Exogenous Phytohormones: Effects on Lettuce Photosynthesis, Antioxidant Response and Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Conditions
2.2. Biometric Measurements
2.3. Determination of Photosynthetic Parameters
2.4. Chlorophyll Fluorescence Imaging Analysis
2.5. Antioxidant Activity and Total Phenolic Content
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Moore, S.; Chen, C.; Lindsey, K. Crosstalk complexities between auxin, cytokinin and ethylene in Arabidopsis root development: From experiments to systems modeling and back again. Mol. Plant 2017, 10, 1480–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Qiangsheng, W.; Yanfeng, D.; Zhenghui, L.; Ganghua, L.; Shaohua, W. Effect of nitrogen and 6-BA on development of tillering bud and its physiological mechanism. Acta Agron. Sin. 2009, 35, 1893–1899. [Google Scholar] [CrossRef]
- Wang, R.F.; Jiwang, Z.; Lu, P.; Shuting, D.; Peng, L.; Bin, Z. Effects of endogenous hormones on tiller development process of different maize varieties. Sci. Agric. Sin. 2012, 45, 840–847. [Google Scholar]
- Cai, T.; Haicheng, X.; Yanping, Y.; Weibing, Y.; Dianliang, P.; Yingli, N.; Cailong, X.; Dongqing, Y.; Zhenlin, W. Mechanisms of tiller occurrence affected by exogenous IAA, GA3, and ABA in wheat with different spike-types. Acta Agron. Sin. 2013, 39, 1835–1842. [Google Scholar] [CrossRef]
- Cai, T.; Meng, X.; Liu, X.; Liu, T.; Wang, H.; Jia, Z.; Yang, D.; Ren, X. Exogenous hormonal application regulates the occurrence of wheat tillers by changing endogenous hormones. Front. Plant Sci. 2018, 9, 1886. [Google Scholar] [CrossRef] [Green Version]
- Seif El-Yazal, A.S.; Seif El-Yazal, A.M.; Dwidar, F.E.M.; Rady, M. Phytohormone crosstalk research: Cytokinin and its crosstalk with other phytohormones. Curr. Protein Pept. Sci. 2015, 16, 395–405. [Google Scholar] [CrossRef]
- Pospíšilová, J. Participation of phytohormones in the stomatal regulation of gas exchange during water stress. Biol. Plant. 2003, 46, 491–506. [Google Scholar] [CrossRef]
- Chow, B.; Mccourt, P. Hormone signalling from a developmental context. J. Exp. Bot. 2004, 55, 247–251. [Google Scholar] [CrossRef]
- Todorova, D.; Vaseva, I.; Jiří, M.; Alena; Macháčková, T.; Ivana, N.; Karanov, E. Cytokinin oxidase/dehydrogenase activity as a tool in gibberellic acid/cytokinin cross talk. Biol. Plant. 2007, 51, 579–583. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Y.; Liu, C.; Vonapartis, E.; Liang, J.; Wu, W.; Gazzarrini, S.; He, J.; Yi, M. GhNAC83 inhibits corm dormancy release by regulating ABA signaling and cytokinin biosynthesis in Gladiolus hybridus. J. Exp. Bot. 2019, 70, 1221–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corot, A.; Roman, H.; Douillet, O.; Autret, H.; Perez-Garcia, M.D.; Citerne, S.; Demotes-Mainard, S. Cytokinins and abscisic acid act antagonistically in the regulation of the bud outgrowth pattern by light intensity. Front. Plant Sci. 2017, 8, 1724. [Google Scholar] [CrossRef] [PubMed]
- Masuta, C.; Tanaka, H.; Uehara, K.; Kuwata, S.; Koiwai, A.; Noma, M. Broad resistance to plant viruses in transgenic plants conferred by antisense inhibition of a host gene essential in Sadenosylmethionine- dependent transmethylation reactions. Proc. Natl. Acad. Sci. USA 1995, 92, 6117–6121. [Google Scholar] [CrossRef] [PubMed]
- Argueso, C.T.; Ferreira, F.J.; Epple, P.; To, J.P.C.; Hutchison, C.E.; Schaller, G.E.; Dangl, J.L.; Kieber, J.J. Two-component elements mediate interactions between cytokinin and salicylic acid in plant immunity. PLoS Genet. 2012, 8, e1002448. [Google Scholar] [CrossRef] [Green Version]
- Jogawat, A. Crosstalk among phytohormone signaling pathways during abiotic stress. In Molecular Plant Abiotic Stress: Biology and Biotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 209–220. [Google Scholar]
- Latif, H.H. Physiological responses of Pisum sativum plant to exogenous ABA application under drought conditions. Pak. J. Bot. 2014, 46, 973–982. [Google Scholar]
- Li, G.; Hu, S.; Hou, H.; Kimura, S. Heterophylly: Phenotypic plasticity of leaf shape in aquatic and amphibious plants. Plants 2019, 8, 420. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Hu, S.; Zhao, X.; Kumar, S.; Li, Y.; Yang, J.; Hou, H. Mechanisms of the morphological plasticity induced by phytohormones and the environment in plants. Int. J. Mol. Sci. 2021, 22, 765. [Google Scholar] [CrossRef]
- Moustakas, M.; Hanć, A.; Dobrikova, A.; Sperdouli, I.; Adamakis, I.D.S.; Apostolova, E. Spatial heterogeneity of cadmium effects on Salvia sclarea leaves revealed by chlorophyll fluorescence imaging analysis and laser ablation inductively coupled plasma mass spectrometry. Materials 2019, 12, 2953. [Google Scholar] [CrossRef] [Green Version]
- Guidi, L.; Mori, S.; Degl’innocenti, E.; Pecchia, S. Effects of ozone exposure or fungal pathogen on white lupin leaves as determined by imaging of chlorophyll a fluorescence. Plant Physiol. Biochem. 2007, 45, 851–857. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-EVANS, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH Antioxidant Assay Revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin–Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Miceli, A.; Moncada, A.; Sabatino, L. Effect of gibberellic acid on growth, yield, and quality of leaf lettuce and rocket grown in a floating system. Agronomy 2019, 9, 382. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Cohen, P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature 1982, 296, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.B.; Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic acid to decrease plant stress. Environ. Chem. Lett. 2017, 15, 101–123. [Google Scholar] [CrossRef]
- Alonso-Ramirez, A.; Rodriguez, D.; Reyes, D.; Jimenez, J.A.; Nicolas, G.; Lopez-Climent, M.; Gomez-Cadenas, A.; Nicolas, C. Evidence for a role of gibberellins in salicylic acid-modulated early plant responses to abiotic stress in Arabidopsis seeds. Plant Physiol. 2009, 150, 1335–1344. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Zhang, Z.L.; Hanzlik, S.; Cook, E.; Shen, Q.J. Salicylic acid inhibits gibberellin-induced alpha-amylase expression and seed germination via a pathway involving an abscisic-acid inducible WRKY gene. Plant Mol. Biol. 2007, 64, 293–303. [Google Scholar] [CrossRef]
- Javed, T.; Ali, M.M.; Shabbir, R.; Anwar, R.; Afzal, I.; Mauro, R.P. Alleviation of copper-induced stress in pea (Pisum sativum L.) through foliar application of gibberellic acid. Biology 2021, 10, 120. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Li, Y.; Du, W.; Zhang, Y.; Han, Y.; Liu, C.; Fan, S.; Hao, J. Application of exogenous auxin and gibberellin regulates the bolting of lettuce (Lactuca sativa L.). Open Life Sci. 2022, 17, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Hönig, M.; Plihalova, L.; Husičkova, A.; Nilser, J.; Doležal, K. Role of cytokinins in senescence, antioxidant defence and photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Huh, S.U.; Kojima, M.; Sakakibara, H.; Paek, K.H.; Hwang, I. The cytokinin-activated transcription factor ARR2 promotes plant immunity via TGA3/NPR1-dependent salicylic acid signaling in arabidopsis. Dev. Cell 2010, 19, 284–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhaman, M.S.; Irman, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress. Plants 2020, 10, 37. [Google Scholar] [CrossRef]
- Al-Taey, D.K.; Saadoon, A.H.S.; Alazawi, S.S. Study of kinetin traetment on growth and activity of some antioxidant enzymes of spinach under salt stress. Curr. Trends Nat. Sci. 2021, 10, 457–465. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, L.; Liu, L.; Wang, J.; Li, C.; Wang, Q. Multiple phytohormone signalling pathways modulate susceptibility of tomato plants to Alternaria alternate f. sp. lycopersici. J. Exp. Bot. 2013, 64, 637–650. [Google Scholar] [CrossRef] [Green Version]
- Matilla-Vazquez, M.A.; Matilla, A.J. Ethylene: Role in plants under environmental stress. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Springer: New York, NY, USA, 2014; Volume 2, pp. 189–222. [Google Scholar]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Khan, F.; Ullah, N.; Faiq, M.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Kim, S.H.; Chung, I.M. Exogenous phytohormones increase the accumulation of health-promoting metabolites and influence the expression patterns of biosynthesis related genes and biological activity in Chinese cabbage (Brassica rapa spp. pekinensis). Sci. Hortic. 2015, 193, 136–146. [Google Scholar] [CrossRef]
- Rahimzadeh-Bajgiran, P.; Munehiro, M.; Omasa, K. Relationships between the photochemical reflectance index (PRI) and chlorophyll fluorescence parameters and plant pigment indices at different leaf growth stages. Photosynth. Res. 2012, 113, 261–271. [Google Scholar] [CrossRef]
- Jogawat, A.; Yadav, B.; Chhaya Lakra, N.; Singh, A.K.; Narayan, O.P. Crosstalk between phytohormones and secondary metabolites in the drought stress tolerance of crop plants: A review. Physiol. Plant. 2021, 172, 1106–1132. [Google Scholar] [CrossRef]
- Marcińska, I.; Czyczlo-Mysza, I.; Skrzypek, E.; Grzesiak, M.T.; Janowiak, F.; Filek, M.; Michal, D.; Dziurka, K.; Waligorski, P.; Juzon, K.; et al. Alleviation of osmotic stress effects by exogenous application of salicylic or abscisic acid on wheat seedlings. Int. J. Mol. Sci. 2013, 14, 13171–13193. [Google Scholar] [CrossRef] [PubMed] [Green Version]

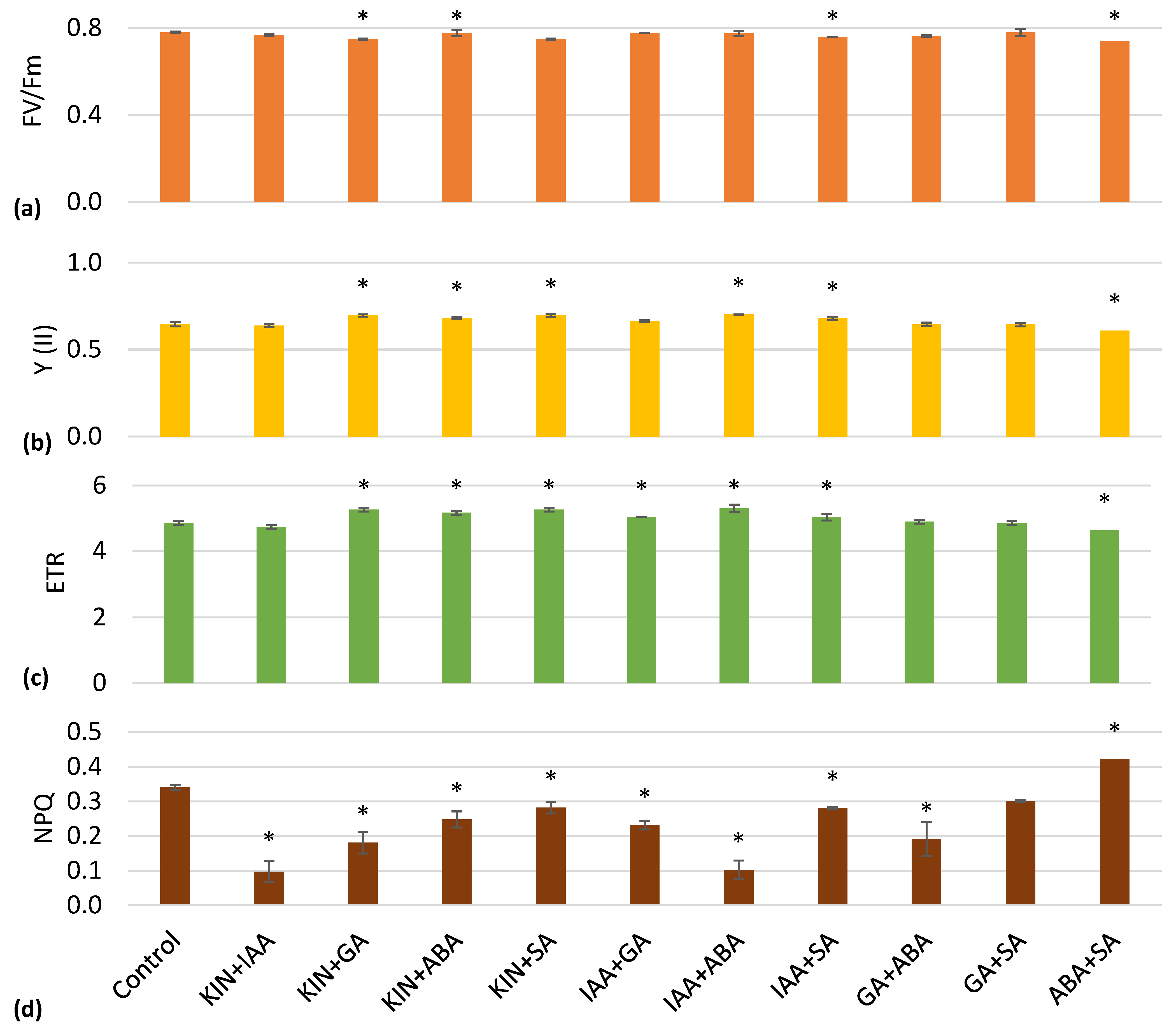
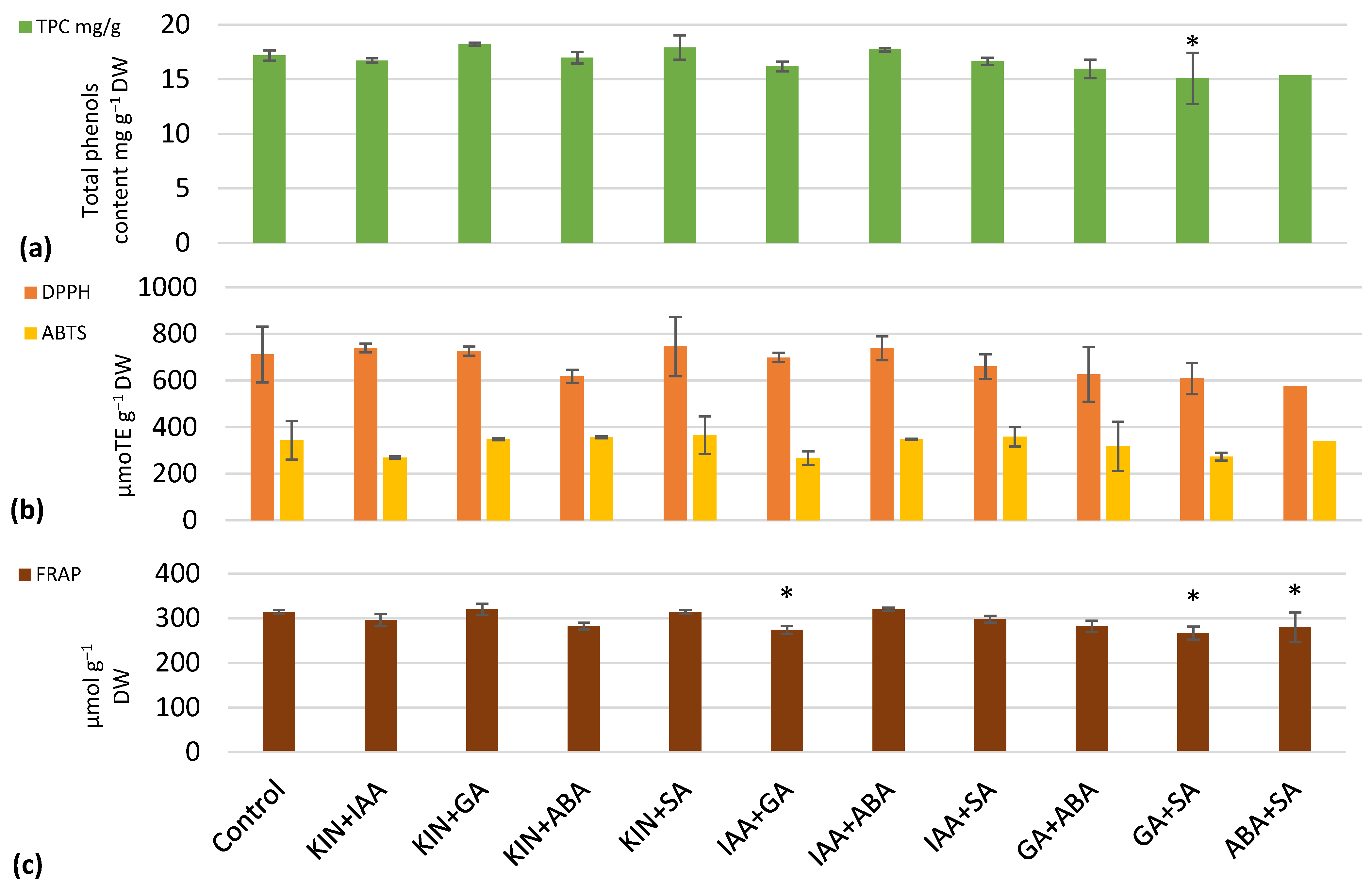
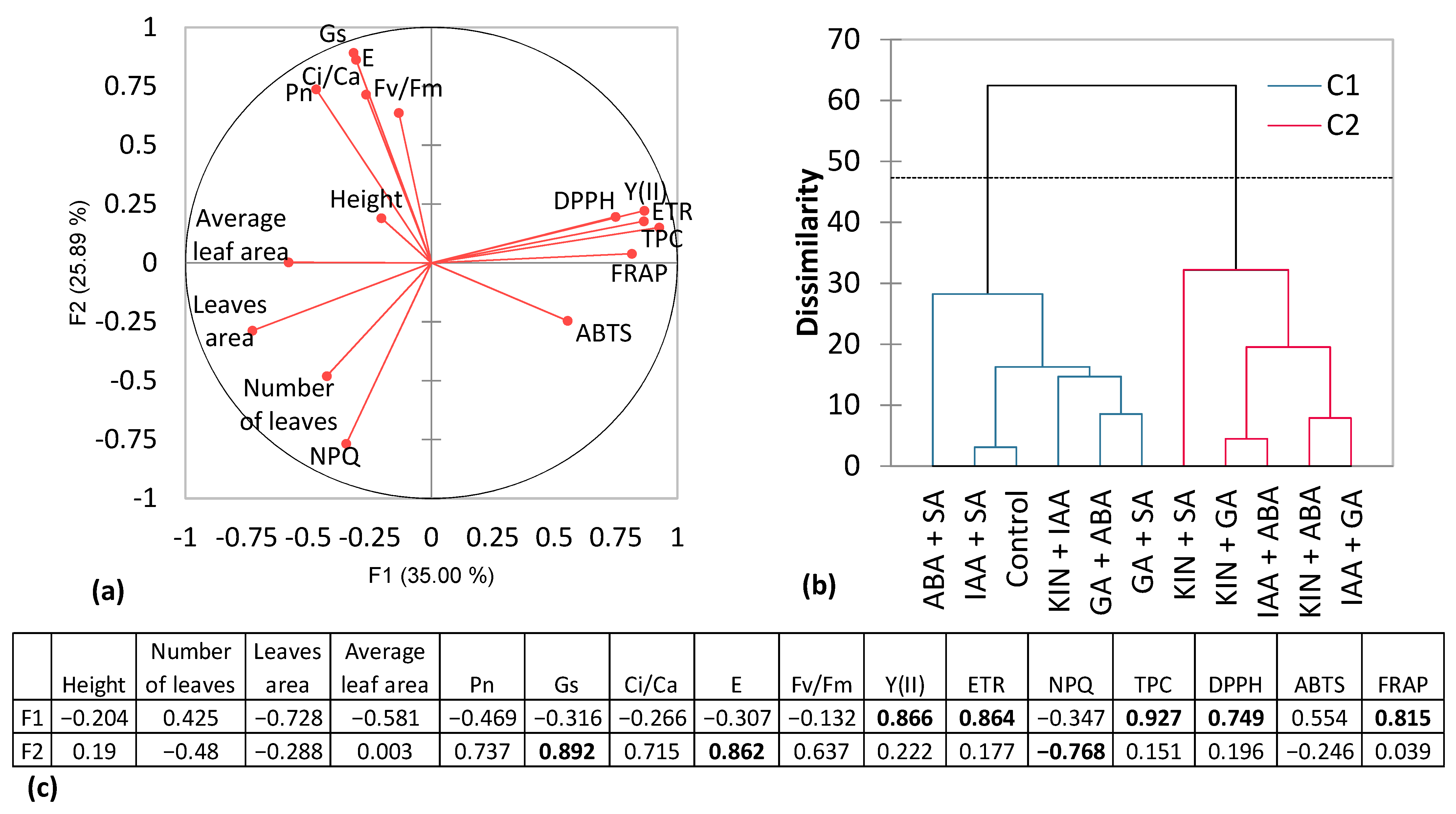
| Lettuce Height, cm | Number of Leaves | Total Leaf Area, cm2 | Plant Fresh Weight, g | Plant Dry Weight, g | |
|---|---|---|---|---|---|
| Control | 12.1 ± 0.1 | 4.3 ± 0.6 | 68.0 ± 7.0 | 2.21 ± 0.1 | 0.15 ± 0.01 |
| KIN + IAA | 11.0 ± 0.9 | 4.7 ± 0.6 | 73.0 ± 7.8 | 2.59 ± 0.5 | 0.16 ± 0.03 |
| KIN + GA | 10.7 * ± 0.5 | 4.3 ± 0.6 | 58.9 ± 9.1 | 1.92 ± 0.2 | 0.14 ± 0.02 |
| KIN + ABA | 11.3 ± 1.0 | 4.0 ± 0.0 | 57.9 ± 4.6 | 2.20 ± 0.4 | 0.12 ± 0.02 |
| KIN + SA | 12.0 ± 0.7 | 4.3 ± 0.6 | 67.5 ± 5.5 | 2.24 ± 0.1 | 0.15 ± 0.03 |
| IAA + GA | 11.3 ± 0.3 | 4.0 ± 0.0 | 54.1 * ± 3.0 | 1.85 ± 0.2 | 0.12 ± 0.02 |
| IAA + ABA | 11.0 ± 0.4 | 4.0 ± 0.0 | 50.3 * ± 3.8 | 1.86 ± 0.3 | 0.12 ± 0.04 |
| IAA + SA | 11.4 ± 0.7 | 5.3 * ± 0.6 | 77.1 ± 2.4 | 2.63 ± 0.1 | 0.19 ± 0.03 |
| GA + ABA | 12.7 ± 0.3 | 4.0 ± 0.0 | 77.5 ± 5.4 | 2.83 * ± 0.4 | 0.16 ± 0.04 |
| GA + SA | 12.7 ± 1.1 | 5.0 ± 0.0 | 79.1 * ± 2.9 | 2.83 * ± 0.2 | 0.17 ± 0.02 |
| ABA + SA | 9.7 * ± 0.1 | 5.0 ± 0.0 | 75.7 ± 2.8 | 2.46 ± 0.3 | 0.18 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbutis, M.; Laužikė, K.; Samuolienė, G. Exogenous Phytohormones: Effects on Lettuce Photosynthesis, Antioxidant Response and Growth. Horticulturae 2023, 9, 792. https://doi.org/10.3390/horticulturae9070792
Urbutis M, Laužikė K, Samuolienė G. Exogenous Phytohormones: Effects on Lettuce Photosynthesis, Antioxidant Response and Growth. Horticulturae. 2023; 9(7):792. https://doi.org/10.3390/horticulturae9070792
Chicago/Turabian StyleUrbutis, Martynas, Kristina Laužikė, and Giedrė Samuolienė. 2023. "Exogenous Phytohormones: Effects on Lettuce Photosynthesis, Antioxidant Response and Growth" Horticulturae 9, no. 7: 792. https://doi.org/10.3390/horticulturae9070792
APA StyleUrbutis, M., Laužikė, K., & Samuolienė, G. (2023). Exogenous Phytohormones: Effects on Lettuce Photosynthesis, Antioxidant Response and Growth. Horticulturae, 9(7), 792. https://doi.org/10.3390/horticulturae9070792







