Abstract
We previously showed that chronic warming plus elevated carbon dioxide (eCO2) causes extreme upward bending of leaflets and petioles (i.e., hyponasty) in tomato (Solanum lycopersicum), which reduces growth. In that study, only two levels of CO2 (400, 700 ppm) and temperature (30, 37 °C) were tested in young vegetative plants, and the underlying mechanism for warming + eCO2 hyponasty was not investigated. In this study, warming + eCO2 hyponasty was evaluated in tomato across a range of temperatures and CO2 concentrations, and at multiple life stages. Based on their roles in thermal hyponasty, ethylene and auxin tomato mutants were examined, and light quality manipulated, to explore the mechanism for warming + eCO2 hyponasty. At eCO2 (800 ppm), the petiole angle increased roughly linearly with temperature from 30 to 38 °C. Under high temperature stress (38 °C), the petiole angle increased similarly at all eCO2 concentrations (600/800/1000 vs. 400 ppm). All life stages examined had an increased petiole angle in leaves developed during warming + eCO2, such that most leaves in juvenile plants exhibited hyponasty but only young growing leaves did so in adults. Auxin-insensitive mutants displayed a reduced petiole angle compared to auxin-sensitive, ethylene-sensitive, ethylene-insensitive, and non-mutant genotypes, indicating that auxin, but not ethylene, is likely a main component of this hyponastic response. Reduced far-red-to-red light plus increased blue light reduced petiole hyponasty compared to non-filtered white light during warming + eCO2. These results indicate that eCO2 affects the well-characterized thermal hyponastic response of leaves, which has implications for future plant responses to climate change.
1. Introduction
Emissions from human activities are increasing carbon dioxide (CO2) and other atmospheric greenhouse gas concentrations, resulting in global warming. Average global temperatures may increase by as much as 3.3–5.7 °C by 2100, causing a direct increase in the frequency, severity, and longevity of heatwaves [1]. As sessile organisms, plants must adapt to climate change via chemical or structural changes or face likely declines in productivity [2,3]. The combined effects of chronic supra-optimal warming and elevated CO2 (eCO2) on plant responses remain partly unknown, as eCO2 may alleviate or exacerbate the effects of heat stress depending on photosynthetic pathway or species [4,5,6]. Elevated CO2 may increase net photosynthesis by reducing photorespiration, and increase water-use efficiency by decreasing stomatal conductance [7,8]. Elevated CO2 can also alter plant hormone concentrations or plant sensitivity to hormones, which could interact with or interfere with plant hormonal changes associated with heat adaptation [9,10,11,12,13].
Warming-related changes to plant development, organization, and/or shape, such as early flowering, stem elongation, altered leaf morphology, and hyponasty (upward bending), are known collectively as thermomorphogenesis [14,15,16,17,18]. Thermomorphogenesis seems to be related to shade-avoidance responses, but instead of suboptimal photosynthetically active radiation (PAR) or low red:far-red (R:FR) light-induced changes in phytochrome B (phyB) activity, higher temperatures increase the rate at which the active far-red form of phyB (Pfr) reverts to the inactive red form (Pr) through thermal reversion [15,19]. Briefly, thermomorphogenesis may occur as thermal reversion of phyB releases the inhibitive pressure of phyB Pfr on the basic helix–loop–helix factor PHYTOCHROME-INTERACTING FACTOR 4 (PIF4), while another unknown protein kinase stabilizes PIF4 at elevated temperatures [14,16]. PIF4 activates the expression of auxin biosynthesis genes such as TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS1 (TAA1), CYTOCHROME P450 FAMILY 79B (e.g., CYP79B2), and the YUCCA (YUC) family of genes. This increase in auxin indirectly increases brassinosteroid (BR) production by disrupting the production of BR inhibitors by BRI-EMS-SUPPRESSOR 1 (BES1), and it has led to hypocotyl/petiole/root elongation, increased specific leaf area (SLA), and increased leaf angle in the model species Arabidopsis thaliana [14,16,17,18,20]. Subsequent increases in BR concentration may contribute to a feedback loop, as BRASSINAZOLE RESISTANT 1 (BZR1) can induce further expression of growth-promoting genes by binding to the promoter of PIF4 [21]. The increase in leaf angle observed at high temperatures results from the accumulation of polar auxin transport on the abaxial side of the petiole, and hyponasty is believed to reduce leaf temperature by moving leaves further from the radiant heat of the soil surface and increasing cooling from transpiration [22,23].
Hyponasty results from differential growth of the abaxial and adaxial surfaces of the petiole or leaf blade and often occurs in response to non-directional unfavorable environmental conditions. The severity of stressors such as submergence, eCO2, chronic shading, and supra-optimal temperatures can trigger changes in plant hormone concentrations or photoreceptor activity, which moderate the extent of the hyponastic responses [14,23,24,25,26]. For example, submergence greatly reduces gas diffusion rates in stems, leading to the accumulation of the gaseous hormone ethylene, which can increase 20-fold within the first hour of submergence. In flood-tolerant species, the increase in ethylene is followed by petiole elongation [24,26,27]. Auxin may also play a role in the timing and amplitude of hyponasty in submerged plants, as inhibition of polar auxin transport can delay the initiation of differential growth in some submerged species [28]. Like submergence, eCO2 increases the production of ethylene and the expression of ethylene-related genes [12], which at extremely high concentrations (>10,000 ppm CO2) can result in leaf hyponasty [29]. Increased ethylene production during over-crowding is associated as a precursor to shade-induced hyponasty in Nicotiana tabacum (tobacco) [30]. Other plants primarily react to sub-optimal PAR, low R:FR ratios, or reduced blue light via auxin signaling and polar auxin transport [25,26,31] to induce hyponasty. Though photosensitivity and ethylene sensitivity have been shown to be involved in shade-induced hyponasty, the two pathways are independent of one another, demonstrating that multiple separate pathways can result in similar phenotypic responses to environmental change [32]. Additionally, ethylene-insensitive A. thaliana mutants showed enhanced sensitivity to heat-induced hyponasty, while all lines with constitutive ethylene responses had a much lower amplitude in hyponastic responses, demonstrating an apparent antagonistic relationship between ethylene and heat-induced hyponasty [33].
In our previous study, a combination of eCO2 and heat stress dramatically increased leaf angle and decreased biomass in juvenile Solanum lycopersicum (tomato) plants [34]. Here, we build upon that research by further evaluating the hyponastic response of tomato plants to eCO2 and chronic warming. First, the threshold temperature and CO2 concentration for hyponastic growth in juvenile tomato plants were determined. The leaf angle was compared among juvenile plants grown at multiple temperatures and a single level of eCO2, and among plants grown at multiple CO2 concentrations and a single level of chronic warming. Second, eCO2 and warming-induced hyponasty was compared between young juvenile, pre-reproductive adults, and flowering adults to establish the relative effect of elevated CO2 plus chronic warming at different life stages. Third, the role of ethylene and auxin in chronic warming + eCO2-induced hyponasty was investigated. Tomato hormone mutants (ethylene sensitive, ethylene insensitive, auxin sensitive, auxin insensitive, respectively) were compared to a heat-tolerant non-mutant genotype. Fourth, tomato plants were grown under blue-enriched or white light to investigate the role of light quality on warming + eCO2-induced hyponasty.
2. Methods
Plant culture: The tomato ‘Celebrity’ (Cel), a semi-determinate heat-resistant commercial cultivar, was used as a non-mutant genotype. Previously characterized auxin and ethylene mutants were obtained from the CM Rick Tomato Genetics Resource Center at the University of California, Davis (https://tgrc.ucdavis.edu/ (accessed 1 June 2021)): never ripe (nr, ethylene insensitive, ‘Platense’ background), epinastic (epi, ethylene sensitive, ‘VFN-8′ background), diageotropica (dgt, auxin insensitive, ‘VFN-8′ background), and entire (e, auxin sensitive, ‘VFNT Cherry’ background) [35,36,37,38]. The plants were germinated in drainable trays containing a calcite clay–peat moss mixture (2:1, v:v) in a greenhouse (ca. 28 °C day/24 °C night with a 14 h photoperiod) and watered daily with tap water. After emergence, seedlings were provided half-strength nutrient solution (see below) weekly until transplant. After the emergence of the first adult leaf, approximately 18 days after sowing, seedlings were transplanted into 2.3 L cylindrical polyvinylchloride (PVC) pots (10 cm diameter × 30 cm height, with mesh bottoms) filled with the calcite clay–peat moss mixture, and fertilized one to three times weekly, according to size, until selected for treatment. The nutrient concentrations of the full-strength solution were 4.5 mM KNO3, 0.5 mM NH4NO3, 2 mM KH2PO4, 2 mM CaCl2, 1 mM MgSO4, 50 μM Fe-EDTA (ethylene-diamine-tetra-acetic acid), 50 μM H3BO3, 10 μM MnCl2, 5 μM ZnSO4, and 0.1 μM NaMoO4; the pH was adjusted to 6.0. Sets of plants of similar size and/or growth stage (e.g., number of leaves, initiation of flowering) were then selected for the treatments and transferred to growth chambers (model E36HO, Percival Scientific Inc., Perry, IA, USA) for treatment. The chamber CO2 concentration was controlled by a computer in combination with a CO2 sensor, control valve and injector, and external tank of pure CO2.
Plants were acclimated to the growth-chamber environments over a 24 h period, at 600 µmol m−2 s−1 PAR at the tops of plants, a 14 h photoperiod, a near-optimal growth temperature of 30/25 °C (day/night), and “ambient” CO2 (400 ppm). Elevated temperature treatments were gradually imposed on the plants by incrementally increasing day/night temperatures over four days to avoid heat shock. After reaching the treatment temperatures, the CO2 treatments were started, and plants were grown for 7–10 days under treatment conditions. During the treatments, pots were flushed with full-strength nutrient solution every other day and watered as needed to prevent drought stress (i.e., until soil was moist throughout).
Measurements: The base of a protractor was aligned with the stem, and the angle between the stem and the adaxial surface of the petiole was measured. The resulting angle was subtracted from 90° to determine the abaxial angle relative to the soil surface for comparison. Every other day during treatment, measurements were made on the three most-recently fully developed adult leaves selected from each plant, and these measurements were averaged to obtain a mean value for the plant for that day. The plants were photographed at the conclusion of the treatment duration to document the visual effects on the petiole angle. Unless otherwise noted, plants were harvested upon completion of the treatments, oven dried at 75 °C for 5 days, and weighed to determine shoot dry mass.
Treatments: Temperature and CO2 gradients—To assess the sensitivity of tomato to warming and eCO2-induced hyponasty, pre-reproductive adult ‘Celebrity’ plants (with 5–7 leaves) were grown across a range of each factor for seven days. In one experiment, plants were grown at daytime temperature treatments of 29, 32, 35, and 38 °C (night temperatures were 5 °C below day temperatures) at eCO2 (800 ppm) (n = 8 plants per temperature). In a separate experiment, plants were grown at CO2 concentrations of 400, 600, 800, and 1000 ppm at a temperature of 38 °C day/33 °C night (n = 6 plants per CO2 concentration).
Life stages—To determine if warming + eCO2-induced hyponasty occurs at all life stages, juvenile (3–5 adult leaves, n = 6 plants), pre-reproductive adult (8–12 leaves, n = 6 plants), and flowering (n = 4 plants) tomato ‘Celebrity’ plants from the same germination cohort were exposed to either 30 °C and 400 ppm CO2 or 38 °C and 800 ppm CO2; the flowering stage also included an additional treatment group at 35 °C and 800 ppm CO2 (n = 8 plants). The plants were grown for 10 days at treatment levels to allow for development under treatment conditions. At harvest, the number of affected leaves and when they developed (before or during treatment exposure) was recorded and calculated as a percentage of total leaves to determine if hyponasty occurred in all leaves or only in leaves developed under treatment conditions.
Ethylene and auxin involvement—To investigate if auxin or ethylene may be involved with warming + eCO2-induced hyponasty, the tomato hormone mutant genotypes described above (nr, epi, e, and dgt) were compared to the tomato ‘Celebrity’ (n = 3–4 plants per genotype per treatment). In this experiment, pre-reproductive plants (5–7 leaves) were grown at 29 °C and 400 ppm CO2 or 35 °C and 800 ppm CO2 for 7 days, as described above.
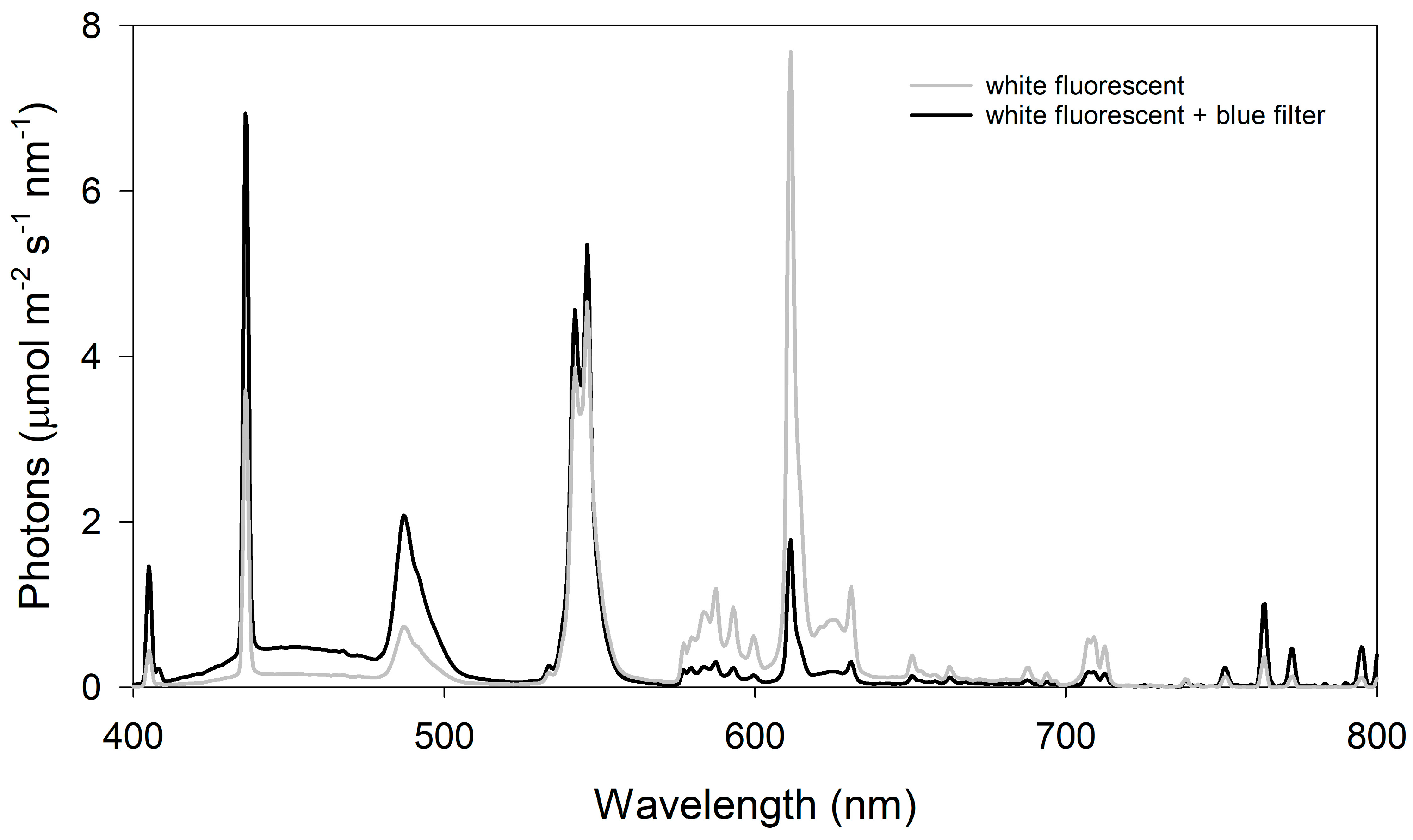
Light quality—To examine the influence of light quality on warming + eCO2-induced hyponasty, transparent blue vinyl film (SKU:VINTRBLUPP.010, ePlastics, San Diego, CA, USA) was suspended below the chamber’s fluorescent lights to reduce red and far-red light transmission and increase blue light transmission [39]. The effects of the light filter on the light quality were measured and compared to the chamber lights using a spectroradiometer (StellarNet PS-200, Apogee Instruments, Logan, UT, USA) and averaging 5 spectroradiometer scans (Figure 1). Unfiltered chamber lights were dimmed using chamber controls to provide equivalent light intensities of 140 µmol m−2 s−1 PAR across the treatments. Pre-reproductive plants (5–7 leaves) were grown under filtered light or white light at 30 °C and 400 ppm CO2 or 38 °C and 800 ppm CO2 for 10 days, as described above.
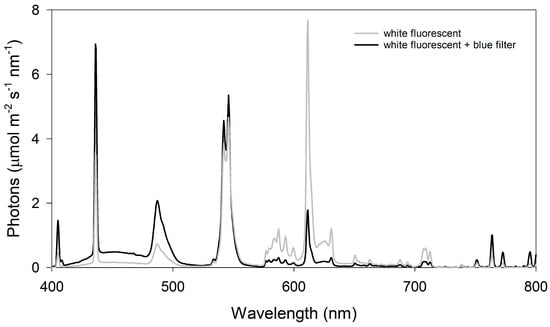
Figure 1.
Comparison of filtered blue light and unfiltered white fluorescent light. The blue filter increased blue light by 216% (40.1:18.6; 430 ± 10 nm), decreased red light by 75% (5.7:23.1; 630 ± 10 nm), and decreased far-red light by 9% (0.70:0.77; 730 ± 10 nm).
Data analysis: The data were analyzed and tested for normality, equal variance, and independence with S-L, normal Q-Q, and residual vs. fitted plots, respectively, using R version 4.1.2 (R Core Team (2021), Vienna, Austria). The results were analyzed with one- or two-way analysis of variance (ANOVA), and Tukey’s post hoc test was used for multiple comparisons if the ANOVA results were significant (p < 0.05). If the data failed normality or equal-variance tests, then the results were analyzed with non-parametric ANOVA and Holm–Sidak tests. In all instances, which were rare, parametric and non-parametric analyses yielded similar conclusions. The data were graphed using Sigma Plot 14.5 (Systat Software, San Jose, CA, USA).
3. Results
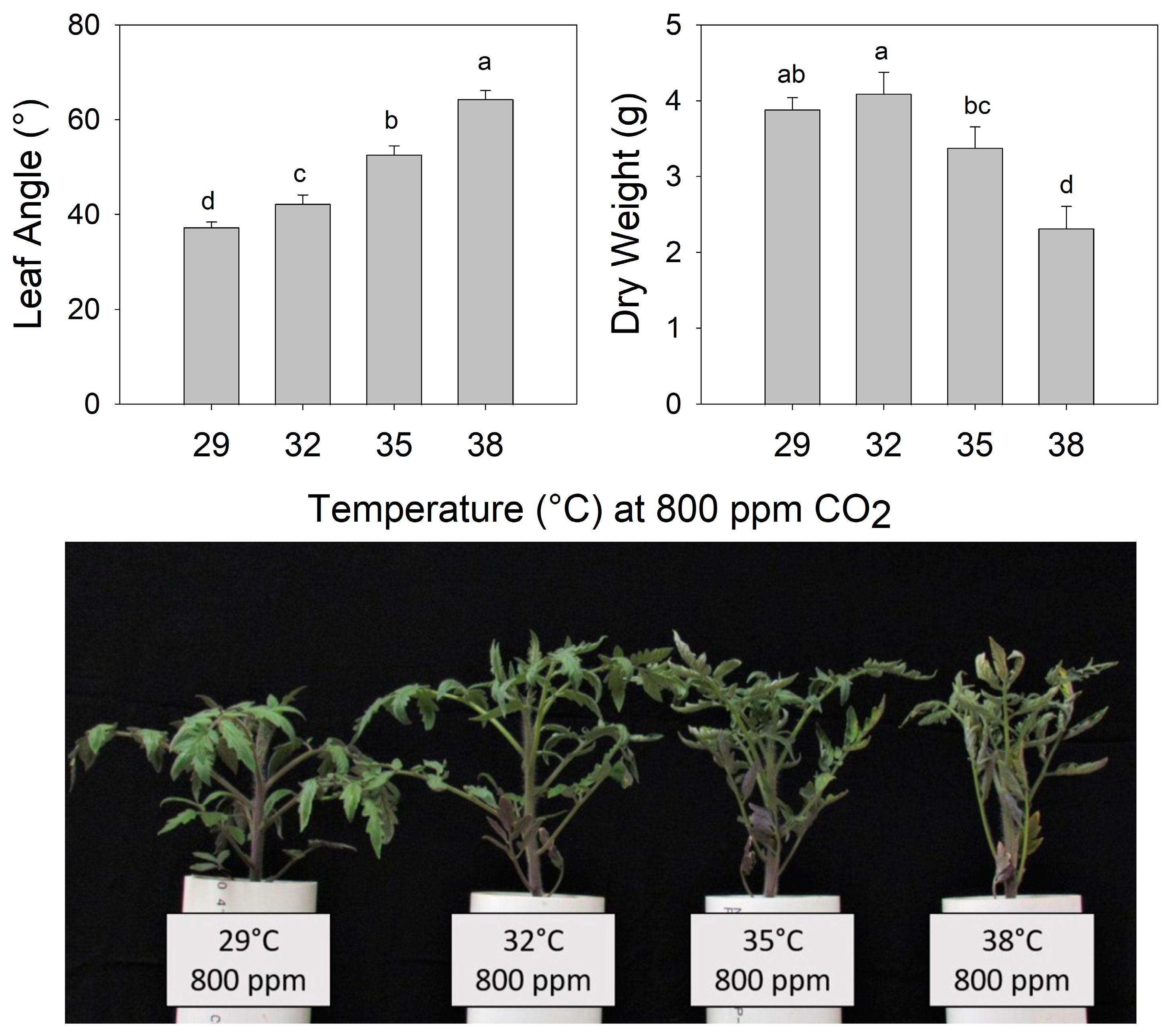
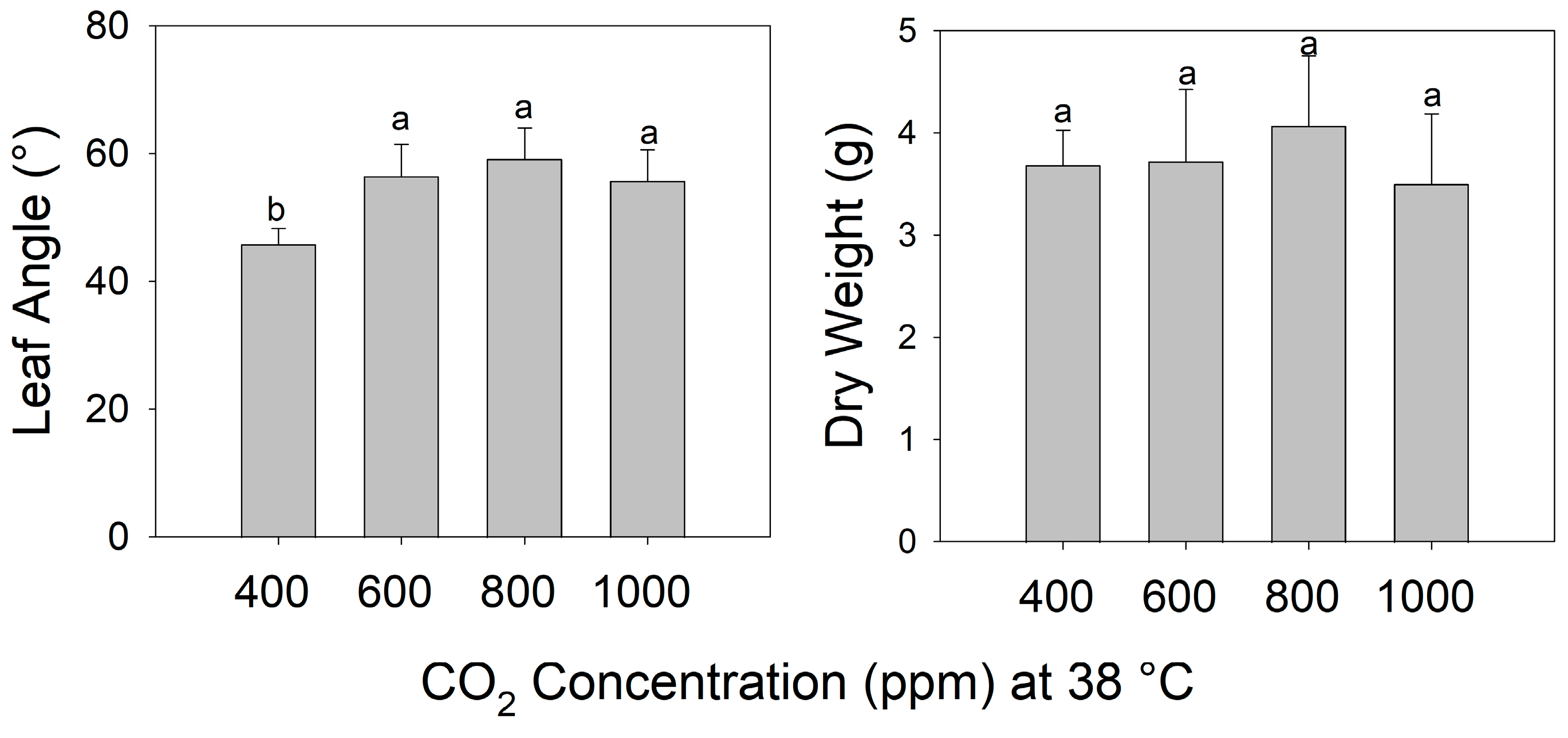
The final petiole angle increased steadily as the temperature increased from 29 °C to 38 °C in pre-reproductive (juvenile) plants grown for 7 days at eCO2 (Figure 2) (F3,26 = 113.5, p < 0.001). Shoot biomass was highest in plants grown at 32 °C and decreased as the temperature increased above 32 °C. Notably, all leaves of these young plants exhibited warming + eCO2 hyponasty (Figure 2) (F3,26 = 19.13, p < 0.001). In contrast, during warming (38 °C), all eCO2 treatments (600, 800, and 1000 ppm) increased the petiole angle to a similar extent compared to ambient CO2 (400 ppm) (F3,19 = 5.725, p < 0.01), but shoot biomass did not differ significantly among CO2 treatments (Figure 3) (F3,19 = 0.647, p > 0.5).
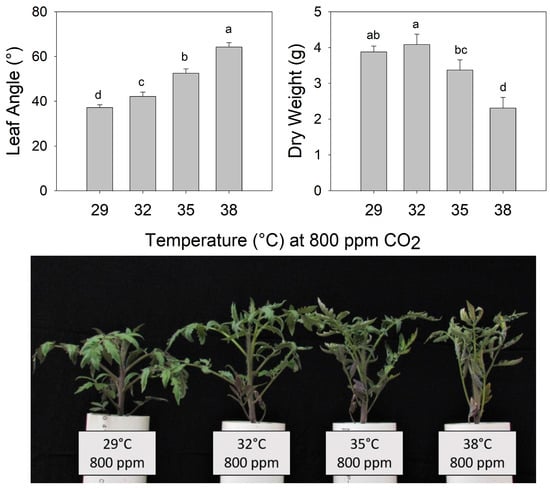
Figure 2.
(Upper) Leaf angle and dry weight of tomato (Solanum lycopersicum ‘Celebrity’) grown at 29/24 °C day/night and 400 ppm carbon dioxide (CO2) and then transferred for 10 days to elevated CO2 (800 ppm) and different temperatures (29, 32, 35, or 38 °C). Error bars show one standard deviation, and different letters indicate significant differences among treatments (Tukey’s HSD, α = 0.05). (Lower) Tomato plants (S. lycopersicum ‘Celebrity’) grown at 29/24 °C day/night and 400 ppm carbon dioxide (CO2) and then transferred for 7 days to different temperatures (29, 32, 35, and 38 °C) at elevated CO2 (800 ppm).
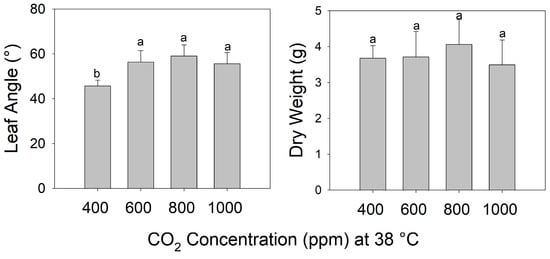
Figure 3.
Leaf angle and dry weight of tomato (S. lycopersicum ‘Celebrity’) grown at 29/24 °C day/night and 400 ppm carbon dioxide (CO2) and then transferred for 10 days to 38 °C and 400 (ambient), 600, 800, or 1000 ppm CO2. Error bars show one standard deviation, and different letters indicate significant differences among treatments (Tukey’s HSD, α = 0.05).
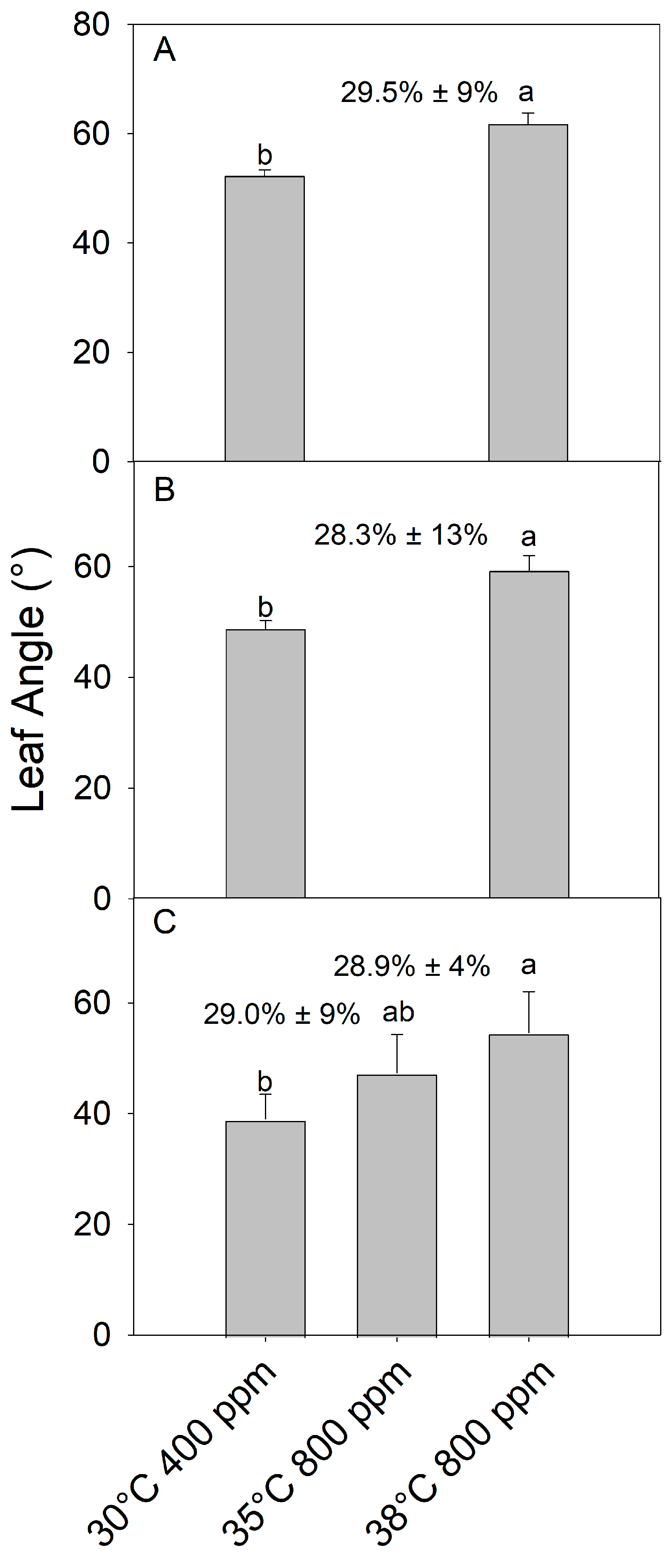
The combination of eCO2 (800 ppm) and severe warming (38 °C) increased the final petiole angle in juvenile (as above) (F1,8 =29.5, p < 0.001), older pre-reproductive adult (F1,8 = 22.27, p < 0.01), and flowering plants (F2,12 = 208.67, p > 01) compared to the control temperature (30 °C) and ambient CO2 (400 ppm) (Figure 4). Additionally, in flowering adults, mild warming (35 °C) + eCO2 increased the petiole angle an intermediate amount between the control and severe warming + eCO2 treatments. As expected, given that hyponasty is a growth response, the petiole angle increased most dramatically in leaves developed during the treatments, with previously fully developed leaves showing little to no change in the petiole angle (visual observation only, data not collected). Since flowering adults were producing many new leaves on axillary stems, hyponasty occurrence (% of leaves) in warming + eCO2 was similar across plants regardless of developmental stage or warming intensity (p > 0.05) (Figure 4; % included above the bars). Additionally, fruits that developed during the 10-day warming + eCO2 (35 °C and 800 ppm) treatment in the flowering plants had reduced pigmentation compared to control plants, causing fruit to appear white (Figure 5).
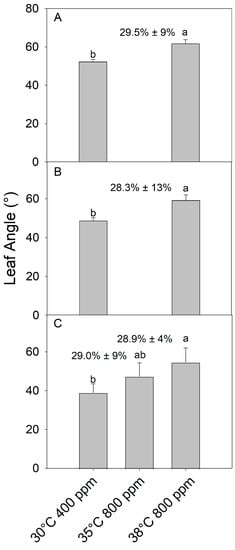
Figure 4.
Leaf angle in developing leaves of (A) juvenile, (B) pre-flowering adult, and (C) flowering tomato plants (S. lycopersicum ‘Celebrity’) grown at 30/25 °C day/night and 400 ppm carbon dioxide (CO2) and then transferred for 10 days to 30 °C and 400 ppm CO2 or 35 or 38 °C and 800 ppm CO2. Juvenile and pre-flowering treatments were not treated at 35 °C and 800 ppm due to growth chamber limitations. Error bars show one standard deviation, and different letters indicate significant differences among treatments (Tukey’s HSD, α = 0.05). The mean (+1 SD) proportion (%) of leaves showing a hyponastic response per plant is indicated above bars.

Figure 5.
Tomato (S. lycopersicum ‘Celebrity’) fruit grown at 30/25 °C day/night and 400 ppm carbon dioxide (CO2) and then transferred for 10 days to 30 °C and 400 ppm CO2 (left two fruit) or 35 °C and 800 ppm CO2 (right two fruit).
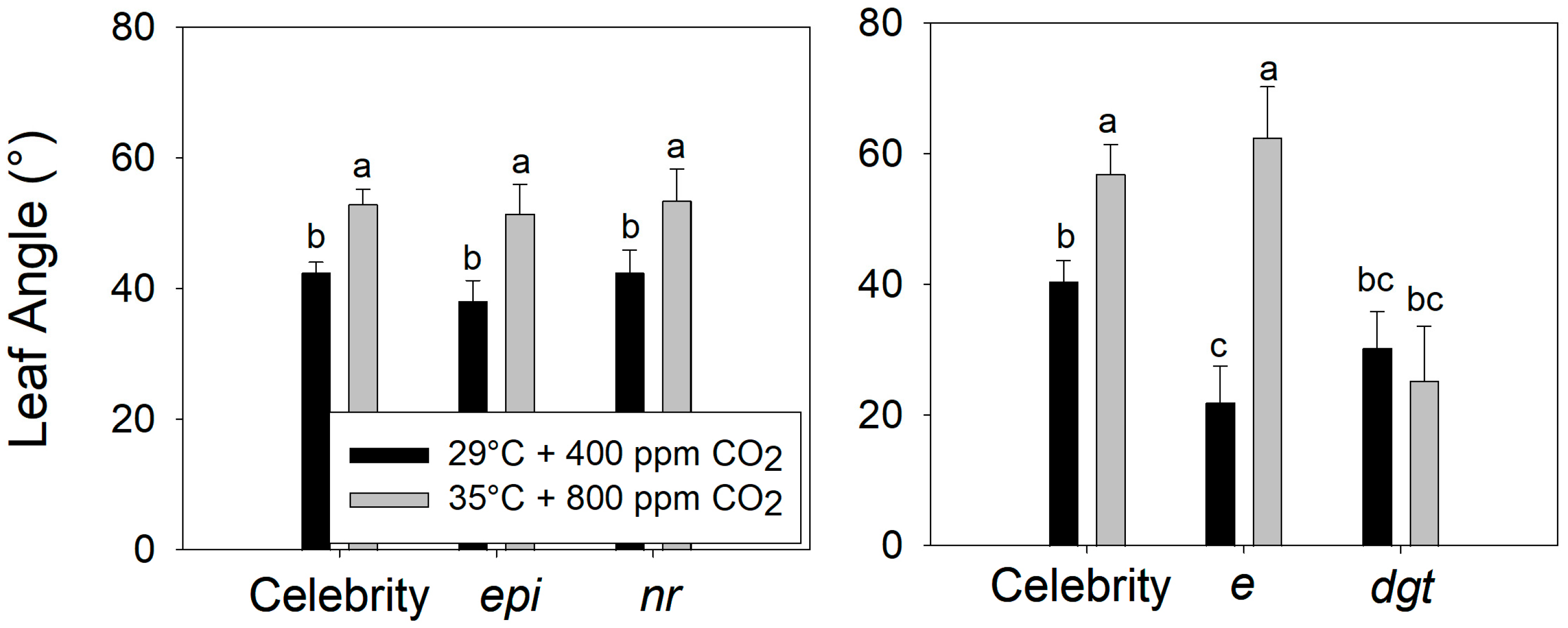
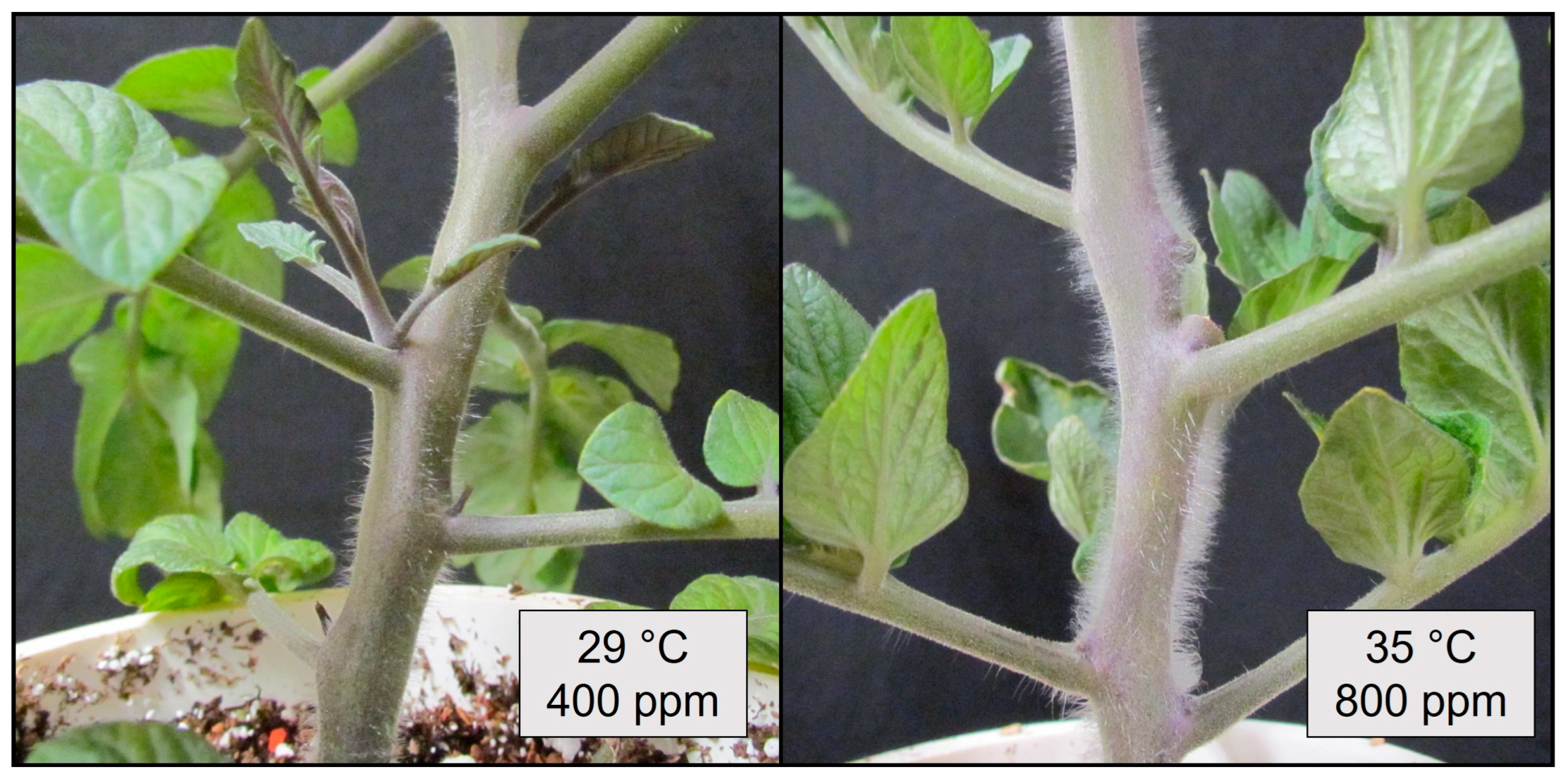
Compared to plants grown at the control conditions (29 °C, 400 ppm CO2), warming + eCO2 increased the petiole angle to a similar extent in the ‘Celebrity’, ethylene-sensitive (epi), and ethylene-insensitive (nr) genotypes, despite their presumed differences in ethylene production or sensitivity (Figure 6) (F2,24 = 0.298, p > 0.5). In contrast, the ‘Celebrity’ and auxin-sensitive plants (e) both exhibited a hyponastic response to warming + eCO2, while the auxin-insensitive (dgt) plants did not (Figure 6 and Figure 7) (F2,12 = 25.31, p < 0.001). Interestingly, trichome production on stems was stimulated in auxin-sensitive plants (e) during warming + eCO2 compared to the control treatment (Figure 8) (visual observation only, data not collected).
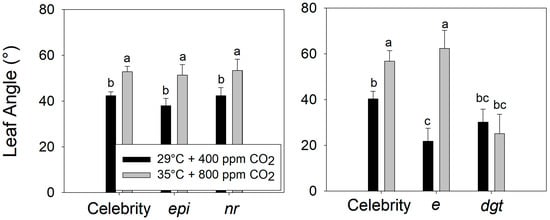
Figure 6.
Leaf angle of reference tomato (S. lycopersicum ‘Celebrity’) vs. ethylene-sensitive (epi) and ethylene-insensitive (nr) mutants (upper), and ‘Celebrity’ vs. auxin-sensitive (e) auxin-insensitive (dgt) mutants (lower). Plants were grown at 29/24 °C day/night and 400 ppm carbon dioxide (CO2) and then transferred for 10 days to control (29 °C and 400 ppm CO2) or warming + elevated carbon dioxide (eCO2) treatments (35 °C and 800 ppm CO2). Error bars show one standard deviation, and different letters indicate significant differences among treatments.

Figure 7.
Comparison of auxin-insensitive (dgt) and auxin-sensitive (e) mutant tomatoes (S. lycopersicum) to reference ‘Celebrity’ at warming + elevated carbon dioxide (eCO2) (35 °C and 800 ppm) treatment.
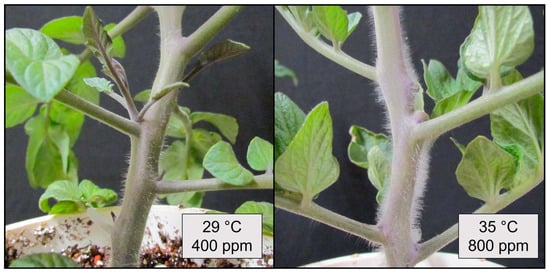
Figure 8.
Trichomes produced during the ambient carbon dioxide (CO2 400 ppm) + optimal (29 °C) temperature treatment (left) and trichomes produced during elevated carbon dioxide (eCO2) + warming (800 ppm, 35 °C) treatment (right) on auxin-sensitive (e) tomato (S. lycopersicum) stems.
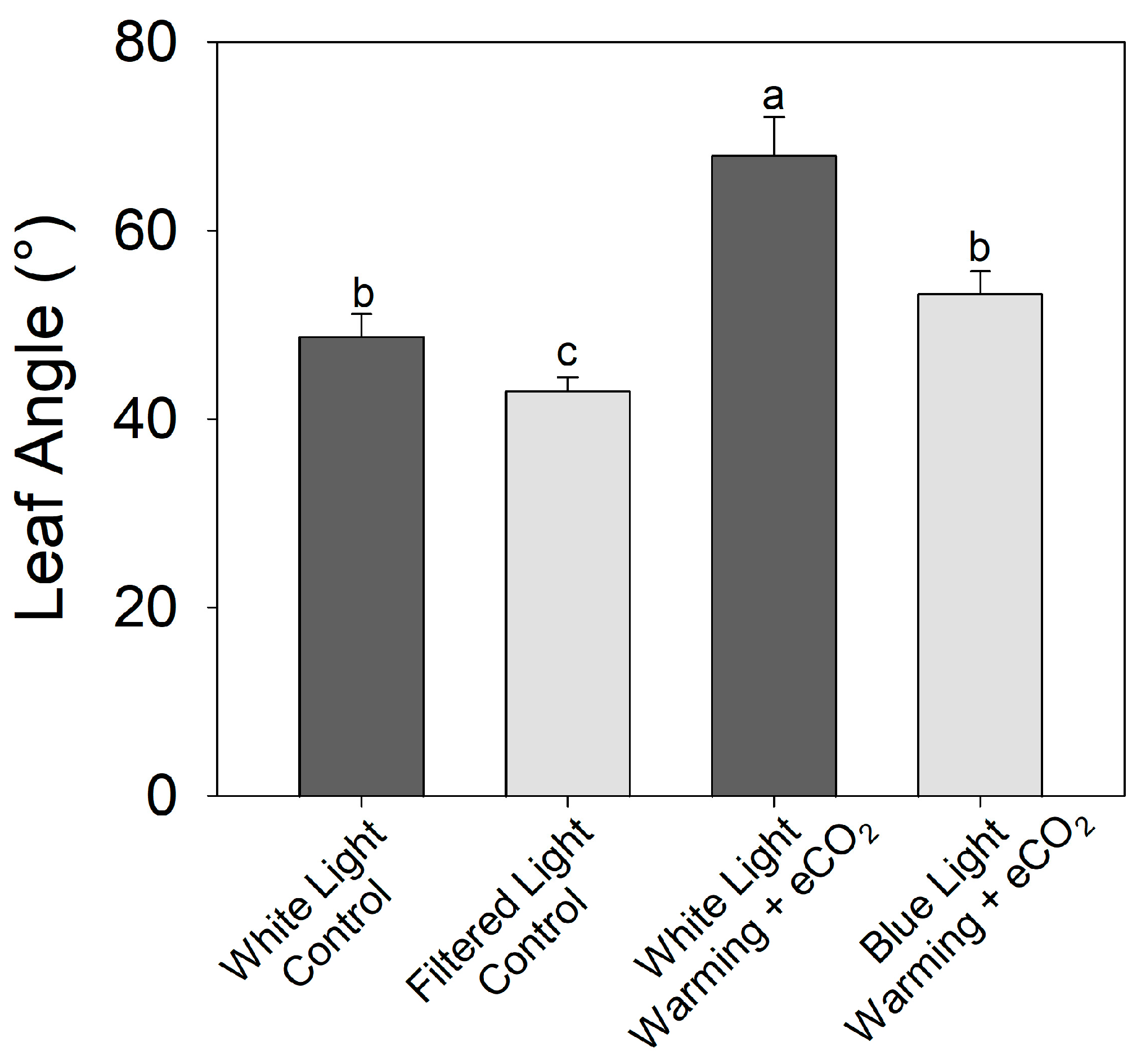
An increase in the proportion of blue light reduced the leaf angle relative to white light counterparts in both the control (30 °C, 400 ppm CO2) and warming + eCO2 (38 °C, 800 ppm CO2) treatments (Figure 9) (F1,16 = 10.87, p < 0.01). Plants grown under blue-light filters and exposed to warming + eCO2 had leaf angles similar to plants grown under white light and at optimal temperature and ambient CO2.
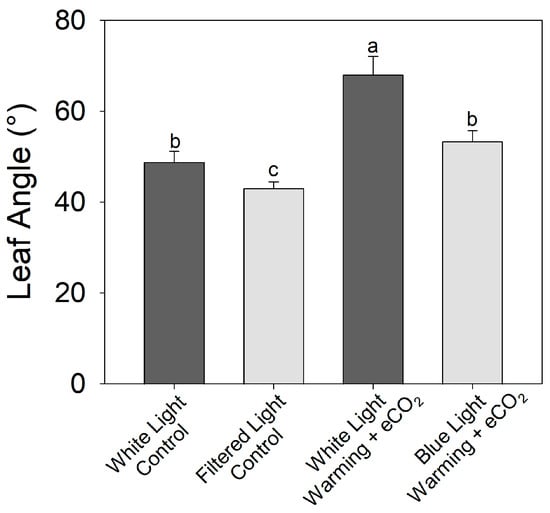
Figure 9.
Effects of light quality on warming + elevated carbon dioxide (eCO2) hyponasty in tomato (S. lycopersicum ‘Celebrity’). Plants were grown at 30/25 °C day/night and 400 ppm CO2 and then transferred for 10 days to the control (30 °C and 400 ppm CO2) or warming + eCO2 treatments (35 °C and 800 ppm CO2) with either unfiltered fluorescent white light or filtered light (with increased blue and reduced far-red-to-red light). Error bars show one standard deviation, and different letters indicate significant differences among treatments (Tukey’s HSD, α = 0.05).
4. Discussion
Though heat-induced leaf hyponasty has been extensively studied, the combined effect of warming and eCO2 in causing leaf hyponasty has been rarely studied and is less understood. Jayawardena et al. [34] first documented warming + eCO2 hyponasty, and they observed that the combined influence of each stressor was greater than the sum of warming or eCO2 alone. However, which stressor more strongly influenced the hyponastic response of tomato plants was not investigated, nor was it determined if hyponasty occurred at all plant growth stages. Further, information on the underlying mechanism causing the warming plus eCO2-induced hyponasty is not available.
In this study, the positive relationship between the petiole angle and temperature at eCO2 (800 ppm) suggests that temperature may either determine the maximum amplitude of the warming + eCO2 hyponastic response or the rate of abaxial vs. adaxial petiole growth through temperature-induced changes in plant hormone concentration or perception [13,21]. A modest increase in the petiole angle associated with mild warming plus eCO2 (32 °C and 800 ppm) did not reduce plant growth compared to the control treatment (29 °C and 800 ppm). However, biomass accumulation was less as the temperature increased beyond 32 °C. This could be the result of lower in situ photosynthesis due to reduced light interception as a result of hyponasty [34], heat inactivation of photosynthesis (e.g., [5]), or a combination of both.
Unlike with temperature, the final petiole angle did not increase steadily with elevated atmospheric CO2 concentration during warming (38 °C), but instead, eCO2 increased the amplitude of hyponasty similarly at all concentrations above ambient (i.e., 600, 800, and 1000 ppm at 38 °C). Hence, the effect of eCO2 on the petiole angle appears to become saturated at modestly elevated CO2, compared to current ambient CO2, presumably via changes in the concentration of, or sensitivity to, hormones and signaling pathways causing leaf hyponasty [9,11,12,13]. Our results suggest that eCO2 may modulate warming-induced hyponasty in tomato via crosstalk between multiple chemical pathways causing the synergistic hyponastic response to warming.
Previous studies suggest that ethylene concentration or sensitivity affects the amplitude of the petiole angle change in response to stressors [30,33,40]. However, the increase in the petiole angle in the ethylene mutants examined in this study (ethylene sensitive, epi, and ethylene insensitive, nr) were similar to the response displayed by the reference genotype ‘Celebrity’ when all were treated with warming + eCO2, indicating that ethylene is not a main component driving warming + eCO2 leaf hyponasty. In contrast, the auxin mutants exhibited dramatically different responses from one another when grown at warming + eCO2. The auxin-sensitive plants (e) exhibited a hyponastic response to warming + eCO2 that was similar to the reference ‘Celebrity’ genotype, while the auxin-insensitive (dgt) plants did not exhibit a hyponastic response to warming + eCO2. It is unlikely that the lack of response seen in the auxin-insensitive plants (dgt) is a result of differences in genetic background, as both the auxin-insensitive and ethylene-sensitive (epi) mutants share the same background but displayed different growth responses. Furthermore, all seven backgrounds used in the warming + eCO2-induced hyponasty experiments have displayed dramatic hyponasty (‘Brandywine’, ‘Celebrity’, ‘Early Girl’, ‘H3406′, ‘Platense’, ‘VFNT cherry’, ‘VFN-8′) except the auxin-insensitive VFN-8 dgt mutant (this study and 34). Additionally, the auxin-sensitive (e) plants grown at warming + eCO2 had a dramatic increase in visual trichome density along their stems, compared to plants grown under control conditions (visual observation only, data not collected), which may be associated with increased auxin perception or signaling [41]. Interestingly, when we compared different developmental stages, fruit development was also affected by the combination of warming + eCO2. No plants produced fruit at severe warming + eCO2 (38 °C and 800 ppm), and fruit developed during mild warming + eCO2 (35 °C and 800 ppm) contained less chlorophyll than those developed at control temperature + ambient CO2. Reduced fruit chlorophyll pigmentation has been associated with warming stress and thermal reversion of phyB in tomato [42], which may implicate the light/temperature sensor phyB in inducing warming + eCO2 hyponasty. Additionally, a change in light quality, which increased the blue proportion and reduced the R/FR ratio, reduced the petiole angle for plants grown at warming + eCO2, further implicating the activity of photosensors in warming + eCO2-induced hyponasty. We cannot yet determine if this inhibition of leaf hyponasty is associated with the reduction in the far-red proportion (FR/(R + FR)) [43] and/or an increase in the proportion of blue light.
As leaf hyponasty is a relatively slow growth response, rather than a rapidly reversible tropism [34], it should be most prominent in young rapidly developing leaves and less evident in older leaves that are already fully expanded or senescing. As expected, in this study, regardless of plant growth stage, warming + eCO2 induced a hyponasty response, compared to control conditions, in young leaves. Importantly, the proportion of hyponastic leaves per plant was similar among plants of different ages. Therefore, warming + eCO2-induced leaf hyponasty has the potential to significantly impact plants throughout their lifetimes, with potentially negative impacts on whole-plant light capture and carbon gain.
Thermal reversion of phyB and increased auxin activity are both associated with warming-induced differential growth and hypocotyl elongation in plants, via a suite of growth responses collectively referred to as thermomorphogenesis [15,16,18,21,23,31,32]. Though we are not yet able to confirm that warming + eCO2 leaf hyponasty is the result of thermomorphogenesis in tomato, evidence of photosensor and auxin involvement in this hyponastic response suggest that CO2 concentration may modulate the thermomorphogenic pathway in this species. We were unable to find any studies which link eCO2 to thermomorphogenesis, so our study is likely the first to suggest that eCO2 modulates thermomorphogenic adaptation in tomato by increasing the amplitude of leaf hyponasty during warming stress. Regardless of mechanism, warming stress + eCO2 will potentially have an impact on production throughout the life of tomato plants via reductions in biomass, which can impact time to flower, fruit quality, and yield. Further investigation into the chemical controls of warming + eCO2-induced hyponasty may provide plant breeders with insights needed to develop more resilient cultivars as atmospheric CO2 and temperatures increase in the coming decades. Furthermore, as our experiments were conducted in growth chambers using fluorescent and incandescent bulbs as the source of light, which provide a light spectrum that mimics but does not exactly match natural sunlight, it will be important to study warming + eCO2 hyponasty under natural light conditions.
5. Conclusions
Chronic warming plus elevated carbon dioxide (eCO2) causes extreme upward bending of leaflets and petioles (i.e., hyponasty) in tomato (Solanum lycopersicum), which reduces growth. Our results indicate that eCO2 affects the well-characterized thermal-hyponastic response of leaves. Future research might target the signalling pathways of this thermomorphogenic response to help develop climate-change-tolerant tomato varieties.
Author Contributions
The project supervisor for this research was S.A.H.; the project design was developed by S.A.H., J.K.B. and M.D.T.; M.D.T. conducted the experiments, analyzed results, and wrote the initial draft of the manuscript; S.A.H. and J.K.B. edited the manuscript and procured funding for the research. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded a grant from the United States Department of Agriculture to SAH (grant ID = NACA 58-5082-9-019). Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Data Availability Statement
Datasets are available on request: The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Acknowledgments
The authors thank Heidi Appel for helpful comments on the manuscript, Reagan Roberts for help with plant care, and Steven Murphy for help with growth chamber maintenance.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2021; pp. 1–31. [Google Scholar]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed]
- Schleussner, C.; Deryng, D.; Müller, C.; Elliott, J.; Saeed, F.; Folberth, C.; Liu, W.; Wang, X.; Pugh, T.; Thiery, W.; et al. Crop productivity changes in 1.5 °C and 2 °C worlds under climate sensitivity uncertainty. Environ. Res. Lett. 2019, 13, 064007. [Google Scholar] [CrossRef]
- Hamilton, E.; Heckathorn, S.; Joshi, P.; Wang, D.; Barua, D. Interactive effects of elevated CO2 and growth temperature on the tolerance of photosynthesis to acute heat stress in C3 and C4 species. J. Integr. Plant Biol. 2008, 50, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Heckathorn, S.; Barua, D.; Joshi, P.; Hamilton, W.; LaCroix, J. Effects of elevated CO2 on the tolerance of photosynthesis to acute heat stress in C3, C4, and CAM species. Am. J. Bot. 2008, 95, 165–176. [Google Scholar] [CrossRef]
- Wang, D.; Heckathorn, S.; Wang, X.; Philpott, S. A meta-analysis of plant physiological and growth response to temperature and elevated CO2. Oecologia 2012, 169, 1–3. [Google Scholar] [CrossRef]
- Wang, D.; Heckathorn, S.; Hamilton, E.; Frantz, J. Effects of CO2 on the tolerance of photosynthesis to heat stress can be affected by photosynthetic pathway and nitrogen. Am. J. Bot. 2014, 101, 34–44. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Y.; Jia, B.; Zhou, G. Elevated-CO2 response of stomata and its dependence on environmental factors. Front. Plant Sci. 2016, 7, 657. [Google Scholar] [CrossRef]
- Wang, Y.; Du, S.; Li, L.; Huang, L.; Fang, P.; Lin, X.; Zhang, Y.; Wang, H. Effect of CO2 elevation on root growth and its relationship with indole acetic acid and ethylene in tomato seedlings. Pedosphere 2009, 19, 570–576. [Google Scholar] [CrossRef]
- Van Zanten, M.; Ritsema, T.; Polko, J.; Leon-Reyes, A.; Voesenek, L.; Millenaar, F.; Pieterse, C.; Peeters, A. Modulation of ethylene- and heat-controlled hyponastic leaf movement in Arabidopsis thaliana by plant defense hormones jasmonate and salicylate. Planta 2012, 235, 677–685. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.; Li, S.; Sun, Z.; Shao, S.; Hu, L.; Ye, M.; Zhou, Y.; Xia, X.; Yu, J.; Shi, K. Anatagonism between phytohormone signaling underlies the variation of disease susceptibility of tomato plants under elevated CO2. J. Exp. Bot. 2015, 66, 1951–1963. [Google Scholar] [CrossRef]
- Pan, C.; Zhang, H.; Ma, Q.; Fan, F.; Fu, R.; Ahammed, G.; Yu, J.; Shi, K. Role of ethyelene biosynthesis and signaling in elevated CO2-induced heat stress response of tomato. Planta 2019, 250, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Euring, D.; Cha, J.; Lin, Z.; Lu, M.; Huang, L.; Kim, W. Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar] [CrossRef]
- Quint, M.; Delker, C.; Franklin, K.; Wigge, P.; Halliday, K.; van Zanten, M. Molecular and genetic control of plant thermomorphogenesis. Nat. Plants 2016, 2, 15190. [Google Scholar] [CrossRef]
- Casal, J.; Balasubramanian, S. Thermomorphogenesis. Annu. Rev. Plant Biol. 2019, 70, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, Y.; Yang, S. Molecular regulation of plant responses to environmental temperatures. Mol. Plant 2020, 13, 544–564. [Google Scholar] [CrossRef]
- Lippmann, R.; Babben, S.; Menger, A.; Delker, C.; Quint, M. Development of wild and cultivated plants under global warming conditions. Curr. Biol. 2019, 29, R1326–R1338. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, J.; Park, Y.; Park, C. Plant Thermomorphogenic adaptation to global warming. J. Plant Biol. 2020, 63, 1–9. [Google Scholar] [CrossRef]
- Klose, C.; Nagy, F.; Schäfer, E. Thermal Reversion of Plant Phytochromes. Mol. Plant 2019, 13, 386–397. [Google Scholar] [CrossRef]
- Fritz, M.; Rosa, S.; Sicard, A. Mechanisms underlying the environmentally induced plasticity of leaf morphology. Front. Genet. 2018, 9, 478. [Google Scholar] [CrossRef]
- Ibañez, C.; Delker, C.; Martinez, C.; Bürstenbinder, K.; Janitza, P.; Lippmann, R.; Ludwig, W.; Sun, H.; James, G.; Klecker, K.; et al. Brassinosteroids dominate hormonal regulation of plant thermomorphogenesis via BZR1. Curr. Biol. 2018, 28, 303–310. [Google Scholar] [CrossRef]
- Crawford, A.; McLachlan, D.; Hethehrinton, A.; Franklin, K. High temperature exposure increases plant cooling capacity. Curr. Biol. 2012, 22, R396–R397. [Google Scholar] [CrossRef]
- Park, Y.; Lee, H.; Gil, K.; Kim, J.; Lee, J.; Lee, H.; Cho, H.; Vu, L.; Smet, I.; Park, C. Developmental programming of thermonastic leaf movement. Plant Physiol. 2019, 180, 1185–1197. [Google Scholar] [CrossRef]
- Voesenek, L.; Colmer, T.; Pierik, R.; Millenaar, F.; Peeters, A. How plants cope with complete submergence. New Phytol. 2006, 170, 213–226. [Google Scholar] [CrossRef]
- Franklin, K. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef]
- Van Zanten, M.; Pons, T.; Janssen, J.; Voesenek, L.; Peeters, A. On the relevance and control of leaf angle. Crit. Rev. Plant Sci. 2010, 29, 300–316. [Google Scholar] [CrossRef]
- Banga, M.; Slaa, E.; Blom, C.; Voesenek, L. Ethylene biosynthesis and accumulation under drained and submerged conditions. Plant Physiol. 1996, 112, 229–237. [Google Scholar] [CrossRef]
- Cox, M.; Benschop, J.; Vreeburg, R.; Wagemaker, C.; Moritz, T.; Peeters, A.; Voesenek, L. The roles of ethylene, auxin, abscisic acid, and gibberellin in the hyponastic growth of submerged Rumex palustris petioles. Plant Physiol. 2004, 136, 2948–2960. [Google Scholar] [CrossRef]
- Dhawan, K.; Bassi, P.; Spencer, M. Effects of carbon dioxide on ethylene production and action in intact sunflower plants. Plant Physiol. 2004, 136, 2948–2960. [Google Scholar] [CrossRef]
- Pierik, R.; Visser, E.; De Kroon, H.; Voesenek, L. Ethylene is required in tobacco to successfully compete with proximate neighbours. Plant Cell Environ. 2003, 26, 1229–1234. [Google Scholar] [CrossRef]
- Küpers, J.; Oskam, L.; Pierik, R. Photoreceptors regulate plant developmental plasticity through auxin. Plants 2020, 9, 940. [Google Scholar] [CrossRef]
- Millenaar, F.; van Zanten, M.; Cox, M.; Pierik, R.; Voesenek, L.; Peeters, A. Differential petiole growth in Arabidopsis thaliana: Photocontrol and hormonal regulation. New Phytol. 2009, 184, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Van Zanten, M.; Voesenek, L.; Peeters, A.; Millenaar, F. Hormone- and light-mediated regulation of heat-induced differential petiole growth in Arabidopsis. Plant Physiol. 2009, 151, 1446–1458. [Google Scholar] [CrossRef]
- Jayawardena, D.; Heckathorn, S.; Bista, D.; Boldt, J. Elevated carbon dioxide plus chronic warming causes dramatic increases in leaf angle in tomato, which correlates with reduced plant growth. Plant Cell Environ. 2019, 42, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Lanahan, M.; Yen, H.; Giovannoni, J.; Klee, H. The never ripe mutation blocks ethylene perception in tomato. Plant Cell 1994, 6, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Barry, C.; Fox, E.; Yen, H.; Lee, S.; Ying, T.; Grierson, D.; Giovannoni, J. Analysis of the ethylene response in the epinastic mutant of tomato. Plant Physiol. 2001, 127, 58–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Batista-Silva, W.; Medeiros, D.; Rodrigues-Salvador, A.; Daloso, D.; Omena-Garcia, R.; Oliveira, F.; Pino, L.; Peres, L.; Nunes-Nesi, A.; Fernie, A.; et al. Modulation of auxin signaling through DIAGETROPICA and ENTIRE differentially affects tomato plant growth via changes in photosynthetic and mitochondrial metabolism. Plant Cell Environ. 2018, 42, 448–465. [Google Scholar] [CrossRef]
- Bhattarai, S.; Harvey, J.; Djidonou, D.; Leskovar, D. Exploring morpho-physiological variation for heat stress tolerance in tomato. Plants 2021, 10, 347. [Google Scholar] [CrossRef]
- Chia, P.; Kubota, C. End-of-day far-red light quality and dose requirements for tomato rootstock hypocotyl elongation. Am. Soc. Hortic. Sci. 2010, 45, 1501–1506. [Google Scholar] [CrossRef]
- Pierik, R.; Djakovic-Petrovic, T.; Keuskamp, D.; de Wit, M.; Voesenek, L. Auxin and ethylene regulate elongation responses to neighbor proximity signals independent of gibberellin and DELLA proteins in Arabidopsis. Plant Physiol. 2009, 149, 1701–1712. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, F.; Tang, Y.; Yuan, Y.; Deng, W.; Li, Z. Auxin response gene SlARF3 plays multiple roles in tomato development and is involved in the formation of epidermal cells and trichomes. Plant Cell Physiol. 2015, 56, 2110–2124. [Google Scholar] [CrossRef]
- Bianchetti, R.; Luca, B.; Haro, L.; Rosado, D.; Demarco, D.; Conte, M.; Bermudez, L.; Freschi, L.; Fernie, A.; Michaelson, L.; et al. Phytochrome-dependent temperature perception modulates isoprenoid metabolism. Plant Physiol. 2020, 183, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, P.; Bugbee, B. Far-red fraction: An improved metric for characterizing phytochrome effects on morphology. J. Am. Soc. Hortic. Sci. 2021, 146, 3–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).