Exogenous Selenium Enhances Cadmium Stress Tolerance by Improving Physiological Characteristics of Cabbage (Brassica oleracea L. var. capitata) Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Handling
2.2. Measurement of Growth Parameters
2.3. Determination of Root Activity
2.4. Determination of Chlorophyll Content
2.5. Determination and Analysis of Leaf Stomata Parameters
2.6. Determination and Analysis of Leaf Chlorophyll Fluorescence Parameters
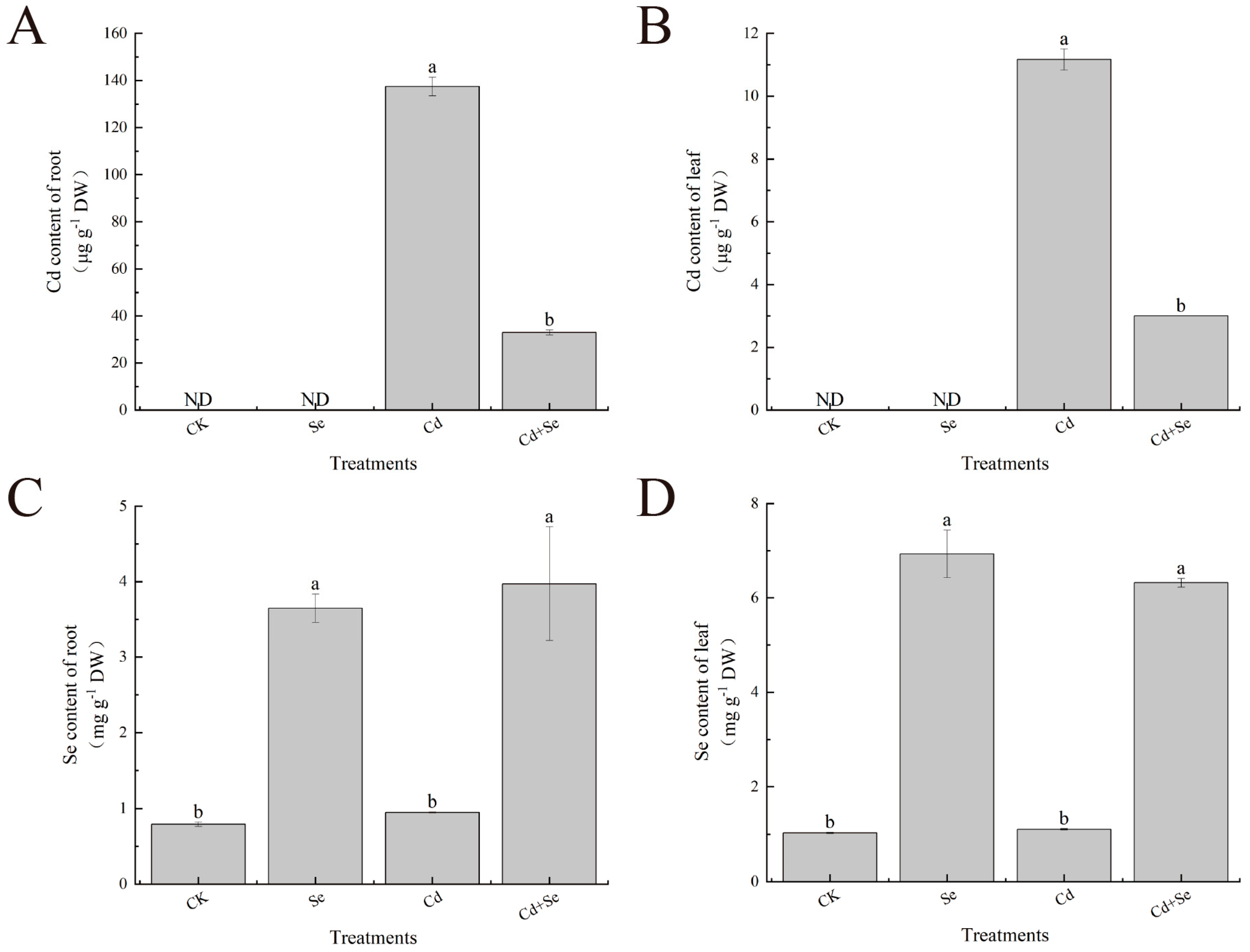
2.7. Determination of Se and Cd Contents
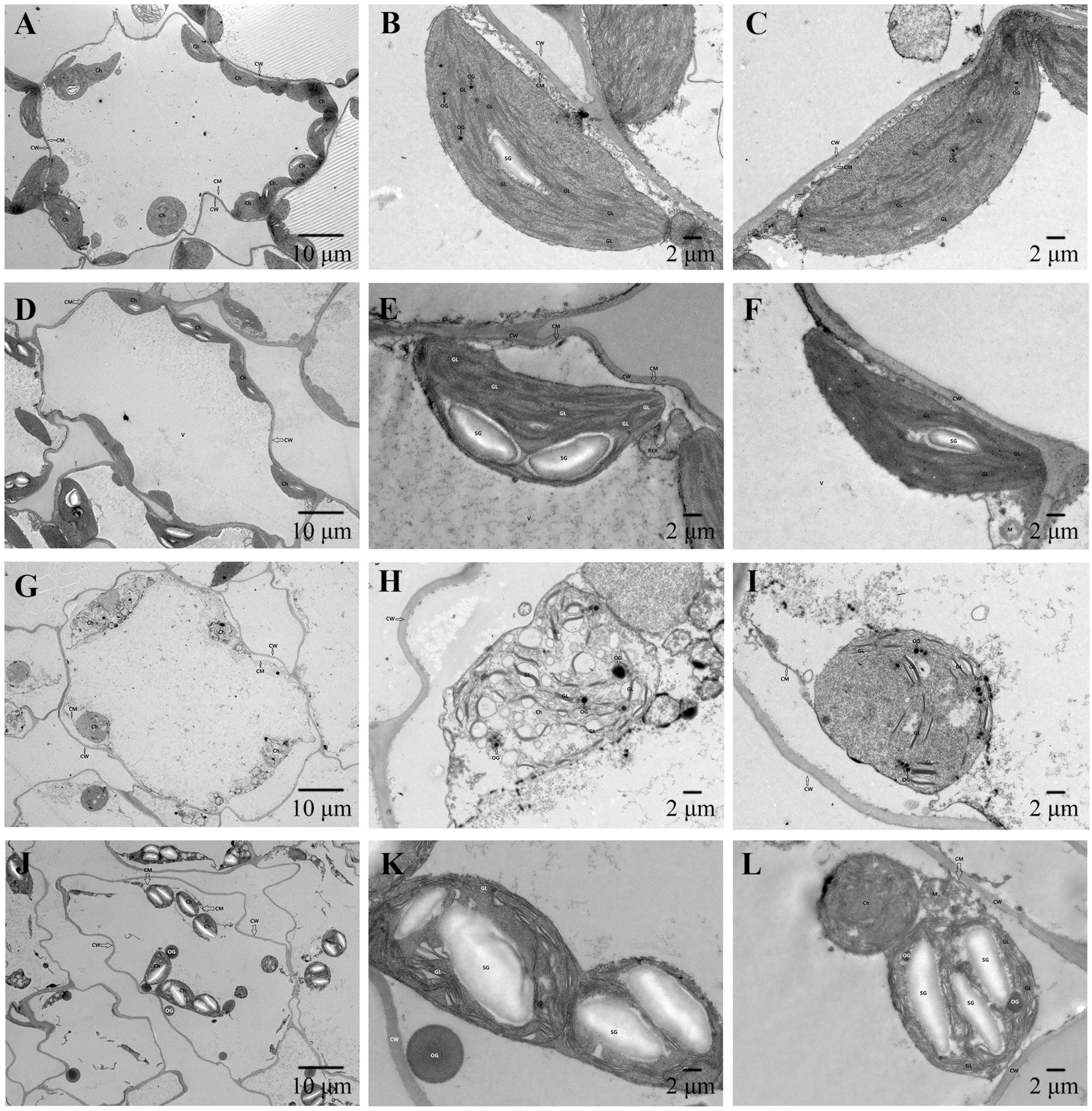
2.8. Chloroplast Ultrastructure Analysis
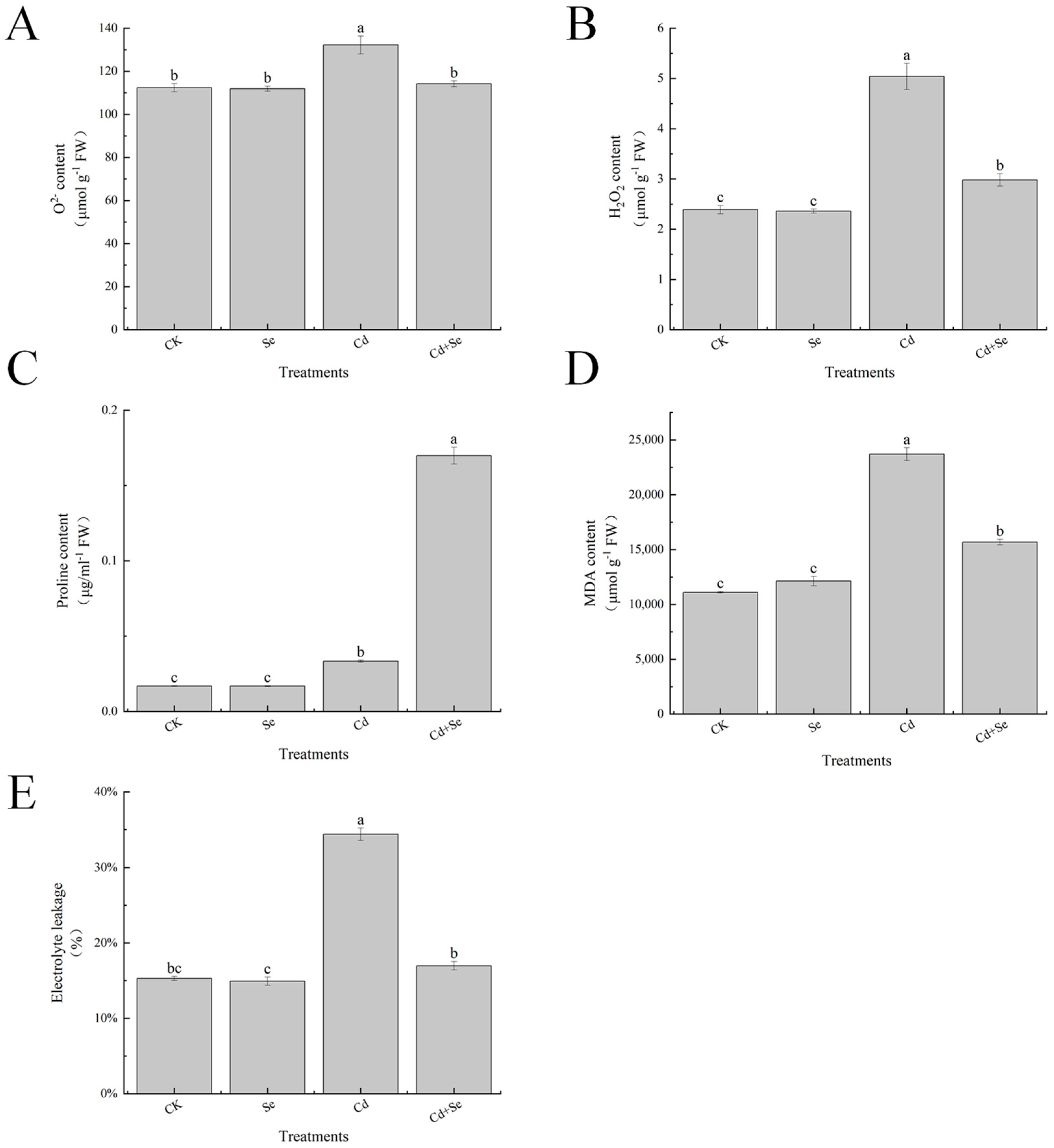
2.9. Determination and Analysis of Electrolyte Leakage
2.10. Determination and Analysis of MDA and Proline Content
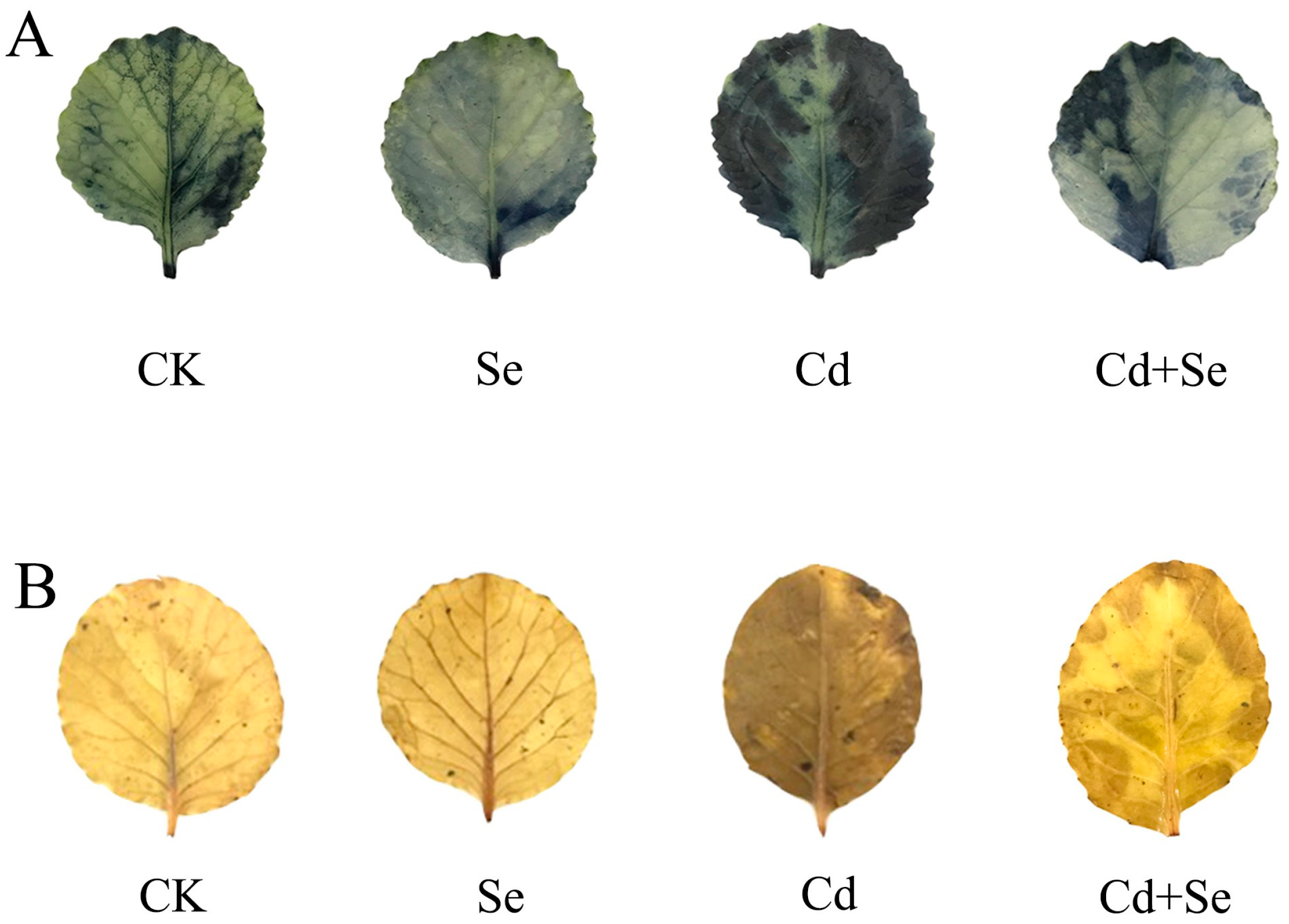
2.11. O2− and H2O2 Histochemical Staining and Content
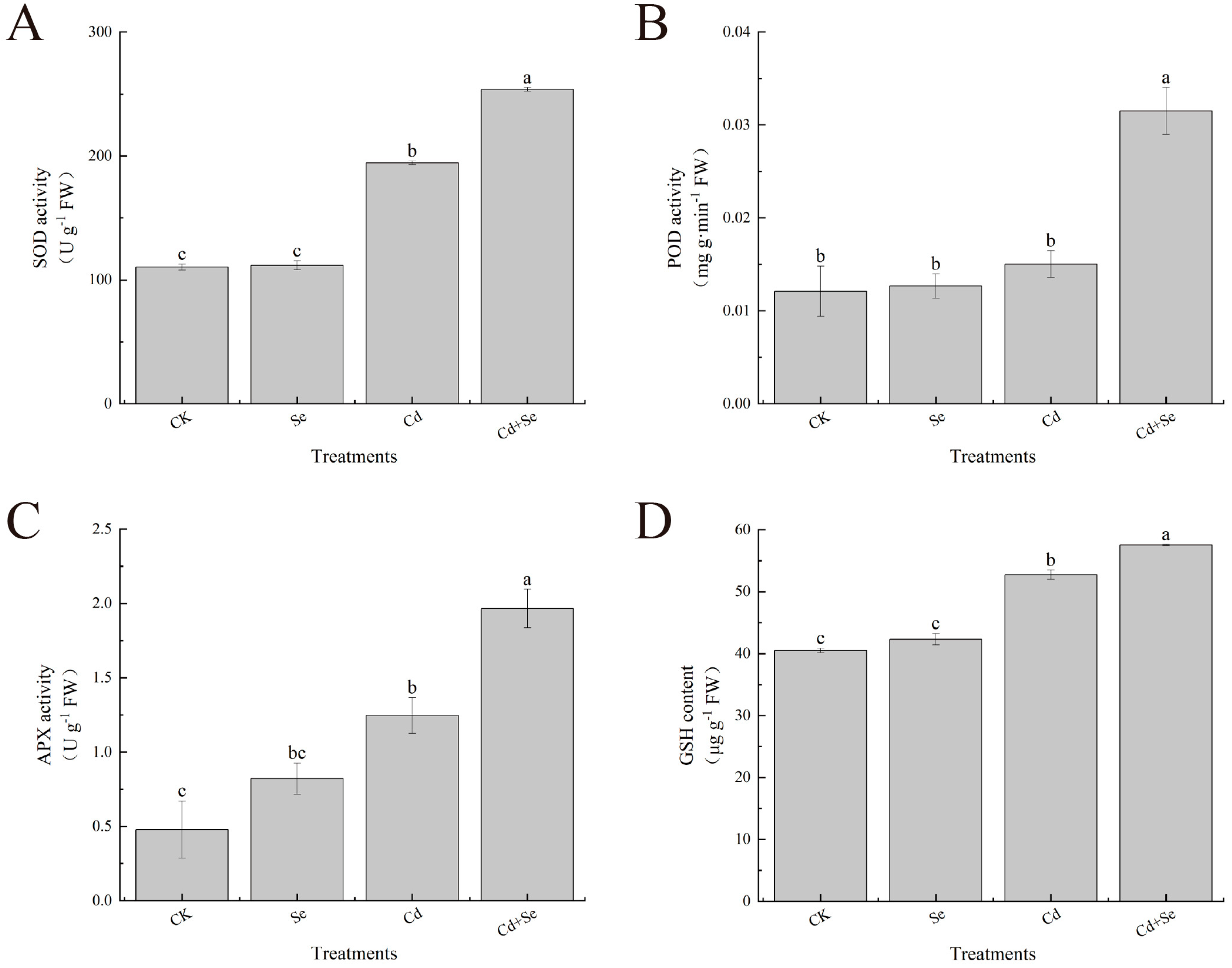
2.12. Antioxidant Enzyme System Determination and Analysis
2.13. Statistical Analysis
3. Results
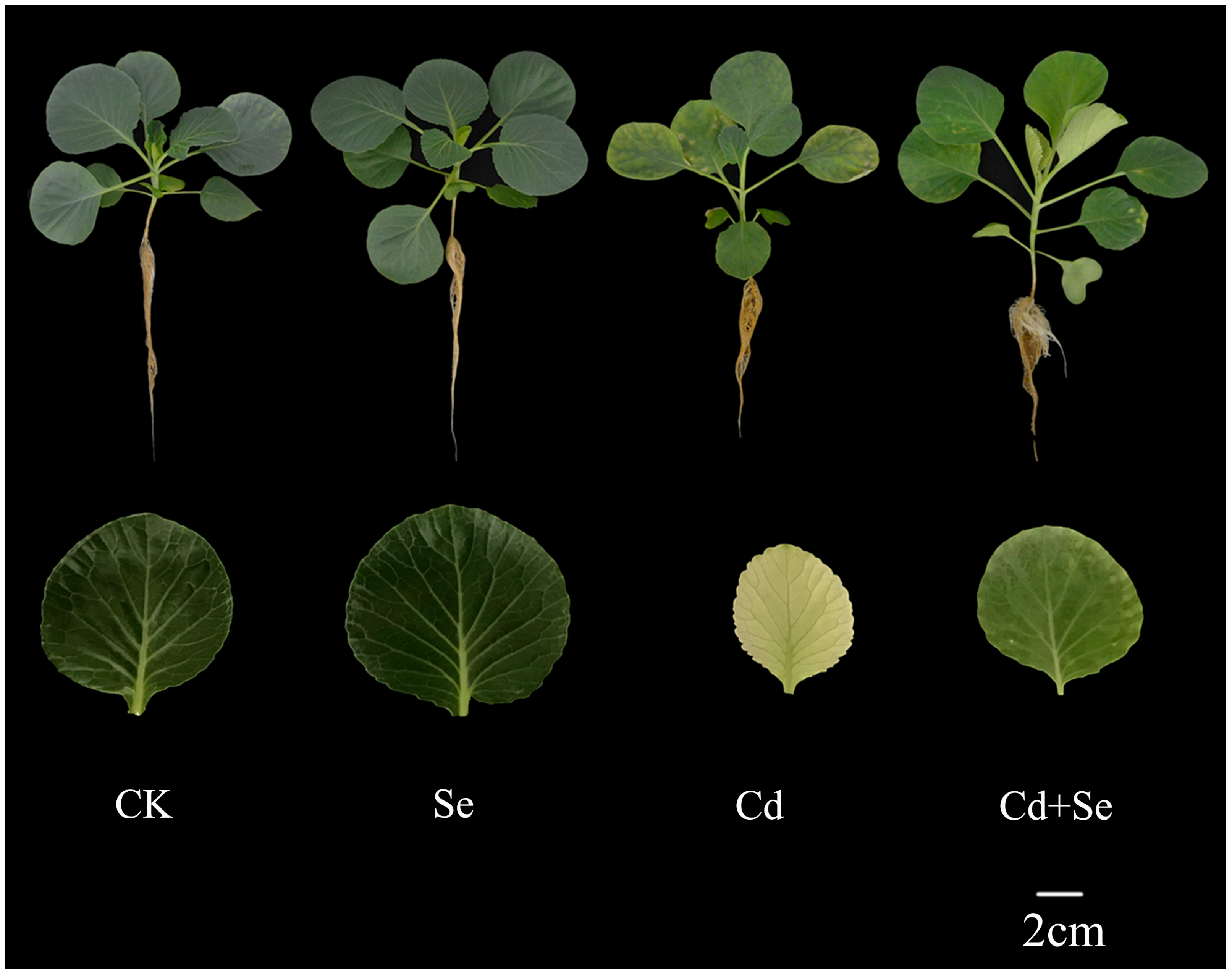
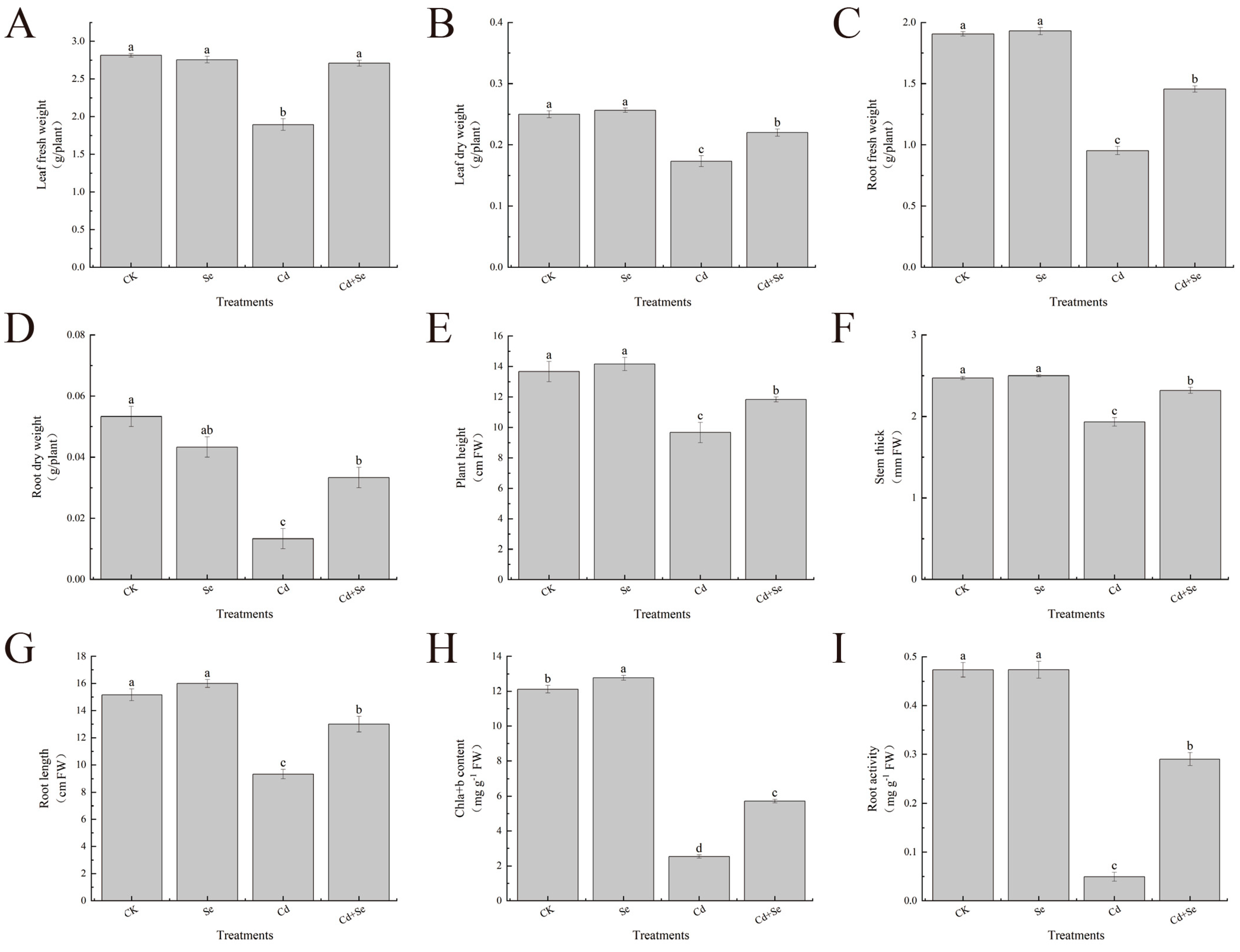
3.1. Exogenous Se Can Promote the Growth of Cabbage Seedlings under Cd Stress
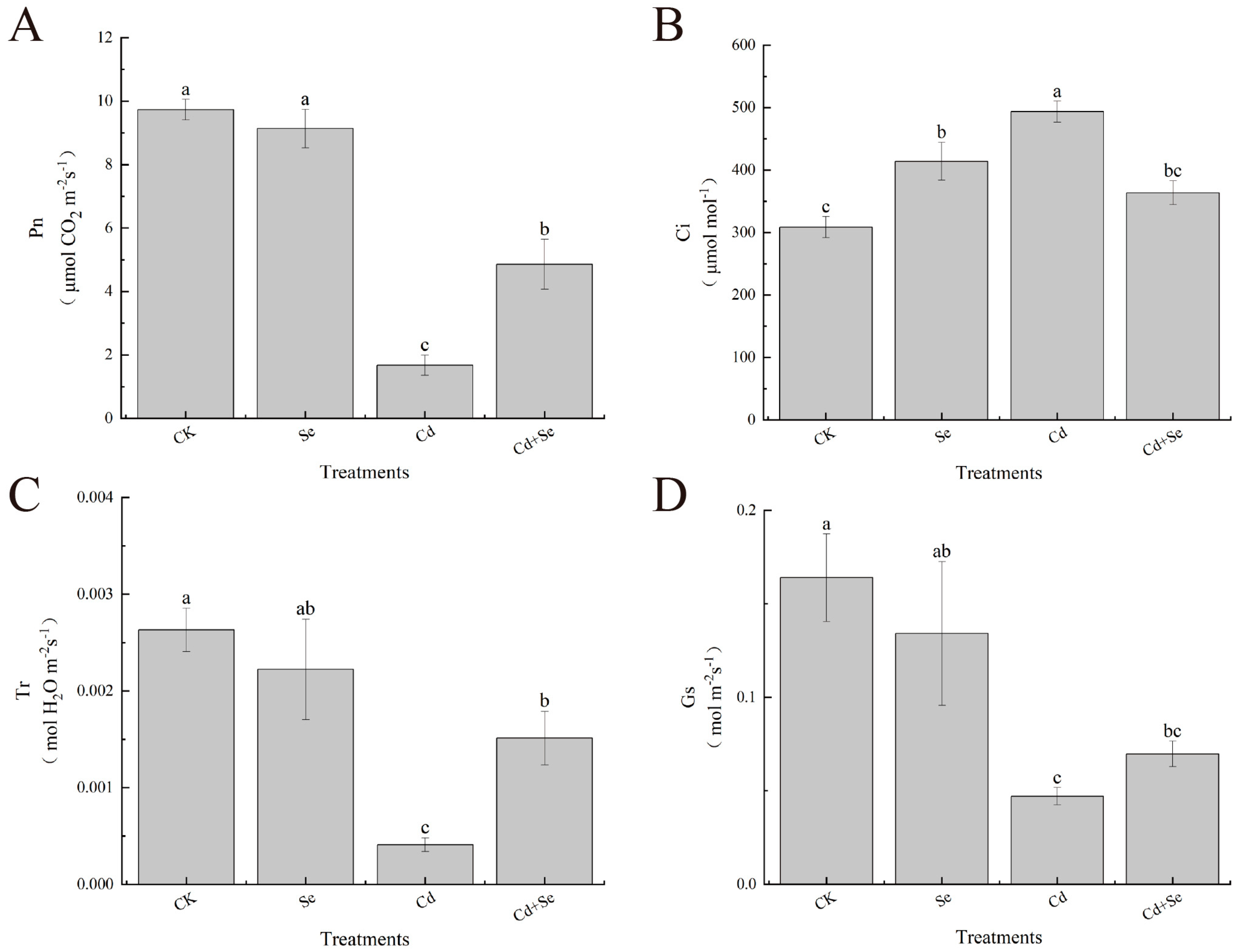
3.2. Effects of Exogenous Se on Stomatal Parameters of Cabbage Seedlings under Cd Stress
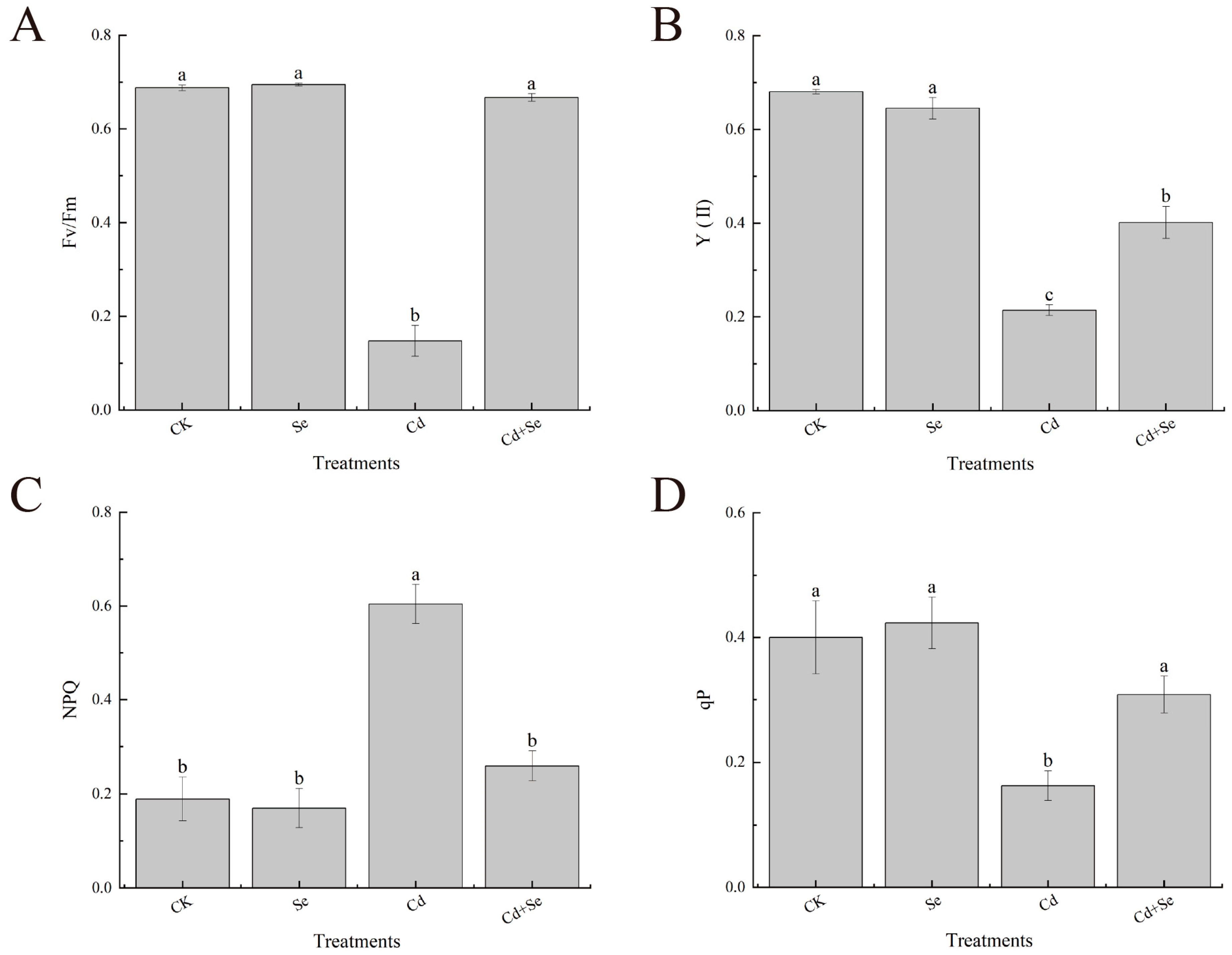
3.3. Effects of Exogenous Se on Chlorophyll Fluorescence Parameters of Cabbage Seedlings under Cd Stress
3.4. Exogenous Se Can Enhance the Tolerance of Cabbage Seedlings under Cd Stress
3.5. Observation of Chloroplast Ultrastructure in Leaves of Cabbage Seedlings
3.6. Effects of Exogenous Se on Reactive Oxygen Species Accumulation and Leaf Membrane Esterification of Cabbage Seedlings under Cd Stress
3.7. Effects of Exogenous Se on Reactive Oxygen Species Accumulation and Leaf Membrane Esterification of Cabbage Seedlings under Cd Stress
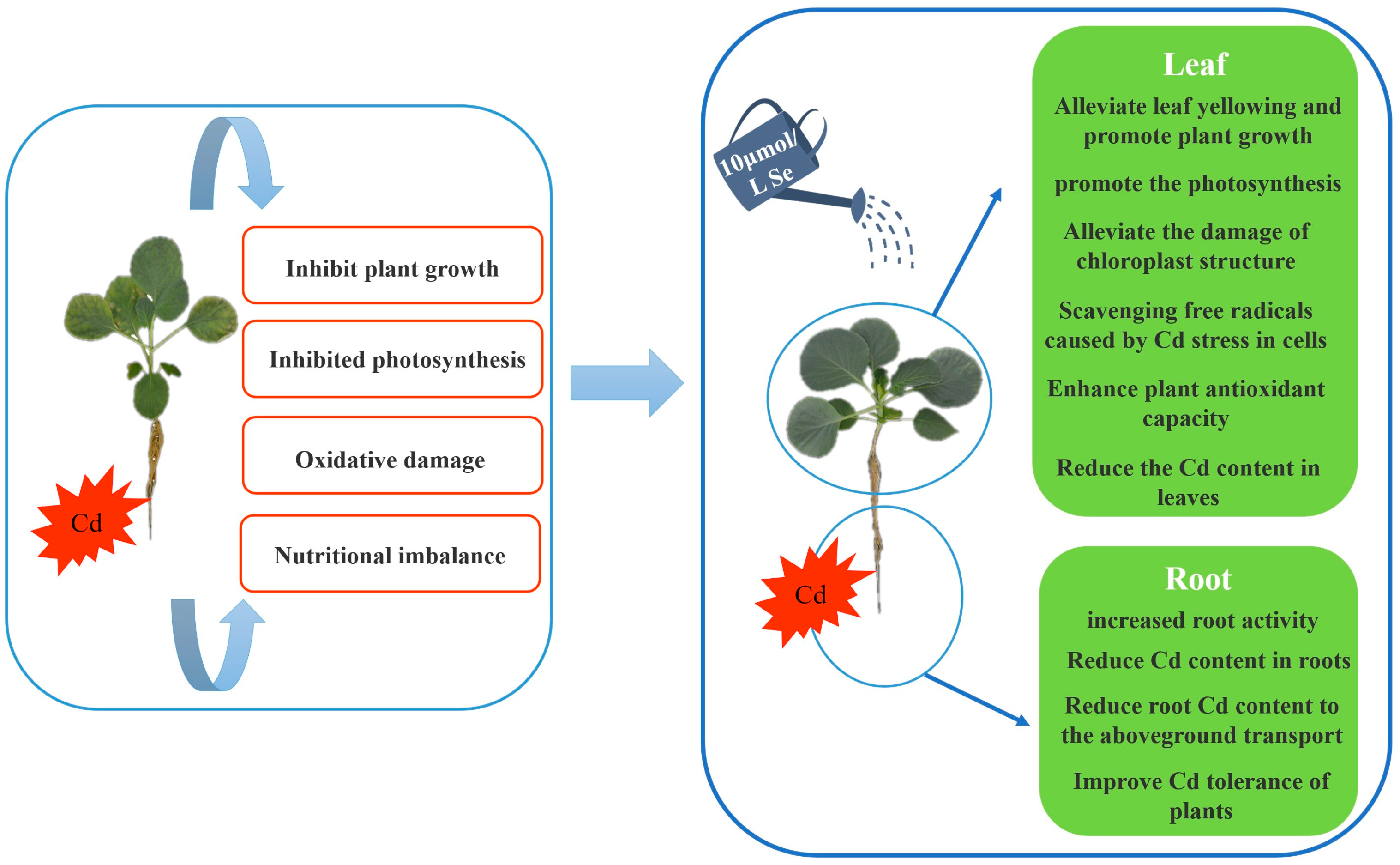
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalid, U.; Sher, F.; Noreen, S.; Lima, E.C.; Rasheed, T.; Sehar, S.; Amami, R. Comparative effects of conventional and nano-enabled fertilizers on morphological and physiological attributes of Caesalpinia bonducella plants. J. Saudi Soc. Agric. Sci. 2022, 21, 61–72. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Rehman, M.Z.U.; Maqbool, A. A critical review on the effects of zinc at toxic levels of Cd in plants. Environ. Sci. Pollut. Res. 2019, 26, 6279–6289. [Google Scholar] [CrossRef] [PubMed]
- Baruah, N.; Mondal, S.C.; Farooq, M.; Gogoi, N. Influence of Heavy Metals on Seed Germination and Seedling Growth of Wheat, Pea, and Tomato. Water Air Soil Pollut. 2019, 230, 273. [Google Scholar] [CrossRef]
- Alves, L.R.; Rossatto, D.R.; Rossi, M.L.; Martinelli, A.P.; Gratão, P.L. Se improves photosynthesis and induces ultrastructural changes but does not alleviate Cd-stress damages in tomato plants. Protoplasma 2020, 257, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.Y.; Wei, X.J.; Wang, H.Z. Cd tolerant and sensitive wheat lines: Their differences in pollutant accumulation, cell damage, and autophagy. Biol. Plant 2018, 62, 379–387. [Google Scholar] [CrossRef]
- Chmielowska-Bąk, J.; Izbiańska, K.; Ekner-Grzyb, A.; Bayar, M.; Deckert, J. Cd Stress Leads to Rapid Increase in RNA Oxidative Modifications in Soybean Seedlings. Front. Plant Sci. 2017, 8, 2219. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Deng, X.; Quan, L.; Xia, Y.; Shen, Z. Metallothioneins BcMT1 and BcMT2 from Brassica campestris enhance tolerance to Cd and copper and decrease production of reactive oxygen species in Arabidopsis thaliana. Plant Soil 2013, 367, 507–519. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Zheng, J.; Shen, Z.; Xu, X. Exogenous foliar application of fulvic acid alleviate Cd toxicity in lettuce (Lactuca sativa L.). Ecotoxicol. Environ. Saf. 2019, 167, 10–19. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, G.; Bao, M.; Wang, L.; Xie, X. Exogenous application of ascorbic acid mitigates Cd toxicity and uptake in Maize (Zea mays L.). Environ. Sci. Pollut. Res. Int. 2019, 26, 19261–19271. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Se Pretreatment Upregulates the Antioxidant Defense and Methylglyoxal Detoxification System and Confers Enhanced Tolerance to Drought Stress in Rapeseed Seedlings. Biol. Trace Elem. Res. 2011, 143, 1758–1776. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Chapter 16—Silicon and Se: Two Vital Trace Elements that Confer Abiotic Stress Tolerance to Plants. In Emerging Technologies and Management of Crop Stress Tolerance; Ahmad, P., Rasool, S., Ahmad, P., Rasool, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 377–422. [Google Scholar]
- Battin, E.E.; Brumaghim, J.L. Antioxidant activity of sulfur and selenium: A review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem. Biophys. 2009, 55, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Dong, Y.Y.; Feng, L.Y.; Deng, Z.L.; Xu, Q.; Tao, Q.; Wang, C.Q.; Chen, Y.E.; Yuan, M.; Yuan, S. Se Enhances Cd Accumulation Capability in Two Mustard Family Species-Brassica napus and B. juncea. Plants 2020, 9, 7. [Google Scholar]
- Silva, V.M.; Boleta, E.H.M.; Lanza, M.G.D.B.; Lavres, J.; Martins, J.T.; Santos, E.F.; Dos Santos, F.L.M.; Putti, F.F.; Junior, E.F.; White, P.J.; et al. Physiological, biochemical, and ultrastructural characterization of Se toxicity in cowpea plants. Environ. Exp. Bot. 2018, 150, 172–182. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Zia Ur Rehman, M.; Rinklebe, J.; Tsang, D.C.W.; Bashir, A.; Maqbool, A.; Tack, F.M.G.; Ok, Y.S. Cd phytoremediation potential of Brassica crop species: A review. Sci. Total Environ. 2018, 631–632, 1175–1191. [Google Scholar] [CrossRef]
- Wang, T.; Song, J.; Liu, Z.; Liu, Z.; Cui, J. Melatonin alleviates cadmium toxicity by reducing nitric oxide accumulation and IRT1 expression in Chinese cabbage seedlings. Environ. Sci. Pollut. Res. 2021, 28, 15394–15405. [Google Scholar] [CrossRef]
- Song, A.; Li, Z.; Zhang, J.; Xue, G.; Fan, F.; Liang, Y. Silicon-enhanced resistance to cadmium toxicity in Brassica chinensis L. is attributed to Si-suppressed cadmium uptake and transport and Si-enhanced antioxidant defense capacity. J. Hazard. Mater. 2009, 172, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Abd Allah, E.F.; Hashem, A.; Sarwat, M.; Gucel, S. Exogenous Application of Se Mitigates Cd Toxicity in Brassica juncea L. (Czern & Cross) by Up-Regulating Antioxidative System and Secondary Metabolites. J. Plant Growth Regul. 2016, 35, 936–950. [Google Scholar]
- Luo, H.H.; Zhang, Y.L.; Zhang, W.F. Effects of water stress and rewatering on photosynthesis, root activity, and yield of cotton with drip irrigation under mulch. Photosynthetica 2016, 5, 65–73. [Google Scholar] [CrossRef]
- Luo, Z.; He, J.; Polle, A.; Rennenberg, H. Heavy metal accumulation and signal transduction in herbaceous and woody plants: Paving the way for enhancing phytoremediation efficiency. Biotechnol. Adv. 2016, 34, 1131–1148. [Google Scholar] [CrossRef]
- Wintermans, J.F.; de Mots, A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim. Biophys. Acta 1965, 109, 448–453. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. BBA Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, X.; Sun, X.; Tan, Q.; Tang, Y.; Nie, Z.; Hu, C. Xylem transport and gene expression play decisive roles in cadmium accumulation in shoots of two oilseed rape cultivars (Brassica napus). Chemosphere 2015, 119, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Nie, Z.; Liu, H.; Zhao, P.; Qin, S.; Shi, Z. Influence of selenium on root morphology and photosynthetic characteristics of winter wheat under cadmium stress. Environ. Exp. Bot. 2018, 150, 232–239. [Google Scholar] [CrossRef]
- Ma, X.; Song, L.; Yu, W.; Hu, Y.; Liu, Y.; Wu, J.; Ying, Y. Growth, physiological, and biochemical responses of Camptotheca acuminata seedlings to different light environments. Front. Plant Sci. 2015, 6, 321. [Google Scholar] [CrossRef] [PubMed]
- Bajji, M.; Kinet, J.M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2004, 36, 61–70. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Mao, R.; Li, W.; He, Z.; Bai, Z.; Xia, P.; Liang, Z.; Liu, Y. Physiological, transcriptional, and metabolic alterations in spaceflight-subjected Senna obtusifolia. Plant Physiol. Biochem. 2019, 139, 33–43. [Google Scholar] [CrossRef]
- Järup, L.; Akesson, A. Current status of Cd as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Current health risk assessment practice for dietary Cd: Data from different countries. Food Chem. Toxicol. 2017, 106, 430–445. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, Y.; Zhu, Y.G.; Tang, Z.; Mcgrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
- Satarug, S.; Baker, J.R.; Urbenjapol, S.; Haswell-Elkins, M.; Reilly, P.E.B.; Williams, D.J.; Moore, M.R. A global perspective on Cd pollution and toxicity in non-occupationally exposed population. Toxicol. Lett. 2003, 137, 65–83. [Google Scholar] [CrossRef]
- Zaki, H.E.M.; Radwan, K.S.A. The use of osmoregulators and antioxidants to mitigate the adverse impacts of salinity stress in diploid and tetraploid potato genotypes (Solanum spp.). Chem. Biol. Technol. Agric. 2022, 9, 19. [Google Scholar] [CrossRef]
- Camara, A.Y.; Wan, Y.; Yu, Y.; Wang, Q.; Li, H. Effect of Se on uptake and translocation of arsenic in rice seedlings (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2018, 148, 869–875. [Google Scholar] [CrossRef]
- Huang, Q.; Xu, Y.; Liu, Y.; Qin, X.; Huang, R.; Liang, X. Se application alters soil Cd bioavailability and reduces its accumulation in rice grown in Cd-contaminated soil. Environ. Sci. Pollut. Res. Int. 2018, 25, 31175–31182. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yin, X.; Bañuelos, G.; Lin, Z.; Liu, Y.; Li, M.; Yuan, L. Indications of Se Protection against Cd and Lead Toxicity in Oilseed Rape (Brassica napus L.). Front. Plant Sci. 2016, 7, 1875. [Google Scholar] [CrossRef]
- Baszyński, T. Interference of Cd2+ in functioning of the photosynthetic apparatus of higher plants. Acta Soc. Bot. Pol. 1986, 55, 291–304. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Wang, G.; Xu, L.; Shen, Z. Cd-induced accumulation of hydrogen peroxide in the leaf apoplast of Phaseolus aureus and Vicia sativa and the roles of different antioxidant enzymes. J Hazard. Mater. 2009, 168, 76–84. [Google Scholar] [CrossRef]
- Komarkova, M.; Kovalikova, Z.; Simek, J.; Skarka, A.; Tuma, J. Physiological and biochemical responses of Brassica napus L. cultivars exposed to Cd stress. Plant Soil Environ. 2022, 68, 431–440. [Google Scholar] [CrossRef]
- Filek, M.; Keskinen, R.; Hartikainen, H.; Szarejko, I.; Janiak, A.; Miszalski, Z.; Golda, A. The protective role of Se in rape seedlings subjected to Cd stress. J. Plant Physiol. 2008, 165, 833–844. [Google Scholar] [CrossRef]
- Qi, W.; Li, Q.; Chen, H.; Liu, J.; Xing, S.; Xu, M.; Yan, Z.; Song, C.; Wang, S. Se nanoparticles ameliorate Brassica napus L. Cd toxicity by inhibiting the respiratory burst and scavenging reactive oxygen species. J. Hazard. Mater. 2021, 417, 125900. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, Z.; Kong, X.; Chen, Y.; Li, J. Exogenous tryptophan application improves Cd tolerance and inhibits Cd upward transport in broccoli (Brassica oleracea var. italica). Front. Plant Sci. 2022, 13, 969675. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Y.; Wang, X.; Lv, J.; Tang, Z.; Hu, L.; Luo, S.; Wang, R.; Ali, B.; Yu, J. Physiological Mechanism of Exogenous 5-Aminolevulinic Acid Improved the Tolerance of Chinese Cabbage (Brassica pekinensis L.) to Cd Stress. Front. Plant Sci. 2022, 13, 845396. [Google Scholar] [CrossRef] [PubMed]
- Alshegaihi, R.M.; Mfarrej, M.F.B.; Saleem, M.H.; Parveen, A.; Ahmad, K.S.; Ali, B.; Abeed, A.H.A.; Alshehri, D.; Alghamdi, S.A.; Alghanem, S.M.S.; et al. Effective citric acid and EDTA treatments in Cd stress tolerance in pepper (Capsicum annuum L.) seedlings by regulating specific gene expression. S. Afr. J. Bot. 2023, 159, 367–380. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.; Wu, Z.; Liu, X.; Cai, M.; Jia, W.; Zhao, X. Se reduces Cd accumulation in seed by increasing Cd retention in root of oilseed rape (Brassica napus L.). Environ. Exp. Bot. 2019, 158, 161–170. [Google Scholar] [CrossRef]
- Hussain, M.I.; Reigosa, M.J. A chlorophyll fluorescence analysis of photosynthetic efficiency, quantum yield and photon energy dissipation in PSII antennae of Lactuca sativa L. leaves exposed to cinnamic acid. Plant Physiol. Biochem. 2011, 49, 1290–1298. [Google Scholar] [CrossRef]
- Su, Y.; Qin, C.; Begum, N.; Ashraf, M.; Zhang, L. Acetylcholine ameliorates the adverse effects of Cd stress through mediating growth, photosynthetic activity and subcellular distribution of Cd in tobacco (Nicotiana benthamiana). Ecotoxicol. Environ. Saf. 2020, 198, 110671. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V.; Johnson, M.P.; Duffy, C.D.P. The photoprotective molecular switch in the photosystem II antenna. Biochim. Biophys. Acta (BBA)—Bioenerg. 2012, 1817, 167–181. [Google Scholar] [CrossRef]
- Wu, H.; Fan, S.; Gong, H.; Guo, J. Roles of salicylic acid in Se-enhanced salt tolerance in tomato plants. Plant Soil 2023, 484, 569–588. [Google Scholar] [CrossRef]
- Hirotsu, N.; Makino, A.; Yokota, S.; Mae, T. The photosynthetic properties of rice leaves treated with low temperature and high irradiance. Plant Cell Physiol. 2005, 46, 1377–1383. [Google Scholar] [CrossRef]
- Vaculík, M.; Pavlovič, A.; Lux, A. Silicon alleviates Cd toxicity by enhanced photosynthetic rate and modified bundle sheath’s cell chloroplasts ultrastructure in maize. Ecotoxicol. Environ. Saf. 2015, 120, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, X.; Li, H.; Bi, J.; Yu, J.; Liu, X.; Zhou, H.; Rong, Z. Se modulates Cd-induced ultrastructural and metabolic changes in cucumber seedlings. RSC Adv. 2020, 10, 17892–17905. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Lian, X.; Zhang, B.; Liu, X.; Yu, J.; Gao, Y.; Zhang, Q.; Sun, H. Nano silicon dioxide reduces Cd uptake, regulates nutritional homeostasis and antioxidative enzyme system in barley seedlings (Hordeum vulgare L.) under Cd stress. Environ. Sci. Pollut. Res. Int. 2023, 30, 67552–67564. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Ma, Z.; Zhu, L.; Xiao, X.; Xie, Y.; Zhu, J.; Wang, J. Rhizobacterial Strain Bacillus megaterium BOFC15 Induces Cellular Polyamine Changes that Improve Plant Growth and Drought Resistance. Int. J. Mol. Sci. 2016, 17, 976. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Malik, Z.; Parveen, A.; Zong, Y.; Abbasi, G.H.; Rafiq, M.T.; Shaaban, M.; Mustafa, A.; Bashir, S.; Rafay, M.; et al. Biochar alleviates Cd phytotoxicity by minimizing bioavailability and oxidative stress in pak choi (Brassica chinensis L.) cultivated in Cd-polluted soil. J. Environ. Manag. 2019, 250, 109500. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, K.; Zhan, Z.; Wang, B.; Wang, W.; Wei, W.; Li, D.; Huang, W.; Xu, Z. Exogenous Selenium Enhances Cadmium Stress Tolerance by Improving Physiological Characteristics of Cabbage (Brassica oleracea L. var. capitata) Seedlings. Horticulturae 2023, 9, 1016. https://doi.org/10.3390/horticulturae9091016
Jia K, Zhan Z, Wang B, Wang W, Wei W, Li D, Huang W, Xu Z. Exogenous Selenium Enhances Cadmium Stress Tolerance by Improving Physiological Characteristics of Cabbage (Brassica oleracea L. var. capitata) Seedlings. Horticulturae. 2023; 9(9):1016. https://doi.org/10.3390/horticulturae9091016
Chicago/Turabian StyleJia, Kaiyue, Zhipeng Zhan, Bingqian Wang, Wuhong Wang, Wenjing Wei, Dawei Li, Wei Huang, and Zhongmin Xu. 2023. "Exogenous Selenium Enhances Cadmium Stress Tolerance by Improving Physiological Characteristics of Cabbage (Brassica oleracea L. var. capitata) Seedlings" Horticulturae 9, no. 9: 1016. https://doi.org/10.3390/horticulturae9091016
APA StyleJia, K., Zhan, Z., Wang, B., Wang, W., Wei, W., Li, D., Huang, W., & Xu, Z. (2023). Exogenous Selenium Enhances Cadmium Stress Tolerance by Improving Physiological Characteristics of Cabbage (Brassica oleracea L. var. capitata) Seedlings. Horticulturae, 9(9), 1016. https://doi.org/10.3390/horticulturae9091016




