Vermicompost Improves Tomato Yield and Quality by Promoting Carbohydrate Transport to Fruit under Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Environment Conditions
2.3. Experimental Design and Treatments
2.4. Determination of Soil Chemical Properties
2.5. Plant Sampling and Measurements
2.6. Determination of Leaf Gas Exchange Parameters
2.7. Determination of Root Activities
2.8. Yield and Fruit Quality Assays
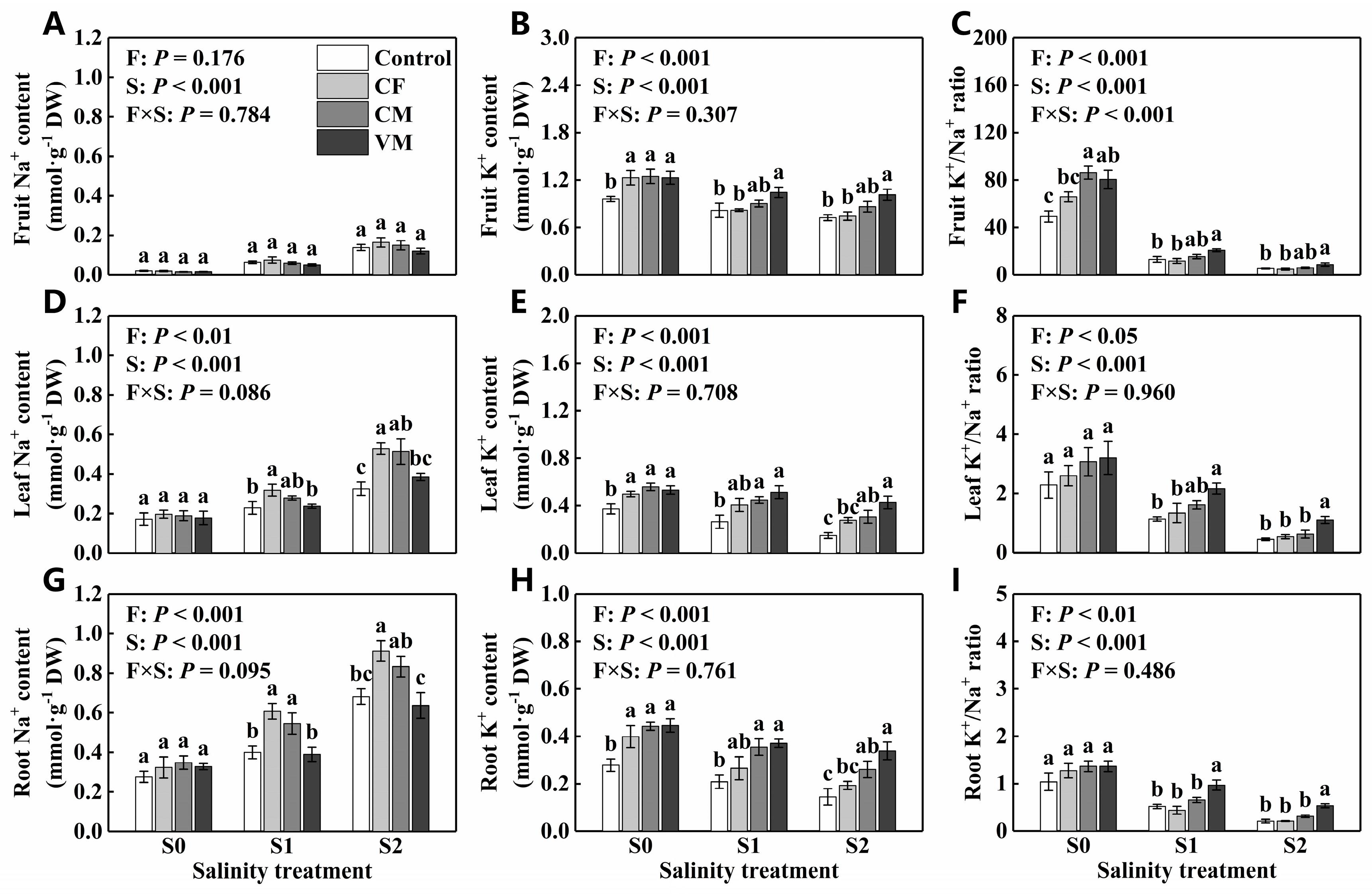
2.9. Determination of N, P, Na+, and K+ in Fruits, Leaves, or Roots
2.10. Determination of Proline and Soluble Protein in Leaves and Roots
2.11. Determination of Soluble Sugars and Starch in Fruits, Leaves, and Roots
2.12. Statistical Analyses
3. Results
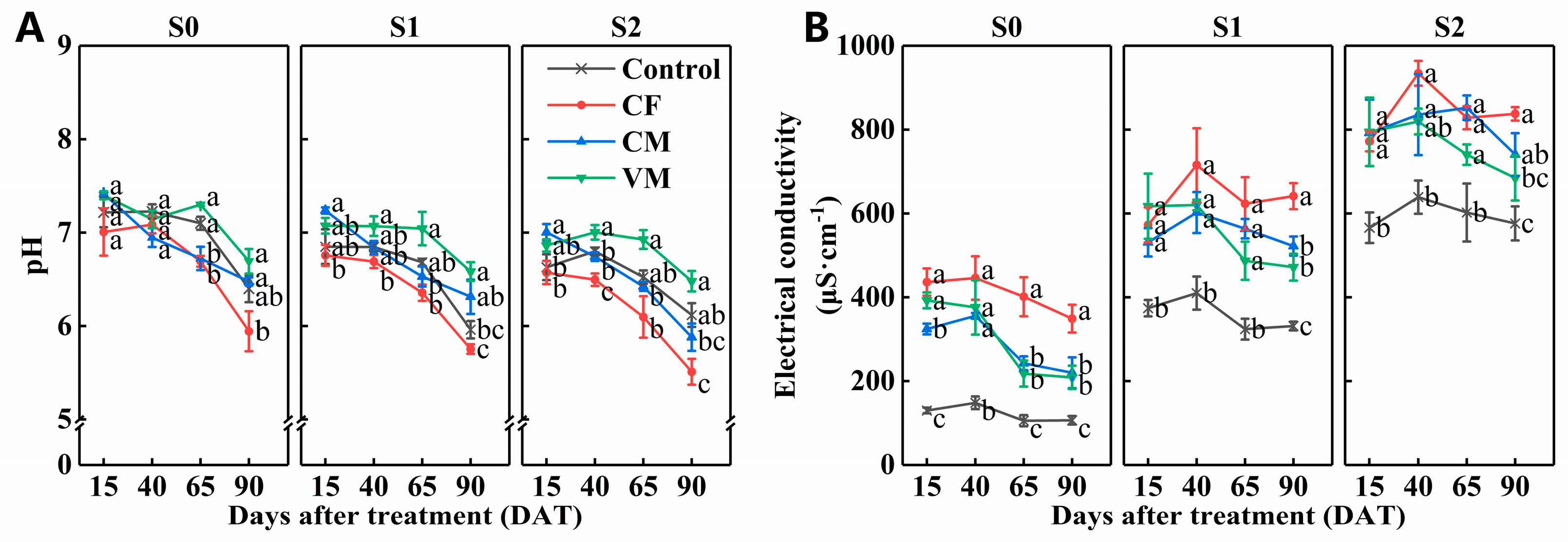
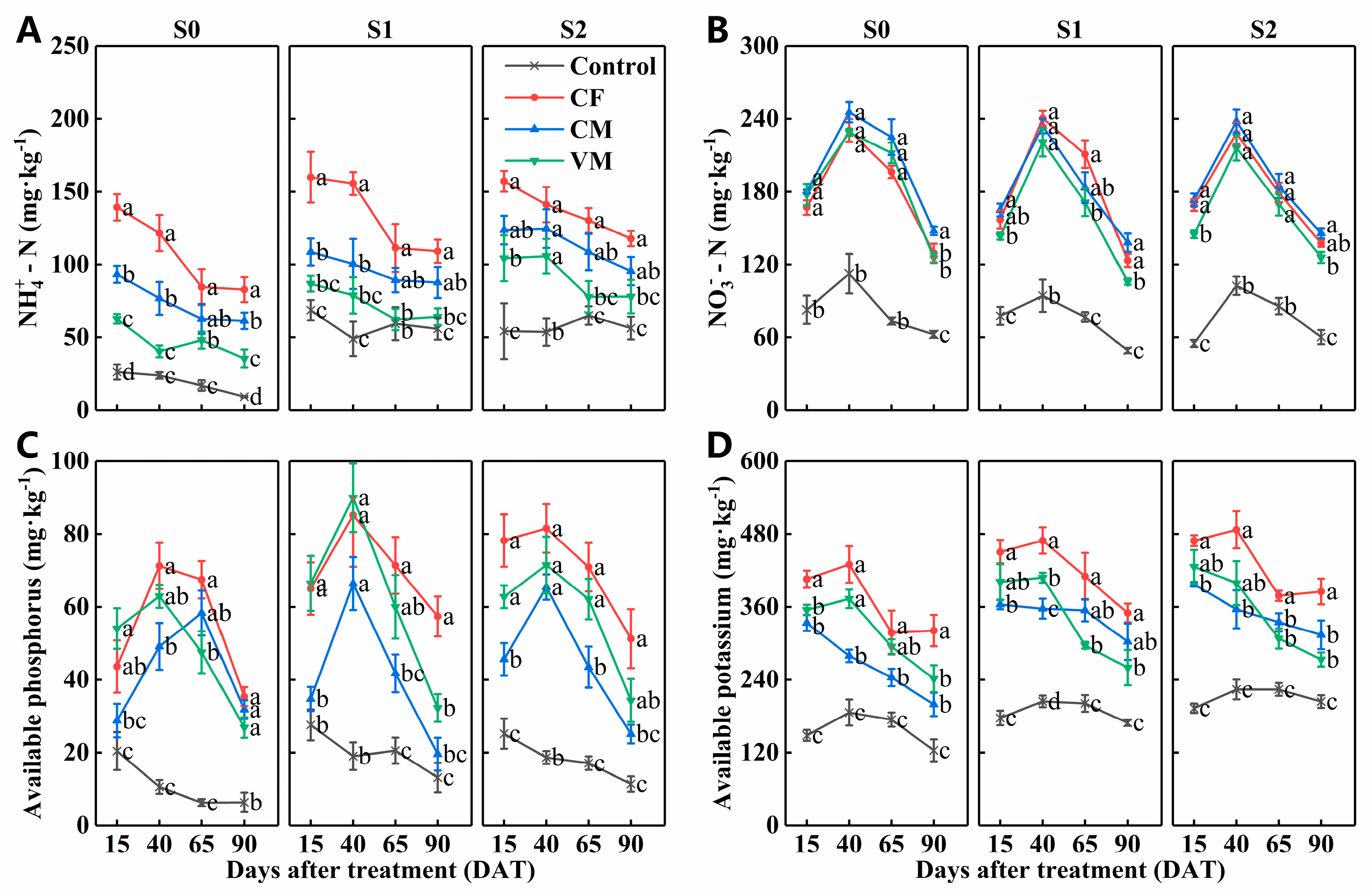
3.1. Effect of Fertilization on Soil Ph, Ec, and Available Nutrients under Salt Stress
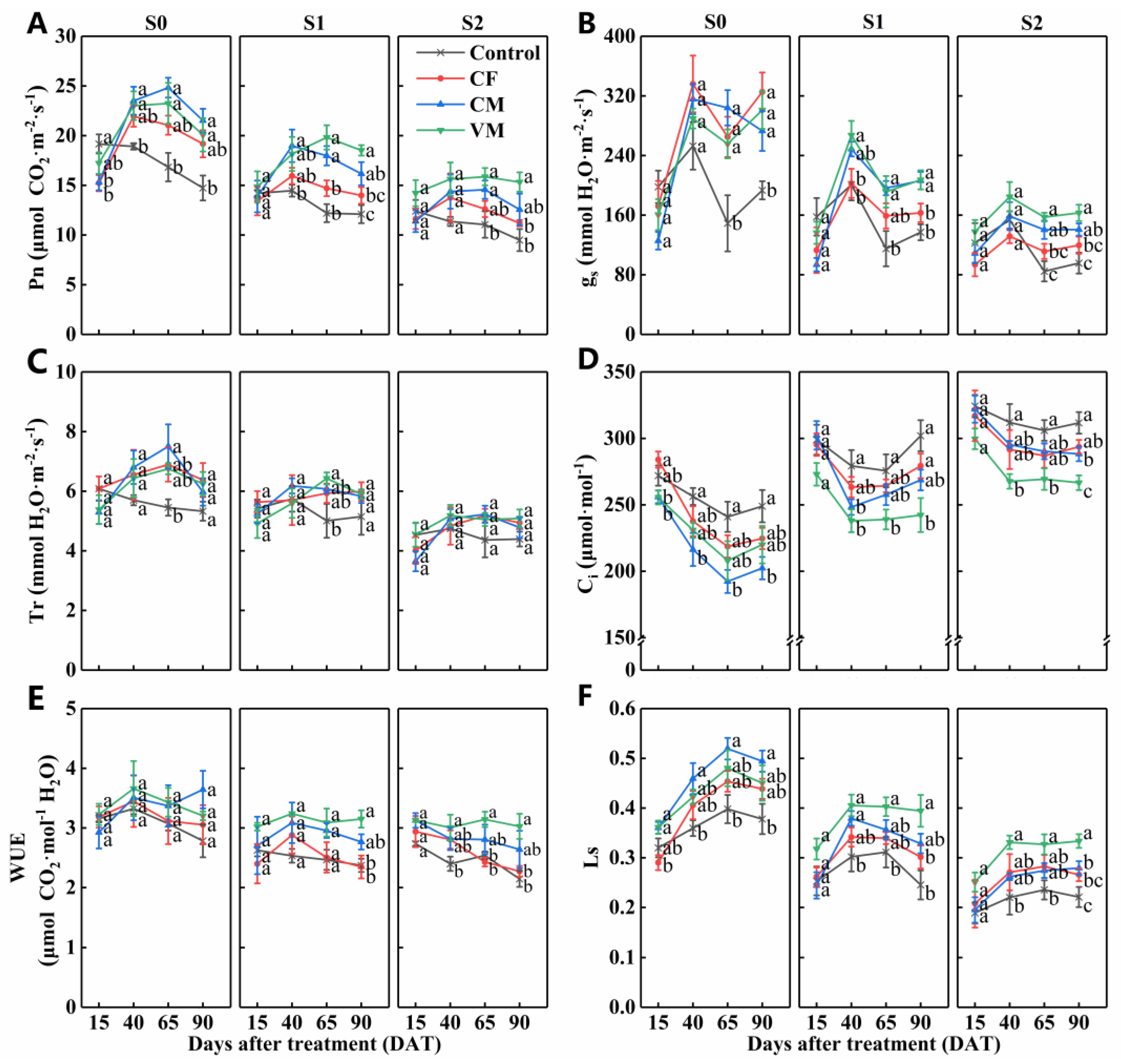
3.2. Effect of Fertilization on Plant Growth, Chlorophyll Content, and Photosynthesis under Salt Stress
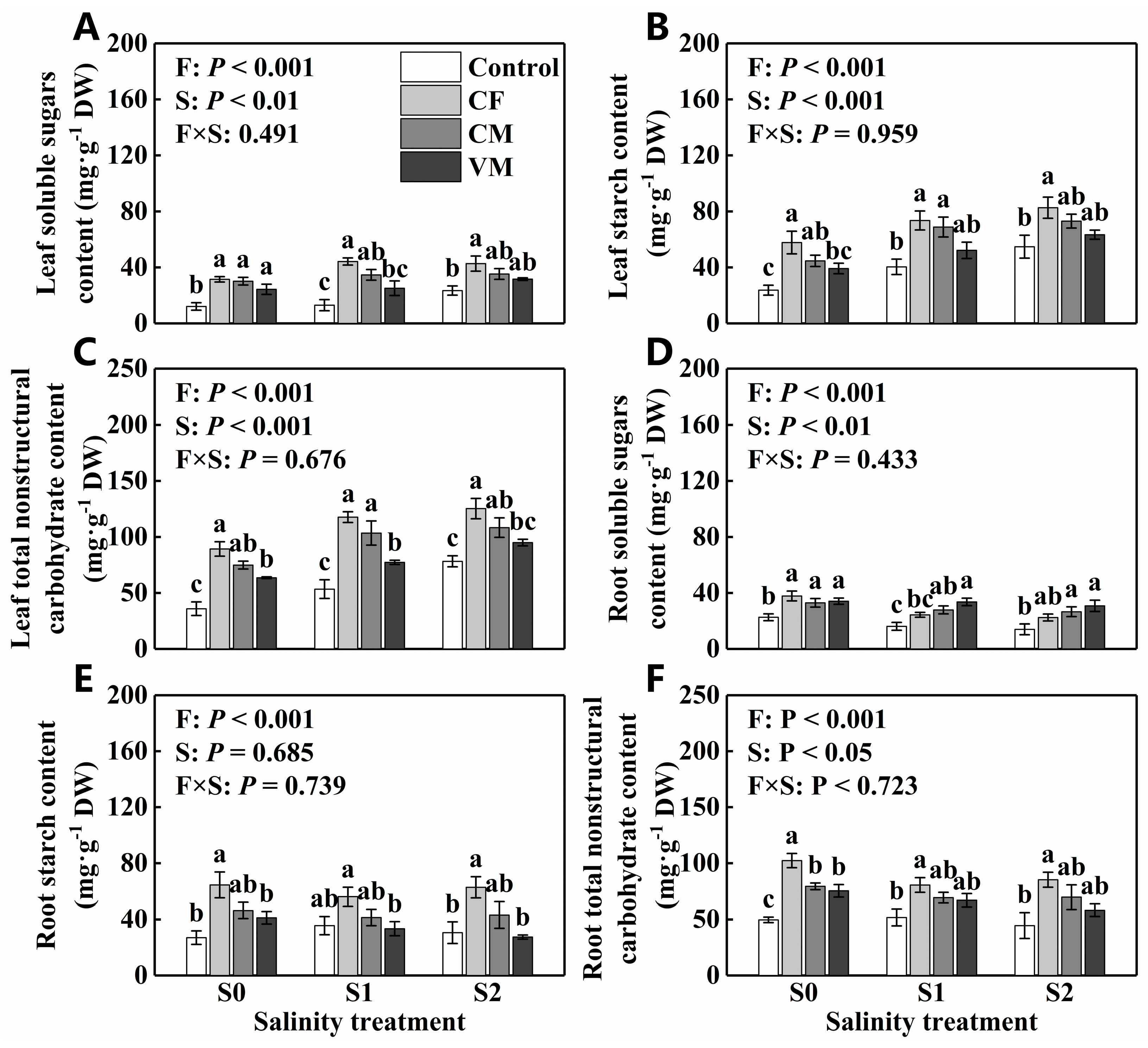
3.3. Effect of Fertilization on Nonstructural Carbohydrates, Proline, and Soluble Protein in Leaves and Roots under Salt Stress
3.4. Effect of Fertilization on Na+, K+, K+ /Na+, N, and P in Fruits, Leaves, and Roots under Salt Stress
3.5. Effect of Fertilization on Yield and Fruit Quality under Salt Stress
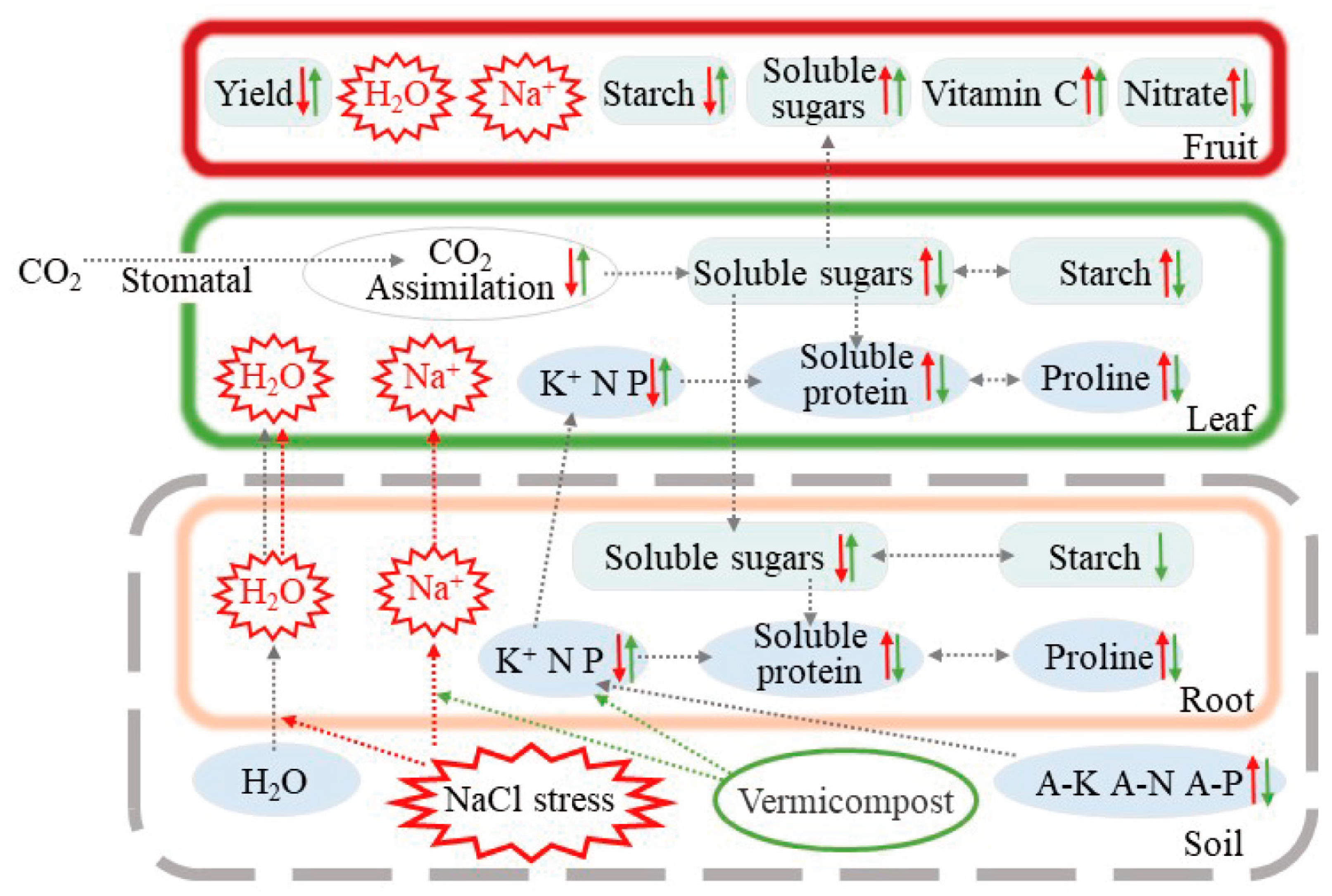
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Properties | Salt Levels | ||
|---|---|---|---|
| S0 | S1 | S2 | |
| Plant dry weight (g) | 38.6 ± 3.65a | 29.8 ± 2.63b | 22.9 ± 2.33b |
| SPAD | 50.0 ± 2.20a | 39.0 ± 2.19b | 34.4 ± 2.27b |
| Root activity (ug TTF·g−1·h−1) | 278 ± 15.6a | 226 ± 11.2b | 184 ± 9.26c |
| Fruit water content (%) | 94.5 ± 0.36a | 92.4 ± 0.54b | 90.0 ± 0.59c |
| Leaf water content (%) | 87.4 ± 0.47a | 86.0 ± 0.39b | 83.9 ± 0.43c |
| Root water content (%) | 86.8 ± 0.24a | 85.4 ± 0.35b | 85.1 ± 0.32b |
| Leaf soluble sugars content (g·kg−1 DW) | 24.5 ± 2.60a | 29.6 ± 3.87a | 33.3 ± 2.63a |
| Leaf starch content (g·kg−1 DW) | 41.4 ± 4.29b | 58.7 ± 4.82a | 68.5 ± 4.14a |
| Leaf total nonstructural carbohydrate content (g·kg−1 DW) | 65.9 ± 6.24b | 88.0 ± 8.06a | 102 ± 5.99a |
| Root soluble sugars content (g·kg−1 DW) | 31.9 ± 2.09a | 25.5 ± 2.19ab | 23.5 ± 2.40b |
| Root starch content (g·kg−1 DW) | 44.8 ± 4.87a | 41.6 ± 3.72a | 41.0 ± 5.19a |
| Root total nonstructural carbohydrate content (g·kg−1 DW) | 76.7 ± 5.97a | 67.2 ± 4.08a | 64.5 ± 5.93a |
| Leaf proline content (ug·g−1 FW) | 115 ± 13.3b | 143 ± 13.2ab | 174 ± 12.61a |
| Leaf soluble protein content (mg·g−1 FW) | 9.88 ± 0.89b | 11.5 ± 0.81ab | 13.8 ± 0.75a |
| Root proline content (ug·g−1 FW) | 41.2 ± 5.49b | 59.7 ± 9.71ab | 67.9 ± 9.05a |
| Root soluble protein content (mg·g−1 FW) | 4.05 ± 0.49b | 5.44 ± 0.41ab | 5.81 ± 0.55a |
| Fruit Na+ content (mmol·g−1 DW) | 0.017 ± 0.01c | 0.062 ± 0.01b | 0.143 ± 0.01a |
| Fruit K+ content (mmol·g−1 DW) | 1.17 ± 0.05a | 0.896 ± 0.04b | 0.836 ± 0.04b |
| Fruit K+/Na+ ratio | 70.5 ± 4.95c | 15.3 ± 1.32b | 6.20 ± 0.61a |
| Leaf Na+ content (mmol·g−1 DW) | 0.184 ± 0.01c | 0.266 ± 0.01b | 0.438 ± 0.03a |
| Leaf K+ content (mmol·g−1 DW) | 0.490 ± 0.03a | 0.408 ± 0.03a | 0.290 ± 0.03b |
| Leaf K+/Na+ ratio | 2.79 ± 0.23a | 1.56 ± 0.15b | 0.678 ± 0.09c |
| Root Na+ content (mmol·g−1 DW) | 0.319 ± 0.021c | 0.486 ± 0.03b | 0.766 ± 0.04a |
| Root K+ content (mmol·g−1 DW) | 0.392 ± 0.02a | 0.300 ± 0.02b | 0.234 ± 0.03c |
| Root K+/Na+ ratio | 1.24 ± 0.07a | 0.647 ± 0.07b | 0.315 ± 0.04c |
| Fruit N content (mmol·g−1 DW) | 1.32 ± 0.12a | 1.21 ± 0.10a | 1.08 ± 0.07a |
| Fruit P content (umol·g−1 DW) | 121 ± 13.51a | 92.3 ± 10.8ab | 66.5 ± 7.13b |
| Leaf N content (mmol·g−1 DW) | 1.80 ± 0.15a | 1.43 ± 0.11b | 1.19 ± 0.09b |
| Leaf P content (umol·g−1 DW) | 95.0 ± 8.63a | 73.5 ± 7.45ab | 63.3 ± 6.16b |
| Root N content (mmol·g−1 DW) | 1.17 ± 0.08a | 0.942 ± 0.07b | 0.828 ± 0.08b |
| Root P content (umol·g−1 DW) | 64.6 ± 5.22a | 47.8 ± 5.20b | 40.4 ± 4.57b |
| Yield (g·plant−1) | 735 ± 52.7a | 464 ± 30.82b | 262 ± 26.10c |
| Sugar acid ratio | 10.6 ± 0.84a | 11.0 ± 0.98a | 9.95 ± 0.77a |
| Vitamin C (mg·100g−1) | 27.5 ± 2.13b | 32.0 ± 2.35ab | 34.8 ± 2.33a |
| Organic acid (%) | 0.404 ± 0.01c | 0.466 ± 0.01b | 0.568 ± 0.02a |
| Soluble solid (%) | 4.47 ± 0.25c | 5.66 ± 0.24b | 6.54 ± 0.26a |
| Nitrate content (mg·kg−1) | 55.3 ± 3.66b | 71.4 ± 5.00a | 74.7 ± 4.54a |
| Fruit soluble sugars content (g·kg−1 FW) | 41.9 ± 2.86b | 50.3 ± 3.31ab | 55.5 ± 3.40a |
| Fruit starch content (g·kg−1 FW) | 15.0 ± 1.07a | 11.2 ± 1.31b | 7.38 ± 1.21c |
| Fruit total nonstructural carbohydrate content (g·kg−1 FW) | 56.9 ± 3.32a | 61.5 ± 4.36a | 62.9 ± 3.85a |
References
- Ivushkin, K.; Bartholomeus, H.; Bregt, A.K.; Pulatov, A.; Kempen, B.; de Sousa, L. Global mapping of soil salinity change. Remote Sens. Environ. 2019, 231, 111260. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, H.M.; Du, H.; Bao, Z.L.; Shi, Q.H. Sugar metabolic and N-glycosylated profiles unveil the regulatory mechanism of tomato quality under salt stress. Environ. Exp. Bot. 2020, 177, 104145. [Google Scholar] [CrossRef]
- Zhao, S.S.; Zhang, Q.K.; Liu, M.Y.; Zhou, H.P.; Ma, C.L.; Wang, P.P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Yang, X.L.; Li, Y.Y.; Chen, H.B.; Huang, J.; Zhang, Y.M.; Qi, M.F.; Liu, Y.F.; Li, T.L. Photosynthetic response mechanism of soil salinity-induced cross-tolerance to subsequent drought stress in tomato plants. Plants 2020, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.W.; Zheng, W.L.; Wang, W.X.; Lv, H.F.; Liang, B.; Li, J.L. Exogenous pig blood-derived protein hydrolysates as a promising method for alleviation of salt stress in tomato (Solanum lycopersicum L.). Sci. Hortic. 2022, 294, 110779. [Google Scholar] [CrossRef]
- Fang, S.M.; Hou, X.; Liang, X.L. Response mechanisms of plants under saline-slkali stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.M.; Horie, T. Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Annu. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Z.Y.; Li, J.; Tang, L.; Jia, S.K.; Feng, X.M.; Wang, C.G.; Yuan, L.Y.; Hou, J.F.; Zhu, S.D. Effects of Ca(NO3)2 stress on mitochondria and nitrogen metabolism in roots of cucumber seedlings. Agronomy 2020, 10, 167. [Google Scholar] [CrossRef]
- Fan, H.F.; Ding, L.; Xu, Y.L.; Du, C.X. Seed germination, seedling growth and antioxidant system responses in cucumber exposed to Ca(NO3)2. Hortic. Environ. Biotechnol. 2017, 58, 548–559. [Google Scholar] [CrossRef]
- Cai, A.D.; Xu, M.G.; Wang, B.R.; Zhang, W.J.; Liang, G.P.; Hou, E.P.; Luo, Y.Q. Manure acts as a better fertilizer for increasing crop yields than synthetic fertilizer does by improving soil fertility. Soil Till. Res. 2019, 189, 168–175. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- de Melo, T.R.; Pereira, M.G.; de Cesare Barbosa, G.M.; da Silva Neto, E.C.; Andrello, A.C.; Tavares Filho, J. Biogenic aggregation intensifies soil improvement caused by manures. Soil Tillage Res. 2019, 190, 186–193. [Google Scholar] [CrossRef]
- Doan, T.T.; Ngo, P.T.; Rumpel, C.; van Nguyen, B.; Jouquet, P. Interactions between compost, vermicompost and earthworms influence plant growth and yield: A one-year greenhouse experiment. Sci. Hortic. 2013, 160, 148–154. [Google Scholar] [CrossRef]
- Liu, M.L.; Wang, C.; Liu, X.L.; Lu, Y.C.; Wang, Y.F. Saline-alkali soil applied with vermicompost and humic acid fertilizer improved macroaggregate microstructure to enhance salt leaching and inhibit nitrogen losses. Appl. Soil Ecol. 2020, 156, 103705. [Google Scholar] [CrossRef]
- Wu, D.; Feng, Y.F.; Xue, L.H.; Liu, M.Q.; Yang, B.; Hu, F.; Yang, L.Z. Biochar combined with vermicompost increases crop production while reducing ammonia and nitrous oxide emissions from a Paddy soil. Pedosphere 2019, 29, 82–94. [Google Scholar] [CrossRef]
- Liu, M.L.; Wang, C.; Wang, F.Y.; Xie, Y.J. Maize (Zea mays) growth and nutrient uptake following integrated improvement of vermicompost and humic acid fertilizer on coastal saline soil. Appl. Soil Ecol. 2019, 142, 147–154. [Google Scholar] [CrossRef]
- Ding, Z.L.; Kheir, A.M.S.; Ali, O.A.M.; Hafez, E.M.; ElShamey, E.A.; Zhou, Z.X.; Wang, B.Z.; Lin, X.; Ge, Y.; Fahmy, A.E.; et al. A vermicompost and deep tillage system to improve saline-sodic soil quality and wheat productivity. J. Environ. Manag. 2021, 277, 111388. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Yu, Z.X.; Xu, L.; Zhao, Y.L.; Yi, S.Q.; Shen, C.; Wang, Y.M.; Li, Y.L.; Zuo, W.G.; Gu, C.H.; et al. Effects of vermicompost application on growth and heavy metal uptake of barley grown in mudflat salt-affected soils. Agronomy 2022, 12, 1007. [Google Scholar] [CrossRef]
- Goswami, L.; Nath, A.; Sutradhar, S.; Bhattacharya, S.S.; Kalamdhad, A.; Vellingiri, K.; Kim, K.H. Application of drum compost and vermicompost to improve soil health, growth, and yield parameters for tomato and cabbage plants. J. Environ. Manag. 2017, 200, 243–252. [Google Scholar] [CrossRef]
- Arthur, G.D.; Aremu, A.O.; Kulkarni, M.G.; Van Staden, J. Vermicompost leachate alleviates deficiency of phosphorus and potassium in tomato seedlings. HortScience 2012, 9, 1304–1307. [Google Scholar] [CrossRef]
- Benazzouk, S.; Dobrev, P.I.; Djazouli, Z.E.; Motyka, V.; Lutts, S. Positive impact of vermicompost leachate on salt stress resistance in tomato (Solanum lycopersicum L.) at the seedling stage: A phytohormonal approach. Plant Soil 2019, 446, 145–162. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Kaiser, E.; Li, T.; Marcelis, L.F.M. NaCl affects photosynthetic and stomatal dynamics by osmotic effects, and reduces photosynthetic capacity by ionic effects in tomato. J. Exp. Bot. 2022, 73, 3637–3650. [Google Scholar] [CrossRef] [PubMed]
- He, W.J.; Yan, K.; Zhang, Y.; Bian, L.X.; Mei, H.M.; Han, G.X. Contrasting photosynthesis, photoinhibition and oxidative damage in honeysuckle (Lonicera japonica Thunb.) under iso-osmotic salt and drought stresses. Environ. Exp. Bot. 2021, 182, 11. [Google Scholar] [CrossRef]
- Katuwal, K.B.; Xiao, B.; Jespersen, D. Physiological responses and tolerance mechanisms of seashore paspalum and centipedegrass exposed to osmotic and iso-osmotic salt stresses. J. Plant Physiol. 2020, 248, 153154. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Al-Khaishany, M.Y.; Khan, M.N.; Al-Amri, A.; Ali, H.M.; Alaraidh, I.A.; Alsahli, A.A. Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int. J. Mol. Sci. 2019, 20, 353. [Google Scholar] [CrossRef]
- Yousfi, S.; Rabhi, M.; Hessini, K.; Abdelly, C.; Gharsalli, M. Differences in efficient metabolite management and nutrient metabolic regulation between wild and cultivated barley grown at high salinity. Plant Biol. 2010, 12, 650–658. [Google Scholar] [CrossRef]
- Lu, S.W.; Qi, F.; Li, T.L. Effect of NaCl, Na+-salt and Cl−-salt stresses on photosynthetic characteristics and sucrose metabolism in tomato leaf. J. Henan Agric. Sci. 2012, 41, 107–112. [Google Scholar] [CrossRef]
- Yang, F.J.; Li, T.L.; Su, Y.; Lu, S.W. Effects of NaCl and Na+, Cl− stress on photosynthetic characteristics of different genotypes of tomato seedlings. Acta Agric. Boreali Sin. 2009, 24, 163–168. [Google Scholar]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann Bot. 2017, 1, 1–11. [Google Scholar] [CrossRef]
- Zhou, Y.; Diao, M.; Chen, X.J.; Cui, J.X.; Pang, S.Q.; Li, Y.Y.; Hou, C.Y.; Liu, H.Y. Application of exogenous glutathione confers salinity stress tolerance in tomato seedlings by modulating ions homeostasis and polyamine metabolism. Sci. Hortic. 2019, 250, 45–58. [Google Scholar] [CrossRef]
- Ciura, J.; Kruk, J. Phytohormones as targets for improving plant productivity and stress tolerance. J. Plant Physiol. 2018, 229, 32–40. [Google Scholar] [CrossRef]
- Xu, L.; Yan, D.; Ren, X.Y.; Wei, Y.Y.; Zhou, J.; Zhao, H.Y.; Liang, M.X. Vermicompost improves the physiological and biochemical responses of blessed thistle (Silybum marianum Gaertn.) and peppermint (Mentha haplocalyx Briq) to salinity stress. Ind. Crops Prod. 2016, 94, 574–585. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhao, F.Y.; Zhang, G.X.; Zhang, Y.Q.; Yang, L.J. Vermicompost improves tomato yield and quality and the biochemical properties of soils with different tomato planting history in a greenhouse study. Front. Plant Sci. 2017, 8, 1978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Y.; Zhang, Y.Y.; Dijkstra, F.A.; Li, Z.J.; Zhang, Y.Q.; Zhang, T.S.; Lu, Y.Q.; Shi, J.W.; Yang, L.J. Effects of amendments on phosphorous status in soils with different phosphorous levels. Catena 2019, 172, 97–103. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Zhang, Y.Y.; Dong, W.G.; Zhang, Y.Q.; Zhang, G.X.; Sun, Z.P.; Yang, L.J. Vermicompost can suppress Fusarium oxysporum f. sp. lycopersici via generation of beneficial bacteria in a long-term tomato monoculture soil. Plant Soil 2019, 440, 491–505. [Google Scholar] [CrossRef]
- Bao, S.D. Analysis of Soil Agro-Chemistry; Agricultural Press Chinese: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Norman, R.Z.; Edberg, J.C.; Stucki, J.W. Determination of nitrate in soil extracts by dual-wavelength ultraviolet spectrophotometry. Soil Sci. Soc. Am. J. 1985, 49, 1182–1185. [Google Scholar] [CrossRef]
- Li, H.S. Principles and Techniques of Plant Physiological Biochemical Experimental; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Bates, L.S. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Shen, J.L.; Wang, Y.; Shu, S.; Jahan, M.S.; Zhong, M.; Wu, J.Q.; Sun, J.; Guo, S.R. Exogenous putrescine regulates leaf starch over-accumulation in cucumber under salt stress. Sci. Hortic. 2019, 253, 99–110. [Google Scholar] [CrossRef]
- Li, S.H.; Li, Y.M.; Gao, Y.; He, X.R.; Zhang, D.L.; Liu, B.B.; Li, Q.M. Effects of CO2 enrichment on non-structural carbohydrate metabolism in leaves of cucumber seedlings under salt stress. Sci. Hortic. 2020, 265, 109275. [Google Scholar] [CrossRef]
- Rufty, T.W.; Huber, S.C. Changes in starch formation and activities of sucrose phosphate synthase and cytoplasmic fructose-1,6-bisphosphatase in response to source-sink alterations. Plant Physiol. 1983, 72, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Parastesh, F.; Alikhani, H.A.; Etesami, H. Vermicompost enriched with phosphate-solubilizing bacteria provides plant with enough phosphorus in a sequential cropping under calcareous soil conditions. J. Clean. Prod. 2019, 221, 27–37. [Google Scholar] [CrossRef]
- Song, X.L.; Li, H.B.; Song, J.X.; Chen, W.F.; Shi, L.H. Biochar/vermicompost promotes Hybrid Pennisetum plant growth and soil in saline soils. Plant Physiol. Bioch. 2022, 183, 96–110. [Google Scholar] [CrossRef]
- Gharbi, E.; Martinez, J.P.; Benahmed, H.; Hichri, I.; Dobrev, P.I.; Motyka, V.; Quinet, M.; Lutts, S. Phytohormone profiling in relation to osmotic adjustment in NaCl-treated plants of the halophyte tomato wild relative species Solanum chilense comparatively to the cultivated glycophyte Solanum lycopersicum. Plant Sci. 2017, 258, 77–89. [Google Scholar] [CrossRef]
- Gong, B.; Wen, D.; VandenLangenberg, K.; Wei, M.; Yang, F.J.; Shi, Q.H.; Wang, X.F. Comparative effects of NaCl and NaHCO3 stress on photosynthetic parameters, nutrient metabolism, and the antioxidant system in tomato leaves. Sci. Hortic. 2013, 157, 1–12. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, Y.C.; Zhu, G.T.; Li, Y.M.; Niu, Q.F.; Yao, J.J.; Hua, K.; Bai, J.J.; Zhu, Y.F.; Shi, H.Z.; et al. Loss of salt tolerance during tomato domestication conferred by variation in a Na+/K+ transporter. EMBO J. 2020, 39, e103256. [Google Scholar] [CrossRef]
- Chakraborty, K.; Bhaduri, D.; Meena, H.N.; Kalariya, K. External potassium (K+) application improves salinity tolerance by promoting Na+-exclusion, K+-accumulation and osmotic adjustment in contrasting peanut cultivars. Plant Physiol. Bioch. 2016, 103, 143–153. [Google Scholar] [CrossRef]
- Ju, F.Y.; Pang, J.L.; Huo, Y.Y.; Zhu, J.J.; Yu, K.; Sun, L.Y.; Loka, D.A.; Hu, W.; Zhou, Z.G.; Wang, S.S.; et al. Potassium application alleviates the negative effects of salt stress on cotton (Gossypium hirsutum L.) yield by improving the ionic homeostasis, photosynthetic capacity and carbohydrate metabolism of the leaf subtending the cotton boll. Field Crops Res. 2021, 272, 108288. [Google Scholar] [CrossRef]
- Kanai, S.; Moghaieb, R.E.; El-Shemy, H.A.; Panigrahi, R.; Mohapatra, P.K.; Ito, J.; Nguyen, N.T.; Saneoka, H.; Fujita, K. Potassium deficiency affects water status and photosynthetic rate of the vegetative sink in green house tomato prior to its effects on source activity. Plant Sci. 2011, 180, 368–374. [Google Scholar] [CrossRef]
- Zeng, Y.L.; Li, L.; Yang, R.R.; Yi, X.Y.; Zhang, B.H. Contribution and distribution of inorganic ions and organic compounds to the osmotic adjustment in Halostachys caspica response to salt stress. Sci. Rep. 2015, 5, 13639. [Google Scholar] [CrossRef]
- He, X.F.; Wang, X.M.; Chen, B.L.; Ma, Z.Y.; Huang, Z.; Shen, X.; Chai, Z.P. The influence of nitrogen (N) input on the sink-source relationship of ‘Korla Fragrant’ pear (Pyrus brestschneideri Rehd.Cv.). Erwerbs-Obstbau 2023, 65, 35–45. [Google Scholar] [CrossRef]
- Zhu, Q.; Ozores-Hampton, M.; Li, Y.C.; Morgan, K.T. Phosphorus application rates affected phosphorus partitioning and use efficiency in tomato production. Agron. J. 2018, 110, 2050–2058. [Google Scholar] [CrossRef]
- Zhang, J.F.; Wang, Z.H.; Fan, B.H.; Hou, Y.S.; Dou, Y.Q.; Ren, Z.L.; Chen, X.J. Investigating the proper application rate of nitrogen under mulched drip irrigation to improve the yield and quality of tomato in saline soil. Agronomy 2020, 10, 1101. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Balibrea, M.E.; Dell’Amico, J.; Bolarín, M.C.; Pérez-Alfocea, F. Carbon partitioning and sucrose metabolism in tomato plants growing under salinity. Physiol. Plantarum 2000, 110, 503–511. [Google Scholar] [CrossRef]
- Nurrahma, A.H.I.; Yabuta, S.; Junaedi, A.; Sakagami, J.I. Analysis of non-structural carbohydrate in relation with shoot elongation of rice under complete submergence. Sustainability 2021, 13, 670. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Chatzieustratiou, E.; Constantopoulou, E.; Kapotis, G. Yield and quality of lettuce and rocket grown in floating culture system. Not. Bot. Horti Agrobot. 2016, 44, 603–612. [Google Scholar] [CrossRef]
- Herencia, J.F.; García-Galavís, P.A.; Dorado, J.A.R.; Maqueda, C. Comparison of nutritional quality of the crops grown in an organic and conventional fertilized soil. Sci. Hortic. 2011, 129, 882–888. [Google Scholar] [CrossRef]
- Çolpan, E.; Zengin, M.; Özbahçe, A. The effects of potassium on the yield and fruit quality components of stick tomato. Hortic. Environ. Biotechnol. 2013, 54, 20–28. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Okorokova-Facanha, A.L.; Façanha, A.R. Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence, and plasma membrane H+-ATPase activity in maize roots. Plant Physiol. 2002, 130, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tan, S.N.; Wong, W.S.; Ng, C.Y.L.; Teo, C.H.; Ge, L.; Chen, X.; Yong, J.W.H. Mass spectrometric evidence for the occurrence of plant growth promoting cytokinins in vermicompost tea. Biol. Fert. Soils 2014, 50, 401–403. [Google Scholar] [CrossRef]
- Guo, M.; Wang, X.S.; Guo, H.D.; Bai, S.Y.; Khan, A.; Wang, X.M.; Gao, Y.M.; Li, J.S. Tomato salt tolerance mechanisms and their potential applications for fighting salinity: A review. Front. Plant Sci. 2022, 13, 949541. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Niu, W.Q.; Cao, X.S.; Wang, J.W.; Zhang, M.Z.; Duan, X.H.; Zhang, Z.X. Effect of soil aeration on root morphology and photosynthetic characteristics of potted tomato plants (Solanum lycopersicum) at different NaCl salinity levels. BMC Plant Biol. 2019, 19, 331. [Google Scholar] [CrossRef]
- Aslani, L.; Gholami, M.; Mobli, M.; Ehsanzadeh, P.; Bertin, N. Decreased sink/source ratio enhances hexose transport in the fruits of greenhouse tomatoes: Integration of gene expression and biochemical analyses. Physiol. Plantarum 2020, 170, 120–131. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Kong, X.Q.; Dong, H.Z. Removal of early fruiting branches impacts leaf senescence and yield by altering the sink/source ratio of field-grown cotton. Field Crops Res. 2018, 216, 10–21. [Google Scholar] [CrossRef]
- Adams, W.W.; Muller, O.; Cohu, C.M.; Demmig-Adams, B. May photoinhibition be a consequence, rather than a cause, of limited plant productivity? Photosynth. Res. 2013, 117, 31–34. [Google Scholar] [CrossRef]
- Khelil, A.; Menu, T.; Ricard, B. Adaptive response to salt involving carbohydrate metabolism in leaves of a salt-sensitive tomato cultivar. Plant Physiol. Biochem. 2007, 45, 551–559. [Google Scholar] [CrossRef]
- Khan, H.A.; Siddique, K.H.M.; Colmer, T.D. Vegetative and reproductive growth of salt-stressed chickpea are carbon-limited: Sucrose infusion at the reproductive stage improves salt tolerance. J. Exp. Bot. 2017, 68, 2001–2011. [Google Scholar] [CrossRef]




| Properties | Vermicompost | Cow Manure | Chemical Fertilizer |
|---|---|---|---|
| pH | 6.58 | 6.92 | - |
| EC (µS cm−1) | 3330 | 1750 | - |
| OM (g kg−1) | 183 | 326 | - |
| Total N (g kg−1) | 11.1 | 9.07 | 106 |
| Total P2O5 (g kg−1) | 19.2 | 20.9 | 66.7 |
| Total K2O (g kg−1) | 4.24 | 6.97 | 107 |
| Total Ca (g kg−1) | 26.2 | 19.5 | 100 |
| Total Mg (g kg−1) | 3.53 | 2.82 | - |
| DAT | p-Value | pH | EC | NH4+-N | NO3−-N | A-P | A-K |
|---|---|---|---|---|---|---|---|
| F | <0.01 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 15 | S | <0.001 | <0.001 | <0.01 | <0.01 | <0.01 | <0.001 |
| F × S | =0.988 | =0.783 | =0.677 | <0.05 | =0.178 | =0.979 | |
| F | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 40 | S | <0.001 | <0.001 | <0.001 | =0.460 | <0.01 | <0.05 |
| F × S | =0.083 | =0.999 | =0.389 | =0.853 | =0.787 | =0.849 | |
| F | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 65 | S | <0.001 | <0.001 | <0.001 | <0.01 | =0.605 | <0.001 |
| F × S | =0.800 | =0.415 | =0.868 | <0.05 | =0.112 | =0.116 | |
| F | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 90 | S | <0.01 | <0.001 | <0.001 | <0.01 | =0.137 | <0.001 |
| F × S | =0.409 | =0.919 | =0.838 | =0.731 | <0.05 | =0.392 |
| DAT | p-Value | Pn | gs | Tr | Ci | WUE | Ls |
|---|---|---|---|---|---|---|---|
| F | =0.069 | <0.05 | =0.282 | <0.05 | =0.360 | <0.05 | |
| 15 | S | <0.001 | <0.01 | <0.001 | <0.001 | <0.05 | <0.001 |
| F × S | =0.617 | =0.683 | =0.442 | =0.595 | =0.678 | =0.592 | |
| F | <0.01 | =0.067 | =0.553 | <0.01 | =0.121 | <0.01 | |
| 40 | S | <0.001 | <0.001 | <0.01 | <0.001 | <0.01 | <0.001 |
| F × S | =0.976 | =0.126 | =0.954 | =0.525 | =0.992 | =0.525 | |
| F | <0.001 | <0.001 | <0.01 | <0.01 | <0.05 | <0.01 | |
| 65 | S | <0.001 | <0.001 | <0.001 | <0.001 | <0.01 | <0.001 |
| F × S | =0.480 | =0.233 | =0.729 | =0.419 | =0.975 | =0.422 | |
| F | <0.001 | <0.001 | <0.05 | <0.001 | <0.01 | <0.001 | |
| 90 | S | <0.001 | <0.001 | <0.001 | <0.001 | <0.01 | <0.001 |
| F × S | =0.434 | <0.05 | =0.996 | =0.332 | =0.481 | =0.336 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Chen, C.; Liu, Y.; Zhang, G.; Yang, L. Vermicompost Improves Tomato Yield and Quality by Promoting Carbohydrate Transport to Fruit under Salt Stress. Horticulturae 2023, 9, 1015. https://doi.org/10.3390/horticulturae9091015
Wu D, Chen C, Liu Y, Zhang G, Yang L. Vermicompost Improves Tomato Yield and Quality by Promoting Carbohydrate Transport to Fruit under Salt Stress. Horticulturae. 2023; 9(9):1015. https://doi.org/10.3390/horticulturae9091015
Chicago/Turabian StyleWu, Di, Chunlan Chen, Yifei Liu, Guoxian Zhang, and Lijuan Yang. 2023. "Vermicompost Improves Tomato Yield and Quality by Promoting Carbohydrate Transport to Fruit under Salt Stress" Horticulturae 9, no. 9: 1015. https://doi.org/10.3390/horticulturae9091015
APA StyleWu, D., Chen, C., Liu, Y., Zhang, G., & Yang, L. (2023). Vermicompost Improves Tomato Yield and Quality by Promoting Carbohydrate Transport to Fruit under Salt Stress. Horticulturae, 9(9), 1015. https://doi.org/10.3390/horticulturae9091015






