Microbiological Activity Affects Post-Harvest Quality of Cocoa (Theobroma cacao L.) Beans
Abstract
1. Introduction
2. Microbiological Activity in Post-Harvest Handling of Cocoa Beans
2.1. Sorting
2.2. Cocoa Pod Ripening
2.3. Cocoa Pods Breaking
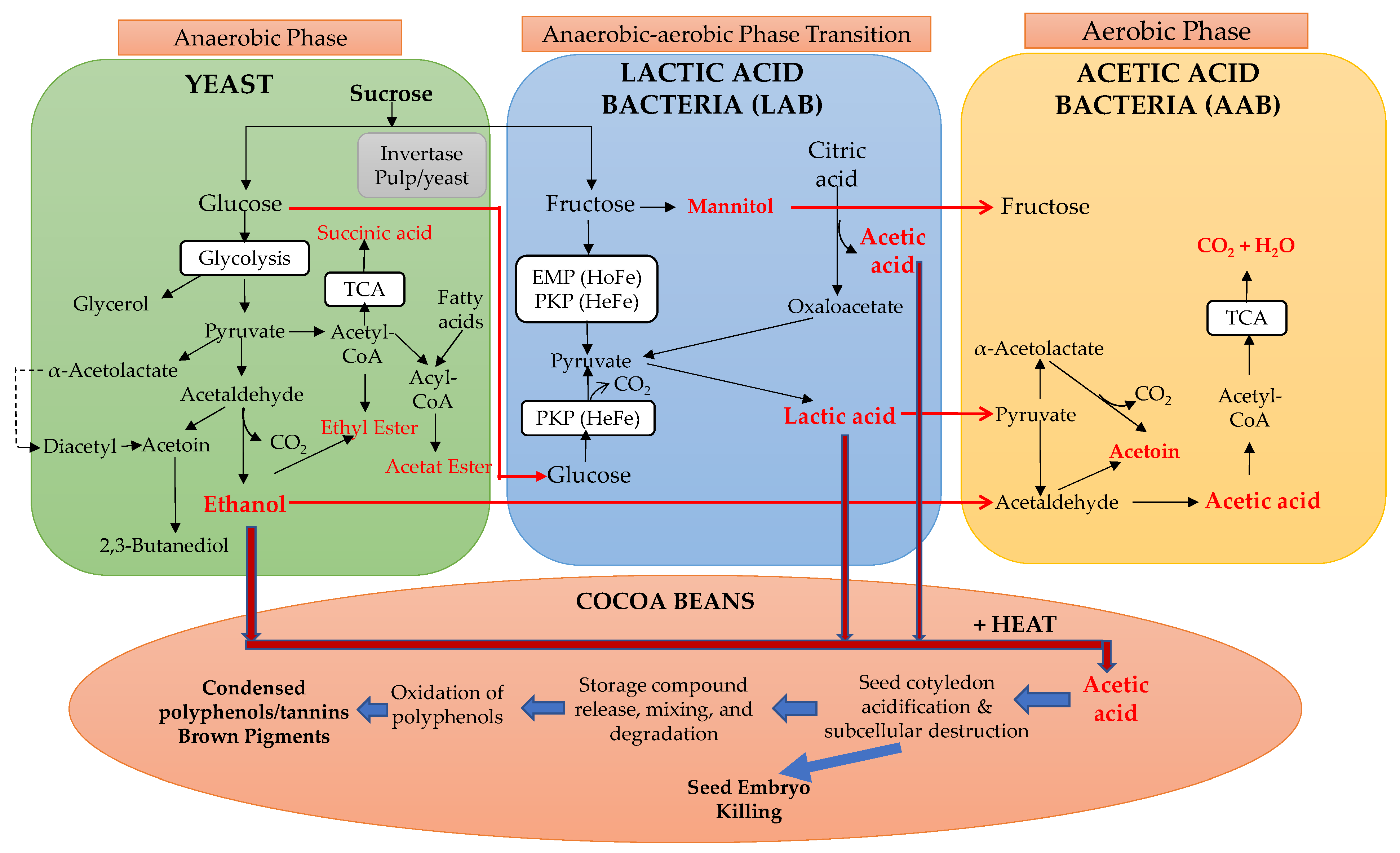
2.4. Fermentation
2.5. Drying
2.6. Storage
3. Effect of Microorganism Activity on Quality of Cocoa Beans
3.1. Physical Properties
3.2. Chemical Properties
4. Quality Improvement of Cocoa Beans
4.1. Post-Harvest Treatments for the Quality Improvement of Cocoa Beans
4.2. Technological Difficulties in Maintaining the Postharvest Quality of Cocoa Beans
5. Conclusions and Future Research
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mihai, R.A.; Landazuri Abarca, P.A.; Tinizaray Romero, B.A.; Florescu, L.I.; Catană, R.; Kosakyan, A. Abiotic Factors from Different Ecuadorian Regions and Their Contribution to Antioxidant, Metabolomic and Organoleptic Quality of Theobroma cacao L. Beans, Variety “Arriba Nacional”. Plants 2022, 11, 976. [Google Scholar] [CrossRef]
- Kongor, J.E.; Hinneh, M.; de Walle, D.V.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—A review. Food Res. Int. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- Dzelagha, B.F.; Ngwa, N.M.; Nde Bup, D. A Review of Cocoa Drying Technologies and the Effect on Bean Quality Parameters. Int. J. Food Sci. 2020, 2020, 8830127. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Currò, S.; Van de Walle, D.; Dewettinck, K.; Mirisola, M.; Fasolato, L.; Carletti, P. Quality Evaluation of Fair-Trade Cocoa Beans from Different Origins Using Portable Near-Infrared Spectroscopy (NIRS). Foods 2023, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Streule, S.; Freimüller Leischtfeld, S.; Galler, M.; Miescher Schwenninger, S. Monitoring of cocoa post-harvest process practices on a small-farm level at five locations in Ecuador. Heliyon 2022, 8, e09628. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.J.R.; Almeida, M.H.; Nout, M.J.R.; Zwietering, M.H. Theobroma cacao L. “The Food of the Gods”: Quality Determinants of Commercial Cocoa Beans, with Particular Reference to the Impact of Fermentation. Crit. Rev. Food Sci. Nutr. 2011, 51, 731–761. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Ríos, H.G.; Suárez-Quiroz, M.L.; Hernández-Estrada, Z.J.; Castellanos-Onorio, O.P.; Alonso-Villegas, R.; Rayas-Duarte, P.; Cano-Sarmiento, C.; Figueroa-Hernández, C.Y.; González-Rios, O. Yeasts as Producers of Flavor Precursors during Cocoa Bean Fermentation and Their Relevance as Starter Cultures: A Review. Fermentation 2022, 8, 331. [Google Scholar] [CrossRef]
- Lembong, E.; Djali, M.; Zaida; Utama, G.L. The potential of dry fermented cocoa (Theobroma cacao L.) variety Lindak bean shell treated at different degrees of roasting as a functional food. IOP Conf. Ser. Earth Environ. Sci. 2021, 733, 12068. [Google Scholar] [CrossRef]
- Sari, A.B.T.; Fahrurrozi; Marwati, T.; Djaafar, T.F.; Hatmi, R.U.; Purwaningsih; Wanita, Y.P.; Lisdiyanti, P.; Perwitasari, U.; Juanssilfero, A.B.; et al. Chemical Composition and Sensory Profiles of Fermented Cocoa Beans Obtained from Various Regions of Indonesia. Int. J. Food Sci. 2023, 2023, 5639081. [Google Scholar] [CrossRef]
- Subroto, E.; Andoyo, R.; Indiarto, R.; Lembong, E. Physicochemical properties, sensory acceptability, and antioxidant activity of chocolate bar fortified by solid lipid nanoparticles of gallic acid. Int. J. Food Prop. 2022, 25, 1907–1919. [Google Scholar] [CrossRef]
- Purbaningrum, K.; Hidayat, C.; Witasari, L.D.; Utami, T. Flavor Precursors and Volatile Compounds Improvement of Unfermented Cocoa Beans by Hydrolysis Using Bromelain. Foods 2023, 12, 820. [Google Scholar] [CrossRef]
- Apriyanto, M.; Sutardi; Supriyanto; Harmayani, E. Study on effect of fermentation to the quality parameter of cocoa bean in Indonesia. Asian J. Dairy Food Res. 2016, 35, 160–163. [Google Scholar] [CrossRef]
- Agyirifo, D.S.; Wamalwa, M.; Otwe, E.P.; Galyuon, I.; Runo, S.; Takrama, J.; Ngeranwa, J. Metagenomics analysis of cocoa bean fermentation microbiome identifying species diversity and putative functional capabilities. Heliyon 2019, 5, e02170. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, E.S.; Triyadi, R.; Khusna, R.N.B.; Djaafar, T.F.; Utami, T.; Marwati, T.; Hatmi, R.U. Indigenous Yeast, Lactic Acid Bacteria, and Acetic Acid Bacteria from Cocoa Bean Fermentation in Indonesia Can Inhibit Fungal-Growth-Producing Mycotoxins. Fermentation 2021, 7, 192. [Google Scholar] [CrossRef]
- Iacumin, L.; Pellegrini, M.; Colautti, A.; Orecchia, E.; Comi, G. Microbial Characterization of Retail Cocoa Powders and Chocolate Bars of Five Brands Sold in Italian Supermarkets. Foods 2022, 11, 2753. [Google Scholar] [CrossRef]
- Schwan, R.F.; Wheals, A.E. The Microbiology of Cocoa Fermentation and its Role in Chocolate Quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Kongor, J.E.; Takrama, J.; Budu, A.S. Changes in nib acidification and biochemical composition during fermentation of pulp pre-conditioned cocoa (Theobroma cacao) beans. Int. Food Res. J. 2013, 20, 1843–1853. [Google Scholar]
- Apriyanto, M.; Umanailo, M.C.B. Decrease polyphenols, ethanol, lactic acid, and acetic acid during fermentation with addition of cocoa beans innoculum. Int. J. Sci. Technol. Res. 2019, 8, 461–465. [Google Scholar]
- De Vuyst, L.; Weckx, S. The cocoa bean fermentation process: From ecosystem analysis to starter culture development. J. Appl. Microbiol. 2016, 121, 5–17. [Google Scholar] [CrossRef]
- De Vuyst, L.; Leroy, F. Functional role of yeasts, lactic acid bacteria and acetic acid bacteria in cocoa fermentation processes. FEMS Microbiol. Rev. 2020, 44, 432–453. [Google Scholar] [CrossRef] [PubMed]
- Camu, N.; De Winter, T.; Addo, S.K.; Takrama, J.S.; Bernaert, H.; De Vuyst, L. Fermentation of cocoa beans: Influence of microbial activities and polyphenol concentrations on the flavour of chocolate. J. Sci. Food Agric. 2008, 88, 2288–2297. [Google Scholar] [CrossRef]
- Wollgast, J.; Anklam, E. Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000, 33, 423–447. [Google Scholar] [CrossRef]
- Díaz-Muñoz, C.; De Vuyst, L. Functional yeast starter cultures for cocoa fermentation. J. Appl. Microbiol. 2022, 133, 39–66. [Google Scholar] [CrossRef]
- Timothy, L.; Maarten, J.; Nicholas, C.; Luc, D.V. Kinetic Analysis of Strains of Lactic Acid Bacteria and Acetic Acid Bacteria in Cocoa Pulp Simulation Media toward Development of a Starter Culture for Cocoa Bean Fermentation. Appl. Environ. Microbiol. 2010, 76, 7708–7716. [Google Scholar] [CrossRef]
- Ardhana, M.M.; Fleet, G.H. The microbial ecology of cocoa bean fermentations in Indonesia. Int. J. Food Microbiol. 2003, 86, 87–99. [Google Scholar] [CrossRef]
- Delgado-Ospina, J.; Molina-Hernández, J.B.; Chaves-López, C.; Romanazzi, G.; Paparella, A. The Role of Fungi in the Cocoa Production Chain and the Challenge of Climate Change. J. Fungi 2021, 7, 202. [Google Scholar] [CrossRef] [PubMed]
- Ackah, E.; Dompey, E. Effects of fermentation and drying durations on the quality of cocoa (Theobroma cacao L.) beans during the rainy season in the Juaboso District of the Western-North Region, Ghana. Bull. Natl. Res. Cent. 2021, 45, 175. [Google Scholar] [CrossRef]
- Atmaja, M.I.P.; Haryadi, H.; Supriyanto, S. Quality Improvement of Non Fermented Cocoa Bean Through Pre-Incubation Treatments. J. Ind. Beverage Crop. 2016, 3, 11–20. [Google Scholar] [CrossRef]
- Kamphuis, H.J. Production and Quality Standards of Cocoa Mass, Cocoa Butter and Cocoa Powder. In Industrial Chocolate Manufacture and Use; Beckett, S.T., Ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2008; pp. 121–141. ISBN 9781444301588. [Google Scholar]
- Delgado-Ospina, J.; Molina-Hernandez, J.B.; Viteritti, E.; Maggio, F.; Fernández-Daza, F.F.; Sciarra, P.; Serio, A.; Rossi, C.; Paparella, A.; Chaves-López, C. Advances in understanding the enzymatic potential and production of ochratoxin A of filamentous fungi isolated from cocoa fermented beans. Food Microbiol. 2022, 104, 103990. [Google Scholar] [CrossRef]
- Cho, W.-I.; Chung, M.-S. Bacillus spores: A review of their properties and inactivation processing technologies. Food Sci. Biotechnol. 2020, 29, 1447–1461. [Google Scholar] [CrossRef]
- Pereira, A.P.M.; Sant’Ana, A.S. Diversity and fate of spore forming bacteria in cocoa powder, milk powder, starch and sugar during processing: A review. Trends Food Sci. Technol. 2018, 76, 101–118. [Google Scholar] [CrossRef]
- Copetti, M.V.; Iamanaka, B.T.; Frisvad, J.C.; Pereira, J.L.; Taniwaki, M.H. Mycobiota of cocoa: From farm to chocolate. Food Microbiol. 2011, 28, 1499–1504. [Google Scholar] [CrossRef]
- Copetti, M.V.; Iamanaka, B.T.; Pitt, J.I.; Taniwaki, M.H. Fungi and mycotoxins in cocoa: From farm to chocolate. Int. J. Food Microbiol. 2014, 178, 13–20. [Google Scholar] [CrossRef]
- Krapf, T.; Gantenbein-Demarchi, C. Thermal inactivation of Salmonella spp. during conching. LWT—Food Sci. Technol. 2010, 43, 720–723. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Phoku, J.Z.; Barnard, T.G.; Potgieter, N.; Dutton, M.F. Mycotoxigenic potentials of the genera: Aspergillus, Fusarium and Penicillium isolated from houseflies (Musca domestica L.). Acta Trop. 2017, 168, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.N.; Vargas, E.A.; Gomes, M.B.; Vieira, C.B.M.; Dos Santos, E.A.; Bicalho, A.A.C.; Silva, S.D.C.; Rezende, R.P.; De Oliveira, I.S.; Luz, E.D.; et al. Aflatoxins and ochratoxin A: Occurrence and contamination levels in cocoa beans from Brazil. Food Addit. Contam. Part A 2019, 36, 815–824. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, M.D.S.; Pena, P.O.; Brum, D.M.; Imazaki, F.T.; Tucci, M.L.S.; Efraim, P. Behavior of Salmonella during fermentation, drying and storage of cocoa beans. Int. J. Food Microbiol. 2013, 167, 363–368. [Google Scholar] [CrossRef]
- Purnamasari, L.; Ali, A.; Cuk, T.N. Assessment of Crude Aflatoksin B1 Production Based on Local Aspergillus flavus Mold Isolate in Corn and Corn + Ground Peanut Media. Bul. Peternak. 2016, 40, 133–137. [Google Scholar] [CrossRef]
- Janssen, S.; Pankoke, I.; Klus, K.; Schmitt, K.; Stephan, U.; Wöllenstein, J. Two underestimated threats in food transportation: Mould and acceleration. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130312. [Google Scholar] [CrossRef]
- Uygun, Y.; Jafri, S.A.I. Controlling risks in sea transportation of cocoa beans. Cogent Bus. Manag. 2020, 7, 1778894. [Google Scholar] [CrossRef]
- Dewayani, W.; Syamsuri, R.; Septianti, E.; Suriany; Halil, W.; Nurlaila; Rahman, A. Good Handling Practice Study to Reduce The Level of Contamination in Cocoa Beans in East Luwu. IOP Conf. Ser. Earth Environ. Sci. 2022, 1024, 12067. [Google Scholar] [CrossRef]
- Leandro-Muñoz, M.E.; Tixier, P.; Germon, A.; Rakotobe, V.; Phillips-Mora, W.; Maximova, S.; Avelino, J. Effects of microclimatic variables on the symptoms and signs onset of Moniliophthora roreri, causal agent of Moniliophthora pod rot in cacao. PLoS ONE 2017, 12, e0184638. [Google Scholar] [CrossRef]
- Ndoumbe-Nkeng, M.; Cilas, C.; Nyemb, E.; Nyasse, S.; Bieysse, D.; Flori, A.; Sache, I. Impact of removing diseased pods on cocoa black pod caused by Phytophthora megakarya and on cocoa production in Cameroon. Crop Prot. 2004, 23, 415–424. [Google Scholar] [CrossRef]
- Bariah, S.K.; Tajul, A.Y. Effect of Cocoa Pods Storage on the Temperature and Physicochemical Changes during Shallow Box Fermentation. IJISET-Int. J. Innov. Sci. Eng. Technol. 2017, 4, 197–203. [Google Scholar]
- Septianti, E.; Salengke; Langkong, J.; Sukendar, N.K.; Hanifa, A.P. Characteristic Quality of Pinrang’s Cocoa Beans During Fermentation Used Styrofoam Containers. Canrea J. Food Technol. Nutr. Culin. J. 2020, 3, 10–25. [Google Scholar] [CrossRef]
- Djaafar, T.F.; Elghina, L.; Widodo, S.; Marwati, T.; Utami, T.; Rahayu, E.S. Study of Good Handling Practices and Critical Control Point Determination of Dried Fermented Cocoa Bean in Gunung Kidul Regency, Yogyakarta. IOP Conf. Ser. Earth Environ. Sci. 2019, 309, 12015. [Google Scholar] [CrossRef]
- Rinaldo, R.; Chozin, M.A. Management of Handling Cocoa Pod (Theobroma cacao L.) in Central Java. Bul. Agrohorti 2016, 4, 210–214. [Google Scholar] [CrossRef]
- Meulemans, C.C.; Consultancy, O.R.; Surapati, T.U.; Tjatjo, A. Colorimetric Measurements of Cocoa Beans (Theobroma cacao). Indones. J. Agric. Sci. 2013, 3, 52. [Google Scholar] [CrossRef]
- Bachtiar, W.F.; Aji, G.K.; Norsita, D.I. Value Chain Performance of Cocoa Beans in Banjaroya, Kulon Progo. Gontor AGROTECH Sci. J. 2019, 5, 1. [Google Scholar] [CrossRef]
- Tee, Y.-K.; Bariah, K.; Hisyam Zainudin, B.; Samuel Yap, K.-C.; Ong, N.-G. Impacts of cocoa pod maturity at harvest and bean fermentation period on the production of chocolate with potential health benefits. J. Sci. Food Agric. 2022, 102, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O.; Quao, J.; Budu, A.S.; Takrama, J.; Saalia, F.K. Effect of pulp preconditioning on acidification, proteolysis, sugars and free fatty acids concentration during fermentation of cocoa (Theobroma cacao) beans. Int. J. Food Sci. Nutr. 2011, 62, 755–764. [Google Scholar] [CrossRef]
- Hartuti, S.; Juanda, J.; Khatir, R. Efforts to Improve Cocoa Bean Quality (Theobroma cacao L.) through the Postharvest Handling Stage (Review). Indones. J. Ind. Res. 2020, 15, 38–52. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Quao, J.; Budu, A.S.; Takrama, J.; Saalia, F.K. Influence of pulp-preconditioning and fermentation on fermentative quality and appearance of Ghanaian cocoa (Theobroma cacao) beans. Int. Food Res. J. 2012, 19, 127–133. [Google Scholar]
- Hamdouche, Y.; Meile, J.C.; Lebrun, M.; Guehi, T.; Boulanger, R.; Teyssier, C.; Montet, D. Impact of turning, pod storage and fermentation time on microbial ecology and volatile composition of cocoa beans. Food Res. Int. 2019, 119, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S.B. Acinetobacter spp. as nosocomial pathogens: Epidemiology and resistance features. Saudi J. Biol. Sci. 2018, 25, 586–596. [Google Scholar] [CrossRef]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef]
- Brisse, S.; Fevre, C.; Passet, V.; Issenhuth-Jeanjean, S.; Tournebize, R.; Diancourt, L.; Grimont, P. Virulent Clones of Klebsiella pneumoniae: Identification and Evolutionary Scenario Based on Genomic and Phenotypic Characterization. PLoS ONE 2009, 4, e4982. [Google Scholar] [CrossRef]
- Pérez Portuondo, I. Bacillus cereus y su papel en las intoxicaciones alimentarias. Rev. Cuba. Salud Pública 2012, 38, 98–108. [Google Scholar] [CrossRef][Green Version]
- Khan, A.R.; Mustafa, A.; Hyder, S.; Valipour, M.; Rizvi, Z.F.; Gondal, A.S.; Yousuf, Z.; Iqbal, R.; Daraz, U. Bacillus spp. as Bioagents: Uses and Application for Sustainable Agriculture. Biology 2022, 11, 1763. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The Significance of Bacillus spp. in Disease Suppression and Growth Promotion of Field and Vegetable Crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef] [PubMed]
- Iyanda, M.O.; Alhassan, E.A.; Adekanye, T.A. Design, Fabrication and Testing of a Cocoa Depodding Machine. Mindanao J. Sci. Technol. 2018, 16, 11–24. [Google Scholar]
- Josué, D.; Bienvenu, K.; Fonsso Josué, D.; Mbetmi Guy-De-Patience, F.; Bonaventure, D. Design and development of cocoa pod breaking and beans extraction machine. Int. J. Eng. Technol. 2019, 8, 357–366. [Google Scholar]
- Lozano Tovar, M.D.; Tibasosa, G.; González, C.M.; Ballestas Alvarez, K.; Lopez Hernandez, M.D.P.; Rodríguez Villamizar, F. Isolation and identification of microbial species found in cocoa fermentation as microbial starter culture candidates for cocoa bean fermentation in Colombia. Pelita Perkeb. 2020, 36, 236–248. [Google Scholar] [CrossRef]
- Mota-Gutierrez, J.; Botta, C.; Ferrocino, I.; Giordano, M.; Bertolino, M.; Dolci, P.; Cannoni, M.; Cocolin, L. Dynamics and Biodiversity of Bacterial and Yeast Communities during Fermentation of Cocoa Beans. Appl. Environ. Microbiol. 2018, 84, e01164-18. [Google Scholar] [CrossRef]
- Steensels, J.; Snoek, T.; Meersman, E.; Nicolino, M.P.; Voordeckers, K.; Verstrepen, K.J. Improving industrial yeast strains: Exploiting natural and artificial diversity. FEMS Microbiol. Rev. 2014, 38, 947–995. [Google Scholar] [CrossRef] [PubMed]
- Djaafar, T.F.; Monika, D.C.; Marwati, T.; Triwitono, P.; Rahayu, E.S. Microbiology, Chemical, and Sensory Characteristics of Cocoa Powder: The Effect of Lactobacillus plantarum HL-15 as Culture Starter and Fermentation Box Variation. Digit. Press Life Sci. 2020, 2, 8. [Google Scholar] [CrossRef]
- Indarti, E.; Widayat, H.P.; Zuhri, N. Effect of fermentation container and thickness of bean mass during fermentation process of cocoa bean (Theobroma cocoa L). Proc. Annu. Int. Conf. Syiah Kuala Univ. 2011, 1, 64–69. [Google Scholar]
- Nielsen, D.S.; Teniola, O.D.; Ban-Koffi, L.; Owusu, M.; Andersson, T.S.; Holzapfel, W.H. The microbiology of Ghanaian cocoa fermentations analysed using culture-dependent and culture-independent methods. Int. J. Food Microbiol. 2007, 114, 168–186. [Google Scholar] [CrossRef]
- Rahardjo, Y.P.; Rahardja, S.; Samsudin; Saidah; Dalapati, A.; Amalia, A.F.; Purwaningsih, H.; Syamsu, K. A literature review on cocoa fermentation techniques to shorten fermentation time. IOP Conf. Ser. Earth Environ. Sci. 2022, 974, 12111. [Google Scholar] [CrossRef]
- Mozzi, F.; Ortiz, M.E.; Bleckwedel, J.; De Vuyst, L.; Pescuma, M. Metabolomics as a tool for the comprehensive understanding of fermented and functional foods with lactic acid bacteria. Food Res. Int. 2013, 54, 1152–1161. [Google Scholar] [CrossRef]
- Ordoñez-Araque, R.; Landines Vera, E.; Urresto-Villegas, J.; Caicedo-Jaramillo, C. Microorganisms during cocoa fermentation: Systematic review. Foods Raw Mater. 2020, 8, 155–162. [Google Scholar] [CrossRef]
- Kustyawati, M.E.; Setyani, S. Effect of the Addition of Mixed Inoculums on the Chemical and Microbiological Changes During Cocoa Fermentation. J. Teknol. Ind. dan Has. Pertan. 2008, 13, 73–84. [Google Scholar]
- Ouattara, D.H.; Ouattara, H.G.; Goualie, B.G.; Kouame, L.M.; Niamke, S.L. Biochemical and functional properties of lactic acid bacteria isolated from Ivorian cocoa fermenting beans. J. Appl. Biosci. 2014, 77, 6489–6499. [Google Scholar] [CrossRef]
- Miguel MG DC, P.; de Castro Reis, L.V.; Efraim, P.; Santos, C.; Lima, N.; Schwan, R.F. Cocoa fermentation: Microbial identification by MALDI-TOF MS, and sensory evaluation of produced chocolate. LWT 2017, 77, 362–369. [Google Scholar] [CrossRef]
- Hatmi, R.U.; Kobarsih, M.; Cahyaningrum, N. Fungi Level Analysis of Cocoa Beans Based on Fermentation Box Type and Duration. Procedia Food Sci. 2015, 3, 371–382. [Google Scholar] [CrossRef]
- Guehi, T.S.; Dadie, A.T.; Koffi, K.P.B.; Dabonne, S.; Ban-Koffi, L.; Kedjebo, K.D.; Nemlin, G.J. Performance of different fermentation methods and the effect of their duration on the quality of raw cocoa beans. Int. J. Food Sci. Technol. 2010, 45, 2508–2514. [Google Scholar] [CrossRef]
- Moreno-Zambrano, M.; Ullrich, M.S.; Hütt, M.-T. Exploring cocoa bean fermentation mechanisms by kinetic modelling. R. Soc. Open Sci. 2022, 9, 210274. [Google Scholar] [CrossRef]
- Azzolini, M.; Fedrizzi, B.; Tosi, E.; Finato, F.; Vagnoli, P.; Scrinzi, C.; Zapparoli, G. Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae mixed cultures on fermentation and aroma of Amarone wine. Eur. Food Res. Technol. 2012, 235, 303–313. [Google Scholar] [CrossRef]
- De Vuyst, L.; Lefeber, T.; Papalexandratou, Z.; Camu, N. The Functional Role of Lactic Acid Bacteria in Cocoa Bean Fermentation. In Biotechnology of Lactic Acid Bacteria; Wiley: Hoboken, NJ, USA, 2010; pp. 301–325. ISBN 9780813820866. [Google Scholar]
- Almeida, O.G.G.; Pinto, U.M.; Matos, C.B.; Frazilio, D.A.; Braga, V.F.; von Zeska-Kress, M.R.; De Martinis, E.C.P. Does Quorum Sensing play a role in microbial shifts along spontaneous fermentation of cocoa beans? An in silico perspective. Food Res. Int. 2020, 131, 109034. [Google Scholar] [CrossRef]
- Freitas, S.R. Cocoa Fermentations Conducted with a Defined Microbial Cocktail Inoculum. Appl. Environ. Microbiol. 1998, 64, 1477–1483. [Google Scholar] [CrossRef]
- Farrera, L.; Colas de la Noue, A.; Strub, C.; Guibert, B.; Kouame, C.; Grabulos, J.; Montet, D.; Teyssier, C. Towards a Starter Culture for Cocoa Fermentation by the Selection of Acetic Acid Bacteria. Fermentation 2021, 7, 42. [Google Scholar] [CrossRef]
- Calvo, A.M.; Botina, B.L.; García, M.C.; Cardona, W.A.; Montenegro, A.C.; Criollo, J. Dynamics of cocoa fermentation and its effect on quality. Sci. Rep. 2021, 11, 16746. [Google Scholar] [CrossRef] [PubMed]
- Zoi, P.; Gwen, F.; Edwina, R.; Carlos, J.J.; Freddy, A.; Heide-Marie, D.; Luc, D.V. Species Diversity, Community Dynamics, and Metabolite Kinetics of the Microbiota Associated with Traditional Ecuadorian Spontaneous Cocoa Bean Fermentations. Appl. Environ. Microbiol. 2011, 77, 7698–7714. [Google Scholar] [CrossRef]
- Pereira, G.V.d.M.; Miguel, M.G.d.C.P.; Ramos, C.L.; Schwan, R.F. Microbiological and Physicochemical Characterization of Small-Scale Cocoa Fermentations and Screening of Yeast and Bacterial Strains to Develop a Defined Starter Culture. Appl. Environ. Microbiol. 2012, 78, 5395–5405. [Google Scholar] [CrossRef] [PubMed]
- Fernández Maura, Y.; Balzarini, T.; Clapé Borges, P.; Evrard, P.; De Vuyst, L.; Daniel, H.-M. The environmental and intrinsic yeast diversity of Cuban cocoa bean heap fermentations. Int. J. Food Microbiol. 2016, 233, 34–43. [Google Scholar] [CrossRef]
- Lagunes Gálvez, S.; Loiseau, G.; Paredes, J.L.; Barel, M.; Guiraud, J.-P. Study on the microflora and biochemistry of cocoa fermentation in the Dominican Republic. Int. J. Food Microbiol. 2007, 114, 124–130. [Google Scholar] [CrossRef]
- Jamili; Yanti, N.A.; Susilowati, P.E. Diversity and the role of yeast in spontaneous cocoa bean fermentation from Southeast Sulawesi, Indonesia. Biodiversitas 2016, 17, 90–95. [Google Scholar] [CrossRef]
- Koff, O.; Samagaci, L.; Goualie, B.; Niamke, S. Diversity of Yeasts Involved in Cocoa Fermentation of Six Major Cocoa-Producing Regions in Ivory Coast. Eur. Sci. J. ESJ 2017, 13, 496–516. [Google Scholar] [CrossRef][Green Version]
- Koné, M.K.; Guéhi, S.T.; Durand, N.; Ban-Koffi, L.; Berthiot, L.; Tachon, A.F.; Brou, K.; Boulanger, R.; Montet, D. Contribution of predominant yeasts to the occurrence of aroma compounds during cocoa bean fermentation. Food Res. Int. 2016, 89, 910–917. [Google Scholar] [CrossRef]
- Daniel, H.-M.; Vrancken, G.; Takrama, J.F.; Camu, N.; De Vos, P.; De Vuyst, L. Yeast diversity of Ghanaian cocoa bean heap fermentations. FEMS Yeast Res. 2009, 9, 774–783. [Google Scholar] [CrossRef]
- Hernández-Hernández, C.; López-Andrade, P.A.; Ramírez-Guillermo, M.A.; Guerra Ramírez, D.; Caballero Pérez, J.F. Evaluation of different fermentation processes for use by small cocoa growers in Mexico. Food Sci. Nutr. 2016, 4, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Adiko, E.; Ouattara, H.; Doué, G.; Niamké, S. Assessment of the Diversity of Lactic Acid Bacteria Involved in Cocoa Fermentation of Six Main Cocoa Producing Regions of Côte d’Ivoire. Annu. Res. Rev. Biol. 2018, 27, 1–16. [Google Scholar] [CrossRef]
- Soumahoro, S.; Ouattara, H.G.; Droux, M.; Nasser, W.; Niamke, S.L.; Reverchon, S. Acetic acid bacteria (AAB) involved in cocoa fermentation from Ivory Coast: Species diversity and performance in acetic acid production. J. Food Sci. Technol. 2020, 57, 1904–1916. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, H.D.; Ouattara, H.G.; Droux, M.; Reverchon, S.; Nasser, W.; Niamke, S.L. Lactic acid bacteria involved in cocoa beans fermentation from Ivory Coast: Species diversity and citrate lyase production. Int. J. Food Microbiol. 2017, 256, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Chagas Junior, G.C.; Ferreira, N.R.; Gloria, M.B.A.; Gobira, R.M.; Maia, F.D.; Lopes, A.S. Identification of Lactic Acid Bacteria on Raw Material for Cocoa Bean Fermentation in the Brazilian Amazon. Fermentation 2022, 8, 199. [Google Scholar] [CrossRef]
- De Bruyne, K.; Camu, N.; De Vuyst, L.; Vandamme, P. Weissella fabaria sp. nov., from a Ghanaian cocoa fermentation. Int. J. Syst. Evol. Microbiol. 2010, 60, 1999–2005. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.G.G.; De Martinis, E.C.P. Metagenome-Assembled Genomes Contribute to Unraveling of the Microbiome of Cocoa Fermentation. Appl. Environ. Microbiol. 2021, 87, e00584-21. [Google Scholar] [CrossRef]
- Magalhães da Veiga Moreira, I.; De Figueiredo Vilela, L.; Da Cruz Pedroso Miguel, M.G.; Santos, C.; Lima, N.; Freitas Schwan, R. Impact of a Microbial Cocktail Used as a Starter Culture on Cocoa Fermentation and Chocolate Flavor. Molecules 2017, 22, 766. [Google Scholar] [CrossRef]
- Illeghems, K.; De Vuyst, L.; Weckx, S. Comparative genome analysis of the candidate functional starter culture strains Lactobacillus fermentum 222 and Lactobacillus plantarum 80 for controlled cocoa bean fermentation processes. BMC Genomics 2015, 16, 766. [Google Scholar] [CrossRef]
- Nadia, A.B.; Jannah, S.N.; Purwantisari, S. Isolation and Characterization of Lactic Acid Bacteria from Apis mellifera Stomach and Their Potential as Antibacterial Using In Vitro Test Against Growth of Staphylococcus aureus and Salmonella typhimurium. NICHE J. Trop. Biol. 2020, 3, 35–44. [Google Scholar]
- Korcari, D.; Fanton, A.; Ricci, G.; Rabitti, N.S.; Laureati, M.; Hogenboom, J.; Pellegrino, L.; Emide, D.; Barbiroli, A.; Fortina, M.G. Fine Cocoa Fermentation with Selected Lactic Acid Bacteria: Fermentation Performance and Impact on Chocolate Composition and Sensory Properties. Foods 2023, 12, 340. [Google Scholar] [CrossRef]
- Lefeber, T.; Papalexandratou, Z.; Gobert, W.; Camu, N.; De Vuyst, L. On-farm implementation of a starter culture for improved cocoa bean fermentation and its influence on the flavour of chocolates produced thereof. Food Microbiol. 2012, 30, 379–392. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Foong, Y.J.; Lee, S.T.; Ramli, N.; Tan, Y.N.; Ayob, M.K. Incorporation of Potential Probiotic Lactobacillus plantarum Isolated from Fermented Cocoa Beans into Dark Chocolate: Bacterial Viability and Physicochemical Properties Analysis. J. Food Qual. 2013, 36, 164–171. [Google Scholar] [CrossRef]
- Zarić, D.B.; Bulatović, M.L.; Rakin, M.B.; Krunić, T.Ž.; Lončarević, I.S.; Pajin, B.S. Functional, rheological and sensory properties of probiotic milk chocolate produced in a ball mill. RSC Adv. 2016, 6, 13934–13941. [Google Scholar] [CrossRef]
- Faccinetto-Beltrán, P.; Gómez-Fernández, A.R.; Santacruz, A.; Jacobo-Velázquez, D.A. Chocolate as Carrier to Deliver Bioactive Ingredients: Current Advances and Future Perspectives. Foods 2021, 10, 2056. [Google Scholar] [CrossRef] [PubMed]
- Chuah, L.-O.; Foo, H.L.; Loh, T.C.; Mohammed Alitheen, N.B.; Yeap, S.K.; Abdul Mutalib, N.E.; Abdul Rahim, R.; Yusoff, K. Postbiotic metabolites produced by Lactobacillus plantarum strains exert selective cytotoxicity effects on cancer cells. BMC Complement. Altern. Med. 2019, 19, 114. [Google Scholar] [CrossRef]
- Gomes, R.J.; Borges, M.D.F.; Rosa, M.D.F.; Castro-Gómez, R.J.H.; Spinosa, W.A. Acetic acid bacteria in the food industry: Systematics, characteristics and applications. Food Technol. Biotechnol. 2018, 56, 139–151. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Afrida, I.R. Detection of Acid Bacteria in the Process of Cocoa Production. Proceeding Biol. Educ. Conf. 2016, 13, 822–826. [Google Scholar]
- Akinfala, T.O.; Houbraken, J.; Sulyok, M.; Adedeji, A.R.; Odebode, A.C.; Krska, R.; Ezekiel, C.N. Moulds and their secondary metabolites associated with the fermentation and storage of two cocoa bean hybrids in Nigeria. Int. J. Food Microbiol. 2020, 316, 108490. [Google Scholar] [CrossRef]
- Dumadi, S.R. The Moisture Content Increase of Dried Cocoa Beans During Storage at Room Temperature. J. Ilm. Teknol. Energi 2011, 1, 45–54. [Google Scholar]
- Marwati, T.; Purwaningsih; Djaafar, T.F.; Sari, A.B.T. Hernani Inhibition the growth of fungi and improving the quality of cocoa beans through fermentation using lactic acid bacteria. IOP Conf. Ser. Earth Environ. Sci. 2021, 807, 22048. [Google Scholar] [CrossRef]
- Beg, M.S.; Ahmad, S.; Jan, K.; Bashir, K. Status, supply chain and processing of—A review. Trends Food Sci. Technol. 2017, 66, 108–116. [Google Scholar] [CrossRef]
- Dina, S.F.; Limbong, H.P.; Rambe, S.M. Design and Performance Test of Solar Assisted Heat Pump Dryer for Cocoa Bean Drying. J. Ris. Teknol. Ind. 2018, 12, 21–33. [Google Scholar] [CrossRef]
- Hayati, R.; Yusmanizar, Y.; Mustafril, M.; Fauzi, H. Study of Fermentation and Drying Temperature in Cacao Quality (Theobroma cacao L.). J. Keteknikan Pertan. 2012, 26, 129–135. [Google Scholar] [CrossRef]
- Komolafe, C.A.; Waheed, M.A.; Kuye, S.I.; Adewumi, B.A.; Oluwaleye, I.O.; Olayanju, T.M.A. Sun drying of cocoa with firebrick thermal storage materials. Int. J. Energy Res. 2020, 44, 7015–7025. [Google Scholar] [CrossRef]
- Guda, P.; Gadhe, S.; Jakkula, S. Drying of Cocoa Beans by Using Different Techniques. Int. J. Agric. Innov. Res. 2017, 5, 859–865. [Google Scholar]
- Salazar, E.; Valenzuela, R.; Aguilar, M.; Aranda, N.; Sotelo, A.; Chire-Fajardo, G.C.; Ureña, M. Physicochemical properties and microbial group behavior of postharvest peruvian cocoa bean (Theobroma cacao L.). Enfoque UTE 2020, 11, 48–56. [Google Scholar] [CrossRef]
- Sangronis, E.; Soto, M.J.; Valero, Y.; Buscema, I. Cascarilla de cacao venezolano como materia prima para infusiones. Arch. Latinoam. Nutr. 2014, 64, 123–130. [Google Scholar]
- ISO 2451:2017; Cocoa Beans—Specification and Quality Requirements. 3rd ed. International Organization for Standardization (ISO): Geneva, Switzerland, 2017; Volume 2017.
- Djaafar, T.F.; Utami, T.; Marwati, T.; Pramesi, P.C.; Wikandari, R.; Rahayu, E.S. The assessment of good manufacturing practices (GMP) implementation and critical control point (CCP) determination on the cocoa powder processing in Agricultural Techno Park Nglanggeran, Yogyakarta. IOP Conf. Ser. Mater. Sci. Eng. 2020, 980, 12034. [Google Scholar] [CrossRef]
- Code of Practice by Codex Alimentarius Commission (CAC). Code of Practice for the Prevention and Reduction of Ochratoxin A Contamination in Cocoa (CAC/RCP 72-2013); Food and Agriculture Organization (FAO): Rome, Italy, 2013. [Google Scholar]
- Delgado-Ospina, J.; Di Mattia, C.D.; Paparella, A.; Mastrocola, D.; Martuscelli, M.; Chaves-Lopez, C. Effect of Fermentation, Drying and Roasting on Colombian Criollo Cocoa Beans and Shells. Foods 2020, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-M.; Kim, S.-Y.; Kim, D.-R.; Jo, S.-C.; Nam, K.C.; Ahn, D.U.; Lee, S.-C. Effect of Heat Treatment on the Antioxidant Activity of Extracts from Citrus Peels. J. Agric. Food Chem. 2004, 52, 3389–3393. [Google Scholar] [CrossRef]
- Waldemar, S.; Julia, D.; André, L. Increased Biomass Production by Mesophilic Food-Associated Bacteria through Lowering the Growth Temperature from 30 °C to 10 °C. Appl. Environ. Microbiol. 2016, 82, 3754–3764. [Google Scholar] [CrossRef]
- Anne, D.-R.; Jean-Philippe, L.; Jean-Marie, P. Enterobacter spp.: Update on Taxonomy, Clinical Aspects, and Emerging Antimicrobial Resistance. Clin. Microbiol. Rev. 2019, 32, e00002-19. [Google Scholar] [CrossRef]
- Norlia, M.; Jinap, S.; Nor-Khaizura, M.A.R.; Radu, S.; Samsudin, N.I.P.; Azri, F.A. Aspergillus section Flavi and Aflatoxins: Occurrence, Detection, and Identification in Raw Peanuts and Peanut-Based Products Along the Supply Chain. Front. Microbiol. 2019, 10, 2602. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Hocking, A.D.; Pitt, J.I.; Fleet, G.H. Growth and mycotoxin production by fungi in atmospheres containing 80% carbon dioxide and 20% oxygen. Int. J. Food Microbiol. 2010, 143, 218–225. [Google Scholar] [CrossRef]
- Fagbohun, E.; Anibijuwon, I.; Egbebi, O.; Lawal, O. Fungi Associated with Spoilage of Dried Cocoa Beans During Storage in Ekti State of Nigeria. J. Microbiol. Biotechnol. food Sci. 2011, 1, 204–214. [Google Scholar]
- Askun, T.; Elfem, R.; Taskin, E. Comparison of Rose Bengal Chloramphenicol Agar and Dichloran Glycerol Agar (DG18) for Enumeration and Isolation of Moulds from Raisins. J. Appl. Biol. Sci. 2007, 1, 71–74. [Google Scholar]
- Koua, B.K.; Koffi, P.M.E.; Gbaha, P. Evolution of shrinkage, real density, porosity, heat and mass transfer coefficients during indirect solar drying of cocoa beans. J. Saudi Soc. Agric. Sci. 2019, 18, 72–82. [Google Scholar] [CrossRef]
- Sánchez-Hervás, M.; Gil, J.V.; Bisbal, F.; Ramón, D.; Martínez-Culebras, P.V. Mycobiota and mycotoxin producing fungi from cocoa beans. Int. J. Food Microbiol. 2008, 125, 336–340. [Google Scholar] [CrossRef]
- Dogan, A.; Erkan, M. Responses of High Carbon Dioxide Concentration on Postharvest Quality of Fresh Fig Fruit during Storage. Horticulturae 2023, 9, 293. [Google Scholar] [CrossRef]
- Jin Choi, H.; Seuk Bae, Y.; Soo Lee, J.; Hea Park, M.; Gang Kim, J. Effects of Carbon Dioxide Treatment and Modified Atmosphere Packaging on the Quality of Long Distance Transporting “Maehyang” Strawberry. Agric. Sci. 2016, 7, 813–821. [Google Scholar] [CrossRef]
- Mabbett, T. Quality control for stored coffee and cocoa. Int. Pest Control. Mag. 2013, 55, 91–94. [Google Scholar]
- Jonfia-Essien, W.A.; Navarro, S.; Villers, P. Hermetic Storage: A Novel Approach to The Protection of Cocoa Beans. African Crop Sci. J. 2010, 18, 59–68. [Google Scholar] [CrossRef]
- McClure, A.P.; Hopfer, H.; Grün, I.U. Optimizing consumer acceptability of 100% chocolate through roasting treatments and effects on bitterness and other important sensory characteristics. Curr. Res. Food Sci. 2022, 5, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Maïmouna Kouamé, L. Cocoa Fermentation from Agnéby-Tiassa: Biochemical Study of Microflora. Am. J. Biosci. 2015, 3, 203. [Google Scholar] [CrossRef]
- Dulce, V.-R.; Anne, G.; Manuel, K.; Carlos, A.-A.; Jacobo, R.-C.; Sergio de Jesús, C.-E.; Eugenia, L.-C. Cocoa bean turning as a method for redirecting the aroma compound profile in artisanal cocoa fermentation. Heliyon 2021, 7, e07694. [Google Scholar] [CrossRef]
- Anvoh, K.; Guéhi, T.; Beugré, G.; Kinimo, J.; Gnakri, D. Comparison of Biochemical Changes During Alcoholic Fermentation of Cocoa Juice Conducted by Spontaneous and Induced Processes for the Production of Ethanol. Afr. J. Food Agric. Nutr. Dev. 2010, 10, 2740–2754. [Google Scholar] [CrossRef]
- Cempaka, L.; Aliwarga, L.; Purwo, S.; Penia Kresnowati, M.T.A. Dynamics of cocoa bean pulp degradation during cocoa bean fermentation: Effects of yeast starter culture addition. J. Math. Fundam. Sci. 2014, 46, 14–25. [Google Scholar] [CrossRef]
- León-Roque, N.; Abderrahim, M.; Nuñez-Alejos, L.; Arribas, S.M.; Condezo-Hoyos, L. Prediction of fermentation index of cocoa beans (Theobroma cacao L.) based on color measurement and artificial neural networks. Talanta 2016, 161, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cortes, T.; Salgado-Cervantes, M.A.; García-Alamilla, P.; García-Alvarado, M.A.; del C Rodríguez-Jimenes, G.; Hidalgo-Morales, M.; Robles-Olvera, V. Relationship between fermentation index and other biochemical changes evaluated during the fermentation of Mexican cocoa (Theobroma cacao) beans. J. Sci. Food Agric. 2013, 93, 2596–2604. [Google Scholar] [CrossRef]
- Indiarto, R.; Pranoto, Y.; Santoso, U. Supriyanto In vitro antioxidant activity and profile of polyphenol compounds extracts and their fractions on cacao beans. Pakistan J. Biol. Sci. 2019, 22, 34–44. [Google Scholar] [CrossRef]
- Indiarto, R.; Subroto, E.; Sukri, N.; Djali, M. Cocoa (Theobroma cacao L.) beans processing technology: A review of flavonoid changes. Asian J. Plant Sci. 2021, 20, 684–693. [Google Scholar] [CrossRef]
- Payne, M.J.; Hurst, W.J.; Miller, K.B.; Rank, C.; Stuart, D.A. Impact of fermentation, drying, roasting, and Dutch processing on epicatechin and catechin content of cacao beans and cocoa ingredients. J. Agric. Food Chem. 2010, 58, 10518–10527. [Google Scholar] [CrossRef] [PubMed]
- Kresnowati, M.T.A.P.; Suryani, L.; Affifah, M. Improvement of Cocoa Beans Fermentation by LAB Starter Addition. J. Med. Bioeng. 2013, 2, 274–278. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Zhao, J.; Fleet, G. The effect of lactic acid bacteria on cocoa bean fermentation. Int. J. Food Microbiol. 2015, 205, 54–67. [Google Scholar] [CrossRef]
- Romero Vergel, A.P.; Camargo Rodriguez, A.V.; Ramirez, O.D.; Arenas Velilla, P.A.; Gallego, A.M. A Crop Modelling Strategy to Improve Cacao Quality and Productivity. Plants 2022, 11, 157. [Google Scholar] [CrossRef]
- Tardzenyuy, M.E.; Jianguo, Z.; Akyene, T.; Mbuwel, M.P. Improving cocoa beans value chain using a local convection dryer: A case study of Fako division Cameroon. Sci. Afr. 2020, 8, e00343. [Google Scholar] [CrossRef]
- Davit, J.M.; Yusuf, R.P.; Yudari, D.A.S. Effect of Fermentation Cocoa Processing and Non Fermentation on The Quality and Pricing Products on Tunjung Sari Unit Produktive in Tabanan District. J. Agribus. Agritour. 2013, 2, 191–203. [Google Scholar]
- Romanens, E.; Pedan, V.; Meile, L.; Miescher Schwenninger, S. Influence of two anti-fungal Lactobacillus fermentum-Saccharomyces cerevisiae co-cultures on cocoa bean fermentation and final bean quality. PLoS ONE 2020, 15, e0239365. [Google Scholar] [CrossRef]
- Santhanam Menon, A.; Hii, C.L.; Law, C.L.; Shariff, S.; Djaeni, M. Effects of drying on the production of polyphenol-rich cocoa beans. Dry. Technol. 2017, 35, 1799–1806. [Google Scholar] [CrossRef]
- Ariyanti, M. Quality Characteristics of Cocoabeans (Theobroma Cacao L.) With Time Fermentation treatment Based on Iso 2323-2008. Indones. J. Ind. Res. 2017, 12, 34–42. [Google Scholar] [CrossRef]
- Chire, G.C.; Valdivia, R.A.; Milber, O.U. Ochratoxin A in Cacao and Derivatives. Prev. Meas. 2014, 17, 9–15. [Google Scholar] [CrossRef]
- Dano, S.D.; Manda, P.; Dembélé, A.; Kouassi Abla, A.M.; Bibaud, J.H.; Gouet, J.Z.; Ze Maria Sika, C.B. Influence of Fermentation and Drying Materials on the Contamination of Cocoa Beans by Ochratoxin A. Toxins 2013, 5, 2310–2323. [Google Scholar] [CrossRef] [PubMed]
- Sukmawati, D.; Arman, Z.; Hasanah, R.; Balqis, M.; Setiawan, A.; Tafrijiyyah, F.; Sari, R.; Supiyani, A.; Prihantini, N.B.; Husna, S.N.A.; et al. Application of yeasts isolated from fermented cocoa beans for biocontrol of pathogenic mold in chocolate fruit. J. Phys. Conf. Ser. 2021, 1869, 012042. [Google Scholar] [CrossRef]
- Zainuri; Sjah, T.; Prameswari, N.; Werdiningsih, W.; Tarmizi. Good agricultural and postharvest handling practices of Cocoa pods in Lombok to meet Cocoa bean quality for the global market. IOP Conf. Ser. Earth Environ. Sci. 2021, 712, 12028. [Google Scholar] [CrossRef]
- Praseptiangga, D.; Guevara Zambrano, J.M.; Sanjaya, A.P.; Muhammad, D.R.A. Challenges in the development of the cocoa and chocolate industry in Indonesia: A case study in Madiun, East Java. AIMS Agric. Food 2020, 5, 920–937. [Google Scholar] [CrossRef]


| Country | Yeast Species | Characteristics | References |
|---|---|---|---|
| Ecuador | S. cerevisiae, R. minuta, P. manshurica, P. kudriavzevii, P. kluyveri, K. marxianus, H. opuntiae, C. tropicalis, C. sorbosivorans-like, and T. delbrueckii | P. manshurica, P. kudriavzevii, and S. cerevisiae were the dominant ethanol producers. | [86] |
| Brazil | P. kundriavzevii, C. orthopsilosis, K. ohmeri, D. etchellsii, I. orientalis, H. uvarum, P. kluyveri, and S. cerevisiae | These species adapted well to both fermentation box and the stainless steel used in large-scale fermentation. | [87] |
| P. Kluyver, C. magnoliae, and S. cerevisiae | [76] | ||
| Cuba | T. delbruekii, P. terricola, C. ortopsilosis, P. occidentalis, C. tropicalis, P. kluyveri, P. kundriavzevii, H. opuntiae, and P. manshurica | Pichia kudriavzevii was the most common. Some yeasts undergo mutations caused by natural processes, such as transposons genetic recombination, changes in ploidy, and sexual reproduction. | [67,88] |
| Dominican Republic | C. zeylanoides, Y. lipolytica, H. guillermondii, and C. inconspicua | Candida inconspicua was the most common and dominant because it could survive up to the 36th hour of fermentation. | [89] |
| Indonesia | S. Cerevisiae, Kloeckera sp., S. fibuligera, C. tropicalis, and C. krusei | Yeast found in Indonesia could generally live in tropical environments. Saccharomyces cerevisiae and Candida tropicalis were resistant to high temperatures (>40 °C). | [90] |
| C. tropicalis, S. cerevisiae, and Kl. apis | [25] | ||
| Ivory Coast | G. geotrichum, W. anomalus, P. galeiforms, P. kudriavzevii, C. tropicalis, S. cerevisiae, P. kluyveri, and P. kundriavzevii | Pichia kudriavzevii, Pichia kluyveri, and Saccharomyces cerevisiae were the most common and had intraspecific diversity. | [91] |
| P. fermentans, P. klyvera, Candida sp., C. insectorum, P. kudriavezii, I. hanoiensis, P. sporocuriosa, P. manshurica, and H. opuntiae | [92] | ||
| P. kudriavezii, I. hanoiensis, P. sporocuriosa, P. manshurica, and H. opuntiae | [56] | ||
| Ghana | H. guilliermondii, P. membranifaciens, Sc. cerevisiae, S. crataegensis, P. Pijperi, I. Hanoiensis, C. zemplinina, C. michaelii, C. diversa, C. ethanolica, Schiz. pombe, and I. orientalis | H. guilliermondii was the most common species at the beginning of fermentation (0–24 h), while P. membranifaciens was the dominant species at the end of fermentation (36–144 h). | [70] |
| P. manshurica M.(P.) carribica, K. ohmeri, C. orthopsilosis, C. carpophila, H. opuntiae, S. cerevisiae, P. kundriavzevii | S. cerevisiae and P. kundriavzevii were the most common species. Hanseniaspora opuntiae was able to live at a fairly low pH. | [93] | |
| Saccharomyces cerevisiae, Kluyveromyces lactis, Candida glabrata, | S. cerevisiae and K. lactis were the most common species. | [13] | |
| Mexico | H. guilliermondii, S. crataegensis, S. cerevisiae, and P. kundriavzevii | S. cerevisiae was the most important species because it had the best survival. | [94] |
| Country | Bacterial Species | Characteristics | References |
|---|---|---|---|
| Ecuador | Lb. fermentum, A. pasteurianus, Leu. pseudomesenteroides, Lb. plantarum, A. fabarum, F. tropaeoli-like, Lb fabifermentans, Lac. lactis, Lb. nagelii, Lb. cacaonum, E. casseliflavus, A. peroxydans, A. cibinongensis, and A. malorum/indonesiensis | Lb. fermentum, A. pasteurianus, and Leu. Pseudomesenteroides were the most commonly found in fermented cocoa beans. | [86] |
| Indonesia | B. licheniformis, B. pumilus, A. pasteurianus L. plantarum, and L. cellobiosus | L. plantarum was the most consistent bacteria, while lactic acid bacteria had a dominant role in the microbial ecology of cocoa beans fermentation. | [25] |
| Ghana | Ent. faecium, Ent. casseliflavus, W. ghanensis, Leuc. Mesenteroides, Leuc. pseudomesenteroides, L. mali, L. brevis, L. plantarum, and L. Fermentum | L. plantarum was the most commonly found in fermented cocoa beans. Weisella ghanensis was known as the first-line divergent in the genus Weisella. | [99] |
| Lactiplantibacillus plantarum, Lactobacillus nagelii, Liquorilactobacillus cacaonum, Limosilactobacillus fermentum, and Leuconostoc pseudomesenteroides | [100] | ||
| Lb. plantarum, A. pasteurianus, Leu. mesenteroides, G. oxydans, G. diazotrophicus, G. hansenii, Leu. citreum, Lb. fermentum, Lb. brevis, and E. coli | [13] | ||
| Lb. Plantarum, Pd. acidilactici, Lb. hilgardii, Lc. pseudoficulneum, Lb. fermentum, G. oxydans, A. malorum, A. tropicalis, A. syzygii, and A. pasteurianus | [70] | ||
| A. tropicalis-like, A. tropicalis, A. syzygii-like, A. senegalensis, A. senegalensis, and A. pasteurianus | [21] | ||
| Dominican Republic | L. paracasei subsp. paracasei, L. brevis, L. pentosus, and L. plantarum | L. plantarum was most commonly found in fermented cocoa beans. | [89] |
| Ivory Coast | W. cibaria, W. paramesenteroide, L. casei, F. pseudoficulneus, Ent. faecium, L. curieae, Leuc. mesenteroides, and L. plantarum, | Leuconostoc mesenteroides was most commonly found in fermented cocoa beans. Leuconostoc mesenteroides could catabolize citrate more efficiently. | [101] |
| A. malorum, A. ghanensis, A. okinawensis, A. tropicalis, A. pasteurianus, and G. oxydans | A. pasteurianus, A. okinawensis, and A. tropicalis were the most commonly found acetic acid bacteria in fermented cocoa beans. | [96] | |
| Lactobacilli sp., Lactococci sp. | Lactobacilli and lactococci could metabolize sucrose, fructose, and glucose during fermentation. Lactobacilli strains were unable to metabolize citrate, while lactococci strains could use citrate as a carbon source. | [75] | |
| Brazil | Oenococcus oeni, P. acidilactici, S. salivarius, F. pseudoficulneus, Lc. mesenteroides, Lc. lactis, L. reuteri, L. amylovo-rus, P. dextrinicus, L. brevis, L. acidophilus, L. delbrueckii, L. lactis, L. rhamnosus, L. casei, L. fermentum, and L. plantarum. | L. plantarum was the most commonly found in fermented cocoa beans. L. plantarum adapted well to cocoa ecosystem by responding to changes in ethanol concentration, temperature, and acid stress. | [92] |
| Acetobacter senegalensis, Bacillus subtilis, Limosilactobacillus fermentum, Brevundimonas, Pseudomonas, and Kozakia baliensis. | [100] | ||
| G. saccharivorans, G. xylinus, Ga. oxydans, A. peroxydans, A. cerevisiae, A. malorum, A. indonesiensis, A. fabarum, A. lovaniensis, A. senegalensis, A. ghanensis, A. pasteurianus, and A. aceti | [102] | ||
| Ecuador | W. fabaria, W. cibaria, L. satsumensis, F. ficulneus, E. saccharolyticus, L. amylovorus, L. cacaonum, L. nagelii, Lc. lactis subsp. lactis, L. fabifermentans, F. tropaeoli-like, Leuc. pseudomesenteroides, and L. fermentum, | L. fermentum was the dominant and widely studied lactic acid bacteria. L. fermentum lived at the beginning of fermentation of cocoa beans and could change citrate. Assimilation of citric acid increased the pH levels, thereby allowing the growth of less acid-fast lactic acid bacteria species, facilitating acetic acid bacteria growth, and optimizing the expression of some microbial activity, such as pectinolytic activity by yeast. | [86] |
| Microorganism | Lifetime | pH | Temperature | Role | References |
|---|---|---|---|---|---|
| Yeast | Lived at the beginning of fermentation, and then the population increased in the 24th hour. | 3.1–3.3 | 30–35 °C | Yeast converted glucose from the pulp into ethanol. Its decomposed pectin compounds into pectin acids and alcohols in the presence of proto-pectinase enzymes, then decomposed pectin acids into arabinose, galactose, and acetic acid using pectinase enzymes. Converted citric acid contained in the pulp. | [20,75] |
| Lactic acid bacteria | Grew from the beginning of fermentation, and then became dominant at 36 to 72 h. | 3.3–4.0 | 30–40 °C | Broke down sugar into lactic acid, pyruvate, and mannitol, and then lowered the pH. | [20,81,105] |
| Acetic acid bacteria | Grew from the beginning of fermentation, and then became dominant at 72 h. | 4.0–5.0 | 28–30 °C | Played a role in the process of oxidation of alcohol compounds (ethanol) to acetic acid. | [20,113] |
| Mold | Grew at moisture content > 8% | 2.0–8.5 | 25–30 °C | It caused the rotting of cocoa beans and produced toxins and other secondary metabolites. | [114,115] |
| Post-Harvest Stage | Treatments | Conditions | Characteristics | References |
|---|---|---|---|---|
| Sorting and fermentation | Determining cocoa pod maturity and fermentation time to increase healthful bioactive compounds. | Pod harvest: mature and ripe. Post fermentation: 1, 3, 5 days. | Mature cocoa pods and fermentation for 3 days could produce cocoa beans with a high content of bioactive compounds, high antioxidant activity, and the desired flavor. | [52] |
| Fermentation | Fermentation by administering anti-fungal strains. | The anti-fungal strain of L. fermentum and S. cerevisiae were added to 180 kg box. | Culture of L. fermentum 223 and S. cerevisiae H290 had good anti-fungi activity and produced less off-flavor, a good percentage of fermented beans, less astringency, and the best cocoa taste. | [156] |
| Fermentation | Fermentation with the addition of a mixture of LAB and AAB starter cultures | Starter culture: A. pasteurianus 386B, L. fermentum 222, S. cerevisiae H5S5K23 (3 heaps and 1 box). | The addition of lactic acid bacteria and acetic acid bacteria starter culture mixture accelerated carbohydrate fermentation by increasing the conversion of lactic acid and citric acid and was proven to improve the chocolate flavor produced. | [105] |
| Fermentation | Controlled temperature and pH during fermentation. | Temperature: 12–28 °C, 20–26 °C, and 16–28 °C. RH: 80–85%, 80–85%, and 70–75%. | Controlled fermentation of cocoa beans at a pH between 4.75 to 5.19 and a temperature below 40 °C could optimize activity of microbes and enzymes forming flavor compounds, as well as other sensory attributes. | [85] |
| Fermentation | Cocoa bean turning start times on fermentation | Cocoa beans: Criollo, turning start times: 24 and 48 h). | Turning start of 48 h could stimulate flavor-forming microbes, such as M. carpophila, P. manshurica, and H. opuntiae in cocoa beans fermentation. | [143] |
| Fermentation | Addition of acetic acid bacterial culture starter in fermentation | Culture starter: 130 AAB from 3 countries (French Guiana, Ivory Coast, and Mexico). | The addition of A. pasteurianus starter culture increased the conversion of ethanol and lactic acid into acetoin or acetic acid, thereby improving quality of cocoa beans. | [84] |
| Fermentation | Addition of LAB culture starter in fermentation to inhibit the growth of fungi. | Starter LAB: L. fermentum and L. plantarum. Fermentation periods: 5 days. | The addition of L. fermentum, L. plantarum, and a combination of L. plantarum with A. aceti and S. cerevisiae provided suitable pH and temperature, and inhibited the growth of fungi. | [116] |
| Fermentation | Addition of indigenous LAB, yeast, AAB in fermentation to inhibit mycotoxins produced by fungi. | Starter: indigenous Acetobacter spp. HA-37, L. plantarum HL-15, C. famata HY-37 at concentration of 109 CFU/mL. Fermentation periods: 5 days. | The use of indigenous L. plantarum HL-15 or in combination with Acetobacter spp. and C. famata inhibited ochratoxin-A produced by fungi. | [14] |
| Fermentation | Addition of BAL starter L. plantarum in the fermentation of cocoa beans. | Starter L. plantarum: 103 CFU per gram cocoa beans. | The addition of BAL starter accelerated the growth of lactic acid bacteria and acetic acid bacteria. There was also an increase in the amount of acetic acid, lactic acid, and ethanol produced. Fermentation index increased and the time was shorter. | [151] |
| Fermentation and drying | Determining fermentation and drying times in the rainy season. | Fermentation periods: 5–8 days. Drying: 4–6 days. | Fermentation for 8 days and drying for 6 days using sunlight in the rainy season produced the best quality cocoa beans. | [27] |
| Drying | Drying cocoa beans with adsorption, vacuum drying, and freeze drying to get high antioxidant cocoa beans. | Pressure of freeze dryer: 0.015 mbar, condenser dimensions: 31.3 cm × 34.5 cm × 46.0 cm. | Freeze-drying produced cocoa beans with the highest antioxidant activity (71.8 mg Trolox/g) and polyphenol content (126.3 mg GAE/mg). | [157] |
| Drying | Drying cocoa beans with solar power equipped with a heat pump. | Temperature: 32–48 °C and RH: 35–80%. | A solar dryer with a heat pump can speed up the drying of cocoa beans from 6 days to 5 days. | [118] |
| Storage | Re-fermentation of dry non-fermented cocoa beans by administering pure cultures to fermented the non-fermented of dry cocoa beans. | Moisture: 15%. Fermentation periods: 120 h. | The addition of pure culture of A. aceti, L. lactis, and S. cerevisiae improved quality of dry cocoa beans by facilitating the fermentation process and increasing the fermentation index up to 1.03. | [12] |
| Advantages | |||
|---|---|---|---|
| No. | Role | Microorganism | References |
| 1. | It decomposed pectin compounds into pectin acids and alcohol, and the pulp was crushed and released due to the decomposition of pectin. Sugars were then converted into alcohol compounds and citric acid was broken down. | Yeast | [25,75] |
| 2. | Broke down citric acid in cocoa fermentation sugar in the pulp through homofermentative and heterofermentative pathways. | Lactic acid bacteria | [75,105] |
| 3. | Played a role in the process of oxidation of alcohol compounds to acetic acid. | Acetic acid bacteria | [19,20] |
| 4. | Killed the seeds to ensure changes, such as the formation of color and flavor precursors in fermentation. | Acetic acid bacteria, yeast, and lactic acid bacteria | [158] |
| Disadvantages | |||
| No. | Role | Microorganism | References |
| 1. | The growth of mold was a risk to public health due to the toxins it produced. | Aspergillus, Penicillium, and Fusarium | [159,160] |
| 2. | It caused typhus with symptoms of diarrhea, nausea, and dizziness if cocoa beans were contaminated. | Salmonella | [39] |
| 3. | The cause of weathering, reduced nutrition, and the presence of mycotoxins, which could cause health problems in cocoa beans. | Mold | [34] |
| 4. | Contamination by bacteria could cause nosocomial infections when cocoa pods were ripened. | Acinetobacter sp., Klebsiella pneumoniae | [57] |
| 5. | There was damage to the color and flavor of cocoa beans. | Kapang | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subroto, E.; Djali, M.; Indiarto, R.; Lembong, E.; Baiti, N. Microbiological Activity Affects Post-Harvest Quality of Cocoa (Theobroma cacao L.) Beans. Horticulturae 2023, 9, 805. https://doi.org/10.3390/horticulturae9070805
Subroto E, Djali M, Indiarto R, Lembong E, Baiti N. Microbiological Activity Affects Post-Harvest Quality of Cocoa (Theobroma cacao L.) Beans. Horticulturae. 2023; 9(7):805. https://doi.org/10.3390/horticulturae9070805
Chicago/Turabian StyleSubroto, Edy, Mohamad Djali, Rossi Indiarto, Elazmanawati Lembong, and Nur Baiti. 2023. "Microbiological Activity Affects Post-Harvest Quality of Cocoa (Theobroma cacao L.) Beans" Horticulturae 9, no. 7: 805. https://doi.org/10.3390/horticulturae9070805
APA StyleSubroto, E., Djali, M., Indiarto, R., Lembong, E., & Baiti, N. (2023). Microbiological Activity Affects Post-Harvest Quality of Cocoa (Theobroma cacao L.) Beans. Horticulturae, 9(7), 805. https://doi.org/10.3390/horticulturae9070805








