Impact of Cold Stress on Physiological Responses and Fruit Quality of Shiranuhi Mandarin in Response to Cold Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Freezing Treatments
2.2. Lipid Peroxidation Analysis
2.3. Electrolyte Leakage Analyses
2.4. Fruit Properties Analyses
2.5. Free Sugars, Organic Acids, and Ascorbic Acid Determination
2.6. Volatile Compound Analysis Using GC-MS
2.7. Statistical Analysis
3. Results
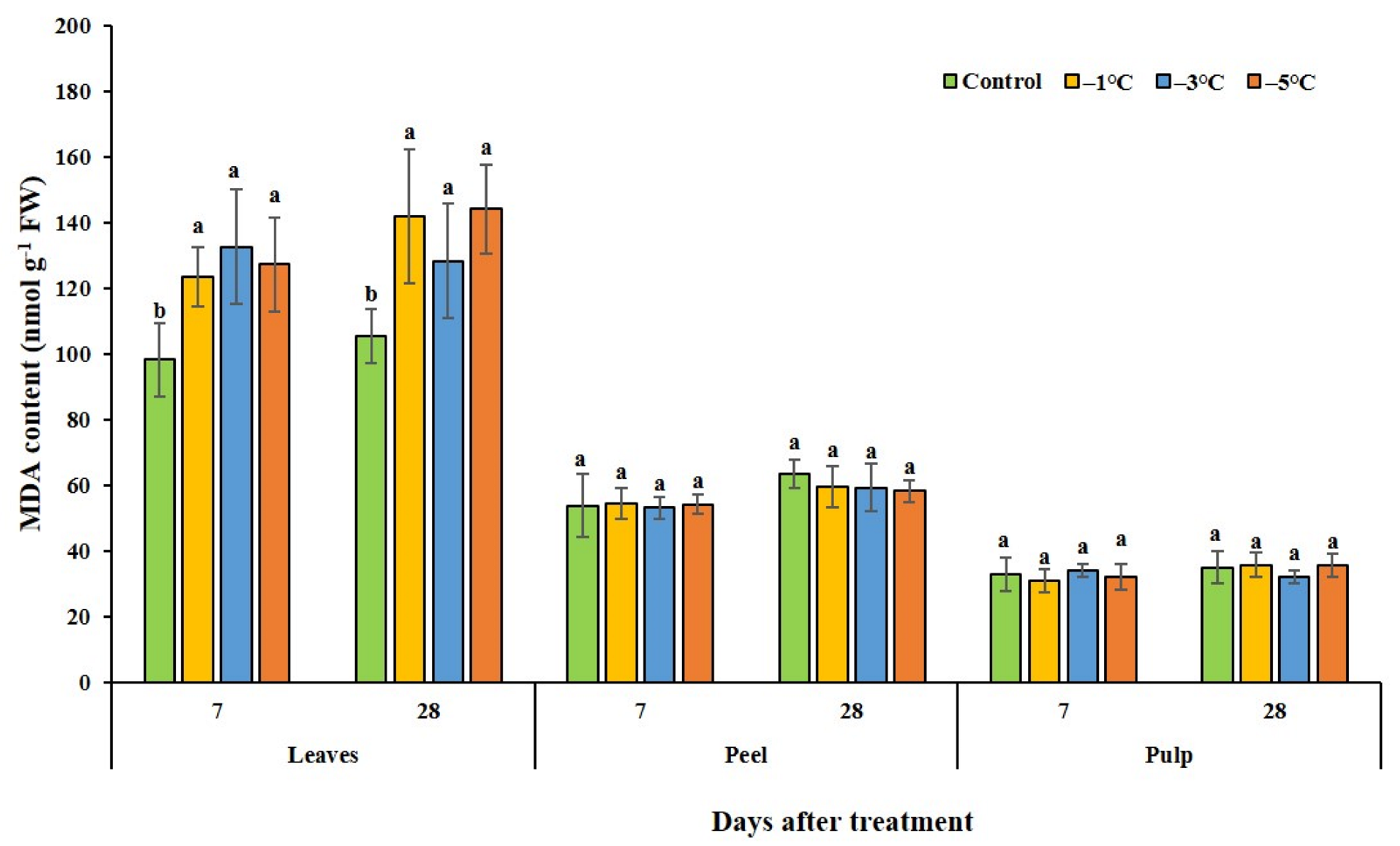
3.1. Plant Response to Cold Treatment in Relation to Lipid Peroxidation
3.2. Changes in Fruit Quality
3.3. Changes in Free Sugars, Organic Acids, and Ascorbic Acid
3.4. Changes in Volatile Compounds
3.5. Correlation between Fruit Quality Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Spiegel-Roy, P.; Goldschmidt, E.E. Biology of Citrus; Cambridge University Press: Cambridge, UK, 1996; pp. 15–17. [Google Scholar] [CrossRef]
- Yelenosky, G. Cold hardiness in citrus stems. Plant Physiol. 1975, 56, 540–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yelenosky, G. Cold hardiness in citrus. Hortic. Rev. 1985, 7, 201–238. [Google Scholar]
- Nesbitt, M.L.; Ebel, R.C.; Findley, D.; Wilkins, B.; Woods, F.; Himelrick, D. Assays to assess freeze injury of Satuma mandarin. HortScience 2002, 37, 871–877. [Google Scholar] [CrossRef] [Green Version]
- Jeon, M.J.; Cho, Y. An analysis of a winter-time temperature change and an extreme cold waves frequency in Korea. J. Clim. Chang. Res. 2015, 6, 87–97. (In Korean) [Google Scholar] [CrossRef]
- Jeung, S.J.; Park, J.Y.; Yang, D.M.; Kim, B.S. The future of extreme climate change in the Korean peninsula using national standard climate change scenarios and the ETCCDI index. J. Korean Soc. Hazard Mitig. 2019, 19, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.W.; Jeung, S.J.; Kim, B.S. Future Extreme Climate Analysis of Jeju Using Climate Change Scenario and ETCCDI Index. J. Korean Soc. Hazard Mitig. 2021, 21, 13–21. (In Korean) [Google Scholar] [CrossRef]
- Korea Meteorological Administration (KMA). Available online: https://data.kma.go.kr/cmmn/main.do (accessed on 31 May 2023).
- Matsumoto, R. ‘Shiranuhi’, a late-maturing citrus cultivar. Bull. Natl. Inst. Fruit Tree Sci. 2001, 35, 115–120. [Google Scholar]
- Jeju Special Self-Governing Province (JSGP). Citrus Annual Distribution Processing Analysis 2021. Available online: http://www.citrus.or.kr (accessed on 13 October 2022). (In Korean).
- Moon, Y.E.; Kang, S.B.; Lee, H.; Choi, Y.H.; Son, I.C.; Lee, D.H.; Kim, S.K.; An, M.I. Projection of potential cultivation region of Satsuma mandarin and ‘Shiranuhi’ mandarin hybrid based on RCP 8.5 emission scenario. Korean J. Agric. For. Meteorol. 2017, 19, 215–222. (In Korean) [Google Scholar] [CrossRef]
- Mckersie, B.D.; Leshem, Y.Y. Freezing stress. In Stress and Stress Coping in Cultivated Plants; Springer: Dordrecht, The Netherlands, 1994. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef]
- Health, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar]
- Taulavuori, E.; Hellström, E.-K.; Taulavuori, K.; Laine, K. Comparison of two methods used to analyze lipid peroxidation from Vaccinium Myrtillus (L.) during snow removal, reacclimation, and cold acclimation. J. Exp. Bot. 2001, 52, 2375–2380. [Google Scholar] [CrossRef] [Green Version]
- Jakhar, S.; Mukherjee, D. Chloroplast pigments, proteins, lipid peroxidation and activities of antioxidative enzymes during maturation and senescence of leaves and reproductive organs of Cajanus cajan L. Physiol. Mol. Biol. Plants 2014, 20, 171–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Yun, S.K.; Kim, S.S.; Park, Y.; Joa, J.; Han, S.; Shin, K.; Song, K.J. Response of citrus to freezing tolerance differs depending on genotypes and growing conditions. Hortic. Environ. Biotechnol. 2021, 62, 181–189. [Google Scholar] [CrossRef]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- De Vega, J.J.; Teshome, A.; Klaas, M.; Grant, J.; Finnan, J.; Barth, S. Physiological and transcriptional response to drought stress among bioenergy grass Miscanthus species. Biotechnol. Biofuels 2021, 14, 60. [Google Scholar] [CrossRef]
- Maul, F.; Sargent, S.A.; Sims, C.A.; Baldwin, E.A.; Balaban, M.O.; Huber, D.J. Tomato flavor and aroma quality as affected by storage temperature. J. Food. Sci. 2000, 65, 1228–1237. [Google Scholar] [CrossRef]
- Zhang, B.; Tieman, D.M.; Jiao, C.; Xu, Y.; Chen, K.; Fe, Z.; Giovannoni, J.J.; Klee, H.J. Chilling-induced tomato flavor loss is associated with altered volatile synthesis and transient changes in DNA methylation. Proc. Natl. Acad. Sci. USA 2016, 113, 12580–12585. [Google Scholar] [CrossRef]
- Kim, M.; Yun, S.K.; Kim, S.S.; Park, Y.; Joa, J.; Han, S. Influence of freezing temperatures on metabolite composition and antioxidant activity in Shiranuhi mandarin. Sci. Hortic. 2021, 288, 110397. [Google Scholar] [CrossRef]
- Obenland, D.M.; Aung, L.H.; Bridges, D.L.; Mackey, B.E. Volatile emissions of Navel oranges as predictors of freeze damage. J. Agric. Food Chem. 2003, 51, 3367–3371. [Google Scholar] [CrossRef]
- Tan, E.S.; Slaughter, D.; Thompson, J.F. Freeze damage detection in oranges using gas sensors. Postharvest Biol. Technol. 2005, 35, 177–182. [Google Scholar] [CrossRef]
- Jimenez-Cuesta, M.; Cuquerella, J.; Martínez-Jávega, J.M. Determination of a color index for citrus fruit degreening. Int. Soc. Citric. 1981, 2, 750–753. [Google Scholar]
- Azuma, R.; Ito, N.; Nakayama, N.; Suwa, R.; Nguyen, N.T.; Larrinaga-Mayoral, J.Á.; Esaka, M.; Fujiyama, H.; Saneoka, H. Fruit are more sensitive to salinity than leaves and stems in pepper plants (Capsicum annum L.). Sci. Hortic. 2010, 125, 17–178. [Google Scholar] [CrossRef]
- Kim, M.; Park, Y.; Yun, S.K.; Kim, S.S.; Joa, J.; Moon, Y.-E.; Do, G.-R. The anatomical differences and physiological responses of sunburned satsuma mandarin (Citrus unshiu Marc.) fruits. Plants 2022, 11, 1801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ji, S.; Wei, B.; Cheng, S.; Wang, Y.; Hao, J.; Wang, S.; Zhou, Q. Transcriptome analysis of postharvest blueberries (Vaccinium corybosum ‘Duke’) in response to cold stress. BMC Plant Biol. 2020, 20, 80. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [Green Version]
- Syvertsen, J.P. Dehydration of freeze-damaged oranges. HortScience 1982, 17, 803–804. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Z.; Li, B.; Qin, G.; Tian, S. Molecular basis for optimizing sugar metabolism and transport during fruit development. Abiotech 2021, 2, 330–340. [Google Scholar] [CrossRef]
- Nagy, S. Vitamin C contents of Citrus Fruit and Their Products: A Review. J. Agric. Food Chem. 1980, 28, 8–18. [Google Scholar] [CrossRef]
- Rey, F.; Zacaría, L.; Rodrigo, M.J. Carotenoids, vitamin C, and antioxidant capacity in the peel of mandarin fruit in relation to the susceptibility to chilling injury during postharvest cold storage. Antioxidants 2020, 9, 1296. [Google Scholar] [CrossRef]
- Berlinet, C.; Brat, P.; Brillouet, J.-M.; Ducruet, V. Ascorbic acid, aroma compounds and browning of orange juices related to PET packaging materials and pH. J. Sci. Food Agric. 2006, 86, 2206–2212. [Google Scholar] [CrossRef]
- Miller, E.V.; Heilman, A.S. Ascorbic Acid and Physiological Breakdown in the Fruits of the Pineapple (Ananas conzosus L. Merr.). Science 1952, 116, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Ren, J.; Fan, G.; Reineccius, G.A.; Li, X.; Zhang, N.; An, Q.; Wang, Q.; Pan, S. Citrus juice off-flavor during different processing and storage: Review of odorants, formation pathways, and analytical techniques. Crit. Rev. Food Sci. Nutr. 2022, 1–26. [Google Scholar] [CrossRef]
- Lin, J.; Rouseff, R.L. Characterization of aroma-impact compounds in cold-pressed grapefruit oil using time–intensity GC–olfactometry and GC–MS. Flavour Fragr. J. 2001, 16, 457–463. [Google Scholar] [CrossRef]
- Miyazawa, N.; Fujita, A.; Kubota, K. Aroma character impact compounds in Kinokuni mandarin orange (Citrus kinokuni) compared with Satsuma mandarin orange (Citrus unshiu). Biosci. Biotechnol. Biochem. 2010, 74, 835–842. [Google Scholar] [CrossRef]
- Sawamura, M.; Onishi, Y.; Ikemoto, J.; Tu, N.T.M.; Phi, N.T.L. Characteristic odour components of bergamot (Citrus bergamia Risso) essential oil. Flavour Fragr. J. 2016, 21, 609–615. [Google Scholar] [CrossRef]





| Days after Treatment (DAT) | Temperature Treatments | Fructose | Glucose | Sucrose | Total |
|---|---|---|---|---|---|
| 7 | control | 24.5 ± 2.0 a | 26.6 ± 3.1 a | 60.5 ± 8.0 a | 111.6 ± 11.0 a |
| −1 °C | 22.0 ± 2.2 ab | 24.3 ± 3.8 a | 61.7 ± 4.5 a | 107.9 ± 8.7 a | |
| −3 °C | 23.5 ± 2.2 ab | 26.1 ± 3.7 a | 60.0 ± 3.3 a | 109.6 ± 8.1 a | |
| −5 °C | 21.4 ± 0.9 b | 23.4 ± 2.7 a | 56.6 ± 2.2 a | 101.3 ± 4.2 a | |
| 14 | control | 23.9 ± 2.5 a | 25.4 ± 2.6 ab | 64.5 ± 3.2 a | 113.8 ± 7.3 a |
| −1 °C | 20.7 ± 1.1 a | 22.8 ± 1.0 b | 57.2 ± 4.5 b | 101.0 ± 5.1 b | |
| −3 °C | 24.2 ± 2.3 a | 28.2 ± 3.1 a | 59.6 ± 2.1 ab | 112.0 ± 5.3 ab | |
| −5 °C | 22.4 ± 2.1 a | 24.9 ± 3.3 ab | 59.8 ± 3.7 ab | 107.0 ± 8.0 ab | |
| 21 | control | 20.1 ± 1.0 b | 19.4 ± 1.6 c | 66.8 ± 1.7 a | 106.3 ± 3.2 ab |
| −1 °C | 21.1 ± 2.3 b | 21.0 ± 2.4 bc | 58.2 ± 4.2 b | 100.3 ± 6.6 b | |
| −3 °C | 24.6 ± 1.9 a | 25.0 ± 2.3 a | 59.4 ± 2.1 b | 109.1 ± 4.3 ab | |
| −5 °C | 23.5 ± 2.1 ab | 23.4 ± 2.7 ab | 62.7 ± 2.6 ab | 109.6 ± 6.3 a | |
| 28 | control | 20.4 ± 1.8 bc | 23.9 ± 3.7 bc | 65.3 ± 3.8 a | 109.7 ± 6.1 ab |
| −1 °C | 19.7 ± 0.9 c | 23.2 ± 1.0 c | 61.8 ± 4.3 a | 104.7 ± 5.4 b | |
| −3 °C | 22.2 ± 2.2 ab | 28.2 ± 3.9 a | 56.1 ± 4.7 b | 106.6 ± 8.9 ab | |
| −5 °C | 22.9 ± 1.2 a | 28.0 ± 3.4 ab | 62.6 ± 2.4 a | 113.5 ± 5.6 a |
| Days after Treatment | Temperature Treatments | Organic Acids (mg/mL Fresh Juice) | Ascorbic Acid (mg/100 mL Fresh Juice) | ||
|---|---|---|---|---|---|
| Malic Acid | Citric Acid | Total | |||
| 7 | Control | 2.5 ± 0.4 a | 11.4 ± 0.9 ab | 13.9 ± 1.0 ab | 43.2 ± 4.0 a |
| −1 °C | 2.7 ± 0.2 a | 10.7 ± 1.2 ab | 13.4 ± 1.1 ab | 41.1 ± 3.0 ab | |
| −3 °C | 2.6 ± 0.2 a | 11.7 ± 1.2 a | 14.3 ± 1.1 a | 43.7 ± 6.5 a | |
| −5 °C | 2.6 ± 0.2 a | 10.0 ± 0.9 b | 12.6 ± 0.8 b | 35.7 ± 6.2 b | |
| 14 | control | 2.6 ± 0.3 a | 11.8 ± 0.6 a | 14.4 ± 0.7 a | 44.0 ± 2.6 a |
| −1 °C | 2.5 ± 0.3 a | 11.3 ± 1.0 ab | 13.8 ± 0.9 ab | 42.3 ± 4.7 a | |
| −3 °C | 2.4 ± 0.1 a | 11.1 ± 0.9 ab | 13.5 ± 0.9 ab | 39.7 ± 2.9 a | |
| −5 °C | 2.3 ± 0.1 a | 10.2 ± 1.0 b | 12.5 ± 1.0 b | 32.1 ± 4.4 b | |
| 21 | control | 2.3 ± 0.2 ab | 12.8 ± 1.0 a | 15.1 ± 1.0 a | 44.1 ±4.1 a |
| −1 °C | 2.5 ± 0.2 a | 10.5 ± 0.7 b | 13.0 ± 0.5 b | 43.0 ±5.3 a | |
| −3 °C | 2.4 ± 0.2 a | 10.3 ± 0.6 b | 12.7 ± 0.6 b | 41.6 ± 4.8 a | |
| −5 °C | 2.0 ± 0.1 b | 9.8 ± 1.1 b | 11.9 ± 1.1 b | 26.0 ± 5.0 b | |
| 28 | control | 2.5 ± 0.4 a | 11.2 ± 1.5 a | 13.6 ± 1.3 a | 46.0 ± 3.3 a |
| −1 °C | 2.4 ± 0.1 a | 9.2 ± 0.5 b | 11.6 ± 0.5 b | 42.8 ± 3.1 a | |
| −3 °C | 2.3 ± 0.1 a | 9.8 ± 0.7 b | 12.1 ± 0.7 b | 43.6 ± 5.2 a | |
| −5 °C | 1.8 ± 0.2 b | 9.4 ± 0.9 b | 11.2 ± 0.8 b | 35.1 ± 3.7 b | |
| Quality Parameters | TSS | Acidity | FN | CCI | TFS | RSU | NRSU | MA | CC | TOA |
|---|---|---|---|---|---|---|---|---|---|---|
| Acidity | 0.227 *** | |||||||||
| FN | 0.056 | 0.261 *** | ||||||||
| CCI | 0.174 ** | −0.63 | 0.406 *** | |||||||
| TFS | −0.109 | −0.083 | −0.096 | 0.064 | ||||||
| RSU | −0.028 | −0.217 * | −0.019 | 0.068 | 0.737 *** | |||||
| NRSU | 0.185 * | 0.083 | −0.124 | 0.028 | 0.772 *** | 0.139 | ||||
| MA | 0.023 | 0.058 | −0.180 * | −0.418 *** | −0.047 | −0.143 | 0.065 | |||
| CC | 0.003 | 0.044 | −0.065 | −0.256 ** | 0.086 | −0.003 | 0.128 | 0.092 | ||
| TOA | 0.014 | 0.055 | −0.102 | −0.336 *** | 0.075 | −0.033 | 0.141 | 0.317 *** | 0.973 *** | |
| AA | 0.020 | −0.140 | −0.133 | −0.179 * | 0.148 | 0.075 | 0.146 | 0.272 ** | 0.476 *** | 0.512 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Moon, Y.-E.; Han, S.G.; Yun, S.K.; Joa, J.-H.; Park, J.-S. Impact of Cold Stress on Physiological Responses and Fruit Quality of Shiranuhi Mandarin in Response to Cold Conditions. Horticulturae 2023, 9, 906. https://doi.org/10.3390/horticulturae9080906
Kim M, Moon Y-E, Han SG, Yun SK, Joa J-H, Park J-S. Impact of Cold Stress on Physiological Responses and Fruit Quality of Shiranuhi Mandarin in Response to Cold Conditions. Horticulturae. 2023; 9(8):906. https://doi.org/10.3390/horticulturae9080906
Chicago/Turabian StyleKim, Misun, Young-Eel Moon, Seung Gab Han, Seok Kyu Yun, Jae-Ho Joa, and Jee-Soo Park. 2023. "Impact of Cold Stress on Physiological Responses and Fruit Quality of Shiranuhi Mandarin in Response to Cold Conditions" Horticulturae 9, no. 8: 906. https://doi.org/10.3390/horticulturae9080906
APA StyleKim, M., Moon, Y.-E., Han, S. G., Yun, S. K., Joa, J.-H., & Park, J.-S. (2023). Impact of Cold Stress on Physiological Responses and Fruit Quality of Shiranuhi Mandarin in Response to Cold Conditions. Horticulturae, 9(8), 906. https://doi.org/10.3390/horticulturae9080906





