Effects of Ethylene and 1-Methylcyclopropene on the Quality of Sweet Potato Roots during Storage: A Review
Abstract
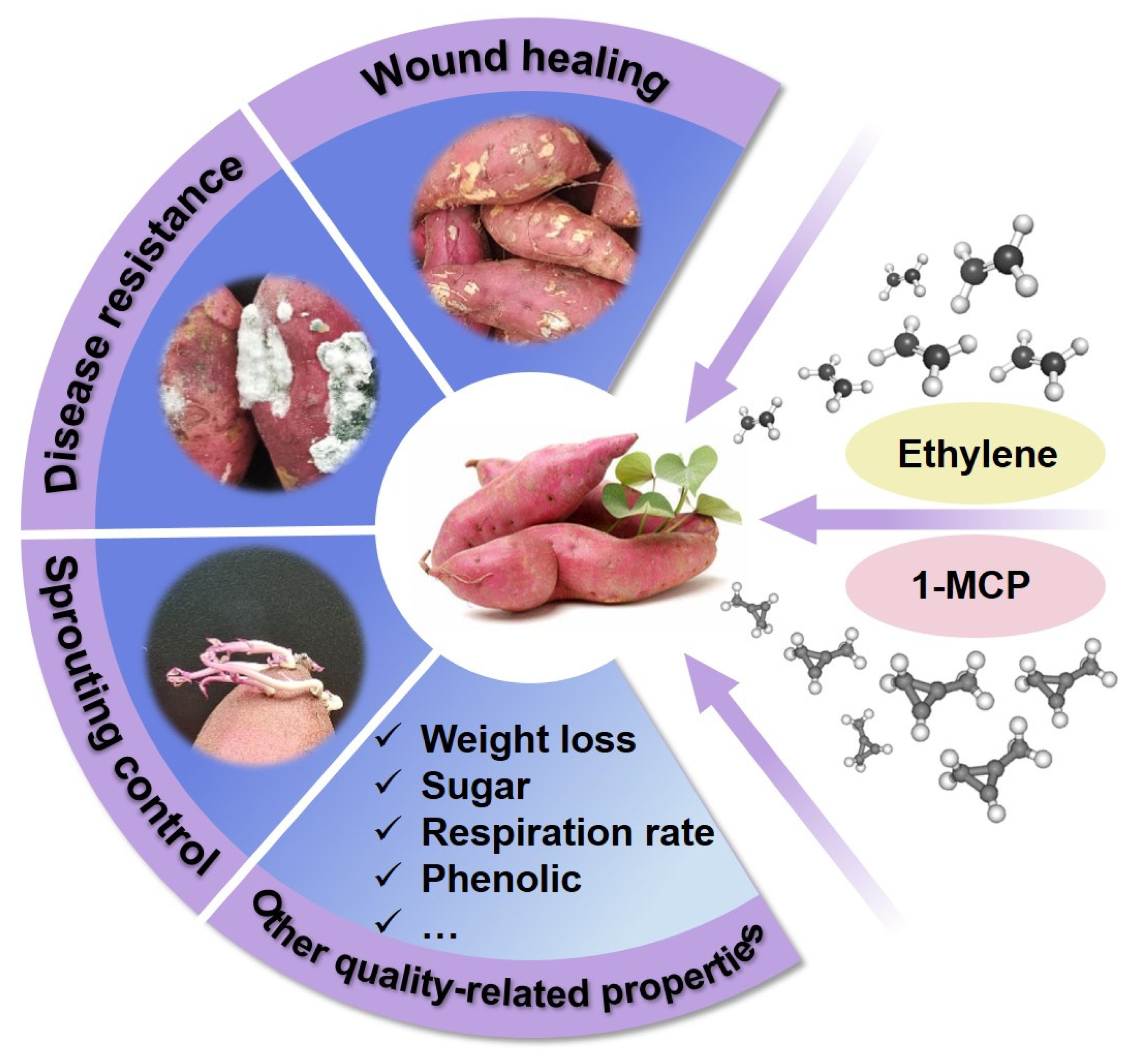
1. Introduction
2. Effects of Ethylene and 1-Methylcyclopropene (1-MCP) on Sprouting Control of Sweet Potato Roots
3. Effects of Ethylene and 1-Methylcyclopropene (1-MCP) on Disease Resistance of Sweet Potato Roots
4. Effects of Ethylene and 1-Methylcyclopropene (1-MCP) on Wound Healing of Sweet Potato Roots
5. Effects of Ethylene and 1-Methylcyclopropene (1-MCP) on Other Quality-Related Properties of Sweet Potato Roots
5.1. Weight Loss
5.2. Respiration Rate
5.3. Sugar Content
5.4. Phenolic Compounds
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Behera, S.; Chauhan, V.B.S.; Pati, K.; Bansode, V.; Nedunchezhiyan, M.; Verma, A.K.; Monalisa, K.; Naik, P.K.; Naik, S.K. Biology and biotechnological aspect of sweet potato (Ipomoea batatas L.): A commercially important tuber crop. Planta 2022, 256, 40. [Google Scholar] [CrossRef]
- Xu, X.; Wu, S.; Chen, K.; Zhang, H.; Zhou, S.; Lv, Z.; Chen, Y.; Cui, P.; Cui, Z.; Lu, G. Comprehensive Evaluation of Raw Eating Quality in 81 Sweet Potato (Ipomoea batatas (L.) Lam) Varieties. Foods 2023, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Drapal, M.; Gerrish, C.; Fraser, P.D. Changes in carbon allocation and subplastidal amyloplast structures of specialised Ipomoea batatas (sweet potato) storage root phenotypes. Phytochemistry 2022, 203, 113409. [Google Scholar] [CrossRef]
- Tang, C.; Lu, Y.; Jiang, B.; Chen, J.; Mo, X.; Yang, Y.; Wang, Z. Energy, Economic, and Environmental Assessment of Sweet Potato Production on Plantations of Various Sizes in South China. Agronomy 2022, 12, 1290. [Google Scholar] [CrossRef]
- Pazos, J.; Zema, P.; Corbino, G.B.; Gabilondo, J.; Borioni, R.; Malec, L.S. Growing location and root maturity impact on the phenolic compounds, antioxidant activity and nutritional profile of different sweet potato genotypes. Food Chem. Mol. Sci. 2022, 5, 100125. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Puentes, A.A.; Palomo, I.; Rodríguez, L.; Fuentes, E.; Villegas-Ochoa, M.A.; González-Aguilar, G.A.; Olivas-Aguirre, F.J.; Wall-Medrano, A. Sweet potato (Ipomoea batatas L.) phenotypes: From agroindustry to health effects. Foods 2022, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Ye, S.; Liao, W.; Wu, M.; He, J.; Mateus, N.; Oliveira, H. The botanical profile, phytochemistry, biological activities and protected-delivery systems for purple sweet potato (Ipomoea batatas (L.) Lam.): An up-to-date review. Food Res. Int. 2022, 161, 111811. [Google Scholar] [CrossRef]
- Alam, M.K. A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- FAOSTAT. Statistics Division of Food and Agriculture Organization of the United Nations. 2021. Available online: http://www.fao.org/faostat/zh/#data/QC (accessed on 10 January 2023).
- Sugri, I.; Maalekuu, B.K.; Kusi, F.; Gaveh, E. Quality and shelf-life of sweet potato as influenced by storage and postharvest treatments. Trends Hortic. Res. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Dash, S.K.; Rayaguru, K. Post-harvest processing and utilization of sweet potato: A review. Food Rev. Int. 2019, 35, 726–762. [Google Scholar]
- Sanchez, P.D.C.; Hashim, N.; Shamsudin, R.; Nor, M.Z.M. Laser-light backscattering imaging approach in monitoring and classifying the quality changes of sweet potatoes under different storage conditions. Postharvest Biol. Technol. 2020, 164, 111163. [Google Scholar] [CrossRef]
- Zaccari, F.; Cabrera, M.; Saadoun, A. Sweet potato and squash storage. Encycl. Food Secur. Sustain. 2018, 2, 464–472. [Google Scholar]
- Ishiguro, K.; Yahara, S.; Yoshimoto, M. Changes in polyphenolic content and radical-scavenging activity of sweetpotato (Ipomoea batatas L.) during storage at optimal and low temperatures. J. Agric. Food Chem. 2007, 55, 10773–10778. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, N.O.; de Sousa Santos, M.N.; de Araujo, F.F.; Véras, M.L.M.; de Jesus Tello, J.P.; da Silva Arruda, R.; Fugate, K.K.; Finger, F.L. Balance between oxidative stress and the antioxidant system is associated with the level of cold tolerance in sweet potato roots. Postharvest Biol. Technol. 2021, 172, 111359. [Google Scholar] [CrossRef]
- Huang, C.; Xu, M.; Lo, S.; Lai, Y. Effects of treatments of sprouting inhibitor CIPC and Co60 on sprouting and decaying rates of the storage roots of sweet potato. J. Taiwan Agric. Res. 2018, 67, 73–81. [Google Scholar]
- Yang, S.F.; Hoffman, N.E. Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Schotsmans, W.C.; Prange, R.K.; Binder, B.M. 1-Methylcyclopropene: Mode of action and relevance in postharvest horticulture research. Hortic. Res. 2009, 35, 263–313. [Google Scholar]
- Blankenship, S.M.; Dole, J.M. 1-Methylcyclopropene: A review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Pankomera, P. Effects of Postharvest Treatments on Sweet Potato (Ipomoea batatas) Storage Quality. Ph.D. Thesis, Massey University, Auckland, New Zealand, 2015. [Google Scholar]
- Amoah, R.; Terry, L.A. Biochemical and physiological changes in stored sweet potatoes as mediated by 1-methylcyclopropene (1-MCP). In VII International Postharvest Symposium 1012; International Society for Horticultural Science: Leuven, Belgium, 2012; pp. 345–351. [Google Scholar]
- Ballesteros-Mártinez, L.; Pérez-Cervera, C.; Andrade-Pizarro, R. Effect of glycerol and sorbitol concentrations on mechanical, optical, and barrier properties of sweet potato starch film. NFS J. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Alamar, M.C.; Anastasiadi, M.; Lopez-Cobollo, R.; Bennett, M.H.; Thompson, A.J.; Turnbull, C.G.; Mohareb, F.; Terry, L.A. Transcriptome and phytohormone changes associated with ethylene-induced onion bulb dormancy. Postharvest Biol. Technol. 2020, 168, 111267. [Google Scholar] [CrossRef]
- Yang, X.; Chen, L.; Yang, Y.; Guo, X.; Chen, G.; Xiong, X.; Dong, D.; Li, G. Transcriptome analysis reveals that exogenous ethylene activates immune and defense responses in a high late blight resistant potato genotype. Sci. Rep. 2020, 10, 21294. [Google Scholar] [CrossRef]
- Ji, C.Y.; Kim, Y.H.; Lee, C.J.; Park, S.U.; Lee, H.U.; Kwak, S.S.; Kim, H.S. Comparative transcriptome profiling of sweetpotato storage roots during curing-mediated wound healing. Gene 2022, 833, 146592. [Google Scholar] [CrossRef]
- Geng, S.; Liu, Z.; Golding, J.B.; Pristijono, P.; Lv, Z.; Lu, G.; Yang, H.; Ru, L.; Li, Y. Transcriptomic analyses of carvone inhibited sprouting in sweet potato (Ipomoea batatas (L.) Lam cv ‘Yan 25’) storage roots. Postharvest Biol. Technol. 2023, 195, 112142. [Google Scholar] [CrossRef]
- Xie, Z.; Zhou, Z.; Li, H.; Yu, J.; Jiang, J.; Tang, Z.; Ma, D.; Zhang, B.; Han, Y.; Li, Z. High throughput sequencing identifies chilling responsive genes in sweetpotato (Ipomoea batatas Lam.) during storage. Genomics 2019, 111, 1006–1017. [Google Scholar] [CrossRef]
- Ji, C.Y.; Kim, H.S.; Lee, C.J.; Kim, S.E.; Lee, H.U.; Nam, S.S.; Li, Q.; Ma, D.F.; Kwak, S.S. Comparative transcriptome profiling of tuberous roots of two sweet potato lines with contrasting low temperature tolerance during storage. Gene 2020, 727, 144244. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Cai, Z.; Huang, J.; Wang, A.; Ntambiyukuri, A.; Chen, B.; Zheng, G.; Li, H.; Huang, Y.; Zhan, J.; et al. Transcriptomic analysis of tuberous root in two sweet potato varieties reveals the important genes and regulatory pathways in tuberous root development. BMC Genom. 2022, 23, 473. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Jang, M.; Nie, H.; Lee, J.; Hong, E.; Kim, S.J.; Kim, S.H. Differential expression pattern of novel MADS-box genes in early root formation and differentiation of sweet potato. Gene Expr. Patterns 2022, 43, 119216. [Google Scholar] [CrossRef] [PubMed]
- Dako, E.; Jankowski, C.K.; Gnimassou, Y.M.; Lebeau, D. Study of inhibition of germination of potato by ethylene. Heliyon 2021, 7, e06175. [Google Scholar] [CrossRef]
- Foukaraki, S.G.; Cools, K.; Chope, G.A.; Terry, L.A. Impact of ethylene and 1-MCP on sprouting and sugar accumulation in stored potatoes. Postharvest Biol. Technol. 2016, 114, 95–103. [Google Scholar] [CrossRef]
- Lv, J.; Bai, L.; Han, X.; Xu, D.; Ding, S.; Li, C.; Ge, Y.; Li, J. Effects of 1-MCP treatment on sprouting and preservation of ginger rhizomes during storage at room temperature. Food Chem. 2021, 349, 129004. [Google Scholar] [CrossRef] [PubMed]
- Downes, K.; Chope, G.A.; Terry, L.A. Postharvest application of ethylene and 1-methylcyclopropene either before or after curing affects onion (Allium cepa L.) bulb quality during long term cold storage. Postharvest Biol. Technol. 2010, 55, 36–44. [Google Scholar] [CrossRef]
- Cheema, M.U.A.; Rees, D.; Colgan, R.J.; Taylor, M.; Westby, A. The effects of ethylene, 1-MCP and AVG on sprouting in sweetpotato roots. Postharvest Biol. Technol. 2013, 85, 89–93. [Google Scholar] [CrossRef]
- Pankomera, P.; Heyes, J.A.; Lewthwaite, S.L.; Roskruge, N. Effects of ethylene and 1-methylcyclopropene on sweetpotato storage root quality. Acta Hortic. 2016, 1118, 163–169. [Google Scholar] [CrossRef]
- Amoah, R.S.; Landahl, S.; Terry, L.A. The timing of exogenous ethylene supplementation differentially affects stored sweetpotato roots. Postharvest Biol. Technol. 2016, 120, 92–102. [Google Scholar] [CrossRef]
- Villordon, A.; Clark, C.; LaBonte, D.; Firon, N. 1-Methylcyclopropene has a variable effect on adventitious root emergence from cuttings of two sweetpotato cultivars. HortScience 2012, 47, 1764–1767. [Google Scholar] [CrossRef]
- Cheema, M.U.A.; Rees, D.; Westby, A.; Taylor, M. Hormonal control of sprouting of sweetpotatoes in storage. In III International Conference Postharvest Unlimited; International Society for Horticultural Science: Leuven, Belgium, 2008; Volume 858, pp. 173–177. [Google Scholar]
- Paul, N.C.; Park, S.; Liu, H.; Lee, J.G.; Han, G.H.; Kim, H.; Sang, H. Fungi Associated with Postharvest Diseases of Sweet Potato Storage Roots and In Vitro Antagonistic Assay of Trichoderma harzianum against the Diseases. J. Fungi 2021, 7, 927. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.C.; Ravi, V. Postharvest spoilage of sweetpotato in tropics and control measures. Crit. Rev. Food Sci. 2005, 45, 623–644. [Google Scholar] [CrossRef]
- Holmes, G.J.; Stange, R.R. Influence of Wound Type and Storage Duration on Susceptibility of Sweetpotatoes to Rhizopus Soft Rot. Plant Dis. 2002, 86, 345–348. [Google Scholar] [CrossRef]
- Bagam, P.H.; Kamble, S.S. Sensitivity of Sclerotium rolfsii sacc., causing tuber rot of sweet potato, to carbendazim. BIOINFOLET-Q. J. Life Sci. 2022, 19, 71. [Google Scholar]
- Paul, N.C.; Nam, S.S.; Park, W.; Yang, J.W.; Kachroo, A. First report of storage tuber rot in sweetpotato (Ipomoea batatas) caused by Plenodomus destruens in Korea. Plant Dis. 2019, 103, 1020. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, M.; Pan, S.; Pan, C.; Li, Y.; Tian, J. Perillaldehyde controls postharvest black rot caused by Ceratocystis fimbriata in sweet potatoes. Front. Microbiol. 2018, 9, 1102. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Li, T.J.; Liu, Y.F.; Zhang, J.; Zhang, Y.; Shen, X.Q.; Li, X.Y.; Miao, X.M.; Feng, Z.Z.; Peng, X.; et al. Antifungal and eliciting properties of chitosan against Ceratocystis fimbriata in sweet potato. Food Chem. 2018, 268, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gao, B.; Ma, J.; Li, X.; Li, J.; Chen, S. Mottle necrosis caused by Globisporangium ultimum var. ultimum on sweet potato roots during harvest and storage in China. J. Phytopathol. 2022, 170, 693–699. [Google Scholar]
- Pan, C.; Yang, K.; Erhunmwunsee, F.; Li, Y.X.; Liu, M.; Pan, S.; Yang, D.; Lu, G.; Ma, D.; Tian, J. Inhibitory effect of cinnamaldehyde on Fusarium solani and its application in postharvest preservation of sweet potato. Food Chem. 2023, 408, 135213. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Y.; Golding, J.B.; Geng, S.; Chen, G.; Yang, H. Metabolomic and Transcriptomic Analyses of Quality Deterioration in Fusarium solani-Infected Sweet Potato (Ipomoea batatas (L.) Lam cv Xinxiang) Storage Roots. J. Agric. Food Chem. 2022, 70, 7258–7266. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Wang, Y.Z.; Chu, Z.H.; Wang, P.S.; Liu, B.Y.; Li, B.Y.; Li, Y.X.; Luan, B.H. Endophytic Bacillus amyloliquefaciens YTB1407 elicits resistance against two fungal pathogens in sweet potato (Ipomoea batatas (L.) Lam.). J. Plant Physiol. 2020, 253, 153260. [Google Scholar] [CrossRef]
- Stahmann, M.A.; Clare, B.G.; Woodbury, W. Increased disease resistance and enzyme activity induced by ethylene and ethylene production of black rot infected sweet potato tissue. Plant Physiol. 1966, 41, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, R.A.; Main, J.L.; Clark, C.A. Sweetpotato Tip Rot Incidence Is Increased by Preharvest Applications of Ethephon and Reduced by Curing. Horttechnology 2013, 23, 288–293. [Google Scholar] [CrossRef]
- Cao, J.; Liu, P.; Wang, X.; Wang, Q.; Shi, J. Combination of wound healing with 1-methylcyclopropene and wound detection by iodine solution to maintain the quality of sweet potato during long-term storage. Int. J. Agric. Biol. Eng. 2021, 14, 241–246. [Google Scholar] [CrossRef]
- Amoah, R.S.; Terry, L.A. 1-Methylcyclopropene (1-MCP) effects on natural disease resistance in stored sweet potato. J. Sci. Food Agric. 2018, 98, 4597–4605. [Google Scholar] [CrossRef]
- van Loon, L.C.; Geraats, B.P.; Linthorst, H.J. Ethylene as a modulator of disease resistance in plants. Trends Plant Sci. 2006, 11, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Harper, G. 1-Methylcyclopropene: A Review of Its Use on Potato Tubers; Sutton Bridge Crop Storage Research, Potato Council: Warwickshire, UK, 2015; p. 8. [Google Scholar]
- Morris, L.; Mann, L. Wound healing, keeping quality, and compositional changes during curing and storage of sweet potatoes. Hilgardia 1955, 24, 143–183. [Google Scholar] [CrossRef]
- Sugri, I.; Maalekuu, B.K.; Gaveh, E.; Kusi, F. Compositional and shelf-life indices of sweet potato are significantly improved by pre-harvest dehaulming. Ann. Agric. Sci. 2019, 64, 113–120. [Google Scholar] [CrossRef]
- Van Oirschot, Q.E.A.; Rees, D.; Aked, J.; Kihurani, A. Sweetpotato cultivars differ in efficiency of wound healing. Postharvest Biol. Technol. 2006, 42, 65–74. [Google Scholar] [CrossRef]
- Rees, D.; van Oirschot, Q.E.; Aked, J. The role of carbohydrates in wound-healing of sweetpotato roots at low humidity. Postharvest Biol. Technol. 2008, 50, 79–86. [Google Scholar] [CrossRef]
- Atuna, R.A.; Carey, E.E.; Low, J.W.; Amagloh, F.K. Wound healing and dry matter content of orange-fleshed sweetpotato cultivars as influenced by curing methods. Open Agric. 2017, 2, 274–279. [Google Scholar] [CrossRef]
- Artschwager, E.; Starrett, R.C. Suberization and wound-periderm formation in sweetpotato and gladiolus as affected by temperature and relative humidity. J. Agric. Res. 1931, 43, 353–364. [Google Scholar]
- Walter, W.M.; Schadel, W.E. A Rapid Method for Evaluating Curing Progress in Sweet Potatoes1. J. Am. Soc. Hortic. Sci. 1982, 107, 1129–1133. [Google Scholar] [CrossRef]
- Randle, W.M.; Woodson, W.R. The effect of storage and wounding on ethylene production by sweet potato. HortScience 1986, 21, 1018–1019. [Google Scholar] [CrossRef]
- Saltveit, M.E.; Locy, R.D. Cultivar Differences in Ethylene Production by Wounded Sweet Potato Roots1. J. Am. Soc. Hortic. Sci. 1982, 107, 1114–1117. [Google Scholar] [CrossRef]
- Cabrera, R.M.; Saltveit, M.E. Survey of wound-induced ethylene production by excised root segments. Physiol. Plant. 2003, 119, 203–210. [Google Scholar] [CrossRef]
- Amand, P.S.; Randle, W.M. Ethylene production and wound healing in sweet potato roots. HortScience 1989, 24, 805–807. [Google Scholar] [CrossRef]
- Wang, S.J.; Lan, Y.C.; Chen, S.F.; Chen, Y.M.; Yeh, K.W. Wound-response regulation of the sweet potato sporamin gene promoter region. Plant Mol. Biol. 2002, 48, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.H.; Yousef, G.G.; Gustafson, S.J.; Truong, V.D.; Yencho, G.C.; Lila, M.A. Phytochemical changes in phenolics, anthocyanins, ascorbic acid, and carotenoids associated with sweetpotato storage and impacts on bioactive properties. Food Chem. 2014, 145, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Dandago, M.A.; Gungula, D.T. Effects of various storage methods on the quality and nutritional composition of sweet potato (Ipomea batatas L.) in Yola Nigeria. Int. Food Res. J. 2011, 18, 271–278. [Google Scholar]
- Cheema, M.U.A. Dormancy and Sprout Control in Root and Tuber Crops. Ph.D. Thesis, University of Greenwich, London, UK, 2010. [Google Scholar]
- Lima, P.C.C.; Santos, M.N.D.S.; Guimaraes, M.E.D.S.; Araujo, N.O.D.; Krause, M.R.; Finger, F.L. Ethylene and its inhibitors affect the quality of processed sweet potatoes. Food Sci. Technol. 2021, 41, 825–832. [Google Scholar] [CrossRef]
- Sowe, S. The Effects of Controlled Atmosphere and Ethylene on the Postharvest Quality of Sweet Potato during Storage. Ph.D. Thesis, Cranfield University, Cranfield, UK, 2018. [Google Scholar]
- Villordon, A.Q. Differential response of ‘Beauregard’ sweetpotato storage roots to ethephon, 1-methylcyclopropene and water submergence treatments. HortScience 2012, 47, S324. [Google Scholar]
- Han, S.H.; Jang, H.D.; Lee, S.J. Modeling respiration rates of Ipomoea batatas (sweet potato) under hermetic storage system. Food Sci. Biotechnol. 2020, 29, 227–234. [Google Scholar] [CrossRef]
- Jung, J.K.; Lee, S.U.; Kozukue, N.; Levin, C.E.; Friedman, M. Distribution of phenolic compounds and antioxidative activities in parts of sweet potato (Ipomoea batata L.) plants and in home processed roots. J. Food Compos. Anal. 2011, 24, 29–37. [Google Scholar] [CrossRef]
| Cultivars | Storage Conditions | Applications | Treatments | Quality-Related Properties | Reference |
|---|---|---|---|---|---|
| Organic ‘Covington’ and Portuguese-derived ‘Covington’ | Cold storage at 15 °C | Sprouting control and disease resistance | 1-MCP (1 µL L−1, 24 h) | Reduced sprouting and decay, phenolic compounds; no effect on respiration rate and sugar, maintained saleable weight | Amoah et al., 2012 [20] |
| ‘Covington’ | Cured (30 ºC, 90% relative humidity, 7 days) then stored at 25 °C | Sprouting control | Ethylene (10 μL L−1, applied continuously) | Reduced sugar, phenolic compounds and phytohormones (abscisic acid and zeatin riboside); suppressed sprout growth, doubled root respiration; increased weight loss and incidence of proximal rots | Amoah et al., 2016 [37] |
| ‘Covington’ | Cold storage at 15 °C | Disease resistance | 1-MCP (1 µL L−1, 24 h) | Reduced decay, weight loss; no effect on respiration rate and carbohydrates | Amoah et al., 2018 [54] |
| ‘Bushbuck’ | 25 °C in incubators | Sprouting control | 1-MCP (625 nl L−1, 24 h) | Inhibited sprouting; reduced respiration rate, weight loss, sugar content (sucrose, glucose and fructose) | Cheema et al., 2010 [71]; Cheema et al., 2013 [35], |
| Ethylene (10 ppm, applied continuously) | Inhibited sprouting; increased respiration rate (3-fold), weight loss (slightly), sucrose; reduced glucose and fructose | ||||
| ‘Beijing 553’ and ‘Chuanshanzi’ | Curing at 29 °C for 4 days, then stored at (13 ± 0.5) °C, 90% relative humidity | Sprouting control and disease resistance | 0.045% 1-MCP cyclodextrin powder, 1.6 g/case | Improved wound healing; inhibited sprouting and decay; increased sugar content; decreased starch content, no color change | Cao et al., 2021 [53] |
| ‘BRS Rubissol’ | Curing at 30 °C and 90% relative humidity for 7 days, stored in chambers at 25 °C and 90% relative humidity | Sprouting control | 1-MCP 1 mg·L−1 in 90 L chamber for 24 h | Reduced sprouting, weight loss; increased dry matter content; processed fried chips showed less browning | Lima et al., 2021 [72] |
| Ethylene 10 μL·L−1 in 90 L chamber for 48 h | Reduced sprouting, weight loss; increased dry matter content | ||||
| ‘Owairaka Red’ | Curing at 30 °C and 90% relative humidity for 4 days then stored at 25 °C and 85% relative humidity | Sprouting control | 1-MCP (1 µL L−1, 24 h) and continuous ethylene (10 µL L−1) | Inhibited sprout growth; increased root respiration rates and weight loss; no color change after cook | Pankomera et al., 2016 [36] |
| 1-MCP (1 µL L−1, 24 h) | No significantly differ from the control | ||||
| Ethylene (10 µL L−1, applied continuously) | Inhibited sprout growth; increased root respiration rates and weight loss; darken cooked flesh color | ||||
| ‘Belle Vue’ | Curing for 4 days (25–30 °C) then stored at 20 °C | Sprouting control | Ethylene (0.001 kPa, applied continuously) with controlled atmosphere | Reduced sprouting; increased phenolics contents, sugars, weight loss and respiration rates | Sowe et al., 2018 [73] |
| ‘Beauregard’ | Curing at 85 °F and 85% relative humidity for 5 days then stored at 60 °F and 75–85% relative humidity | Wound healing | Dipping 1 h in 1-MCP (1 ppm) or ethephon (2.6 mM) | Breakdown-related features on skin appeared after ethephon treatment, not detected in 1-MCP treated roots | Villordon et al., 2012 [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kou, J.; Zang, X.; Li, M.; Li, W.; Zhang, H.; Chen, Y.; Zhu, G. Effects of Ethylene and 1-Methylcyclopropene on the Quality of Sweet Potato Roots during Storage: A Review. Horticulturae 2023, 9, 667. https://doi.org/10.3390/horticulturae9060667
Kou J, Zang X, Li M, Li W, Zhang H, Chen Y, Zhu G. Effects of Ethylene and 1-Methylcyclopropene on the Quality of Sweet Potato Roots during Storage: A Review. Horticulturae. 2023; 9(6):667. https://doi.org/10.3390/horticulturae9060667
Chicago/Turabian StyleKou, Jingjing, Xueqian Zang, Maofu Li, Wenxing Li, Hongna Zhang, Yanli Chen, and Guopeng Zhu. 2023. "Effects of Ethylene and 1-Methylcyclopropene on the Quality of Sweet Potato Roots during Storage: A Review" Horticulturae 9, no. 6: 667. https://doi.org/10.3390/horticulturae9060667
APA StyleKou, J., Zang, X., Li, M., Li, W., Zhang, H., Chen, Y., & Zhu, G. (2023). Effects of Ethylene and 1-Methylcyclopropene on the Quality of Sweet Potato Roots during Storage: A Review. Horticulturae, 9(6), 667. https://doi.org/10.3390/horticulturae9060667






