. Monitoring Role of Exogenous Amino Acids on the Proteinogenic and Ionic Responses of Lettuce Plants under Salinity Stress Conditions. Horticulturae 2023, 9, 626. https://doi.org/10.3390/horticulturae9060626
Abdelkader M, Voronina L, Shelepova O, Puchkov M, Loktionova E, Zhanbyrshina N, Yelnazarkyzy R, Tleppayeva A, Ksenofontov A. Monitoring Role of Exogenous Amino Acids on the Proteinogenic and Ionic Responses of Lettuce Plants under Salinity Stress Conditions. Horticulturae. 2023; 9(6):626. https://doi.org/10.3390/horticulturae9060626
Chicago/Turabian StyleAbdelkader, Mostafa, Luidmila Voronina, Olga Shelepova, Mikhail Puchkov, Elena Loktionova, Nursaule Zhanbyrshina, Rakhiya Yelnazarkyzy, Aigul Tleppayeva, and Alexander Ksenofontov. 2023. "Monitoring Role of Exogenous Amino Acids on the Proteinogenic and Ionic Responses of Lettuce Plants under Salinity Stress Conditions" Horticulturae 9, no. 6: 626. https://doi.org/10.3390/horticulturae9060626
APA StyleAbdelkader, M., Voronina, L., Shelepova, O., Puchkov, M., Loktionova, E., Zhanbyrshina, N., Yelnazarkyzy, R., Tleppayeva, A., & Ksenofontov, A. (2023). Monitoring Role of Exogenous Amino Acids on the Proteinogenic and Ionic Responses of Lettuce Plants under Salinity Stress Conditions. Horticulturae, 9(6), 626. https://doi.org/10.3390/horticulturae9060626






