Integrated Transcriptome and Metabolome Analysis Revealed the Molecular Mechanism of Anthocyanin Synthesis in Purple Leaf Pepper (Capsicum annuum L.) under Different Light Intensities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
2.2. Determination of Physiological Index Parameters
2.3. RNA Extraction, Library Preparation, and Sequencing
2.4. Transcriptome Analysis
2.5. Metabolite Extraction
2.6. Quantitative Real-Time PCR
3. Results
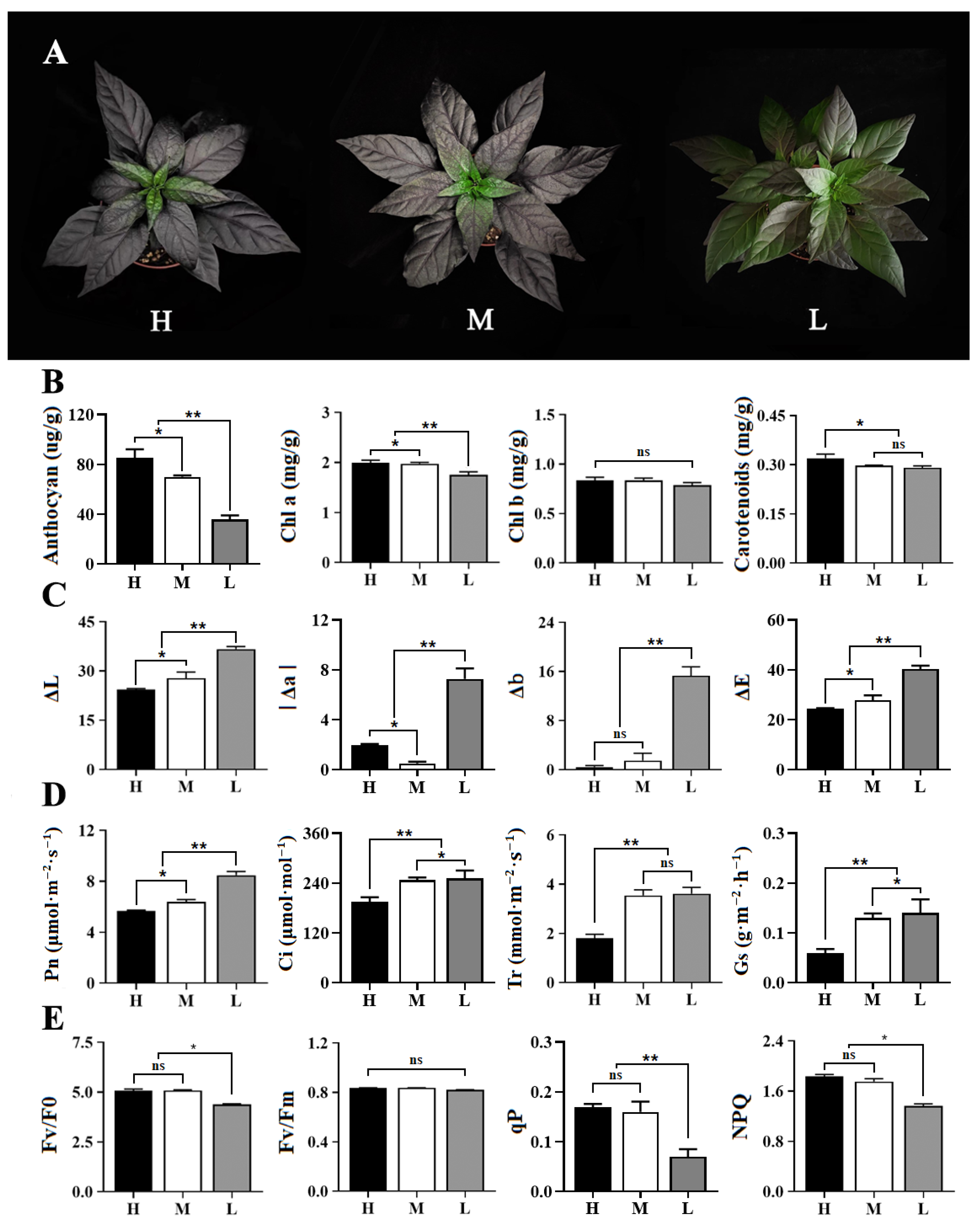
3.1. Phenotype Identification and Pigment Content Analysis
3.2. Transcriptome Analysis of Different Light Intensities
3.3. Analysis of Differentially Expressed Genes (DEGs)
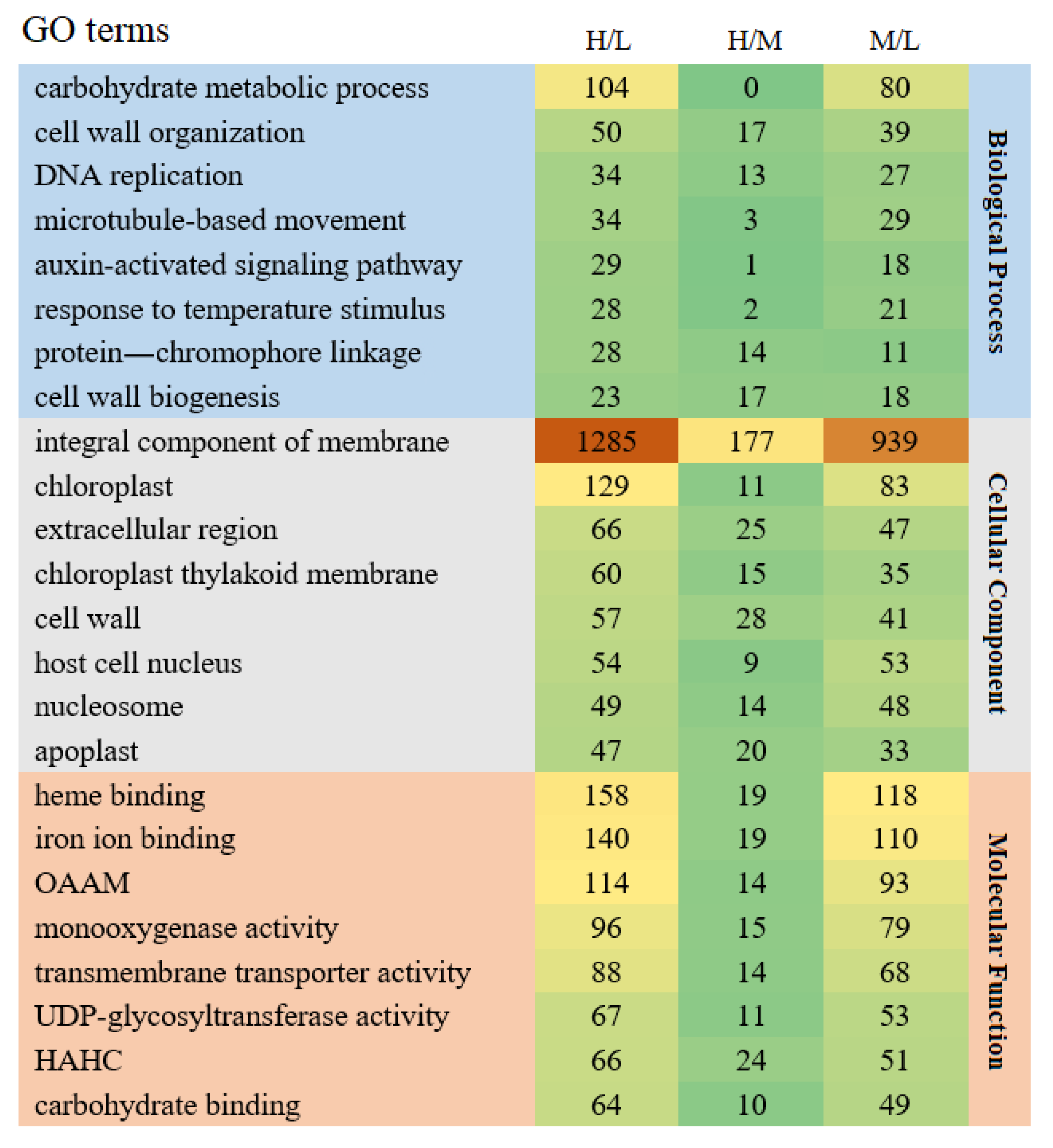
3.4. GO Analyses of DEGs
3.5. KEGG Pathway Analyses of DEGs
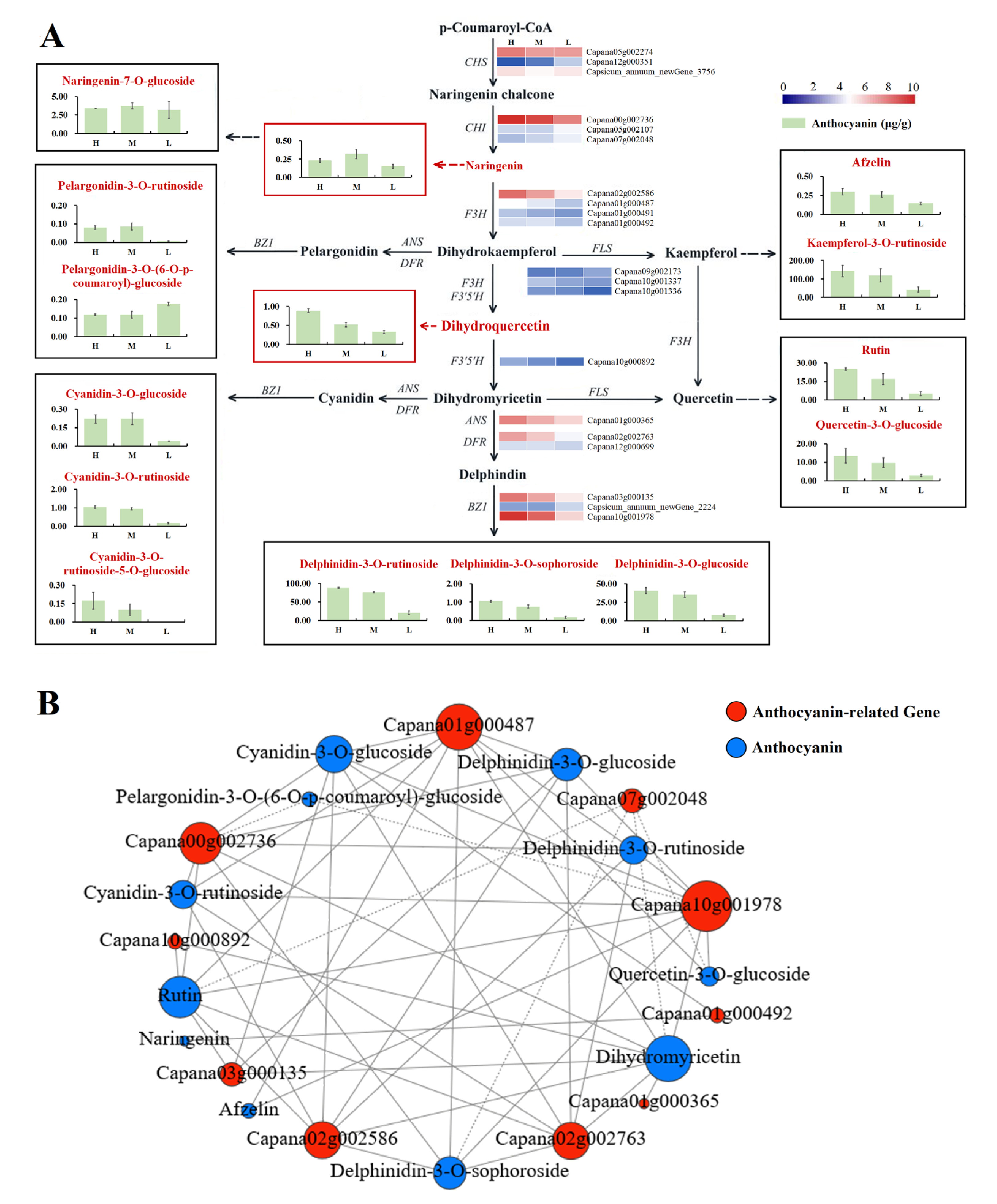
3.6. Metabolome Analysis of Anthocyanins
3.7. Analysis of Genes Involved in Anthocyanin Biosynthesis
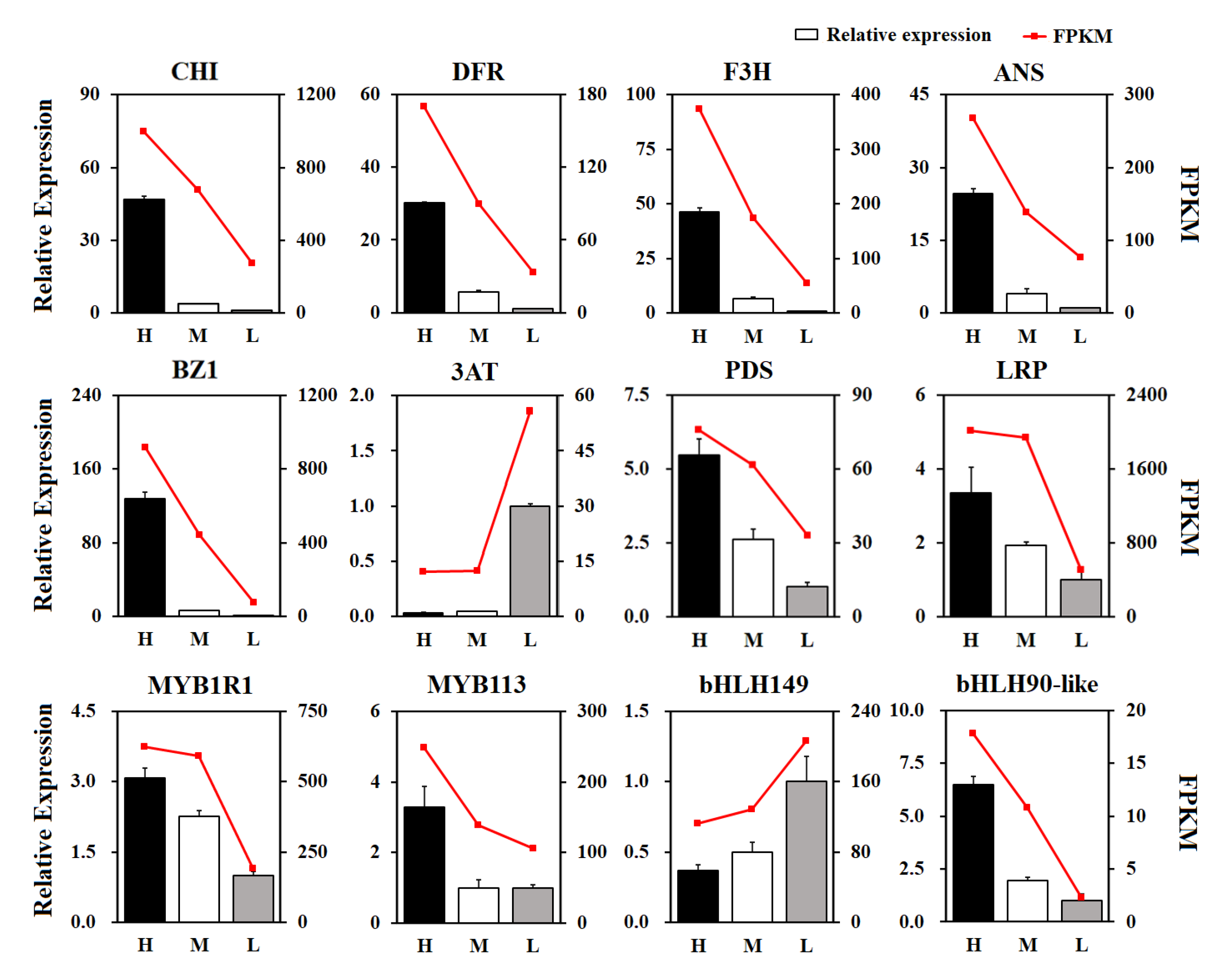
3.8. Analysis of Transcription Factors Involved in Anthocyanin Biosynthesis
3.9. Real-Time PCR Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, Z.; Zou, X. Geographical and Ecological Differences in Pepper Cultivation and Consumption in China. Front. Nutr. 2021, 8, 718517. [Google Scholar] [CrossRef]
- Zhao, M.-H.; Li, X.; Zhang, X.-X.; Zhang, H.; Zhao, X.-Y. Mutation Mechanism of Leaf Color in Plants: A Review. Forests 2020, 11, 851. [Google Scholar] [CrossRef]
- Holton, T.A.; Cornish, E.C. Genetics and Biochemistry of Anthocyanin Biosynthesis. Plant Cell 1995, 7, 1071–1083. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of Plant Pigments: Anthocyanins, Betalains and Carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; DI Gaspero, G. Transcriptional Regulation of Anthocyanin Biosynthesis in Ripening Fruits of Grapevine under Seasonal Water Deficit. Plant Cell Env. 2007, 30, 1381–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Giusti, M.M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, Y.-J.; Zhang, F.-J.; Zhang, G.-Z.; Jiang, X.-Y.; Yu, H.-M.; Hou, B.-K. The Arabidopsis UDP-Glycosyltransferases UGT79B2 and UGT79B3, Contribute to Cold, Salt and Drought Stress Tolerance via Modulating Anthocyanin Accumulation. Plant J. 2017, 89, 85–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarma, A.D.; Sharma, R. Anthocyanin-DNA Copigmentation Complex: Mutual Protection against Oxidative Damage. Phytochemistry 1999, 52, 1313–1318. [Google Scholar] [CrossRef]
- Sivankalyani, V.; Feygenberg, O.; Diskin, S.; Wright, B.; Alkan, N. Increased Anthocyanin and Flavonoids in Mango Fruit Peel Are Associated with Cold and Pathogen Resistance. Postharvest Biol. Technol. 2016, 111, 132–139. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Systematic Identification and Characterization of Anthocyanins by HPLC-ESI-MS/MS in Common Foods in the United States: Fruits and Berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A Colorful Model for the Regulation and Evolution of Biochemical Pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Roles of R2R3-MYB Transcription Factors in Transcriptional Regulation of Anthocyanin Biosynthesis in Horticultural Plants. Plant Mol. Biol. 2018, 98, 1–18. [Google Scholar] [CrossRef]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The Flavonoid Biosynthetic Pathway in Arabidopsis: Structural and Genetic Diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Ke, X.; Yang, X.; Liu, Y.; Hou, X. Plants Response to Light Stress. J. Genet. Genom. 2022, 49, 735–747. [Google Scholar] [CrossRef]
- Maier, A.; Hoecker, U. COP1/SPA Ubiquitin Ligase Complexes Repress Anthocyanin Accumulation under Low Light and High Light Conditions. Plant Signal Behav. 2015, 10, e970440. [Google Scholar] [CrossRef] [Green Version]
- Niu, S.-S.; Xu, C.-J.; Zhang, W.-S.; Zhang, B.; Li, X.; Lin-Wang, K.; Ferguson, I.B.; Allan, A.C.; Chen, K.-S. Coordinated Regulation of Anthocyanin Biosynthesis in Chinese Bayberry (Myrica rubra) Fruit by a R2R3 MYB Transcription Factor. Planta 2010, 231, 887–899. [Google Scholar] [CrossRef]
- Zhou, Y.; Singh, B.R. Effect of Light on Anthocyanin Levels in Submerged, Harvested Cranberry Fruit. J. Biomed. Biotechnol. 2004, 2004, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Uleberg, E.; Rohloff, J.; Jaakola, L.; Trost, K.; Junttila, O.; Häggman, H.; Martinussen, I. Effects of Temperature and Photoperiod on Yield and Chemical Composition of Northern and Southern Clones of Bilberry (Vaccinium myrtillus L.). J. Agric. Food Chem. 2012, 60, 42. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Y.; Chen, C.-T.; Wang, C.Y. The Influence of Light and Maturity on Fruit Quality and Flavonoid Content of Red Raspberries. Food Chem. 2009, 112, 676–684. [Google Scholar] [CrossRef]
- Løvdal, T.; Olsen, K.; Slimestad, R.; Verheul, M.; Lillo, C. Synergetic Effects of Nitrogen Depletion, Temperature, and Light on the Content of Phenolic Compounds and Gene Expression in Leaves of Tomato. Phytochemistry 2010, 71, 605–613. [Google Scholar] [CrossRef]
- Li, J.; He, Y.-J.; Zhou, L.; Liu, Y.; Jiang, M.; Ren, L.; Chen, H. Transcriptome Profiling of Genes Related to Light-Induced Anthocyanin Biosynthesis in Eggplant (Solanum melongena L.) before Purple Color Becomes Evident. BMC Genom. 2018, 19, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaak, C.K.; Petkau, J.C.; Blewett, H.; Karmin; Siow, Y. L. Lingonberry Anthocyanins Protect Cardiac Cells from Oxidative-Stress-Induced Apoptosis. Can. J. Physiol. Pharmacol. 2017, 95, 904–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Dai, Y.; Mao, L.; Yang, S.; Yang, B.; Sun, Y.; Zhou, Y.; Wang, S.; Zou, X.; Ou, L. Integrated Metabolome and Transcriptome Analysis Revealed Carotenoid Metabolism Difference in Pepper (Capsicum annuum L.) Yellowing Mutants under Different Light Quality. Veg. Res. 2022, 2, 21. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Di Paola-Naranjo, R.D.; Sánchez-Sánchez, J.; González-Paramás, A.M.; Rivas-Gonzalo, J.C. Liquid Chromatographic-Mass Spectrometric Analysis of Anthocyanin Composition of Dark Blue Bee Pollen from Echium Plantagineum. J. Chromatogr. A 2004, 1054, 205–210. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global Metabolic Profiling of Animal and Human Tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Qi, Y.; Lou, Q.; Li, H.; Yue, J.; Liu, Y.; Wang, Y. Anatomical and Biochemical Studies of Bicolored Flower Development in Muscari Latifolium. Protoplasma 2013, 250, 1273–1281. [Google Scholar] [CrossRef]
- Taylor, S.C.; Nadeau, K.; Abbasi, M.; Lachance, C.; Nguyen, M.; Fenrich, J. The Ultimate QPCR Experiment: Producing Publication Quality, Reproducible Data the First Time. Trends Biotechnol. 2019, 37, 761–774. [Google Scholar] [CrossRef] [Green Version]
- Mestdagh, P.; Van Vlierberghe, P.; De Weer, A.; Muth, D.; Westermann, F.; Speleman, F.; Vandesompele, J. A Novel and Universal Method for MicroRNA RT-QPCR Data Normalization. Genome Biol. 2009, 10, R64. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Bashandy, H.; Ainasoja, M.; Kontturi, J.; Pietiäinen, M.; Laitinen, R.A.E.; Albert, V.A.; Valkonen, J.P.T.; Elomaa, P.; Teeri, T.H. Functional Diversification of Duplicated Chalcone Synthase Genes in Anthocyanin Biosynthesis of Gerbera Hybrida. New Phytol. 2014, 201, 1469–1483. [Google Scholar] [CrossRef]
- Dong, X.; Braun, E.L.; Grotewold, E. Functional Conservation of Plant Secondary Metabolic Enzymes Revealed by Complementation of Arabidopsis Flavonoid Mutants with Maize Genes. Plant Physiol. 2001, 127, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Albert, N.W.; Lewis, D.H.; Zhang, H.; Irving, L.J.; Jameson, P.E.; Davies, K.M. Light-Induced Vegetative Anthocyanin Pigmentation in Petunia. J. Exp. Bot. 2009, 60, 2191–2202. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.H.; Kim, J.-S.; Kim, S.; Soh, H.Y.; Shin, H.; Jang, H.; Ryu, J.H.; Kim, A.; Yun, K.-Y.; Kim, S.; et al. De Novo Transcriptome Analysis to Identify Anthocyanin Biosynthesis Genes Responsible for Tissue-Specific Pigmentation in Zoysiagrass (Zoysia japonica Steud.). PLoS ONE 2015, 10, e0124497. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, Y.; Zhao, M.; Hu, Y.; Meng, F.; Song, X.; Tigabu, M.; Chiang, V.L.; Sederoff, R.; Ma, W.; et al. Molecular and Metabolic Insights into Anthocyanin Biosynthesis for Leaf Color Change in Chokecherry (Padus virginiana). Int. J. Mol. Sci. 2021, 22, 10697. [Google Scholar] [CrossRef]
- Lou, Q.; Liu, Y.; Qi, Y.; Jiao, S.; Tian, F.; Jiang, L.; Wang, Y. Transcriptome Sequencing and Metabolite Analysis Reveals the Role of Delphinidin Metabolism in Flower Colour in Grape Hyacinth. J. Exp. Bot. 2014, 65, 3157–3164. [Google Scholar] [CrossRef] [Green Version]
- Baudry, A.; Heim, M.A.; Dubreucq, B.; Caboche, M.; Weisshaar, B.; Lepiniec, L. TT2, TT8, and TTG1 Synergistically Specify the Expression of BANYULS and Proanthocyanidin Biosynthesis in Arabidopsis Thaliana. Plant J. 2004, 39, 366–380. [Google Scholar] [CrossRef]
- Terrier, N.; Torregrosa, L.; Ageorges, A.; Vialet, S.; Verriès, C.; Cheynier, V.; Romieu, C. Ectopic Expression of VvMybPA2 Promotes Proanthocyanidin Biosynthesis in Grapevine and Suggests Additional Targets in the Pathway. Plant Physiol. 2009, 149, 1028–1041. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Liu, W.; Zhang, T.; Jiang, S.; Xu, H.; Wang, Y.; Zhang, Z.; Wang, C.; Chen, X. Transcriptomic Analysis of Red-Fleshed Apples Reveals the Novel Role of MdWRKY11 in Flavonoid and Anthocyanin Biosynthesis. J. Agric. Food Chem. 2018, 66, 7076–7086. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional Control of Flavonoid Biosynthesis by MYB-BHLH-WDR Complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, J.; Guo, H.; Yang, C.; Li, Y.; Shen, S.; Zhan, C.; Qu, L.; Liu, X.; Wang, S.; et al. OsRLCK160 Contributes to Flavonoid Accumulation and UV-B Tolerance by Regulating OsbZIP48 in Rice. Sci. China Life Sci. 2022, 65, 1380–1394. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, X.; Gong, Q.; Cao, J.; Shen, W.; Yin, X.; Grierson, D.; Zhang, B.; Xu, C.; Li, X.; et al. Three AP2/ERF Family Members Modulate Flavonoid Synthesis by Regulating Type IV Chalcone Isomerase in Citrus. Plant Biotechnol. J. 2021, 19, 671–688. [Google Scholar] [CrossRef]
- Takos, A.M.; Jaffé, F.W.; Jacob, S.R.; Bogs, J.; Robinson, S.P.; Walker, A.R. Light-Induced Expression of a MYB Gene Regulates Anthocyanin Biosynthesis in Red Apples. Plant Physiol. 2006, 142, 1216–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, L.-W.; Zhou, X.; Burke, S.; Wu, X.; Prior, R.L.; Li, L. The Purple Cauliflower Arises from Activation of a MYB Transcription Factor. Plant Physiol. 2010, 154, 1470–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Chiu, L.-W.; Li, L. Transcriptional Regulation of Anthocyanin Biosynthesis in Red Cabbage. Planta 2009, 230, 1141–1153. [Google Scholar] [CrossRef]
- Xu, Z.-S.; Yang, Q.-Q.; Feng, K.; Xiong, A.-S. Changing Carrot Color: Insertions in DcMYB7 Alter the Regulation of Anthocyanin Biosynthesis and Modification. Plant Physiol. 2019, 181, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Palapol, Y.; Ketsa, S.; Lin-Wang, K.; Ferguson, I.B.; Allan, A.C. A MYB Transcription Factor Regulates Anthocyanin Biosynthesis in Mangosteen (Garcinia mangostana L.) Fruit during Ripening. Planta 2009, 229, 1323–1334. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, X.; Li, X.; Yin, X.; Grierson, D.; Li, F.; Chen, K. A Novel BHLH Transcription Factor Involved in Regulating Anthocyanin Biosynthesis in Chrysanthemums (Chrysanthemum morifolium Ramat.). PLoS ONE 2015, 10, e0143892. [Google Scholar] [CrossRef]
- Liu, X.-F.; Yin, X.-R.; Allan, A.C.; Lin-Wang, K.; Shi, Y.-N.; Huang, Y.-J.; Ferguson, I.B.; Xu, C.-J.; Chen, K.-S. The Role of MrbHLH1 and MrMYB1 in Regulating Anthocyanin Biosynthetic Genes in Tobacco and Chinese Bayberry (Myrica rubra) during Anthocyanin Biosynthesis. Plant Cell Tiss. Organ. Cult. 2013, 115, 285–298. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Urías-Orona, V.; Muy-Rangel, M.D.; Heredia, J.B. Structure and Content of Phenolics in Eggplant (Solanum melongena)—A Review. S. Afr. J. Bot. 2017, 111, 161–169. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ohmiya, A. Seeing Is Believing: Engineering Anthocyanin and Carotenoid Biosynthetic Pathways. Curr. Opin. Biotechnol. 2008, 19, 190–197. [Google Scholar] [CrossRef]
- Park, C.H.; Bong, S.J.; Lim, C.J.; Kim, J.K.; Park, S.U. Transcriptome Analysis and Metabolic Profiling of Green and Red Mizuna (Brassica rapa L. Var. Japonica). Foods 2020, 9, 1079. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Young, J.C.; Rabalski, I. Anthocyanin Composition in Black, Blue, Pink, Purple, and Red Cereal Grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef]
- Jin, S.-W.; Rahim, M.A.; Jung, H.-J.; Afrin, K.S.; Kim, H.-T.; Park, J.-I.; Kang, J.-G.; Nou, I.-S. Abscisic Acid and Ethylene Biosynthesis-Related Genes Are Associated with Anthocyanin Accumulation in Purple Ornamental Cabbage (Brassica oleracea Var. Acephala). Genome 2019, 62, 513–526. [Google Scholar] [CrossRef] [Green Version]
- Anthocyanin Composition of Different Wild and Cultivated Berry Species. Available online: https://www.cabdirect.org/cabdirect/abstract/20143407037 (accessed on 10 June 2023).
- Ponder, A.; Hallmann, E.; Kwolek, M.; Średnicka-Tober, D.; Kazimierczak, R. Genetic Differentiation in Anthocyanin Content among Berry Fruits. Curr. Issues Mol. Biol. 2021, 43, 36–51. [Google Scholar] [CrossRef]
- Sadilova, E.; Stintzing, F.C.; Carle, R. Anthocyanins, Colour and Antioxidant Properties of Eggplant (Solanum melongena L.) and Violet Pepper (Capsicum annuum L.) Peel Extracts. Z. Für Naturforschung C 2006, 61, 527–535. [Google Scholar] [CrossRef]
- Liu, C.; Yao, X.; Li, G.; Huang, L.; Xie, Z. Transcriptomic Profiling of Purple Broccoli Reveals Light-Induced Anthocyanin Biosynthetic Signaling and Structural Genes. PeerJ 2020, 8, e8870. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Mao, L.; Zhou, Y.; Sun, Y.; Liu, Z.; Liang, C. Integrated Transcriptome and Metabolome Analysis Revealed the Molecular Mechanism of Anthocyanin Synthesis in Purple Leaf Pepper (Capsicum annuum L.) under Different Light Intensities. Horticulturae 2023, 9, 814. https://doi.org/10.3390/horticulturae9070814
Shen Y, Mao L, Zhou Y, Sun Y, Liu Z, Liang C. Integrated Transcriptome and Metabolome Analysis Revealed the Molecular Mechanism of Anthocyanin Synthesis in Purple Leaf Pepper (Capsicum annuum L.) under Different Light Intensities. Horticulturae. 2023; 9(7):814. https://doi.org/10.3390/horticulturae9070814
Chicago/Turabian StyleShen, Yiyu, Lianzhen Mao, Yao Zhou, Ying Sun, Zhoubin Liu, and Chengliang Liang. 2023. "Integrated Transcriptome and Metabolome Analysis Revealed the Molecular Mechanism of Anthocyanin Synthesis in Purple Leaf Pepper (Capsicum annuum L.) under Different Light Intensities" Horticulturae 9, no. 7: 814. https://doi.org/10.3390/horticulturae9070814
APA StyleShen, Y., Mao, L., Zhou, Y., Sun, Y., Liu, Z., & Liang, C. (2023). Integrated Transcriptome and Metabolome Analysis Revealed the Molecular Mechanism of Anthocyanin Synthesis in Purple Leaf Pepper (Capsicum annuum L.) under Different Light Intensities. Horticulturae, 9(7), 814. https://doi.org/10.3390/horticulturae9070814





