Magnetic Properties and the Superatom Character of 13-Atom Platinum Nanoclusters
Abstract
:1. Introduction
2. The Concept of Superatoms


3. Results and Discussion
3.1. The EPR Spectrum of Pt13Hm Clusters

3.2. High-Spin Cluster States

| Property | SQUID Measurements, 1.8 K | XMCD Measurements, 7 K | |||
|---|---|---|---|---|---|
| Pt13 | Pt13Hm | Pt13 | Pt13Hm | Pt Foil | |
| µ/µB per Pt13 | 5.9(1) | 5.6(1) | 3.7(4) | 3.0(4) | - |
| 5.8(6) b | 8.4(6) b | ||||
| f/% | 14(1) | 10(1) | 14(10) | 20(10) | - |
| 103 mL/µB | - | - | 5.49(9) | 6.39(7) | 2.1 |
| 103 mS/µB | - | - | 17.1(6) | 21.8(6) | 0.38 |
| mL/mS | - | - | 0.32(2) | 0.29(2) | 0.38 |
3.3. EPR Observation of a Blocking Temperature

3.4. Diamagnetic Clusters
3.5. Super-Diamagnetism
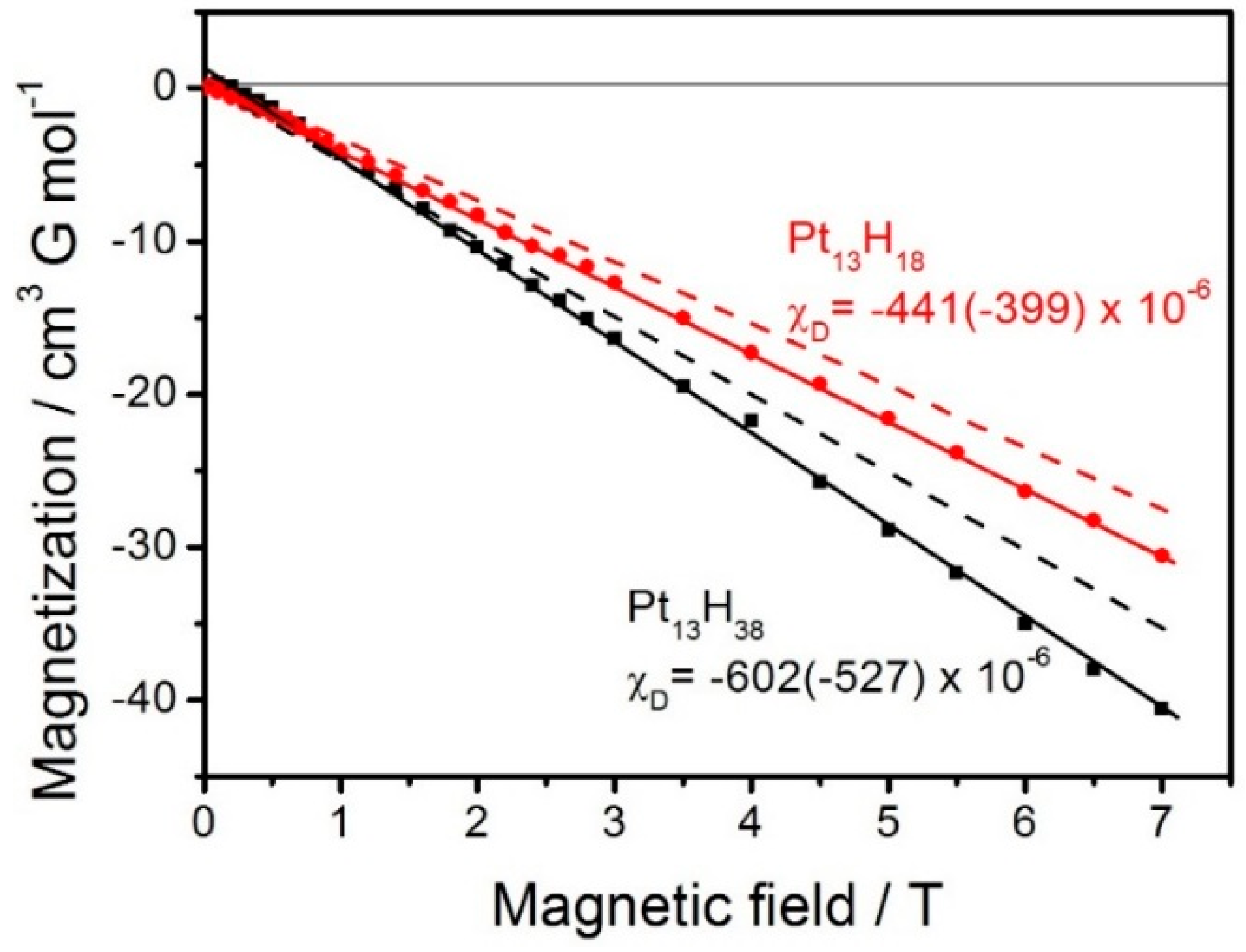

3.6. Potential Superconductivity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roduner, E. Nanoscopic Materials: Size-Dependent Phenomena and Growth Principles, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 2014; Chapter 5. [Google Scholar]
- Yamamoto, Y.; Miura, T.; Nakae, Y.; Teranishi, T.; Miyake, M.; Hori, H. Magnetic Properties of the Noble Metal Nanoparticles Protected by Polymer. Physica B 2003, 329–333, 1183–1184. [Google Scholar] [CrossRef]
- Nakae, Y.; Seino, T.; Teranishi, T.; Miyake, M.; Hori, H. Anomalous spin polarization in Pd and Au nano-particles. Physica B 2000, 284, 1758–1759. [Google Scholar] [CrossRef]
- Moseler, M.; Häkkinen, H.; Barnett, R.N.; Landman, U. Structure and Magnetism of Neutral and Anionic Palladium Clusters. Phys. Rev. Lett. 2001, 86, 2545–2548. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Jena, P. Physics and Chemistry of Finite Size Systems: From Clusters, to Crystals; Jena, P., Khanna, S.N., Rao, B.K., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 1992; Volume I, pp. 709–720. [Google Scholar]
- Kresin, V.V. Electronic Structure of Small Metal Clusters: Thomas-Fermi Statistical Theory. Phys. Rev. B 1988, 38, 3741–3746. [Google Scholar] [CrossRef]
- Roduner, E.; Jensen, C.; van Slageren, J.; Rakoczy, R.A.; Larlus, O.; Hunger, M. Anomalous Diamagnetic Susceptibility in 13-Atom Platinum Superatoms. Angew. Chem. Int. Ed. 2014, 53, 4318–4321. [Google Scholar] [CrossRef] [PubMed]
- Schmauke, T.; Eichel, R.-A.; Schweiger, A.; Roduner, E. Electron Paramagnetic Resonance Studies of a Platinum Cluster in Linde L and Faujasite Zeolites. Phys. Chem. Chem. Phys. 2003, 5, 3076–3084. [Google Scholar] [CrossRef]
- Liu, X.; Dilger, H.; Eichel, R.A.; Kunstmann, J.; Roduner, E. A Small Paramagnetic Platinum Cluster in a NaY Zeolite: Characterization and Hydrogen Adsorption and Desorption. J. Phys. Chem. B 2006, 110, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bauer, M.; Bertagnolli, H.; Roduner, E.; van Slageren, J.; Phillipp, F. Structure and Magnetization of Small Monodisperse Platinum Clusters. Phys. Rev. Lett. 2006. Erratum in 2009, 102, 049902E. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, J.; Bartolomé, F.; García, L.M.; Roduner, E.; Akdogan, Y.; Wilhelm, F.; Rogalev, A. Magnetization of Pt13 clusters supported in a NaY zeolite: A XANES and XMCD study. Phys. Rev. B 2009, 80, 014404:1–014404:10. [Google Scholar] [CrossRef]
- Longo, R.C.; Gallego, L.J. Structures of 13-Atom Clusters of FCC Transition Metals by ab initio and Semi-Empirical Calculations. Phys. Rev. B 2006, 74, 193409:1–193409:4. [Google Scholar] [CrossRef]
- Akdogan, Y.; Anantharaman, S.; Liu, X.; Lahiri, G.K.; Bertagnolli, H.; Roduner, E. Reconstruction of Pt13 Clusters into Pt2(CO)m on CO Addition in NaY Zeolite. J. Phys. Chem. C 2009, 113, 2352–2359. [Google Scholar] [CrossRef]
- Reveles, J.U.; Clayborne, P.A.; Reber, A.C.; Khanna, S.N.; Pradhan, K.; Sen, P.; Pederson, M.R. Designer magnetic superatoms. Nat. Chem. 2009, 1, 310–315. [Google Scholar] [CrossRef] [PubMed]
- King, R.B. Magnetic superatoms. Nat. Chem. 2009, 1, 260–261. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, K.; Reveles, J.U.; Sen, P.; Khanna, S.N. Enhanced magnetic moments of alkali metal coated Sc clusters: New magnetic superatoms. J. Chem. Phys. 2010, 132, 124302:1–124302:5. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.P.; Johnston, R.L.; Rao, C.N.R. On the Size-Induced Metal-Insulator Transition in Clusters and Small Particles. In Metal Clusters in Chemistry; Braunstein, P., Oro, L.A., Raithby, P.R., Eds.; Wiley: Weinheim, Germany, 1999; Volume 3, pp. 1454–1478. [Google Scholar]
- Kreibig, U.; Volmer, M. Optical Properties of Metal Clusters; Springer Series in Materials Science; Springer: Berlin, Germany, 1995; Volume 25, pp. 275–436. [Google Scholar]
- Busani, R.; Folkers, M.; Cheshnovsky, O. Direct Observation of Band-Gap Closure in Mercury Clusters. Phys. Rev. Lett. 1998, 81, 3836–3839. [Google Scholar] [CrossRef]
- De Heer, W.A. The Physics of Simple Metal Clusters: Experimental Aspects and Simple Models. Rev. Mod. Phys. 1993, 65, 611–676. [Google Scholar] [CrossRef]
- Watanabe, H.; Inoshita, T. Superatoms: A Novel Concepts in Materials Science. Optoelectron. Devices Technol. 1986, 1, 33–39. [Google Scholar]
- Castleman, A.W.; Khanna, S.N. Clusters, Superatoms, and Building Blocks of New Materials. J. Phys. Chem. C 2009, 113, 2664–2675. [Google Scholar] [CrossRef]
- Leuchtner, R.E.; Harms, A.C.; Castleman, A.W. Thermal Metal Cluster Anion Reactions: Behavior of Aluminum Clusters with Oxygen. J. Chem. Phys. 1989, 91, 2753–2755. [Google Scholar] [CrossRef]
- Bergeron, D.E.; Castleman, A.W., Jr.; Morisato, T.; Khanna, S.N. Formation of Al13I−: Evidence for the Superhalogen Character of Al13. Science 2004, 304, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Neukermans, S.; Janssens, E.; Chen, Z.F.; Silverans, R.E.; Schleyer, P.V.R.; Lievens, P. Extremely Stable Metal-Encapsulated AlPb10+ and AlPb12+ Clusters: Mass-Spectrometric Discovery and Density Functional Theory Study. Phys. Rev. Lett. 2004, 92, 163401:1–163401:4. [Google Scholar] [CrossRef] [PubMed]
- Roduner, E. Size Matters—Why Nanomaterials Are Different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Akola, J.; Lopez-Acevedo, O.; Jadzinsky, P.D.; Calero, G.; Ackerson, C.J.; Whetten, R.L.; Grönbeck, H.; Häkkinen, H. A unified view of ligand-protected gold clusters as superatom complexes. Proc. Natl. Acad. Sci. USA 2008, 105, 9157–9162. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, H. Atomic and electronic structure of gold clusters: Understanding flakes, cages and superatoms from simple concepts. Chem. Soc. Rev. 2008, 37, 1847–1859. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Aikens, C.M.; Hendrich, M.P.; Gupta, R.; Qian, H.; Schatz, G.C.; Jin, R. Reversible switching of magnetism in thiolate-protected Au25 superatoms. J. Am. Chem. Soc. 2009, 131, 2490–2492. [Google Scholar] [CrossRef] [PubMed]
- Vanquickenborne, L.G.; Haspeslagh, L. On the Meaning of the Spin-Pairing Energy in Transition-Metal Ions. Inorg. Chem. 1982, 21, 2448–2454. [Google Scholar] [CrossRef]
- Christensen, N.E. Spin-orbit projected d-densities of states of Pd, Ag, Pt, Au. J. Phys. F Met. Phys. 1978, 8, L51–L55. [Google Scholar] [CrossRef]
- Varns, R.; Strange, P. Super-Atom Properties of 13-Atom Clusters of Group 13 Elements. Phys. Status Solidi B 2012, 249, 2179–2189. [Google Scholar] [CrossRef]
- Watari, N.; Ohnishi, S. Electronic Structure of H Adsorbed on Pt13 Clusters. J. Chem. Phys. 1997, 106, 7531–7540. [Google Scholar] [CrossRef]
- Watari, N.; Ohnishi, S. Atomic and Electronic Structures of Pd13 and Pt13 Clusters. Phys. Rev. B Condens. Matter 1998, 58, 1665–1677. [Google Scholar] [CrossRef]
- Jensen, C.; Buck, D.; Dilger, H.; Bauer, M.; Phillipp, F.; Roduner, E. Maximum hydrogen chemisorption on KL zeolite supported Pt clusters. Chem. Commun. 2013, 49, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Keppeler, M.; Roduner, E. Platinum-Hydrogen Vibrations and Low Energy Electronic Excitations of 13-Atom Pt Nanoclusters. Phys. Chem. Chem. Phys. 2014, 16, 26613–26616. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; van Slageren, J.; Jakes, P.; Eichel, R.-A.; Roduner, E. Support Effects on Hydrogen Desorption, Isotope Exchange, Chemical Reactivity, and Magnetism of Platinum Nanoclusters in KL Zeolite. J. Phys. Chem. B 2013, 117, 22732–22745. [Google Scholar] [CrossRef]
- Morton, J.R.; Preston, K.F. Atomic Parameters for Paramagnetic Resonance Data. J. Magn. Reson. 1978, 30, 577–582. [Google Scholar] [CrossRef]
- Buneãu, O.; Bartolomé, J.; Bartolomé, F.; García, L.M. Large Orbital Magnetic Momentum in Pt13 Clusters. J. Phys. Condens. Matter 2014, 26, 196006:1–196006:11. [Google Scholar]
- Zhang, I.D.; Budnick, J.D.; Hynes, W.A.; Chien, C.L.; Xiao, J.Q. Effect of the magnetic field on the superparamagnetic relaxation in granular Co-Ag samples. Appl. Phys. Lett. 1998, 72, 2053–2055. [Google Scholar] [CrossRef]
- Hickey, J.B.; Howson, M.A.; Greig, D.; Wiser, N. Enhanced magnetic anisotropy energy density for superparamagnetic samples of cobalt. Phys. Rev. B 1996, 53, 32–33. [Google Scholar] [CrossRef]
- Jensen, C. Elektronische Struktur, Magnetismus und Chemische Reaktivität von Pt13-Nanoclustern im KL-Zeolith. Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 2013. [Google Scholar]
- Blundell, S. Magnetism in Condensed Matter; Oxford Masters Series in Condensed Matter Physics; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- König, R.; Schindler, A.; Hermannsdörfer, T. Superconductivity of Compacted Platinum Powder at Very Low Temperatures. Phys. Rev. Lett. 1999, 82, 4528–4531. [Google Scholar] [CrossRef]
- Schindler, A.; König, R.; Hermansdörfer, T.; Braun, H.F.; Esca, G.; Günther, G.; Meissner, M.; Mertig, M.; Wahl, R.; Pompe, W. Superconductivity at 20 mK in compacted submicrometer platinum powders. Physica B 2003, 329–333, 1427–1428. [Google Scholar] [CrossRef]
- Kresin, V.Z.; Ovchinnikov, Y.N. Shell structure and strengthening of superconductivity pair correlation in nanoclusters. Phys. Rev. B 2006, 74, 024514:1–024514:11. [Google Scholar] [CrossRef]
- Kresin, V.Z.; Ovchinnikov, Y.N. “Giant” strengthening of superconducting pairing in metallic nanoclusters: Large enhancement of Tc and potential for room-temperature superconductivity. Phys. Uspekhi 2008, 51, 427–435. [Google Scholar] [CrossRef]
- Drozdov, A.P.; Eremets, M.I.; Trojan, I.A.; Ksenofontov, V.; Shylin, S.I. Conventional superconductivity at 203 Kelvin at high pressures in the sulfur hydride system. Nature 2015, 525, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Szezȩśniak, D.; Zemła, T.P. On the high-pressure superconducting phase in platinum hydride. Supercond. Sci. Technol. 2015, 28, 085018:1–085018:5. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roduner, E.; Jensen, C. Magnetic Properties and the Superatom Character of 13-Atom Platinum Nanoclusters. Magnetochemistry 2015, 1, 28-44. https://doi.org/10.3390/magnetochemistry1010028
Roduner E, Jensen C. Magnetic Properties and the Superatom Character of 13-Atom Platinum Nanoclusters. Magnetochemistry. 2015; 1(1):28-44. https://doi.org/10.3390/magnetochemistry1010028
Chicago/Turabian StyleRoduner, Emil, and Christopher Jensen. 2015. "Magnetic Properties and the Superatom Character of 13-Atom Platinum Nanoclusters" Magnetochemistry 1, no. 1: 28-44. https://doi.org/10.3390/magnetochemistry1010028