In Vitro Study of the Compatibility of Four Species of Trichoderma with Three Fungicides and Their Antagonistic Activity against Fusarium solani
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Characterization of the Rate of Development and Growth
- y = Distance;
- m = Slope;
- x = Time;
- b = Constant factor.
2.3. Dual Test on Poisoned Culture Medium In Vitro
- PIRG = Percentage inhibition of radial growth;
- R1 = Radial growth (mm) of F. solani without Trichoderma spp.;
- R2 = Radial growth (mm) of F. solani with Trichoderma spp.
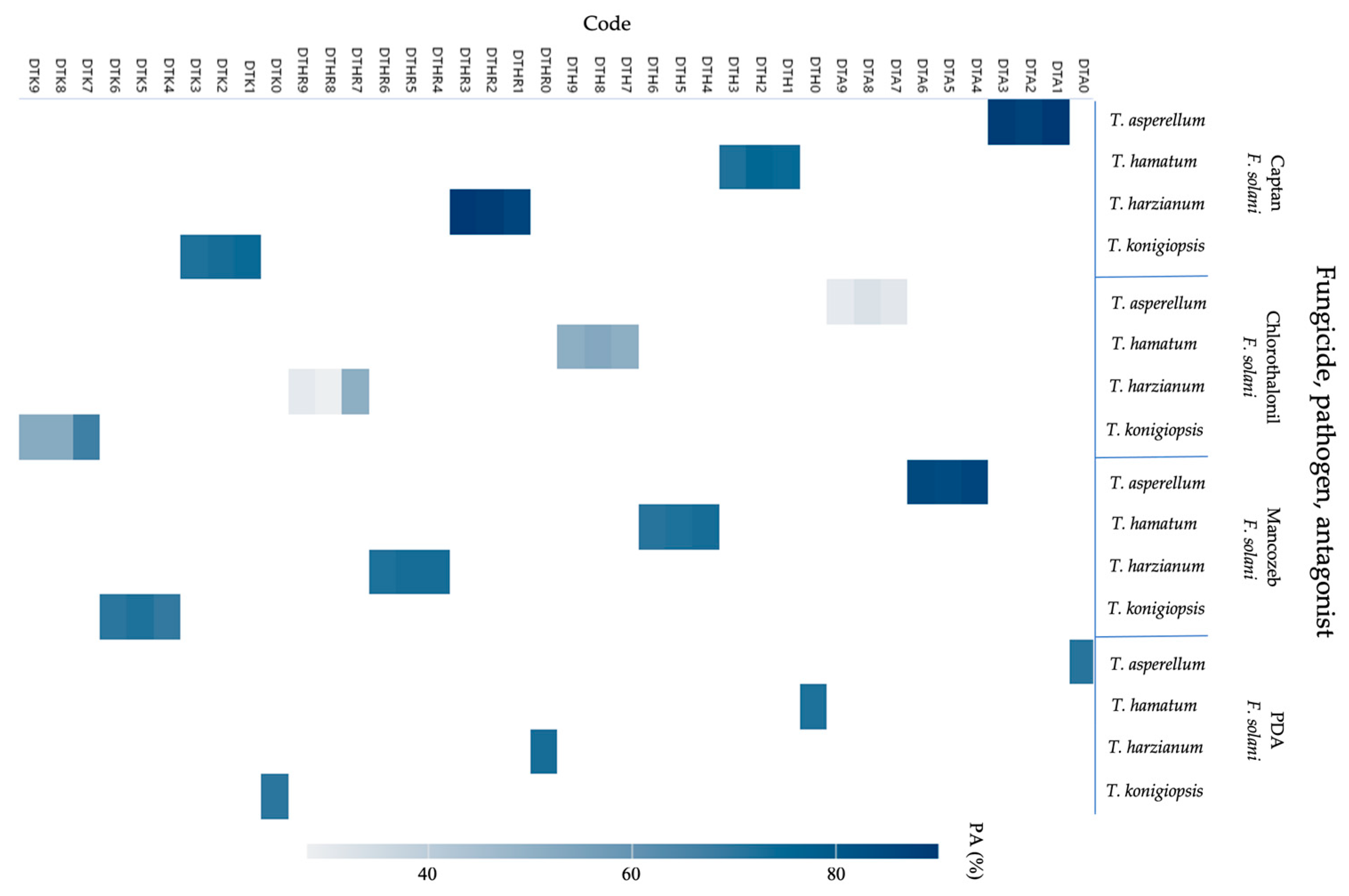
2.4. Potential Antagonism
- PA = Potential antagonism expressed as a percentage;
- Bell = Trichoderma mycoparasitism against F. solani;
- PIRG = Inhibition of radial growth of F. solani.
2.5. Statistical Analysis
- Cγj = Value of the response variable of the experimental unit associated with the γ-th treatment and the j-th repetition;
- μ = Corresponds to the overall mean of the response variable in the experiment;
- ti = Effect of the γ-th treatment;
- εγj = Error of the experimental unit associated with the γ-th treatment;
- j = The j-th repetition;
- γ = 1, 2, 3, 4, …, 40;
- j = 1, 2, 3, 4;
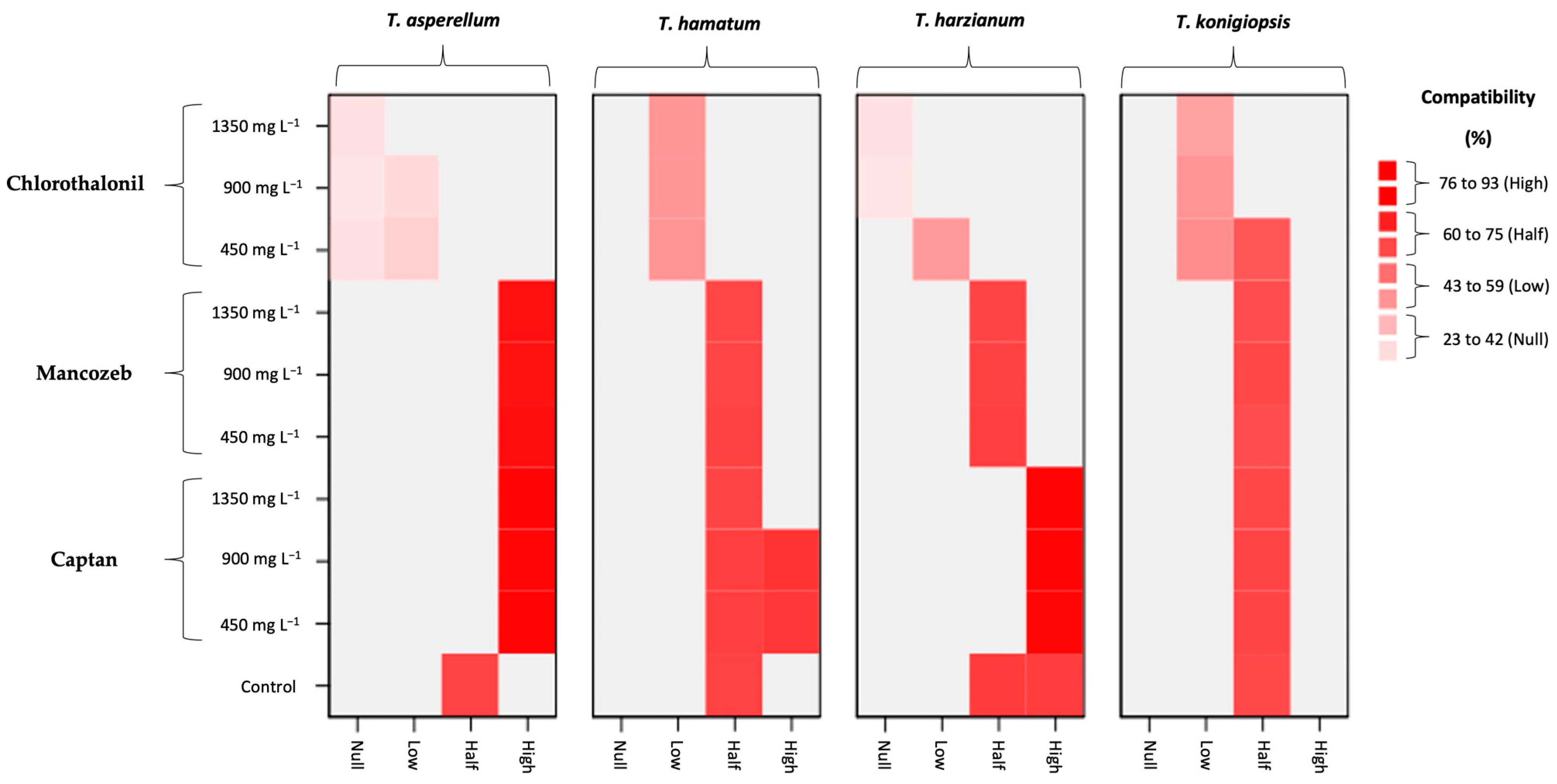
- C = Compatibility (C%).
3. Results
4. Discussion
5. Conclusions
6. Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, X.L.; Phillips, D.; Li, H.; Sivasithamparam, K.; Barbetti, M.J. Severity of strawberry crown and root diseases and associated fungal and oomycete pathogenesis in Western Australia. Australas Plant Pathol. 2011, 40, 109–119. [Google Scholar] [CrossRef]
- Hassan, O.; Chang, T. Morphological and molecular characteristics of fungal species associated with strawberry crown rot in South Korea. Mol. Biol. Rep. 2022, 49, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Martínez, K.; Ortiz, M.; Albis, A.; Gilma Gutiérrez Castañeda, C.; Valencia, M.E.; Grande Tovar, C.D. The effect of edible chitosan coatings incorporated with Thymus capitatus essential oil on the shelf-life of strawberry (Fragaria x ananassa) during cold storage. Biomolecules 2018, 8, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.; Kim, T.Y.; Won, S.-J.; Moon, J.-H.; Ajuna, H.B.; Kim, K.Y.; Ahn, Y.S. Control of fungal diseases and fruit yield improvement of strawberry using Bacillus velezensis CE 100. Microorganisms 2022, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Coronel, A.C.; Parraguirre-Lezama, C.; Pacheco-Hernández, Y.; Santiago-Trinidad, O.; Rivera-Tapia, A.; Romero-Arenas, O. Efficacy of four In vitro fungicides for control of wilting of strawberry crops in Puebla-Mexico. Appl. Sci. 2022, 12, 3213. [Google Scholar] [CrossRef]
- Ruiz-Romero, P.; Valdez-Salas, B.; González-Mendoza, D.; Mendez-Trujillo, V. Antifungal effects of silver phyto-nanoparticles from Yucca shilerifera against strawberry soil-borne pathogens: Fusarium solani and Macrophomina phaseolina. Mycobiology 2018, 46, 47–51. [Google Scholar] [CrossRef] [Green Version]
- De la Lastra, E.; Villarino, M.; Astacio, J.D.; Larena, I.; De Cal, A.; Capote, N. Genetic diversity, and vegetative compatibility of Fusarium solani species complex of strawberry in Spain. Phytopathology 2019, 109, 2142–2151. [Google Scholar] [CrossRef]
- Villarino, M.; De la Lastra, E.; Basallote-Ureba, M.J.; Capote, N.; Larena, I.; Melgarejo, P.; De Cal, A. Characterization of Fusarium solani populations associated with Spanish strawberry crops. Plant Dis. 2019, 103, 1974–1982. [Google Scholar] [CrossRef]
- Manici, L.M.; Caputo, F.; Baruzzi, G. Additional experiences to elucidate the microbial component of soil suppressiveness towards strawberry black root rot complex. Ann. App. Biol. 2005, 146, 421–431. [Google Scholar] [CrossRef]
- Ayoubi, N.; Soleimani, M.J. Morphological and molecular identification of pathogenic Fusarium spp. on strawberry in Iran. Sydowia 2016, 68, 163–171. [Google Scholar]
- Ceja-Torres, L.F.; Mora-Aguilera, G.; Téliz, D.; Mora-Aguilera, A.; Sánchez-García, P.; Muñoz-Ruíz, C.; Tlapal-Bolaños, B.; De La Torre-Almaraz, R. Ocurrencia de hongos y etiología de la secadera de la fresa con diferentes sistemas de manejo agronómico. Agrociencia 2008, 42, 451–461. [Google Scholar]
- Zhang, Y.; Yu, H.; Hu, M.; Wu, J.; Zhang, C. Fungal pathogens associated with strawberry crown rot disease in China. J. Fungi 2022, 8, 1161. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining Biocontrol Agents with Chemical Fungicides for Integrated Plant Fungal Disease Control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- James, J.E.; Santhanam, J.; Cannon, R.D.; Lamps, E. Voriconazole treatment induces a conserved regulatory network of sterol/pleiotropic drug resistance, including an alternative pathway of ergosterol biosynthesis, in the clinically important FSSC species, Fusarium keratoplasticum. J. Fungi 2022, 8, 1070. [Google Scholar] [CrossRef]
- Slaboch, J.; Čechura, L.; Malý, M.; Mach, J. The Shadow Values of Soil Hydrological Properties in the Production Potential of Climatic Regionalization of the Czech Republic. Agriculture 2022, 12, 2068. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Xue, M.; Liu, Z.; Zhang, Q.; Hou, J.; Xing, M.; Wang, R.; Liu, T. The combination of a biocontrol agent Trichoderma asperellum SC012 and hymexazol reduces the effective fungicide dose to control Fusarium Wilt in Cowpea. J. Fungi 2021, 7, 685. [Google Scholar] [CrossRef] [PubMed]
- Rabølle, M.; Spliid, N.H.; Kristensen, K.; Kudsk, P. Determination of fungicide residues in field-grown strawberries following different fungicide strategies against gray mold (Botrytis cinerea). J. Agric. Food Chem. 2006, 54, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Mayo-Prieto, S.; Squarzoni, A.; Carro-Huerga, G.; Porteous-Álvarez, A.J.; Gutiérrez, S.; Casquero, P.A. Organic and conventional bean pesticides in the development of autochthonous strains of Trichoderma. J. Fungi 2022, 8, 603. [Google Scholar] [CrossRef]
- Omar, I.; O’neill, T.M.; Rossall, S. Biological control of Fusarium crown and root rot of tomato with antagonistic bacteria and integrated control when combined with the fungicide carbendazim. Plant Pathol. 2006, 55, 92–99. [Google Scholar] [CrossRef]
- Spadaro, D.; Gullino, M.L. State of the art and future prospects of the biological control of postharvest fruit diseases. Int. J. Food Microbiol. 2004, 91, 185–194. [Google Scholar] [CrossRef]
- Wisniewski, M.E.; Wilson, C.L.; Hershherger, W. Characterization of inhibition of Rhizopus stolonifer germination and growth by Enterobacter cloacae. Can. J. Bot. 1989, 67, 2317–2323. [Google Scholar] [CrossRef]
- Abd-El-Khair, H.; Elshahawy, I.E.; Haggag, H.E. Field application of Trichoderma spp. combined with thiophanate-methyl for controlling Fusarium solani and Fusarium oxysporum in dry bean. Bull. Natl. Res. Cent. 2019, 43, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Terrero-Yépez, P.I.; Peñaherrera-Villafuerte, S.L.; Solís-Hidalgo, Z.K.; Vera-Coello, D.I.; Navarret- Cedeño, J.B.; Herrera-Defaz, M.A. Compatibilidad in vitro de Trichoderma spp. con fungicidas de uso común en cacao (Theobroma cacao L.). Investig. Agrar. 2018, 20, 146–151. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; Arjona-Girona, I.; López-Herrera, C.J. Integrated control of avocado white root rot combining low concentrations of fluazinam and Trichoderma spp. Crop Prot. 2018, 112, 363–370. [Google Scholar] [CrossRef]
- Wang, H.; Chang, K.F.; Hwang, S.F.; Turnbull, G.D.; Howard, R.J.; Blade, S.F.; Callan, N.W. Fusarium root rot of coneflower seedlings and integrated control using Trichoderma and fungicides. BioControl 2005, 50, 317–329. [Google Scholar] [CrossRef]
- Zeravakis, G.; Philippoussis, A.; Ioannidou, S.; Diamantopoulou, P. Mycelium growth kinetics and optimal temperature conditions for the cultivation of edible mushroom species on lignocellulosic substrates. Folia Microbiol. 2001, 46, 231–234. [Google Scholar] [CrossRef]
- Azza, R.E.; Hala, M.I.; Saná, A.M. The role of storage in Mancozeb fungicidal formulations and its antifungal activity against Fusarium oxysporium and Rhizoctonia solani. Árab. J. Chem. 2021, 14, 103322. [Google Scholar] [CrossRef]
- Andrade-Hoyos, P.; Luna-Cruz, L.; Osorio-Hernández, E.; Molina-Gayosso, E.; Landero-Valenzuela, N.; Barrales-Cureño, H.J. Antagonismo de Trichoderma spp. vs. Hongos Asociados a la Marchitez de Chile. Rev. Mex. Cienc. Agric. 2019, 10, 1259–1272. [Google Scholar] [CrossRef] [Green Version]
- Bell, D.K.; Wells, H.D.; Markham, C.R. In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathology 1982, 72, 379–382. [Google Scholar] [CrossRef]
- Reyes-Figueroa, O.; Ortiz-García, C.F.; Torres de la Cruz, M.; Lagunes-Espinoza, L.D.C.; Valdovinos-Ponce, G. Especies de Trichoderma del agroecosistema cacao con potencial de biocontrol sobre Moniliophthora roreri. Chapingo Ser. Cienc. Ambiente 2016, 22, 149–163. [Google Scholar] [CrossRef]
- De Mendiburu, F.; Simon, R. Agricolae-Ten years of an open-source statistical tool for experiments in breeding, agriculture and biology. Peer J. PrePrints 2015, 3, e1404v1. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.; Han, G.; Ren, C.; Zhao, S.; Wu, X.; Bian, T. Fusarium solani infection depressed photosystem performance by inducing foliage wilting in apple seedlings. Front. Plant Sci. 2018, 9, 479. [Google Scholar] [CrossRef] [Green Version]
- Gu, K.X.; Canción, X.S.; Xiao, X.M.; Duan, X.X.; Wang, J.X.; Duan, Y.B.; Hou, Y.P.; Zhou, M.G. A β2-tubulin dsRNA derived from Fusarium asiaticum confers plant resistance to multiple phytopathogens and reduces fungicide resistance. Pestic. Biochem. Physiol. 2019, 153, 36–46. [Google Scholar] [CrossRef]
- Essa, T. Response of some commercial strawberry cultivars to infection by wilt diseases in Egypt and their control with fungicides. Egypt. J. Pathol. 2015, 43, 113–127. [Google Scholar] [CrossRef]
- Robledo-Buriticá, J.; Ángel-García, C.; Castaño-Zapata, J. Microscopía electrónica de barrido ambiental del proceso de infección de Fusarium solani f. sp. passiflorae en plántulas de maracuyá (Passiflora edulis f. flavicarpa). Rev. Acad. Cienc. Exactas 2017, 41, 213–221. [Google Scholar]
- Morales-Mora, L.A.; Andrade-Hoyos, P.; Valencia-de Ita, M.A.; Romero-Arenas, O.; Silva-Rojas, H.V.; Contreras-Paredes, C.A. Caracterización de hongos asociados a fresa y efecto antagonista in vitro de Trichoderma harzianum. Rev. Mex. Fitopatol. 2020, 38, 434–449. [Google Scholar] [CrossRef]
- Miguel-Ferrer, L.; Romero-Arenas, O.; Andrade-Hoyos, P.; Sánchez-Morales, P.; Rivera-Tapia, J.A.; Fernández-Pavía, S.P. Antifungal activity of Trichoderma harzianum and T. koningiopsis against Fusarium solani in seed germination and vigor of Miahuateco chili seedlings. Rev. Mex. Fitopatol. 2021, 39, 228–247. [Google Scholar] [CrossRef]
- Guigón-López, C.; Guerrero-Prieto, V.; Vargas-Albores, F.; Carvajal-Millán, E.; Ávila-Quezada, G.D.; Bravo-Luna, L.; Ruocco, M.; Lanzuise, S.; Woo, S.; Lorito, M. Identificación molecular de cepas nativas de Trichoderma spp. su tasa de crecimiento in vitro y antagonismo contra hongos fitopaógenos. Rev. Mex. Fitopatol. 2010, 28, 87–96. [Google Scholar]
- Suárez, C.; Fernández, R.; Valero, N.; Gámez, R.; Páez, A. Antagonismo in vitro de Trichoderma harzianum Rifai sobre Fusarium solani (Mart.) Sacc., asociado a la marchitezen maracuyá. Rev. Colomb. Biotecnol. 2008, 2, 35–43. [Google Scholar] [CrossRef]
- Michel-Aceves, A.C.; Otero-Sánchez, M.A.; Rebolledo-Domínguez, O.; Lezama-Gutiérrez, R.; Ariza-Flores, R.; Barrios-Ayala, A. Producción y efecto antagónico de quitinasas y glucanasas por Trichoderma spp. en la inhibiciónde Fusarium subglutinans y Fusarium oxysporum in vitro. Rev. Chapingo Ser. Hortic. 2005, 11, 273–278. [Google Scholar] [CrossRef]
- Manzar, N.; Kashyap, A.S.; Goutam, R.S.; Rajawat, M.V.S.; Sharma, P.K.; Sharma, S.K.; Singh, H.V. Trichoderma: Advent of Versatile Biocontrol Agent, Its Secrets and Insights into Mechanism of Biocontrol Potential. Sustainability 2022, 14, 12786. [Google Scholar] [CrossRef]
- Peláez-Álvarez, A.; Santos-Villalobos, S.D.L.; Yépez, E.A.; Parra-Cota, F.I.; Reyes-Rodríguez, R.T. Synergistic effect of Trichoderma asperelleum T8A and Captan against Colletotrichum gloeosporioides (Penz.). Rev. Mex. Cienc. Agric. 2016, 7, 1401–1412. [Google Scholar]
- Ruocco, M.; Lanzuise, S.; Vinale, F.; Marra, R.; Turrà, D.; Woo, S.L.; Lorito, M. Identification of a new biocontrol gene in Trichoderma atroviride: The role of anABC transporter membrane pump in the interaction with different plant-pathogenic fungi. Mol. Plant-Microbe Interact. 2009, 22, 291–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.; Varma, R.K. Compatibility of fungicides and neem products against Fusariu solani f.sp. glycines causing root rot of soybean and Trichoderma spp. J. Mycopathol. Res. 2005, 43, 211–214. [Google Scholar]
- Gonzalez, M.F.; Magdama, F.; Galarza, L.; Sosa, D.; Romero, C. Evaluation of the sensitivity and synergistic effect of Trichoderma reesei and Mancozeb to inhibit under in vitro conditions the growth of Fusarium oxysporum. Commun. Integr. Biol. 2020, 13, 160–169. [Google Scholar] [CrossRef]
- Huilgol, S.N.; Pratibha, M.P.; Hegde, G.M.; Banu, H. Evaluation, and compatibility of new fungicides with Trichoderma harzianum for managing the charcoal rot of soybean. Pharma Innov. J. 2022, 11, 659–664. [Google Scholar]
- Maheshwary, N.; Gangadhara-Naik, B.; Amoghavarsha-Chittaragi, M.; Naik, S.K.; Nandish, M. Compatibility of Trichoderma asperellum with fungicides. Pharma Innov. J. 2020, 9, 136–140. [Google Scholar]
- Hirpara, D.G.; Gajera, H.P. Molecular heterozygosity and genetic exploitations of Trichoderma interfusants enhancing tolerance to fungicides and mycoparasitism against Sclerotium rolfsii Sacc. Infect. Genet. Evol. 2018, 66, 26–36. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, T.; Li, Y.; Wang, X.; Chen, J. Functional characterization of the ABC transporter TaPdr2 in the tolerance of biocontrol the fungus Trichoderma atroviride T23 to dichlorvos stress. Biological. Control 2019, 129, 102–108. [Google Scholar] [CrossRef]
- Hu, X.; Roberts, D.P.; Xie, L.; Yu, C.; Li, Y.; Qin, L.; Liao, X. Use of formulated Trichoderma sp. Tri-1 in combination with reduced rates of chemical pesticide for control of Sclerotinia sclerotiorium on oilseed rape. Crop Prot. 2016, 79, 124–127. [Google Scholar] [CrossRef]
- Elshahawy, I.E.; Haggag, K.H.E.; Abd-El-Khaira, H. Compatibility of Trichoderma spp. with seven chemical fungicides used in the control of soil borne plant pathogens. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1772–1785. [Google Scholar]
- Gangopadhyay, S.; Gopal, R.; Godara, S.L. Effect of Fungicides and antagonists on Fusarium wilt of Cumin. J. Mycol. Plant Pathol. 2009, 39, 331–334. [Google Scholar]
- Monadjemi, S.; El Roz, M.; Richard, C.; Ter Halle, A. Photoreduction of Chlorothalonil fungicide in plant leaf models. Entorno Sci. Technol. 2011, 45, 9582–9589. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Chlorothalonil (accessed on 12 April 2023).


| Fungicide (Tradename) | Active Ingredient | Molecular Formula | Concentration (mg L−1) | ||
|---|---|---|---|---|---|
| Low | Recommended | High | |||
| Control | Water | H2O | - | - | - |
| Captan 50® | Captan | C9H8Cl3NO2S | 450 | 900 | 1350 |
| Mancosol 80® | Mancozeb | C4H6MnN2S4 | |||
| Talonil 75® | Chlorothalonil | C8Cl4N2 | |||
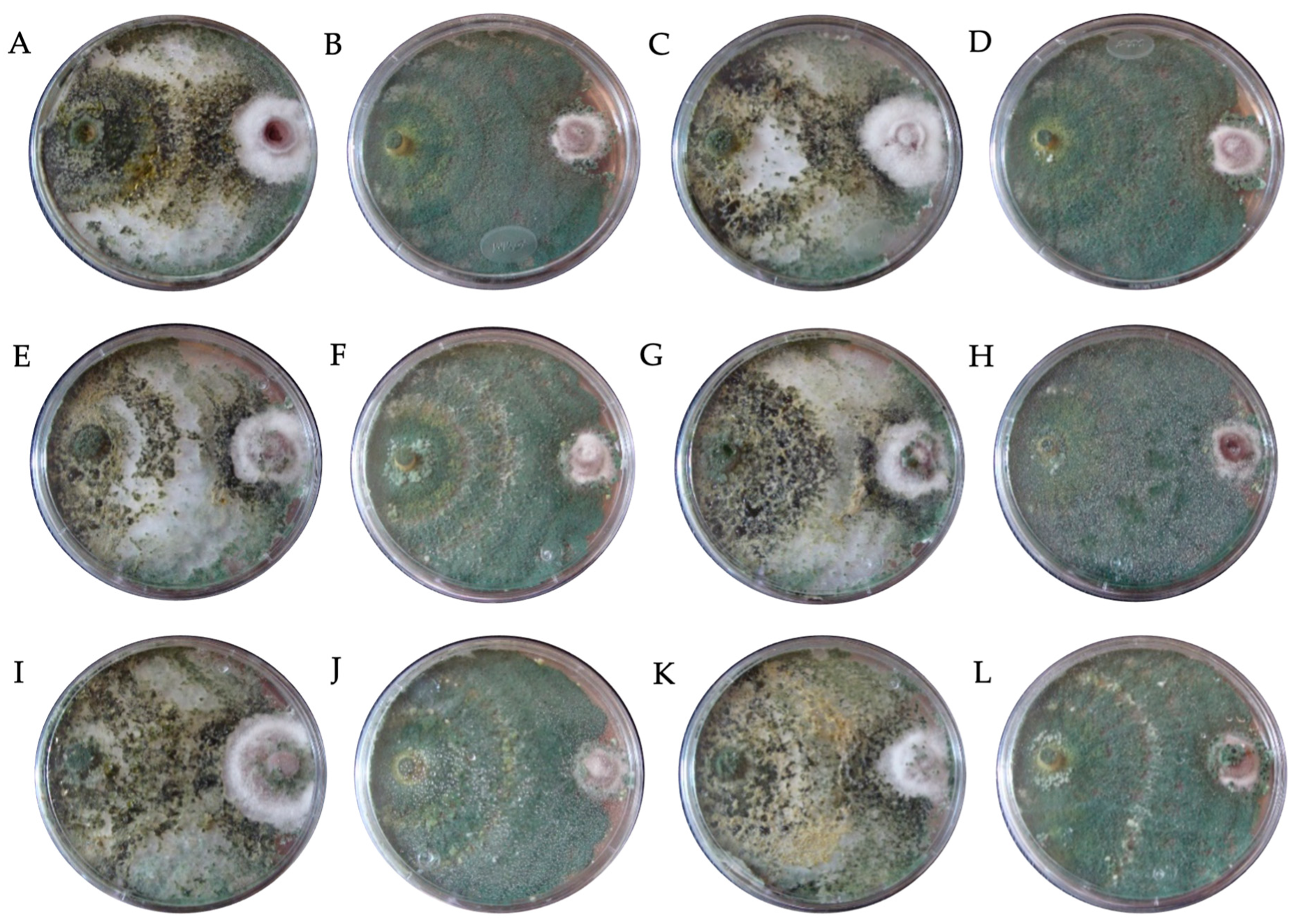
| Class | Mycoparasitism (%) | Characteristics |
|---|---|---|
| I | 100 | Trichoderma grew completely over F. solani and covered the entire mid-surface. |
| II | 75 | Trichoderma grew over at least two-thirds of the mid-surface. |
| III | 50 | Trichoderma and F. solani colonized approximately half of the mid-surface. |
| IV | 25 | F. solani colonized at least two-thirds of the mid-surface. |
| V | 0 | F. solani grew completely over Trichoderma and occupied the entire mid-surface. |
| Grade | Ranges (%) | Compatibility |
|---|---|---|
| I | 23 to 42 | Null |
| II | 43 to 59 | Low |
| III | 60 to 75 | Medium |
| IV | 76 to 93 | High |
| Code | Species | Strains | * Development Rate (mm h−1) | * Growth Rate (cm d−1) |
|---|---|---|---|---|
| DTF0 | F. solani | MA-FC120 | 0.28 ± 0.08 e | 6.71 ± 0.08 e |
| DTH0 | T. hamatum | T-A12 | 1.02 ± 0.02 d | 26.19 ± 0.53 b |
| DTA0 | T. asperellum | T-AS1 | 1.07 ± 0.05 c | 24.92 ± 1.34 d |
| DTK0 | T. konigiopsis | T-K11 | 1.17 ± 0.02 a | 26.82 ± 0.6 a |
| DTHR0 | T. harzianum | T-H4 | 1.14 ± 0.5 b | 25.20 ± 0.02 c |
| Code | Fungicide | Concentration (mg L−1) | Species | * PIRG (%) | SE | Bell Scale |
|---|---|---|---|---|---|---|
| DTH0 | Control (PDA) | - | T. hamatum | 66.95 bcde ± 0.34 | II | |
| DTA0 | T. asperellum | 66.91 bcde ± 0.26 | II | |||
| DTK0 | T. konigiopsis | 64.34 def ± 0.59 | II | |||
| DTHR0 | T. harzianum | 67.31 bcde ± 1.16 | II | |||
| DTH1 | Captan | 450 | T. hamatum | 71.11 bcde ± 1.63 | II | |
| DTA1 | T. asperellum | 76.33 a ± 1.16 | I | |||
| DTK1 | T. konigiopsis | 67.37 bcde ± 1.31 | II | |||
| DTHR1 | T. harzianum | 74.82 b ± 1.01 | I | |||
| DTH2 | 900 | T. hamatum | 69.19 bcde ± 3.16 | II | ||
| DTA2 | T. asperellum | 73.68 bc ± 1.34 | I | |||
| DTK2 | T. konigiopsis | 66.30 bcde ± 0.32 | II | |||
| DTHR2 | T. harzianum | 74.58 b ± 1.11 | I | |||
| DTH3 | 1350 | T. hamatum | 68.37 bcde ± 2.19 | II | ||
| DTA3 | T. asperellum | 72.84 bcd ± 1.08 | I | |||
| DTK3 | T. konigiopsis | 64.84 cde ± 2.32 | II | |||
| DTHR3 | T. harzianum | 72.15 bcd ± 2.51 | I | |||
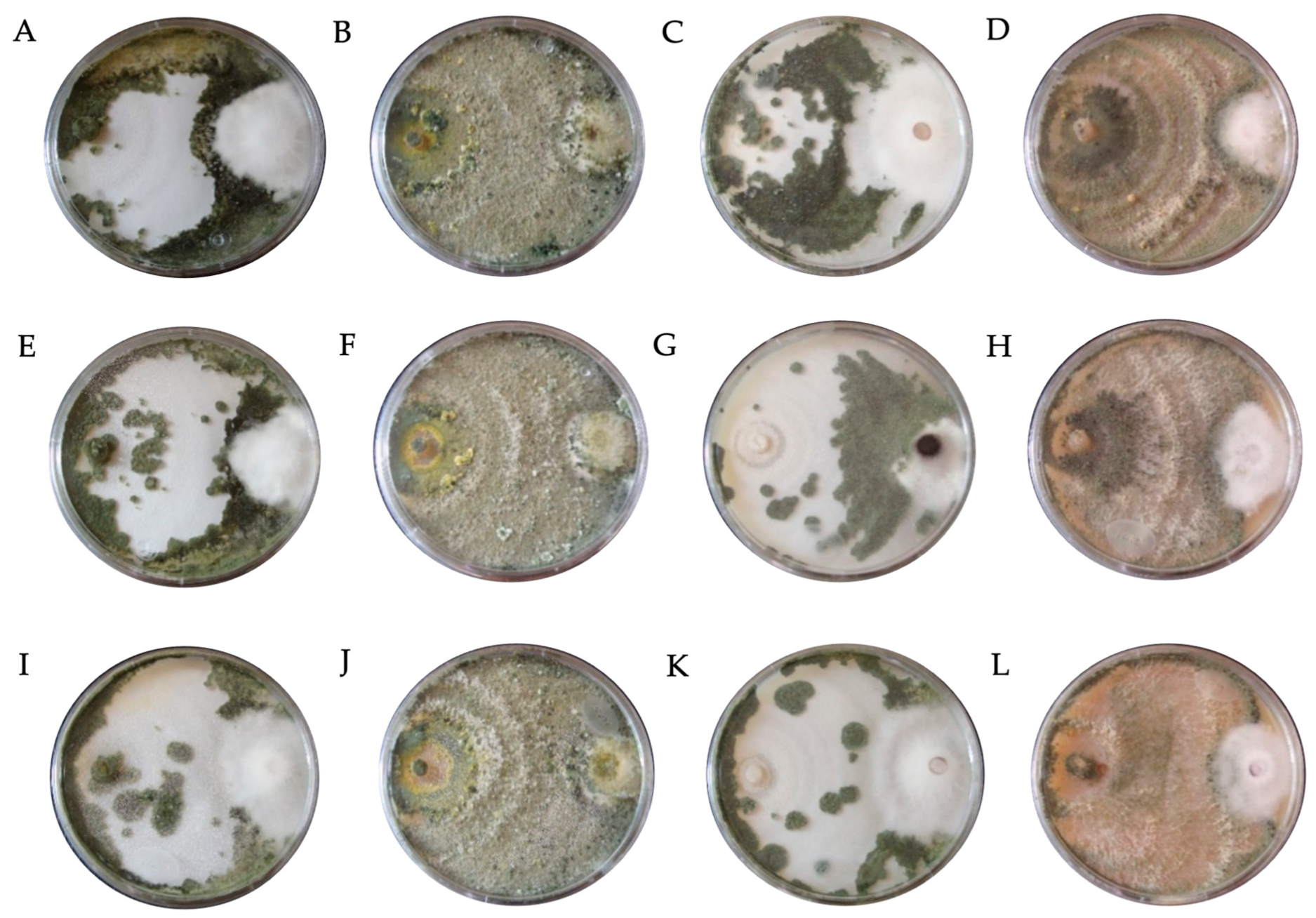
| DTH4 | Mancozeb | 450 | T. hamatum | 67.96 bcde ± 0.85 | II | |
| DTA4 | T. asperellum | 68.56 bcde ± 1.40 | I | |||
| DTK4 | T. konigiopsis | 64.76 cde ± 1.09 | II | |||
| DTHR4 | T. harzianum | 69.60 bcde ± 1.27 | II | |||
| DTH5 | 900 | T. hamatum | 65.88 bcde ± 1.00 | II | ||
| DTA5 | T. asperellum | 67.93 bcde ± 1.14 | I | |||
| DTK5 | T. konigiopsis | 62.82 efg ± 0.82 | II | |||
| DTHR5 | T. harzianum | 68.21 bcde ± 1.80 | II | |||
| DTH6 | 1350 | T. hamatum | 65.72 bcde ± 0.82 | II | ||
| DTA6 | T. asperellum | 66.16 bcde ± 0.91 | I | |||
| DTK6 | T. konigiopsis | 62.47 efg ± 0.15 | II | |||
| DTHR6 | T. harzianum | 67.29 bcde ± 0.60 | II | |||
| DTH7 | Chlorothalonil | 450 | T. hamatum | 49.52 h ± 1.22 | III | |
| DTA7 | T. asperellum | 28.71 i ± 2.24 | IV | |||
| DTK7 | T. konigiopsis | 55.38 fgh ± 1.88 | III | |||
| DTHR7 | T. harzianum | 46.33 h ± 2.36 | III | |||
| DTH8 | 900 | T. hamatum | 48.38 h ± 0.68 | III | ||
| DTA8 | T. asperellum | 28.62 i ± 2.94 | IV | |||
| DTK8 | T. konigiopsis | 50.91 gh ± 1.96 | III | |||
| DTHR8 | T. harzianum | 28.15 i ± 1.83 | IV | |||
| DTH9 | 1350 | T. hamatum | 48.05 h ± 3.65 | III | ||
| DTA9 | T. asperellum | 24.35 i ± 0.97 | IV | |||
| DTK9 | T. konigiopsis | 49.62 h ± 1.26 | III | |||
| DTHR9 | T. harzianum | 26.72 i ± 1.19 | IV | |||
| Origin | Dependent Variable | Sum of Squares (Type III) | gL | Mean Square | F | Sig. |
|---|---|---|---|---|---|---|
| Corrected mode | X1 = PIRG | 27,481.902 a | 39 | 704.664 | 64.55 | <0.001 |
| X2 = Bell | 64,000.000 b | 39 | 1641.026 | 157.538 | <0.001 | |
| X3 = PA | 41,743.713 c | 39 | 1070.352 | 204.495 | <0.001 | |
| Intersection | X1 | 589,547.5 | 1 | 589,547.5 | 54,004.82 | <0.001 |
| X2 | 702,250 | 1 | 702,250 | 67,416 | <0.001 | |
| X3 | 644,671.1 | 1 | 644,671.1 | 123,166.8 | <0.001 | |
| Compatibility (C%) | X1 | 27,481.9 | 39 | 704.664 | 64.55 | <0.001 |
| X2 | 64,000 | 39 | 1641.026 | 157.538 | <0.001 | |
| X3 | 41,743.71 | 39 | 1070.352 | 204.495 | <0.001 | |
| Error | X1 | 1309.989 | 120 | 10.917 | ||
| X2 | 1250 | 120 | 10.417 | |||
| X3 | 628.095 | 120 | 5.234 | |||
| Total | X1 | 618,339.4 | 160 | |||
| X2 | 767,500 | 160 | ||||
| X3 | 687,043 | 160 | ||||
| Total corrected | X1 | 28,791.89 | 159 | |||
| X2 | 65,250 | 159 | ||||
| X3 | 42,371.81 | 159 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parraguirre Lezama, C.; Romero-Arenas, O.; Valencia de Ita, M.D.L.A.; Rivera, A.; Sangerman Jarquín, D.M.; Huerta-Lara, M. In Vitro Study of the Compatibility of Four Species of Trichoderma with Three Fungicides and Their Antagonistic Activity against Fusarium solani. Horticulturae 2023, 9, 905. https://doi.org/10.3390/horticulturae9080905
Parraguirre Lezama C, Romero-Arenas O, Valencia de Ita MDLA, Rivera A, Sangerman Jarquín DM, Huerta-Lara M. In Vitro Study of the Compatibility of Four Species of Trichoderma with Three Fungicides and Their Antagonistic Activity against Fusarium solani. Horticulturae. 2023; 9(8):905. https://doi.org/10.3390/horticulturae9080905
Chicago/Turabian StyleParraguirre Lezama, Conrado, Omar Romero-Arenas, Maria De Los Angeles Valencia de Ita, Antonio Rivera, Dora M. Sangerman Jarquín, and Manuel Huerta-Lara. 2023. "In Vitro Study of the Compatibility of Four Species of Trichoderma with Three Fungicides and Their Antagonistic Activity against Fusarium solani" Horticulturae 9, no. 8: 905. https://doi.org/10.3390/horticulturae9080905
APA StyleParraguirre Lezama, C., Romero-Arenas, O., Valencia de Ita, M. D. L. A., Rivera, A., Sangerman Jarquín, D. M., & Huerta-Lara, M. (2023). In Vitro Study of the Compatibility of Four Species of Trichoderma with Three Fungicides and Their Antagonistic Activity against Fusarium solani. Horticulturae, 9(8), 905. https://doi.org/10.3390/horticulturae9080905







