Abstract
Magnesium (Mg) is the most commonly deficient nutrient in tropical banana-growing areas. The effects of different concentrations of Mg on morpho-physiological and biochemical responses on three commercial cultivars (Baxi, Haigong, and Guangfen No.1) of bananas were accessed for 12 weeks. Results showed genotypic variation in the utilization and tolerance to different Mg levels. The optimum Mg level was 1 mM for Baxi and Guangfen No.1 and 3 mM for Haigong. Both the deficiency and excess of Mg caused a severe reduction in plant height, dry weight, total root length, and root surface area of Haigong and Baxi. Mg stress reduced root growth by impairing photosynthate translocation and increased carbohydrate contents in the source leaf. Under Mg stress, more reduction in photosynthetic pigment and photosynthesis rate, higher accumulation of carbohydrates and malondialdehyde contents, and higher activities of antioxidative enzymes were observed in Haigong as compared to other genotypes. Based on the above results, it is concluded that Haigong is most sensitive to Mg stress while Guangfen No. 1 was least affected and tolerant to changes in the Mg levels.
1. Introduction
Magnesium (Mg) is the most prevalent divalent cation in the cytoplasm of plant cells and a crucial macronutrient for the growth and development of higher plants [1]. Mg plays a key role in the photosynthetic fixation of carbon dioxide in addition to serving as the core component of the chlorophyll [2,3]. In addition, Mg control various metabolic processes in plant cells because of being a cofactor of more than 300 classes of carboxylases, kinases, ATPases, phosphatases, topoisomerases, and polymerases enzymes [4,5]. The Mg ion, due to its large hydrated radius, weakly adsorbs to soil colloids and is leached easily in areas with heavy rainfall under low pH and low cation exchange capacity [6]. Thus, in tropical and subtropical areas, plants are more likely to experience Mg shortage [7].
Magnesium deficiency and its responses have been reported in several plants species in forestry [8], horticulture [9,10,11,12], and agriculture [13,14]. At the same time, higher application of Mg could easily cause Mg toxicity as plants could store large Mg concentrations in the vacuole [5] without showing visible toxicity symptoms [15]. £Furthermore, some soil such as serpentine soils that could contain more than 70% Mg and Fe minerals [16] and water stress conditions could cause Mg toxicity by increasing the metabolic pool of Mg in cells [5].
Numerous studies have examined the morphological and biochemical changes that occur in plants when they lack Mg. Results indicate that deficiency causes leaf chlorosis and reduces photosynthetic efficiency. Since it is a mobile element, the typical chlorosis usually shows up first on the lower and older leaves [13,17].The balance of carbohydrates between source and sink organs is also disturbed by Mg shortage [17,18].
Plasma membrane-bound ATPases necessitate Mg–ATP complexes but the binding of Mg by ATP is disturbed. As a result, the photosynthate export through the phloem is inhibited, and the source leaf accumulates carbohydrates [19]. According to reports, some plants, including spinach [20], Arabidopsis [21], barley [22], and Citrus sinensis [10], were affected by Mg deficiency in terms of dry matter production and carbohydrate partitioning.
The reduction in Mg-chelatase activity reduces the insertion of Mg to protoporphyrin, will alternatively reduce the chlorophyll synthesis [23], accumulate protoporphyrin IX [24], and then accelerate the development of chlorosis, leading to an excessive reduction in photosynthetic electron transport and an excessive production of reactiveoxygen species (ROS). ROS, in turn, activates antioxidative activities to avoid oxidative damage [12,25,26].
Banana (Musa spp.) is an important cash and trading food crop [27,28,29]. Most soils from banana-growing areas in tropical and subtropical areas are acidic, have low pH and low cation exchange capacity, and are highly prone to the nutrient leaching risk of high rainfall. Mg is the most commonly deficient nutrient after N and K, especially in areas where banana plantations for a long time without Mg fertilization and/or under high input of K or Ca [30].
The Mg requirement of banana is quite high as banana plants produce high biomass [31]; but in contrast to other crops [32] the effects of Mg stress on the banana crop have not been well understood. Therefore, there is a critical need to comprehend how the physiological and metabolic processes of the banana react to Mg stress. To understand banana responses, we selected three commercial cultivars with different genome composition to analyze how Mg stress affect metal composition, growth, and physiological response of banana, and to evaluate cultivar differences when subjected to Mg stress.
2. Materials and Methods
2.1. Banana Genotypes and Magnesium Treatment
Three cultivars of banana including Baxi (Musa Cavendish AAA), Haigong (Musa paradisiaca AA),and Guangfen No.1 (MusaPisang Awak ABB) were grown in a greenhouse from 2 July to 25 September 2018 at Hainan University, Haikou, China(20°3′33″ N, 110°19′6″ E). Seedlings of the banana (Baxi, Haigong and Guangfen No.1) were derived from tissue-cultured plantlets. Evenly grown seedlings with five expanded leaves were taken, and the roots were carefully washed to eliminate nutrient residue and transferred to pots filled with sand (one seedling per pot). Seedlings were irrigated with nutrient solution (NS) containing the following composition: 4 mM KNO3, 3 mM CaCl2, 1 mM KH2PO4, 1 mM NH4NO3, 0.06 mM Fe-EDTA, 20 μM H3BO3, 10 μM MnSO4·H2O, 3 μM ZnSO4·7H2O, 0.5 μM CuSO4·5H2O, and 0.09 μM H3MO7N6O24·4H2O. Half-strength NS was used for the first seven days to avoid osmotic stress and then full-strength NS was applied during the rest of the experiment.
For Mg treatment, different concentrations of Mg solution (0, 1, 3, 5 mM MgSO4·7H2O) were applied in NS. For each treatment, MgSO4·7H2O in NS was replaced with equivalent Na2SO4 to maintain osmoticum and sulfur content. The temperature of the greenhouse during the experiment ranged from 26 to 38 °C. Each genotype was tested in 5 random blocks each having 2 plants, which totalized 30 pots (3 genotypes × 5 blocks × 2 plants).
2.2. Collection of Samples for Measurements
After 12 weeks of Mg treatments, the four to sixth leaves (from the bottom) were used for pigment analysis and antioxidant enzyme activity measurement. The remaining plantlets were removed from the sand, and washed, and plant height, shoot biomass, leaf nutrients, and root morphological traits were measured as indicated below.
2.3. Photosynthesis-Related Parameters
A portable gas exchange system (Li-6400, LI-COR Biosciences, Lincoln, NE, USA) was used to check the net CO2 assimilation rate (Pn) of the sixth leaf from the bottom of the plantlets from 9:00 to 11:00 a.m. The ambient CO2 concentration was adjusted to 400 µmol mol−1 by CO2 injection. Photosynthetic photon flux density was set to 1200 µmol m−2s−1 by a red/blue LED light source. One leaf per plant was used and two plants per replicate to take measurements.
A monitoring -PAM chlorophyll fluorometer (Walz. Effeltrich, Effeltrich, Germany) was used to take values of the maximum quantum yield of photosystem II (PSII). Four plants were placed in the dark for 15 min and the minimum fluorescence yield of dark-adapted leaves (F0) was measured under measuring light (photosynthetic active radiation (PAR) less than 2 µmol photons m−2 s−1). Maximum fluorescence yield (Fm) of dark-adapted leaves was measured after a 0.6 s saturating pulse light and Fv/Fm ratio was calculated as previously described [33].
PSII = Fv/Fm = (Fm − F0)/Fm
2.4. Photosynthetic Pigments, Membrane Lipid Peroxidation, and Antioxidant Enzymes Activities
The leaves were ground in liquid nitrogen and frozen for the measurement of chlorophyll, lipid peroxidation (malondialdehyde content), and antioxidant enzyme activities. Chlorophyll contents (totala and b chlorophyll) were assayed via spectrophotometric absorbance at 450 nm, 532 nm, and 600 nm (Brain and Solomon, 2007). For quantifying membrane lipid peroxidation, this assay measures malondialdehyde (MDA), and the level was determined from thatof2-thiobarbituric acid(TBA) reactive metabolites.The leaf (0.5 g fresh weight) was homogenized in trichloroacetic acid (5% m/v), and centrifuged at 3000× g for 10 min at 4 °C. The supernatant (2 mL) was heated with thiobarbituric acid (2 mL, 0.67% m/v) at 100 °C for 30 min, and supernatant absorbance was measured after cooling to quantify thiobarbituric acid reactive species at 450 nm, 532 nm, and 600 nm [34].
To determine superoxidediamutase (SOD), ascorbateperoxidase(APX), and glutathione reductase (GR) activities, the leaf (0.5 g fresh weight) was homogenized in 2 mL of 0.1 M potassium phosphate buffer (pH 7.8), centrifuged at 10,000× g for 5 min at 4 °C, and the supernatant was stored at −20 °C. The modified approach of Giannopotitis and Ries [35] was used to measure SOD activity. The photochemical reduction of nitrobluetetrazolium (NBT) at 560 nm was measured and one unit of SOD activity is defined as the amount of enzyme required inhibiting the photoreduction of NBT by 50%. APX was assayed by monitoring the oxidation of As A at 290 nm according to the method of Nakano and Asada [36], used for measuring APX activity, while the Grace and Logan [37] method was used for measuring GR activity. The decrease in absorbance at 340 nm, as a result of oxidation of NADPH, was determined.
2.5. Root and Shoot Dry Weight Morphological Traits
The plant material was divided into roots and shoots. The roots were suspended in water in a transparent glass box (30 × 20 × 2 cm) and scanned at 800 dpi using a scanner (Seiko Epson Expression 1680, Nagano, Japan). The scanned images were processed with WinRhizo v.2009c software (Regent Instrument Inc., Quebec, QC, Canada) to measure total root length and root surface area. After this, roots were oven-dried to measure the total root dry weight (DW).The plant material was dried at 80 °C for 48 h to a content weight to determine the DW.
2.6. Starch and Sucrose Content of Leaves
The dried leaf samples were homogenized with distilled water, extracted for 10 min with ultrasonic extraction, and kept at 80 °C for 20 min. The supernatant was collected after centrifugation at 14,000× g for 10 min. The process was repeated, and the pellet was re-extracted by vacuum drying at 60 °C. The dry solid fraction was suspended in 1.8 mL 80% alcohol and kept at 4 °C for 10 h. Then sediments were centrifuged at 14,000× g for 10 min, evaporated at 60 °C, and dissolved in 0.5 mL methanol for starch and sucrose analyses. Starch was measured in accordance with Li et al. [38], and the sucrose content was assessed as described by Farhat et al. [18].
2.7. Mineral Nutrients of Leaves
The mineral contents N, P, K of the dried and ground leaves were measured according to Bao [39], about 0.05 g powdered sub-samples were digested using the H2SO4-H2O2 method, and then, their content was measured using the standard Kjeldahl method for N, the molybdate-blue colorimetric method for P, and the flame photometer method for K, respectively. For Ca, Mg analysis, about 0.5 g leaf samples were digested using theHNO3-HClO4-HCl method, and their concentration was determined with an atomic absorption spectrometer.
2.8. Statistical Analysis
Statistical analyses were conducted by ANOVA tests using the SPSS version 17.0 software (USA). Mean values were analyzed by Duncan’s test at p ≤ 0.05 level. Graphs were made using Graphpad (California) version 5.0.
3. Results
3.1. Photosynthetic Pigments and Photosynthesis of Leaves of Banana Genotypes
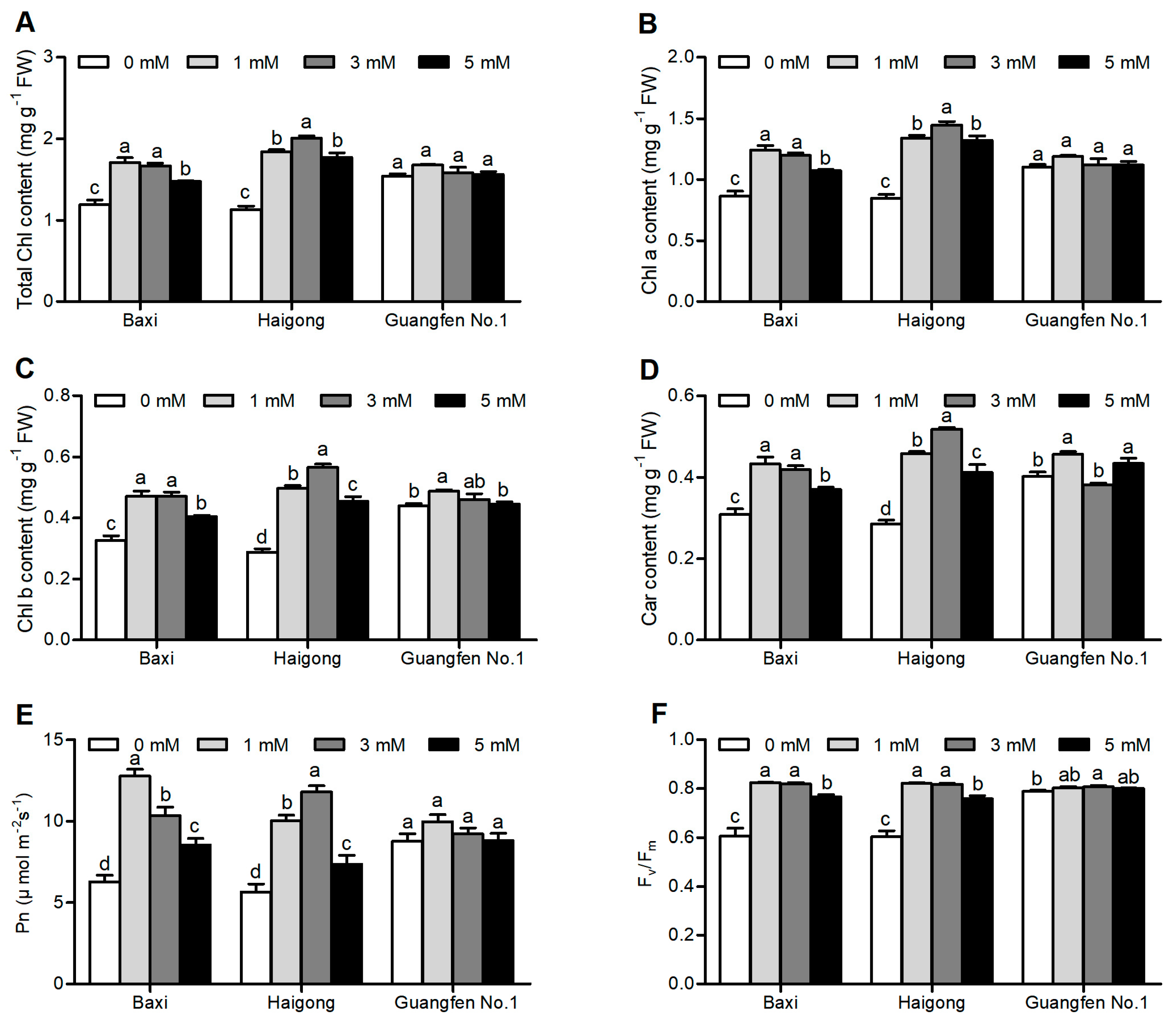
Leaf photosynthetic pigment (chlorophyll and carotenoid) content, photosynthesis (Pn), and maximum quantum yield of PSII (Fv/Fm) of different banana cultivars were significantly affected under different Mg conditions (Table S1). Total chlorophyll content (Figure 1A), chlorophyll a (Figure 1B), chlorophyll b (Figure 1C), and carotenoid content (Figure 1D) were increased to a certain level of Mg (1 or 3 mM) and then decreased at 5 mM of Mgin Baxi and Haigong, but no apparent change was observed in Guangfen No.1 All pigments in Baxi were maximum at 1 mM and were significantly similar to 3 mM. Pigments of Haigong were maximum at 3 mM and were significantly higher than other treatments. Pigments of Guangfen No. 1 were maximum at 1 mM but only chlorophyll band carotenoids were significantly higher than other treatments. Total chlorophyll, chlorophyll a, chlorophyll b, and carotenoid content were maximally increased by 43.5, 43.6, 40.1, 43.5% in Baxi, 71, 96.3, 81.8, 77.4% in Haigong and 7.7, 11.1, 13.4, 8.6% in Guangfen No.1 compared to 0 mM Mg, respectively.
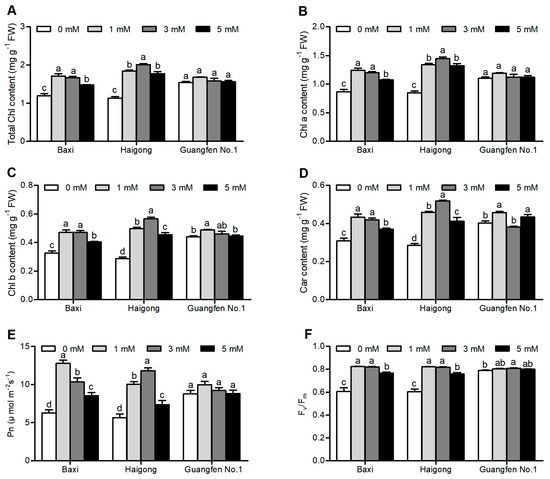
Figure 1.
Effect of different Mg concentrations on (A) Total Chl content, (B) Chl a, (C) Chl b, (D) Car content, (E) Pn, and (F) Fv/Fm of three banana cultivars (Baxi, Haigong, and Guangfen No.1) in the treatments: 0, 1, 3, and 5 (mmol/L). Each bar is mean ± SE (n = 5). Lowercase letters compare cultivars at harvest time within each treatment. Different letters indicate significant differences at p < 0.05.
Pn and Fv/Fm also have the same tendency like pigment content (Figure 1E,F). Pn of Baxi was highest at 1 mM and that of Haigong was maximum at 3 mM. Pn of Guangfen No. 1 was not significantly affected by Mg treatment. Fv/Fm of all three genotypes was statistically similar at 1 and 3 mM and higher than at 0 mM. Pn and Fv/Fm increased by Mg by 103 and 35.8% in Baxi, 108 and 35.5% in Haigong, and 13.8 and 1.7% in Guangfen No.1, respectively, as compared to 0 mM.
3.2. MDA Content and Antioxidant Enzyme Activity
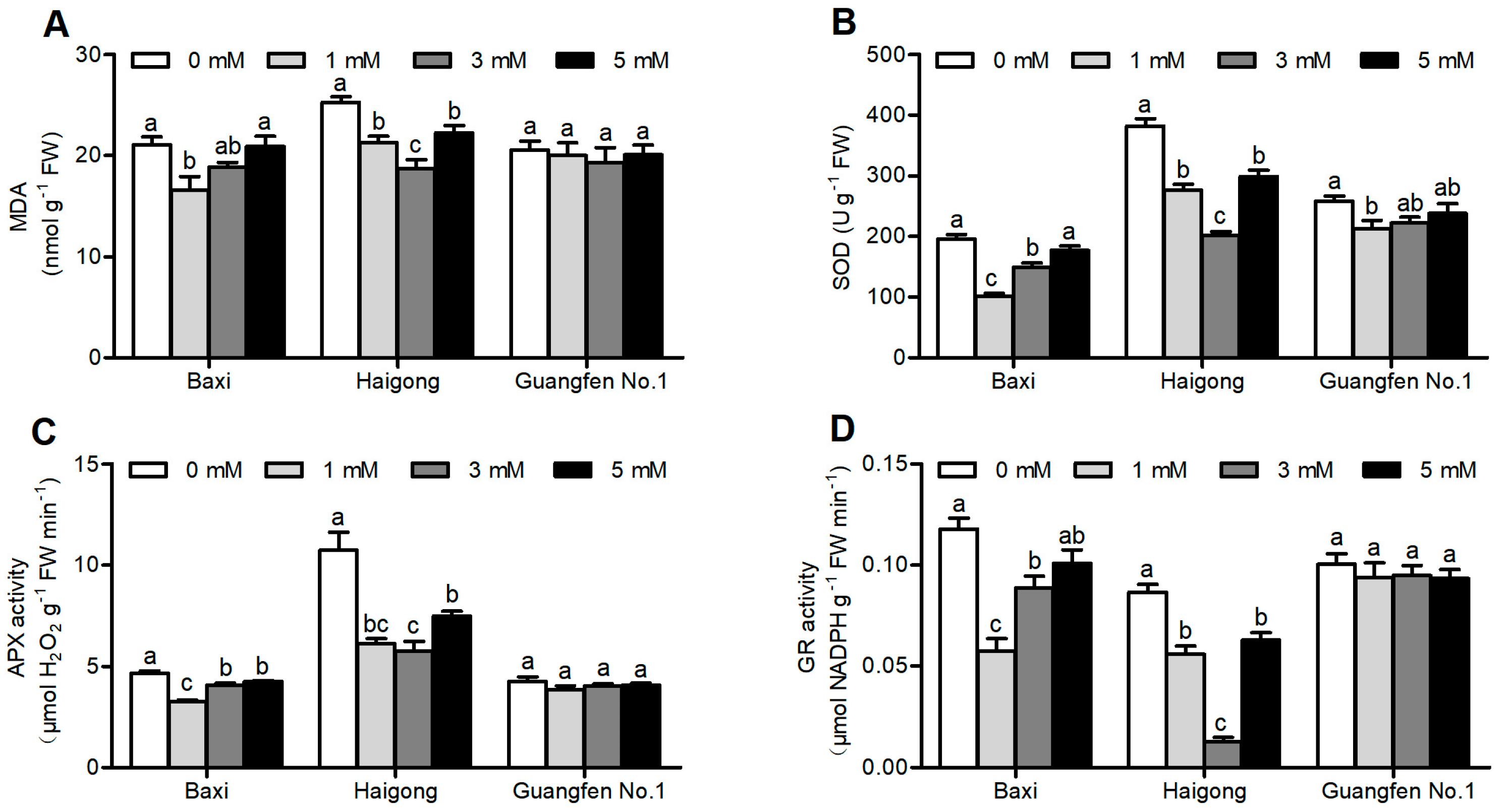
In Baxi and Haigong genotypes, the MDA production was decreased by Mg application as compared to no Mg and then increased again at higher Mg levels (Figure 2A and Table S2). MDA significantly decreased at 1 mM in Baxi and at 3 mM in Haigong in comparison to 0 and 5 mM Mg. Under 0 mM Mg, the level of MDA was increased by 27% for Baxi as compared to 1 mM and by 35% for Haigong as compared to 3 mM Mg. There were no obvious changes in the content of MDA in Guangfen No.1.
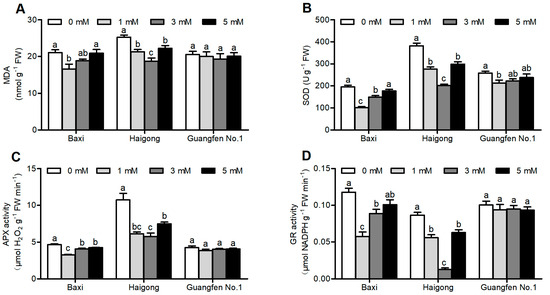
Figure 2.
Effect of different Mg concentrations on (A) MDA,(B) SOD, (C) APX activity and (D) GR activity of three banana cultivars (Baxi, Haigong and Guangfen No.1) in the treatments: 0, 1, 3 and 5 (mmol/L). Each bar is mean ± SE (n = 5). Lowercase letters compare cultivars at harvest time within each treatment. Different letters indicate significant differences at p < 0.05.
Different Mg concentrations significantly influenced the activity of SOD, APX, and GR (Table S2). Under suitable Mg concentration, antioxidant enzymes showed the lowest activities in three banana cultivars (Figure 2B–D). The activity of SOD was decreased by 94.7% for Baxi at 1 mM, by 92.1% for Haigong by 3 mM, and by 17% for Guangfen No.1 at 1 mM compared to 0 mM Mg (Figure 2B). APX and GR activity was observed to be significantly decreased by 43.8 and 86.8% in Baxi at 1 mM, and by 104.5 and 581% in Haigong at 3 mM than at 0 mM Mg, respectively. However, there were no obvious changes in the activity of APX and GR in Guangfen No.1.
3.3. Sucrose and Starch Content of Leaves
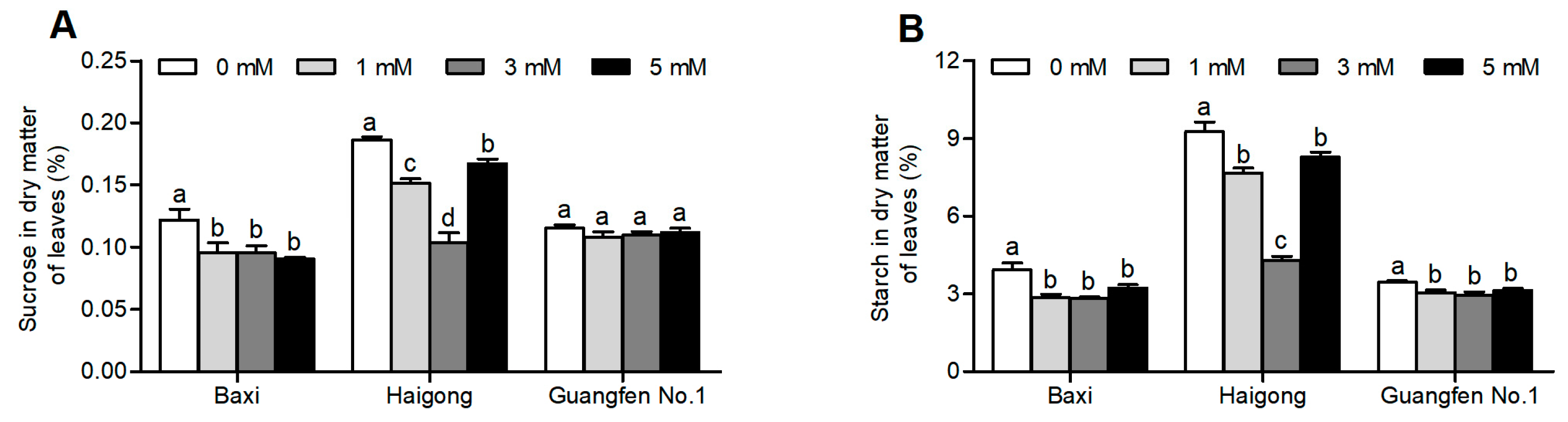
Different Mg concentrations significantly influenced the sucrose and starch content of banana genotypes (Table S2). The sucrose and starch content of Baxi and Haigong were decreased at 1 and 3 mM of Mg than at no Mg (Figure 3A,B). In the Baxi genotype, the sucrose and starch content were similar under 1, 3, and 5 mM Mg. In the Haigong genotype, the sucrose and starch content was decreased by increasing Mg up to 3 mM and then increased again at 5 mM (statistically equal to 1 mM). However, there were no obvious changes in sucrose content in Guangfen No.1.
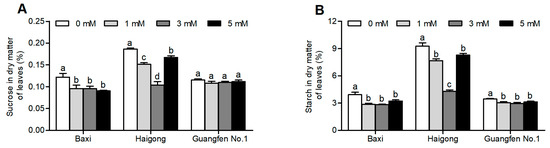
Figure 3.
Effect of different Mg concentrations on (A) sucrose in dry matter of leaf, (B) starch in dry matter of leaf of three banana cultivars (Baxi, Haigong, and Guangfen No.1) in the treatments: 0, 1, 3, and 5 (mmol/L). Each bar is mean ± SE (n = 4). Lowercase letters compare cultivars at harvest time within each treatment. Different letters indicate significant differences at p < 0.05.
3.4. Biomass Accumulation and Changes in Root Morphology
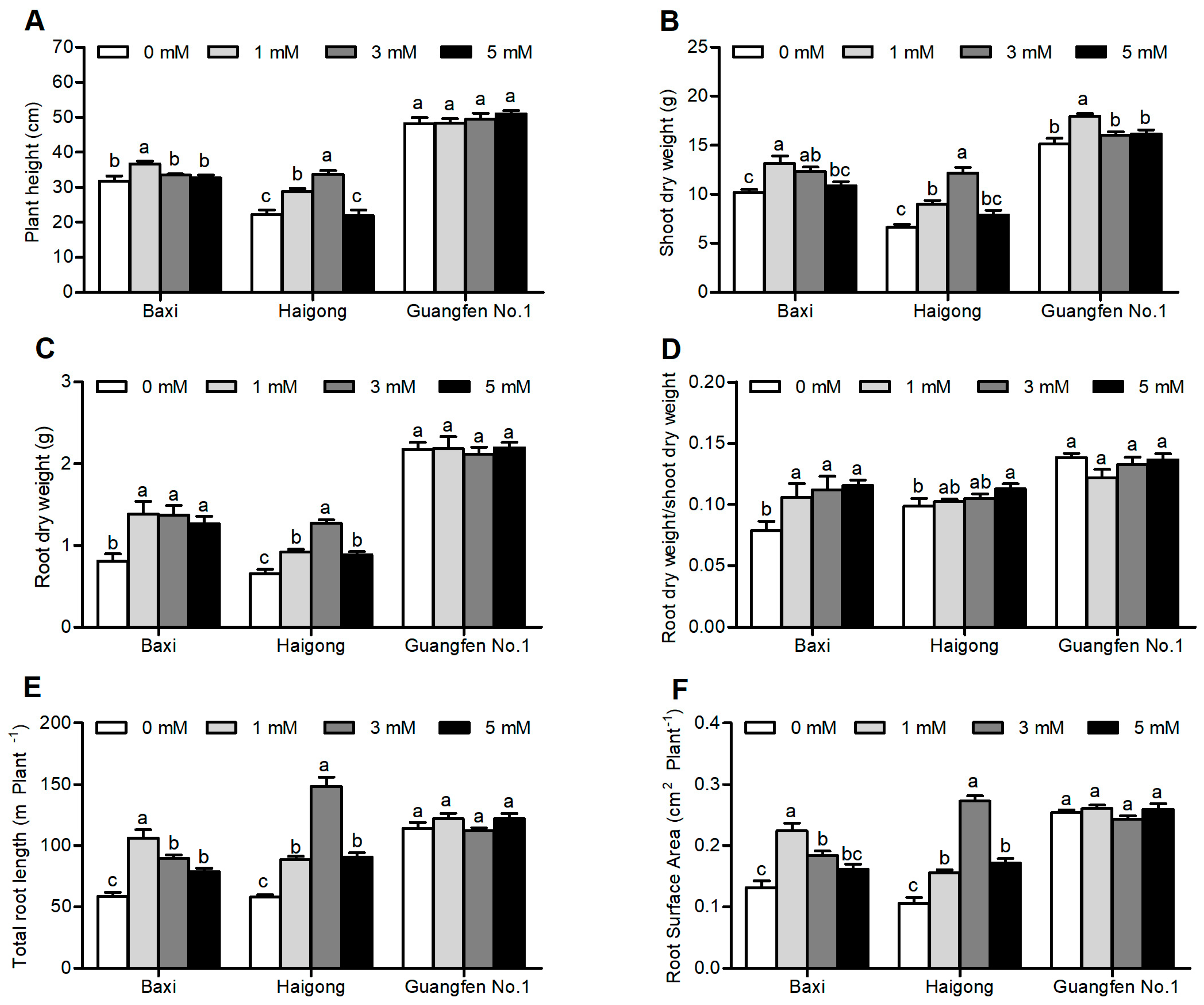
Plant height, shoot DW, root DW, shoot DW/root DW, total root length, and root surface area were significantly influenced by different Mg concentrations (Table S3). These growth traits were firstly increased by increasing Mg concentration and then decreased, except root dry weight and shoot dry weight ratio (Figure 4A–F). The Mg concentration of 1 mM and 3 mM in the NS were significantly optimal for Baxi and Haigong, respectively. Notably, no significant difference was observed in Guangfen No.1 except shoot dry weight when Mg concentration was 1mM. In general, biomass increments, and root morphological alteration were the largest in Haigong among the three cultivars.
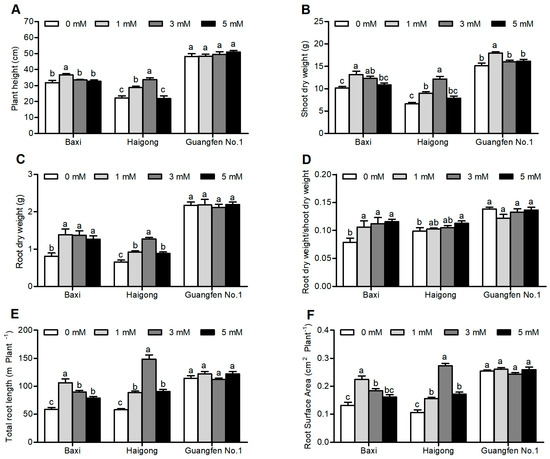
Figure 4.
Effect of different Mg concentrations on (A) plant height, (B) shoot dry weight, (C) root dry weight, and (D) root dry weight/shoot dry weight (E) total root length (F) root surface area of three banana cultivars (Baxi, Haigong, and Guangfen No.1) in the treatments: 0, 1, 3, and 5 (mmol/L). Each bar is mean ± SE (n = 5). Lowercase letters compare cultivars at harvest time within each treatment. Different letters indicate significant differences at p < 0.05.
3.5. Mineral Nutrients of Banana Leaf
The Mg and N, P, K, Ca, Zn content in the leaves of three banana genotypes were evaluated when different concentrations of Mg were applied to the sand (Table 1). The Mg content in the leaf of Mg-treated banana plantlets was found to successively increase in all genotypes with increasing Mg levels from 0 to 5 mM. Haigong accumulated the least Mg at all Mg levels ranging from 1.15 to 2.87 mg g−1, while Baxi accumulated the highest leaf Mg at 0 and 1 M and Guangfen achieved the highest leaf Mg at 3 and 5 mM Mg.

Table 1.
Concentrations of mineral composition of leaf in three cultivars of banana seedlings subjected to different concentrations of Mg. Lowercase letters compare the effects in each Mg condition.
The other mineral nutrients (N, P, K, Ca, and Zn) were significantly influenced by Mg treatment. In contrast to Mg, the N, P, K, Ca, and Zn decreased in the leaf as the Mg level increased in media from 0 to 3 mM in all three genotypes, except P in Guangfen No. 1 which remained unaffected. Increasing Mg to 5 mM increased the N in Baxi and N and K in Guangfen No. 1, statistically equal to 0 mM.
4. Discussion
4.1. Optimum Mg Varied among Banana Cultivars and Altered the Mineral Nutrients of Banana Leaf
To understand the responses of banana cultivars to Mg deficiency and different Mg levels, we have systematically analyzed the nutrient composition, biomass, root morphology, photosynthetic pigments, photosynthesis, carbohydrate content, and antioxidant enzyme activity of three popular commercial banana cultivars.
Nutrient deficiency in plants can often be detected through foliar diagnosis [40]. Critical and commercial standard concentrations of Mg in banana plants usually range between 2–3 mg g−1 [30]. In the present study, supplementing Mg linearly increased the foliar Mg content. The optimal Mg concentration for Baxi and Guangfen No.1 was 1 mM and Haigong was 3 mM at which Mg content was within the critical range of Mg. This showed that the optimal Mg concentration could vary among banana cultivars. The results depicted that at the optimum Mg concentration (1 mM for Baxi and Guangfen No.1 and 3 mM for Haigong), the Mg concentration was 2.44, 2.20, and 2.69 mg g−1 in Baxi, Haigong, and Guangfen No.1, respectively. Baxi and Guangfen No. 1 accumulated >3 mg g−1 Mg at 3 mM and Haigong accumulated 2.87 mg g−1 Mg at 5 mM. Haigong showed a natural tendency of accumulating low Mg as compared to Baxi and Guangfeng No. 1.
4.2. Mg Deficiency Reduced Root Growth by Impairing Photosynthate Translocation
Mineral nutrition plays a major determinant on plant growth allocation. Many differences were observed in the biomass and morphological aspect of banana grown at 0, 1, 3, and 5 mM Mg. Mg deficiency (0 mM) severely hampered the morpho-physiological growth of banana and reduced plant height, dry weight of shoot and root, the ratio of root and shoot dry weight, root length, and root surface area of Baxi and Haigong. Many previous workers [11,13,22,41] also indicated that deficiency of Mg inhibits plant growth.
Our finding that Mg deficiency decreased root dry weight/shoot dry weight ratio is similar to those observed in pepper [42], mulberry [26], and citrus [43]. The reduction in root dry weight/shoot dry weight ratio might be linked with the reduced translocation of sugar from leaves to roots as manifested by the enhanced accumulation of sucrose and starch in leaves by Mg deficiency (Figure 3). High accumulation of sucrose, fructose, and glucose in leaves of Sulla carnosa has been previously observed by Farhat [18] under Mg deficiency. Lack of Mg can reduce H+-ATPase activity in the sieve elements, resulting in abnormal build-up of carbohydrates in leaves, which has been reported in many plant [10,14,41]. Cakmak [44] found a close relationship between root dry weight/shoot dry weight ratio and relative distribution of sugar and starch in root and shoots. Furthermore, the less photosynthesis rate and pigment contents (Figure 1) also verify the theory that the enhanced accumulation of sugars under Mg deficiency is due to the less transportation of sugars from leaves rather than the higher synthesis. This means that lower proportions of photosynthates were translocated from leaves to roots of Mg deficiency seedlings, thus decreasing root dry weight/shoot dry weight ratio. The reduction in total root length and root surface area confirmed that the reduction in root dry weight is linked to Mg deficiency (Figure 4E,F).
The response of three banana cultivars was variable under Mg deficiency. Intriguingly, despite the shoot dry weight of Guangfen No.1 being significantly decreased by Mg deficiency as compared to 1 mM Mg, its seedlings maintained higher shoot and root DW than the other two cultivars. Furthermore, the plant height, root dry weight, ratio of root and shoot dry weight, total root length, and root surface area of Guangfen No.1 were not significantly influenced by Mg deficiency, while these traits were reduced in other cultivars (Figure 4B,C) which indicate genotypic variation for Mg deficiency tolerance. Similarly, at the highest Mg level (5 mM), biomass and root morphological traits of Baxi and Haigong were decreased which showed that these banana cultivars cannot grow well under high Mg concentration. Published data showed that Mg toxicity markedly inhibits plant growth, resulting in shorter root and smaller shoots [45,46,47]. Although the growth traits of Guangfeng No. 1. were not affected. These results demonstrate the Haigong cultivar was most affected by Mg deficiency/toxicity in relation to biomass allocation and root morphology, Guangfen No.1 was minimally affected.
Our results indeed demonstrate that although carbohydrate accumulation was significant in three banana cultivars, this was more pronounced with higher amounts in Haigong as compared to the other two cultivars under a Mg deficiency condition, which made it more sensitive to Mg stress.
4.3. Mg Deficiency Reduces Photosynthetic Pigment Content and Photosynthesis in Leaf of Banana
In the present study, leaves of the Baxi and Haigong cultivars showed significant decrease in chlorophyll a, chlorophyll b, and carotenoids by Mg deficiency and toxicity (Figure 1). The reduction in photosynthetic pigment content in response to Mg deficiency is a common phenomenon as Mg is an essential component of chlorophyll [10,48,49]. Mg deficiency impedes the insertion of Mg into protoporphyrin IX and, thus, chlorophyll synthesis [24].
Both the Mg deficiency and toxicity reduced the Pn and Fv/Fm of Haigong and Baxi. The reduction in Pn and Fv/Fm indicates that Mg stress causes a loss of reaction centers which connect the light-harvesting complex to PSII and change the photosystem stoichiometry of photosystem I (PSI). Among cultivars, Baxi and Haigong showed lower Pn and Fv/Fm under Mg stress conditions as compared to Guangfen No.1 (Figure 1E,F) which could be attributed to a higher reduction in chlorophyll. Huanget al. [11] reported that the reduction of photosynthetic pigment content was accompanied by photosynthetic inhibition. The reduction in photosynthetic pigment and higher accumulation of carbohydrates in leaves reduces the use of absorbed light energy and the electron transport chain becomes saturated and NADPH accumulated [32,41]. High levels of reducing equivalents and components of the saturated electron transport chain facilitate the formation of ROS [50].
Our results indicated that Mg deficiency enhanced the levels of MDA (Figure 2) under Mg stress indicating oxidative damage. Haigong responded to Mg stress by increasing the activity of SOD, APX, and GR in leaves. These results indicated that the antioxidant enzyme activities were elevated under Mg stress. Nevertheless, the antioxidant metabolism (APX and GR) of Guangfen No.1 was less effective in ROS scavenging and oxidative damage was also the least in this cultivar.
Maintaining metal homeostasis is strenuous for plants under Mg stress, as the imbalance can interfere with the plant’s ability to absorption of other ions [11]. The relatively high level of K and Ca metals in banana leaves when seedlings treatment with absolute absence and excess Mg levels (Table 1) are similar to observations reported for Arabidopsis thaliana (L.) Heynh [46], maize (Zea mays L.) [13], and Citrus sinensis [11]. Such a replacement of essential cofactor metals in enzymes of plants under deficiency or toxicity of Mg leads to changes in associated metabolic pathways, which hinder normal cellular functions and growth processes, induce oxidative damage, and weaken the cell membrane integrity of plants.
5. Conclusions
Both Mg deficiency and toxicity affected the morphological, physiological, and biochemical responses of the banana cultivars. Mg stress caused more serious effects on morphological (plant height, plant dry weight, total root length, and root surface area), physiological (photosynthetic pigment and photosynthesis), and biochemical (MDA, SOD, APX, GR, and metals) traits of Haigong and Baxi than in Guangfen No.1. Haigong showed higher accumulation of carbohydrates in leaves, higher membrane damage due to ROS, and more activity of antioxidants enzymes. These data suggested that Haigong is more sensitive to Mg stress than Baxi and Guangfen No.1.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9091017/s1, Table S1: Effect of different Mg concentrations on Chl a, Chl b, Chl a+b, Car, Pn and Fv/Fm.
Author Contributions
Conceptualization, H.H. and Z.S.; Methodology, H.H. and X.W.; Software, X.W.; Validation, Z.S.; Formal analysis, X.W. and J.T.; Resources, Z.S.; Writing—original draft, H.H.; Writing—review & editing, S.K.; Visualization, J.W.; Supervision, H.H. and Z.S.; Project administration, H.H.; Funding acquisition, Z.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (No. 32060382), the Youth Innovation Training Program (CXPY 202110) of Shihezi University, the Research Foundation for Talented Scholars (No. RCZK202044) of Shihezi University, China.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationship that could have appeared to influence the work reported in this paper.
References
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Marschner, H.; Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Portis, J.A. Regulation of Ribulose 1,5-Bisphosphate Carboxylase/Oxygenase Activity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 415–437. [Google Scholar] [CrossRef]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. Biometals 2002, 15, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; pp. 135–189. [Google Scholar] [CrossRef]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Hermans, C.; Johnson, G.N.; Strasser, R.J.; Verbruggen, N. Physiological characterisation of magnesium deficiency in sugar beet: Acclimation to low magnesium differentially affects photosystems I and II. Planta 2004, 220, 344–355. [Google Scholar] [CrossRef]
- Boxler-Baldoma, C.; Lütz, C.; Heumann, H.G.; Siefermann-Harms, D. Structural changes in the vascular bundles of light-exposed and shaded spruce needles suffering from Mg deficiency and ozone pollution. J. Plant Physiol. 2006, 163, 195–205. [Google Scholar] [CrossRef]
- Ruan, J.; Ma, L.; Yang, Y. Magnesium nutrition on accumulation and transport of amino acids in tea plants. J. Sci. Food Agric. 2012, 92, 1375–1383. [Google Scholar] [CrossRef]
- Li, C.-P.; Qi, Y.-P.; Zhang, J.; Yang, L.-T.; Wang, D.-H.; Ye, X.; Lai, N.-W.; Tan, L.-L.; Lin, D.; Chen, L.-S. Magnesium-deficiency-induced alterations of gas exchange, major metabolites and key enzymes differ among roots, and lower and upper leaves of Citrus sinensis seedlings. Tree Physiol. 2017, 37, 1564–1581. [Google Scholar] [CrossRef]
- Huang, J.H.; Xu, J.; Ye, X.; Luo, T.Y.; Ren, L.H.; Fan, G.C.; Qi, Y.P.; Li, Q.; Ferrarezi, R.S.; Chen, L.S. Magnesium deficiency affects secondary lignification of the vascular system in Citrus sinensis seedlings. Trees 2018, 33, 171–182. [Google Scholar] [CrossRef]
- Cai, Y.-T.; Zhang, H.; Qi, Y.-P.; Ye, X.; Huang, Z.-R.; Guo, J.-X.; Chen, L.-S.; Yang, L.-T. Responses of reactive oxygen species and methylglyoxal metabolisms to magnesium-deficiency differ greatly among the roots, upper and lower leaves of Citrus sinensis. BMC Plant Biol. 2019, 19, 76. [Google Scholar] [CrossRef]
- Jezek, M.; Geilfus, C.M.; Bayer, A.; Mühling, K.H. Photosynthetic capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf-application. Front. Plant Sci. 2015, 5, 781. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, Y.; Kutman, U.B.; Mengutay, M.; Cakmak, I. Magnesium applications to growth medium and foliage affect the starch distribution, increase the grain size and improve the seed germination in wheat. Plant Soil 2016, 406, 145–156. [Google Scholar] [CrossRef]
- Shaul, O.; Hilgemann, D.W.; de-Almeida-Engler, J.; Van Montagu, M.; Inzé, D.; Galili, G. Cloning and characterization of a novel Mg2+/H+ exchanger. EMBO J. 2009, 18, 3973–3980. [Google Scholar] [CrossRef]
- Brady, K.U.; Kruckeberg, A.R.; Bradshaw, H.D., Jr. Evolutionary Ecology of Plant Adaptation to Serpentine Soils. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 243–266. [Google Scholar] [CrossRef]
- He, H.; Jin, X.; Ma, H.; Deng, Y.; Huang, J.Q.; Yin, L.Y. Changes of plant biomass partitioning, tissue nutrients and carbohydrates status in magnesium-defcient banana seedlings and remedy potential byfoliar application of magnesium. Sci. Hortic. 2020, 268, 109377. [Google Scholar] [CrossRef]
- Farhat, N.; Rabhi, M.; Krol, M.; Barhoumi, Z.; Ivanov, A.G.; Mccarthy, A.; Abdelly, C.; Smaoui, A.; Hüner, N.P.A. Starch and sugar accumulation in Sulla carnosa leaves upon Mg2+ starvation. Acta Physiol. Plant. 2014, 36, 2157–2165. [Google Scholar] [CrossRef]
- Hermans, C.; Verbruggen, N. Physiological characterization of Mg defificiency in Arabidopsis thaliana. J. Exp. Bot. 2005, 56, 2153–2161. [Google Scholar] [CrossRef]
- Fischer, E.S.; Lohaus, G.; Heineke, D.; Heldt, H.W. Magnesium defificiency results in accumulation of carbohydrates and amino acids in source and sink leaves of spinach. Physiol. Plant. 1998, 102, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Kobayashi, N.I.; Hermans, C.; Ichihashi, Y.; Shibata, A. Short-Term Magnesium Deficiency Triggers Nutrient Retranslocation in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 563. [Google Scholar] [CrossRef]
- Tränkner, M.; Jákli, B.; Tavakol, E.; Geilfus, C.M.; Cakmak, I.; Dittert, K.; Senbayram, M. Magnesium defciency decreases biomasswater-use efciency and increases leaf water-use efciency andoxidative stress in barley plants. Plant Soil 2016, 406, 409–423. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, E.; Yu, X.; Chen, Y.; Yang, Q.; Cao, Y.; Li, X.; Wang, Y.; Fu, A.; Xu, M. Molecular characterization of magnesium chelatase in soybean [Glycine max (L.) Merr.]. Front. Plant Sci. 2018, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Hong, X.; Hu, K.K.; Wang, Y.; Wang, X.X.; Du, S.Y.; Li, Y.; Hu, D.D. Impaired Magnesium Protoporphyrin IX Methyltransferase (ChlM) Impedes Chlorophyll Synthesis and Plant Growth in Rice. Front. Plant Sci. 2017, 8, 1694. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Magnesium deficiency induced oxidative stress and antioxidant responses in mulberry plants. Sci. Hortic. 2006, 108, 7–14. [Google Scholar] [CrossRef]
- Saghaiesh, S.P.; Souri, M.K.; Moghaddam, M. Efects of diferent magnesium levels on some morphophysiological characteristics and nutrient elements uptake in Khatouni melons (Cucumis melo var. inodorus). J. Plant Nutr. 2019, 42, 27–39. [Google Scholar] [CrossRef]
- Perrier, X.; De Langhe, E.; Donohue, M.; Lentfer, C.; Vrydaghs, L.; Bakry, F.; Carreel, F.; Hippolyte, I.; Horry, J.P.; Jenny, C.; et al. Multidisciplinary perspectives on banana (Musa spp.) domestication. Proc. Natl. Acad. Sci. USA 2011, 108, 11311–11318. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Rai, R. Translating the “Banana Genome” to Delineate Stress Resistance, Dwarfing, Parthenocarpy and Mechanisms of Fruit Ripening. Front. Plant Sci. 2016, 7, 1543. [Google Scholar] [CrossRef]
- Mazumdar, P.; Lau, S.E.; Singh, P.; Takhtgahi, H.M.; Harikrishna, J.A. Impact of sea-salt on morpho-physiological and biochemical responses in banana (Musa acuminata cv. Berangan). Physiol. Mol. Biol. Plants 2019, 25, 713–726. [Google Scholar] [CrossRef]
- Robinson, J.; Sauco, V.G. Bananas and Plantains, 2nd ed.; CABI: Cambridge, MA, USA, 2010; Volume 2. [Google Scholar]
- Silva, J.; Silva, I.; Pereira, R. Phosphorus fertilization in banana ‘Prata an’ (AAB) cultivated in two latosols. Rev. Ceres 2011, 58, 238–242. [Google Scholar] [CrossRef]
- Hermans, C.; Bourgis, F.M.; Strasser, R.J.; Delrot, S.; Verbruggen, N. Magnesium deficiency in sugar beets alters sugar partitioning and phloem loading in young mature leaves. Planta 2005, 220, 541–549. [Google Scholar] [CrossRef]
- He, H.S.; Khan, S.; Deng, Y.; Hu, H.; Yin, L.Y.; Huang, J.Q. Supplemental Foliar-Applied Magnesium Reverted Photosynthetic Inhibition and Improved Biomass Partitioning in Magnesium-Deficient Banana. Horticulturae 2022, 8, 1050. [Google Scholar] [CrossRef]
- Camak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase and peroxidase activities in root tips of soyabean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Giannopotitis, C.N.; Ries, S.K. Superoxide dismutases I: Occurrence in higher plants. Plant Physiol. 1997, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascobbate-specific peroxidase in Spinach chloroplasts. Plant Cell. Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Grace, S.C.; Logan, B.A. Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol. 1996, 112, 1631–1640. [Google Scholar] [CrossRef]
- Li, Y.Z.; Zhao, J.Y.; Wu, S.M.; Fan, X.W.; Luo, X.L.; Chen, B.S. Characters related to higher starch accumulation in cassava storage roots. Sci. Rep. 2016, 6, 19823. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agricultural-Chemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Marschner, H.; Kirkby, E.A.; Cakmak, I. Effect of mineral nutritional status on shoot-root partitioning of photoassimilates and cycling of mineral nutrients. J. Exp. Bot. 1996, 47, 1255–1263. [Google Scholar] [CrossRef]
- Meireles da Silva, D.; Brandão, I.R.; Alves, J.D.; de Santos, M.O.; de Souza, K.R.D.; de Silveira, H.R.O. Physiological and biochemical impacts of magnesium-deficiency in two cultivars of coffee. Plant Soil 2014, 382, 133–150. [Google Scholar] [CrossRef]
- Riga, P.; Anza, M. Effect of Magnesium Deficiency on Pepper Growth Parameters: Implications for Determination of Magnesium-Critical Value. J. Plant Nutr. 2003, 26, 1581–1593. [Google Scholar] [CrossRef]
- Yang, G.-H.; Yang, L.-T.; Jiang, H.-X.; Li, Y.; Wang, P.; Chen, L.-S. Physiological impacts of magnesium-deficiency in Citrus seedlings: Photosynthesis, antioxidant system and carbohydrates. Trees 2012, 26, 1237–1250. [Google Scholar] [CrossRef]
- Cakmak, I.; Christine, H.; Marschner, H. Partitioning of shoot and root dry matter and carbohydrates in bean plants suffffering form phosphorus, potassium and magnesium defificiency. J. Exp. Bot. 1994, 45, 1245–1250. [Google Scholar] [CrossRef]
- Venkatesan, S.; Jayaganesh, S. Characterisation of magnesium toxicity, its influence on amino acid synthesis pathway and biochemical parameters of tea. Res. J. Phytochem. 2010, 4, 67–77. [Google Scholar] [CrossRef]
- Niu, Y.; Chai, R.; Liu, L.; Jin, G.; Liu, M.; Tang, C.; Zhang, Y. Magnesium availability regulates the development of root hairs in Arabidopsis thaliana (L.) Heynh. Plant Cell Environ. 2014, 37, 2795–2813. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Cong, Y.; Hussain, N.; Wang, Y.; Liu, Z.; Jiang, L.; Liang, Z.; Chen, K. The remodeling of seedling development in response to long-term magnesium toxicity and regulation by ABA-DELLA signaling in Arabidopsis. Plant Cell Physiol. 2014, 55, 1713–1726. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.Y.; Liao, L.L.; Liu, S.; Nie, M.M.; Li, J.; Zhang, L.D.; Ma, J.F.; Chen, Z.C. Magnesium Deficiency Triggers SGR–Mediated Chlorophyll Degradation for Magnesium Remobilization. Plant Physiol. 2019, 181, 262–275. [Google Scholar] [CrossRef]
- Tang, N.; Li, Y.; Chen, L.-S. Magnesium deficiency-induced impairment of photosynthesis in leaves of fruiting Citrus reticulata trees accompanied by up-regulation of antioxidant metabolism to avoid photo-oxidative damage. J. Plant Nutr. Soil Sci. 2012, 175, 784–793. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).