Abstract
The intrinsic characteristic features of metal–organic frameworks offer unique, great opportunities to develop novel materials with applications in very diverse fields. Aiming to take advantage of these, the application of post-synthetic methodologies has revealed itself to be a powerful approach to the isolation and structuration of metal ions, molecules, or more complex species, either within MOF channels or reticulated at their network, rendering novel and exciting MOFs with new or improved functionalities. Herein, we report the partial post-synthetic metal exchange of Zn(II) metal ions by Co(II) ones in water-stable three-dimensional CaZn6-MOF 1, derived from the amino acid S-methyl-L-cysteine, allowing us to obtain two novel MOFs with increasing contents of the Co(II) ions Co4%@1 and Co8%@1. Remarkably, the presented post-synthetic metal exchange methodology has two relevant implications for us: (i) it allowed us to obtain two novel MOFs, which were not accessible by direct synthesis, and (ii) enabled us to transform physical properties within this family of isoreticular MOFs from the diamagnetic pristine MOF 1 to MOFs Co4%@1 and Co8%@1, exhibiting field-induced, frequency-dependent, alternating current magnetic susceptibility signals, which are characteristic features of single-molecule magnets.
1. Introduction
Single-ion magnets (SIMs) represent the ultimate limit of the miniaturization of single-molecule magnets (SMMs) [1,2,3,4,5,6,7,8,9,10]. Their potential applications as high-density magnetic memories and quantum computing devices in the emerging field of molecular spintronics [11,12,13,14,15] have attracted the attention of many groups working in the fields of molecular magnetism [16,17,18,19,20] and multifunctional magnetic materials [14,21,22,23,24]. In fact, during the last decade, great advances have been achieved in efforts to increase phase memory times and the temperature need to observe magnetic bistability [25,26,27,28]. SIMs have been reported with very distinct metal ions, including lanthanides [8,29] and actinides [30,31], but also with first- [32,33,34] and third-row [35,36] transition metal ions. An interesting family of SIMs are Co(II) complexes. Their inherently large magnetic anisotropy, due to first-order spin–orbit coupling (SOC), allow for the obtention of Co(II)-based SIMs with different coordination geometries and signs of magnetic anisotropy [32,37,38]. However, despite the commendable advances achieved in the field, several daunting challenges remain to be solved, with the controlled spatial organization of magnetic molecules to build solid ordered arrays while retaining their intrinsic individual magnetic properties being one of the greatest ones.
Metal–organic frameworks (MOFs) [39] have emerged as a novel generation of porous materials [40,41] due to their unique differentiating features, such as their modulability and rich host–guest chemistry [42] and high porosity and crystallinity [43,44]. Since their advent, MOFs have shown noteworthy applications in very diverse fields, from gas adsorption and separation [45,46] and catalysis [47,48] to drug delivery [49] and magnetism [50,51], recently being used, for example, in water remediation [52] and chemical nanoreactors as well [53]. This, in part, is a direct consequence of the commendable synthetic efforts to achieve great control of the MOF structure obtained. In fact, even though total control is not always possible to achieve, the different pre- and post-synthetic methodologies [54,55,56,57] used have made it possible to precisely tune the ratio of distinct components and to gain control of MOF dimensionality and topology and, consequently, of the MOF’s final properties.
Thus, MOFs, a priori, represent outstanding frameworks for taking serious steps forward toward gaining control of the spatial organization of magnetic molecules. Indeed, seminal works have been devoted to this end, where the main synthetic strategies can mainly be classified into the following: (i) the direct self-assembly of metal ions with selected polytopic ligands, including mixtures of two distinct metal ions (doping) [37,58,59], (ii) the encapsulation of preformed SMMs [60,61], and (iii) post-synthetic metal exchange [62]. Regarding this last approach, we have already successfully performed it. We used a diamagnetic CaIICoIII 3D MOF as the initial framework, and, post-synthetically, we replaced all CaII ions with different lanthanide metal ions, where the final MOFs with Kramers ions (DyIII and ErIII) exhibited SIM behavior [24]. Also, recently, partial post-synthetic metal exchange was reported in a Zn-based MOF, replacing Zn(II) with Co(II) in different, very low atomic percentages [62]. Here, underpinned in our treasured knowledge of MOFs and SIMs, we further explore the latter route by performing partial post-synthetic metal exchange in a diamagnetic heterobimetallic CaZn6-MOF (Figure 1), replacing Zn(II) with Co(II) metal ions in different controlled atomic percentages (4 and 8%), and we study how this different degree of metal heterogeneity influences the magnetic properties of the obtained CaIIZnIICoII-MOF. The resulting novel mixed-metal MOFs exhibit field-induced, frequency-dependent, alternating relaxation typical of single-molecule magnets.
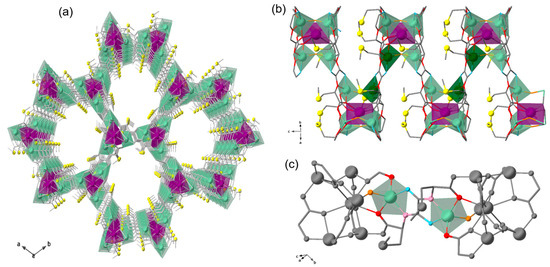
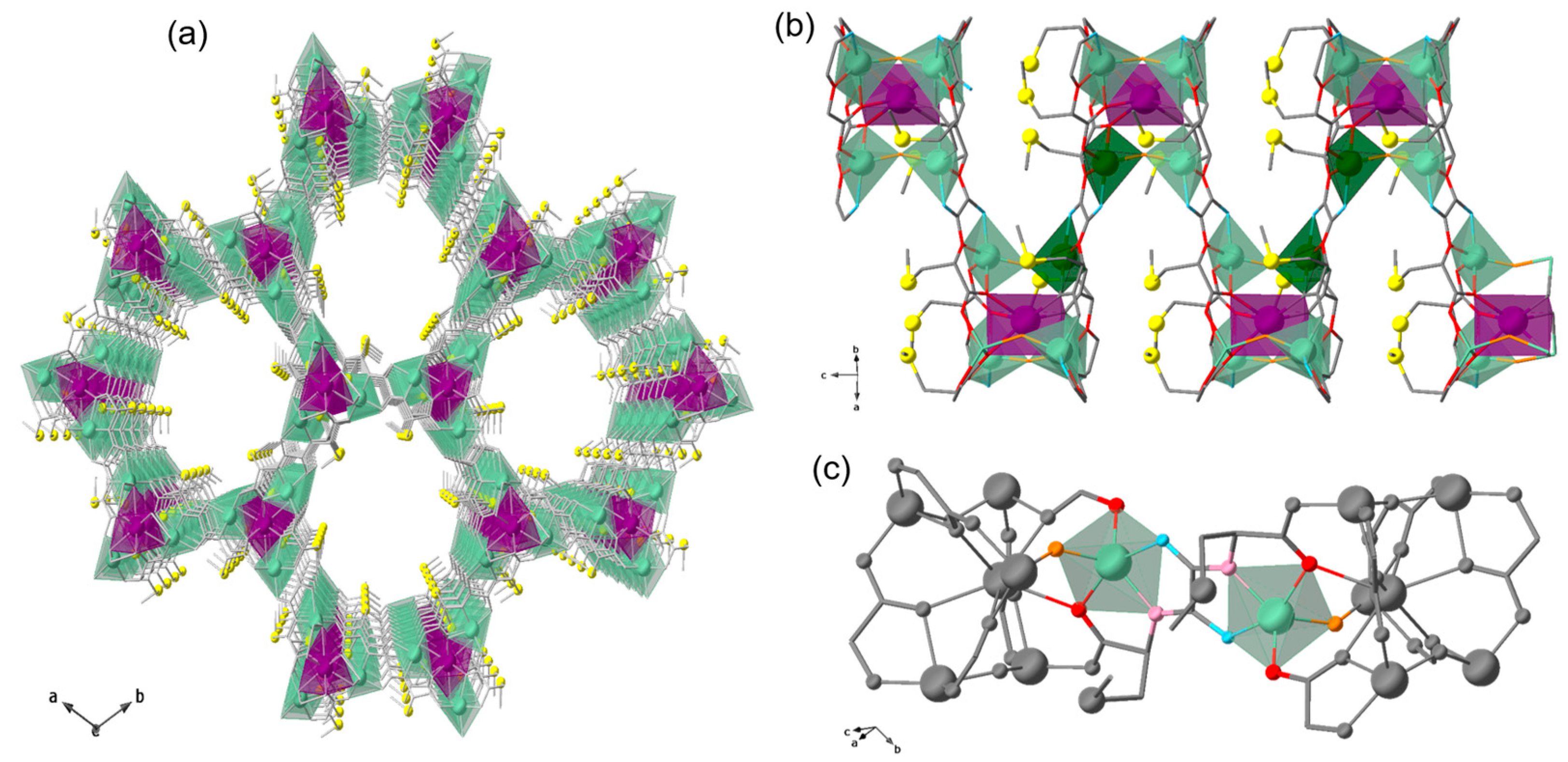
Figure 1.
Crystal structure of 1. (a) Perspective view of 3D porous network of 1 along c crystallographic axis. (b) Fragment of 1 highlighting the connection between dizinc(II) units. Dizinc units are represented by pale and dark polyhedra, whilst Ca and S are depicted as magenta polyhedra and yellow spheres; N, O, and C atoms are depicted as blue, red, and grey sticks. (c) View in detail of the dizinc(II) moiety highlighting the pentacoordinated Zn(II) ions and those atoms involved in their coordination geometry. Color code: Zn, pale green; amidate-N, cyan; carbonyl-O, pale pink; carboxylate-O, red; water/hydroxide-O, orange; rest of the structure is depicted as grey balls and sticks.
2. Materials and Methods
2.1. Materials
All reagents were obtained from commercial sources (Merck-Aldrich, Darmstadt, Germany) and used without further purification unless otherwise indicated. Reactions were performed in conventional round-bottomed flasks or sealed vials equipped with a magnetic stirrer. {CaZn6[(S,S)-Mecysmox]3(OH)2(H2O)}·12 H2O (1) was prepared following a previously reported procedure [63].
2.2. Preparation of {CaCo0.24Zn5.76[(S,S)-Mecysmox]3(OH)2(H2O)}·14 H2O (Co4%@1) and {CaCo0.48Zn5.52[(S,S)-Mecysmox]3(OH)2(H2O)}·16 H2O (Co8%@1)
Polycrystalline powders of 1 (0.2 g, 0.12 mmol) were suspended in water solutions in two independent vials with different amounts of Co(NO3)2·6 H2O (0.01 and 0.10 g for Co4%@1 and Co8%@1, respectively) for 1 h under mild stirring. Then, the resulting solids were isolated by filtration, thoroughly washed with water and methanol, and air dried. Co4%@1: Anal.: calcd for C30H68N6O35S6CaCo0.24Zn5.76 (1689.5): C, 21.32; H, 4.05; S, 11.08; N, 4.84%. Found: C, 21.37; H, 3.99; S, 11.12; N, 5.01%. Co8%@1: Anal.: calcd for C30H72N6O37S6CaCo0.48Zn5.52 (1735.9): C, 20.75; H, 4.18; S, 11.52; N, 5.03%. Found: C, 20.83; H, 4.09; S, 11.56; N, 5.11%.
2.3. Physical Techniques
Elemental (C, H, S, and N) and ICP-MS analyses were performed at the Microanalytical Service of the Universitat de València. The thermogravimetric analysis was performed on crystalline samples under a dry N2 atmosphere with a Mettler Toledo TGA/STDA 851e thermobalance, manufactured by Mettler Toledo, Greifensee, Switzerland, operating at a heating rate of 10 °C min–1. A scanning electron microscopy (SEM) elemental analysis was carried out for 1, Co4%@1, and Co8%@1, using a HITACHI S–4800 electron microscope, sourced from HITACHI High-Technologies Corporation, Tokyo, Japan, coupled with an energy dispersive X-ray (EDX) detector. Data were analyzed with the QUANTAX 400 system manufactured by Bruker AXS, Karlsruhe, Germany.
2.4. Gas Adsorption
The N2 adsorption–desorption isotherms at 77 K were carried out on polycrystalline samples of 1, Co4%@1, and Co8%@1 with a BELSORP-mini-X instrument manufactured by BEL Japan, Inc., Osaka, Japan. The samples were first activated with methanol and then evacuated at 343 K during 16 h under 10–6 Torr prior to their analysis.
2.5. X-Ray Powder Diffraction Measurements
Polycrystalline samples of 1, Co4%@1, and Co8%@1 were introduced into a 0.5 mm borosilicate capillary prior to being mounted and aligned on an Empyrean PANalytical powder diffractometer, manufactured by PANalytical, Almelo, The Netherlands, using Cu Kα radiation (λ = 1.54056 Å). Five repeated measurements were collected at room temperature (2θ = 2–45°) and merged in a single diffractogram.
2.6. Magnetic Measurements
Direct current (dc) magnetic susceptibility measurements (2–300 K) under applied dc magnetic fields of 5000 G (T ≥ 20 K) and 2500 G (2 ≤ T ≤ 20 K) and variable-field (0–5 T) magnetization measurements (2.0–10 K) on finely crushed crystals of Co4%@1 and Co8%@1 (mixed with grease to avoid the crystallite orientation) were carried out with a Quantum Design MPMS-XL7 SQUID magnetometer, manufactured by Quantum Design, San Diego, CA, USA. Variable-temperature (2–8 K) alternating current (ac) magnetic susceptibility measurements for Co4%@1 and Co8%@1 were performed under an applied dc field of 1 and 2.5 T, a ±5.0 G oscillating field, and at frequencies between 0.1 and 10.0 kHz with a Quantum Design Physical Property Measurement System (PPMS) manufactured by Quantum Design, San Diego, CA, USA. The susceptibility data were corrected for the diamagnetism of both the sample holder and the constituent atoms.
The magnetic properties of octahedral cobalt(II) complexes are usually dominated by a first-order spin–orbit coupling (SOC). The coupling between the spin (S = 3/2) and orbital momentum (L = 1)—using the T-P isomorphism [64]—in a distorted octahedral environment generates six Kramers doublets. Among these, the two lowest-energy doublets are usually the only states populated below 300 K. Consequently, a phenomenological approach based on a zero-field splitting (ZFS) of a state S = 3/2, considering only these doublets, was employed. In the spin Hamiltonian (Equation (1)), the parameters D and E represent the axial and rhombic magnetic anisotropy, respectively.
The activation energy (Ea) in Co4%@1 and Co8%@1, based on the phenomenological approach employed, was evaluated using the equation , where the value of E value is always positive.
3. Results and Discussion
3.1. Synthesis and Characterization
In this work, we present the solid-state post-synthetic partial metal exchange of Zn(II) metal ions by Co(II) ones, in a previously reported water-stable three-dimensional MOF, derived from the amino acid S-methyl-L-cysteine, with the formula {CaZn6[(S,S)-Mecysmox]3(OH)2(H2O)}·12 H2O (1) (Mecysmox = bis[S-methylcysteine]oxalyl diamide), allowing us to obtain two novel MOFs with distinct contents of Co(II) ions of formulas {CaCo0.24Zn5.76[(S,S)-Mecysmox]3(OH)2(H2O)}·14 H2O (Co4%@1) and {CaCo0.48Zn5.52[(S,S)-Mecysmox]3(OH)2(H2O)}·16 H2O (Co8%@1). Interestingly, direct synthesis attempts—adapting the previously reported procedure for 1 [63]—using the oxamidate-based ligand H2Me2-(S,S)-Mecysmox in a basic methanolic solution with mixtures of aqueous solutions of Co(NO3)2·6 H2O, Zn(NO3)2·6 H2O and CaCl2 were unsuccessful in synthesizing Co4%@1 and Co8%@1.
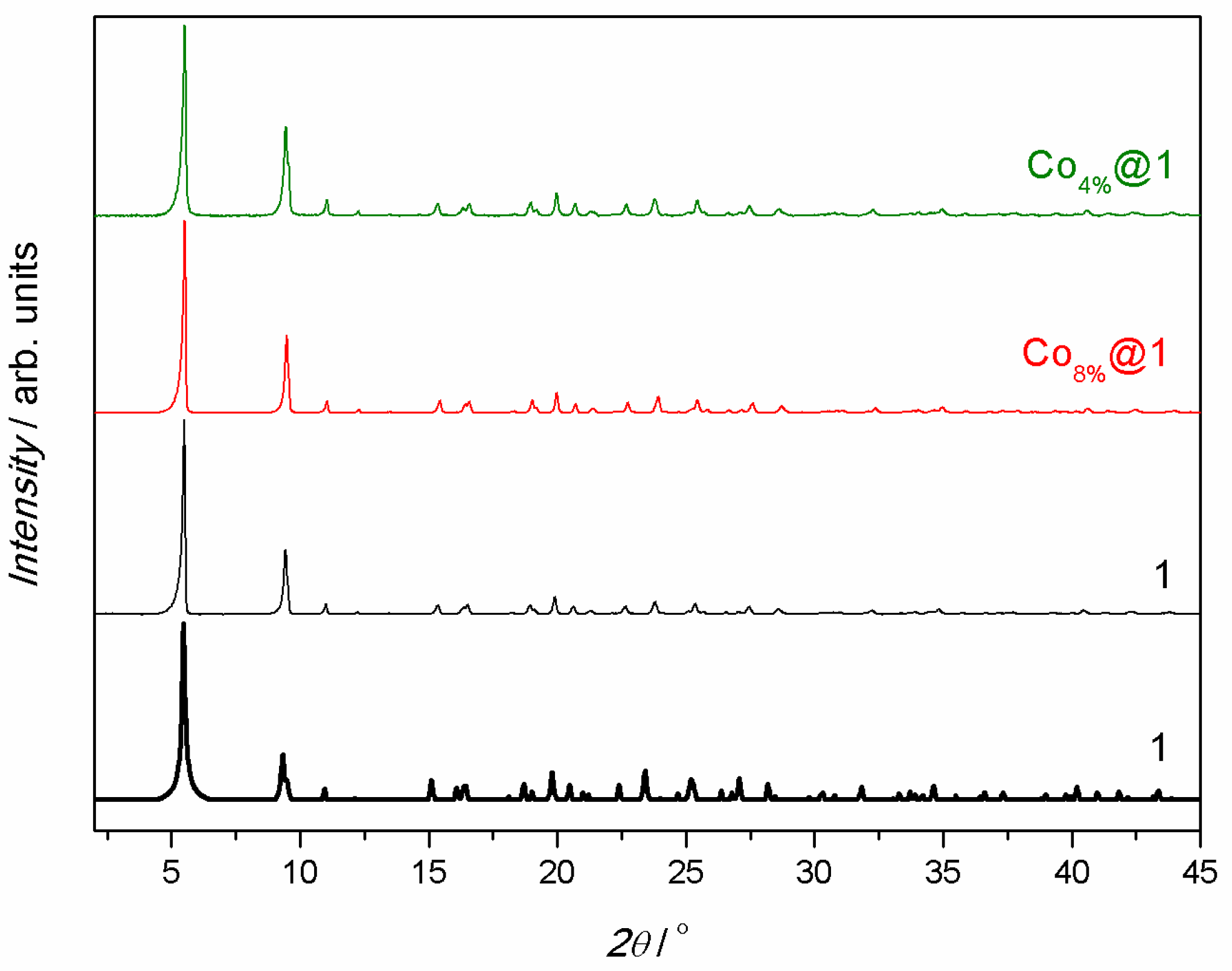
Co4%@1 and Co8%@1 were obtained by immersion of the pristine polycrystalline powder of 1 in aqueous solutions containing different amounts of Co(NO3)2·6H2O (0.01 and 0.10 g for Co4%@1 and Co8%@1, respectively) for 1 h under mild stirring. This process was carefully monitored visually, confirming the absence of powder dissolution and reprecipitation. The nature, pureness. and identity of Co4%@1 and Co8%@1 were conclusively established through a combination of physical techniques, including elemental analyses (C, H, S, and N), inductively coupled plasma mass spectrometry (ICP-MS), scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX), powder X-ray diffraction (PXRD), nitrogen adsorption isotherms at 77 K, and thermogravimetric analyses (TGAs) (see Supporting Information). ICP-MS and SEM-EDX enabled us to determine the atomic percentages of metal ions in each MOF (Tables S1 and S2), and together with elemental analyses and TGAs allowed us to establish their chemical formulas (see Section 2). A comparison of ICP-MS and SEM-EDX data for 1, Co4%@1, and Co8%@1 confirmed that only Zn(II) ions in 1 have been replaced with Co(II) ions, since the atomic ratios between M(II) and Ca(II)—where M = Zn for 1 and Zn + Co for Co4%@1 and Co8%@1—remained unchanged. These results also allowed the quantification of the percentage of substituting Co(II) ions in each compound. We determined Co(II) contents of 4 and 8%, relative to Zn(II)ions, in Co4%@1 and Co8%@1, respectively. SEM micrographs and EDX elemental mapping confirmed a homogeneous distribution of Zn(II), Ca(II), and Co(II) ions in 1, Co4%@1, and Co8%@1 (Figures S1–S3). The TGAs of Co4%@1 and Co8%@1 (Figure S4) revealed a rapid mass loss from room temperature to ca. 100 K, followed by a plateau until decomposition started at ca. 300 K. The calculated percentage weight loss values at 150 K were 14.5% for Co4%@1 and 16.8% for Co8%@1, corresponding to the release of 14 and 16 water molecules, respectively. The PXRD analyses evidenced a match between the theoretical pattern of 1, derived from previously reported single-crystal X-ray diffraction data, and the experimental patterns of each sample (Figure 2). This confirmed the purity of Co4%@1 and Co8%@1, as well as their isoreticular nature with respect to 1. Additionally, the preservation of permanent porosity in Co4%@1 and Co8%@1 after the post-synthetic partial metal exchange was demonstrated by means of nitrogen adsorption isotherms at 77 K (Figure S5). All samples exhibited fully reversible type I isotherms, characteristic of microporous materials with permanent microporosity (Figure S5). The estimated Brunauer–Emmett–Teller (BET) surface areas [706 (1), 694 (Co4%@1), and 686 m2 g−1 (Co8%@1)] as well as the calculated pore widths [0.6622 (1), 0.6387 (Co4%@1), and 0.6589 nm (Co8%@1)] (Figure S6) were nearly identical, confirming the preservation of permanent porosity. Interestingly, the presented post-synthetic partial metal exchange leads to a change in the magnetic properties of the pristine MOF 1, resulting in new MOFs of the oxamidate family, which exhibit SIM behavior (see magnetic properties below).
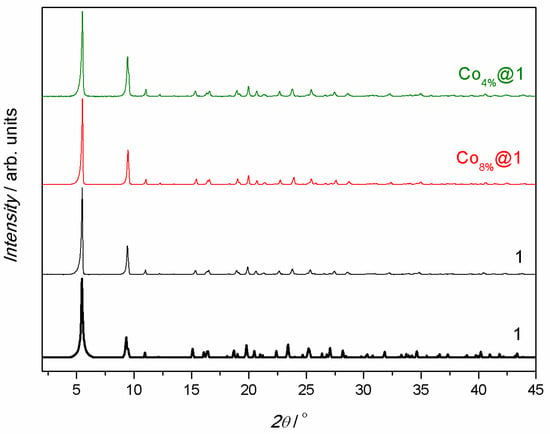
Figure 2.
Calculated (black bold lines) and experimental (solid lines) PXRD patterns of 1 (black), Co8%@1 (red), and Co4%@1 (green) in the 2θ range of 2.0–45.0° at room temperature.
3.2. Description of the Structure
Although the crystal structure of the parent MOF 1 has been previously reported, it is worth describing it here, particularly to understand the possible coordination environments and geometries of the Co(II) ions substituting those of Zn(II) in compounds Co4%@1 and Co8%@1, which, in turn, are responsible for the SIM behavior. MOF 1 crystallizes in the chiral P63 space group and consists of a chiral honeycomb three-dimensional (3D) calcium(II)–zinc(II) network (Figure 1) featuring hexagonal channels. The network is constructed from trans-oxamidato-bridged dizinc(II) units {ZnII2[(S,S)-Mecysmox]} (Figure 1a), which act as linkers between Ca(II) cations through the carboxylate groups and are further interconnected to neighboring dizinc(II) moieties by water–hydroxide groups (Figure 1a). The Zn(II) ions are five-coordinate, exhibiting a trigonal bipyramidal geometry. They coordinate one amidate nitrogen atom, one carbonyl oxygen atom, one carboxylate oxygen atom, one carboxylate oxygen atom from a neighboring dizinc(II) unit, and one water–hydroxide bridging group (in a 1:2 statistical distribution). Thus, a priori, the potential coordination environment of substituting Co(II) ions should be the same as that of the replaced Zn(II) ones. However, with a lack of crystal structure, it is difficult to establish their precise coordination environment, being possible other environments, such as octahedral. Each calcium(II) ion is nine-coordinate in a distorted monocapped square antiprismatic geometry (CaO9), built by six carboxylate oxygen atoms from six oxamidato groups and three oxygen atoms from water–hydroxide groups (Figure 1).
3.3. Magnetic Properties
3.3.1. Static (dc) Magnetic Properties
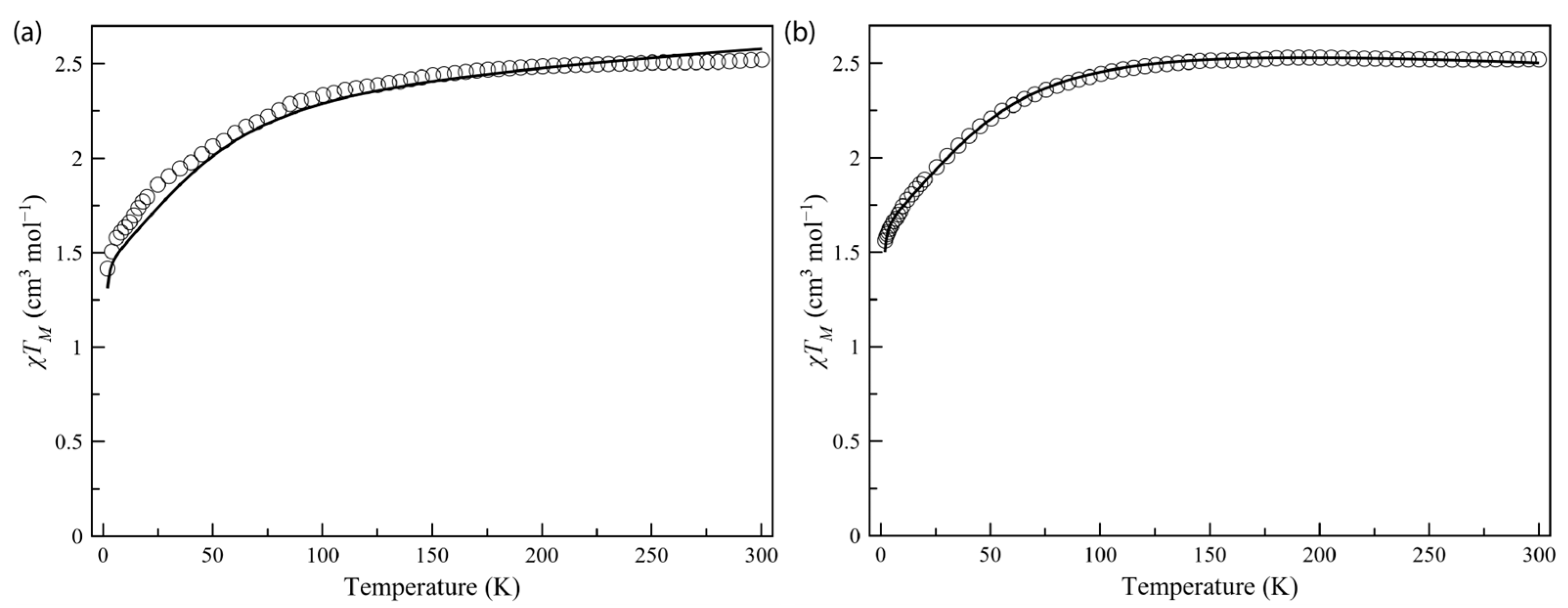
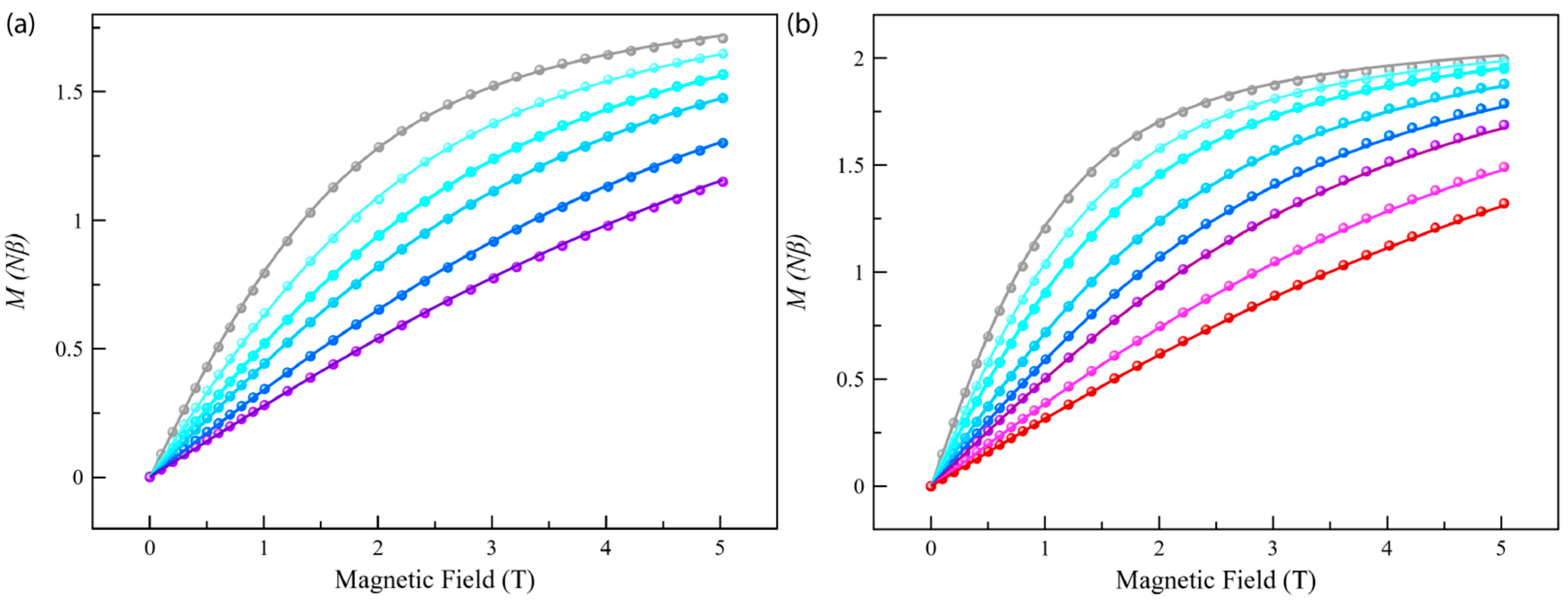
The dc magnetic properties of compounds Co4%@1 and Co8%@1, as the χMT against the T plot, are shown in Figure 3, with χM being the normalized dc molar susceptibility per one cobalt(II) per formula unit (the raw χMT against the T plot is shown in Figure S7). The χMT(T) data for Co4%@1 and Co8%@1 are typical for a high-spin d7 Co(II) site. The χMT value at room temperature is 2.52 cm3 K mol–1 for Co4%@1 and Co8%@1, which is consistent with previously reported mononuclear cobalt(II) complexes with unquenched orbital momentum contributions [37,65]. Upon cooling, χMT decreases smoothly down to 1.42 (Co4%@1) and 1.56 (Co8%@1) cm3 K mol–1 at 2 K, revealing the existence of a significant ZFS arising from the second-order spin–orbit coupling (SOC) of the Co(II) ion (Figure 3). This was further supported by the M vs. H plots, with magnetization values of 1.71 (Co4%@1) and 1.95 Nβ (Co8%@1) at 3 K and 5 T (Figure 4). These values are well below the saturation magnetization for a high-spin d7 Co(II)ion (Msat = gSNβ = 3 Nβ), which suggests the presence of significant axial magnetic anisotropy. Here, where the ground ±3/2 and excited ±1/2 Kramers doublets for D < 0, or the opposite for D > 0, are well separated due to sizeable ZFS effects operating on the quartet ground state, the magnetization for the ground Kramers doublet is the only one to be considered. Additionally, as observed in other highly anisotropic complexes reported [65], the isofield magnetization curves at low temperatures (2–10 K) are nearly superimposable for Co4%@1 and Co8%@1. Thus, M(H/T) curves were not useful in accurately determining the ZFS parameters but allowed us to conclude that the ZFS is quite large.
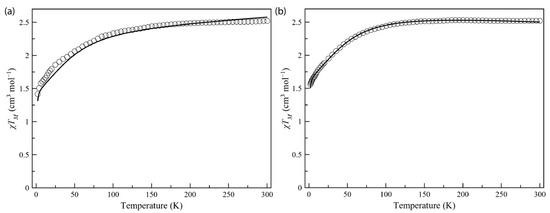
Figure 3.
Temperature dependence of the product of the direct current (dc) molar magnetic susceptibility by the temperature (χMT) for Co4%@1 (a) and Co8%@1 (b). The solid lines correspond to the best-fit curves considering ZFS effects (see text and Tables S3 and S4).
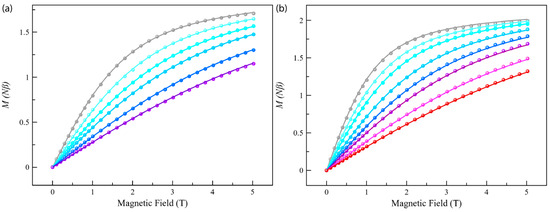
Figure 4.
Field dependence of the magnetization for Co4%@1 (a) and Co8%@1 (b) at low temperatures (T = 2.0–10 K, from grey to red). The solid lines correspond to the best-fit curves considering the ZFS effects (see text and Tables S3 and S4).
In order to quantify the relevant parameters, the experimental data for χMT vs. T and M vs. H of Co4%@1 and Co8%@1 were simultaneously simulated using the PHI program [66] through the spin Hamiltonian considering ZFS parameters (Equation (1)) for an isolated S = 3/2 state (solid line in Figure 3, Figure 4, Figures S7 and S8 and Tables S3 and S4), with axial (D) and rhombic (E) ZFS parameters. As the experimental measurements were performed on finely crushed polycrystalline samples, and components of the g-factor were considered indistinguishable. Thus, only the parallel and perpendicular components were retained ( = = and ). The best-fit values of the parameters were 2.24, 2.28, −55.25 cm–1, 3.06 cm–1, and 0.055 (Co4%@1) and 2.41, 2.35, −50.80 cm–1, 11.94 cm–1, and 0.235 (Co8%@1) cm–1, which are in agreement with the literature values [37,65].
3.3.2. Dynamic (ac) Magnetic Properties
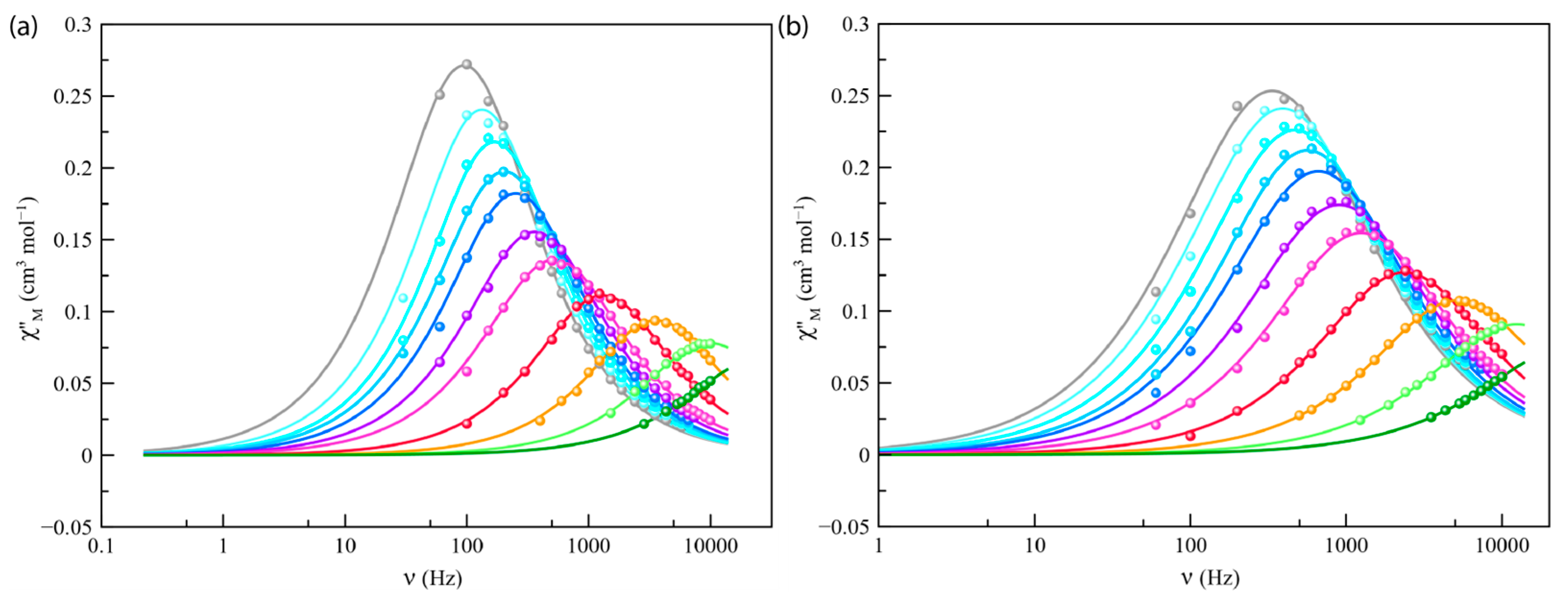
Alternating current (ac) magnetic measurements of Co4%@1 and Co8%@1 were carried out in the temperature range from 2 to 8 K to investigate their magnetization dynamics. No frequency dependence was observed for the ac components in the absence of a magnetic field (Hdc), a feature typically attributed to fast relaxation via quantum tunneling of magnetization. However, when different external static fields (1 and 2.5 kG) were applied, frequency-dependent in-phase (χM′) and out-of-phase (χM′′) signals were observed (Figure 5 and Figures S9–S11), which are consistent with a slow magnetic relaxation process typical of high-spin cobalt(II) SIMs.
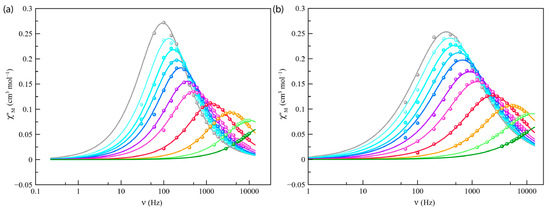
Figure 5.
Frequency dependence of χM′′ for Co4%@1 (a) and Co8%@1 (b) under applied static field of 2500 G with a +5.0 G oscillating field in the temperature range of 2–8 K (from grey to green). The solid lines represent the best-fit curves simulated using the generalized Debye model.
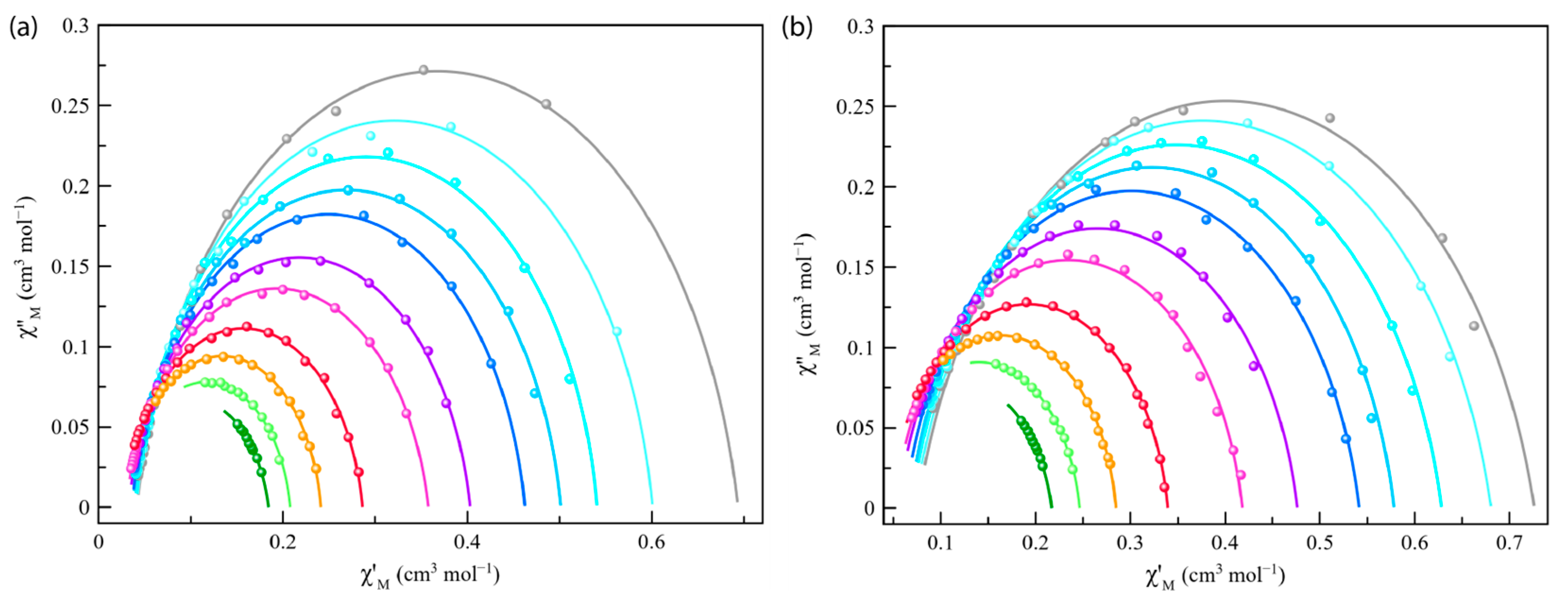
Cole–Cole plots for Co4%@1 and Co8%@1 at 2–8 K under different applied static fields (1.0–2.5 kG) (Figure 6 and Figure S12) showed almost perfect semicircles, which were fitted using the Debye model (solid lines in Figure 6 and Figure S12). The obtained low values of the α parameters at different temperatures and dc magnetic fields [α = 0.099 (8.0 K)–0.121 (2.0 K) and 0.112 (8.0 K)–0.187 (2.0 K) for Co4%@1 and Co8%@1 under applied static field of 1000 G, respectively] (see Tables S3 and S4) support a single relaxation process. Moreover, increasing values of α with decreasing temperature reveal the appearance of intermolecular interactions within both frameworks of Co4%@1 and Co8%@1. The semicircles in the Cole–Cole plots are slightly more flattened in Co8%@1 than in Co4%@1 (Figure 6 and Figure S12). Thus, slightly larger α parameters were obtained for Co8%@1 (Table S4). However, the α parameters are well below the values for spin-glass behavior (α ≥ 0.4).
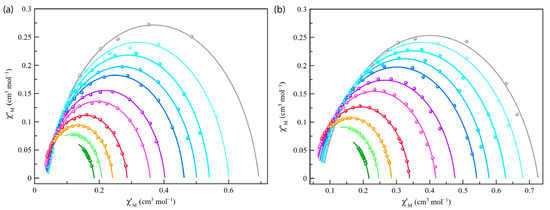
Figure 6.
Cole–Cole plots of Co4%@1 (a) and Co8%@1 (b) at 2–8 K (from grey to green) under applied static field of 2500 G. The solid lines represent the best-fit curves.
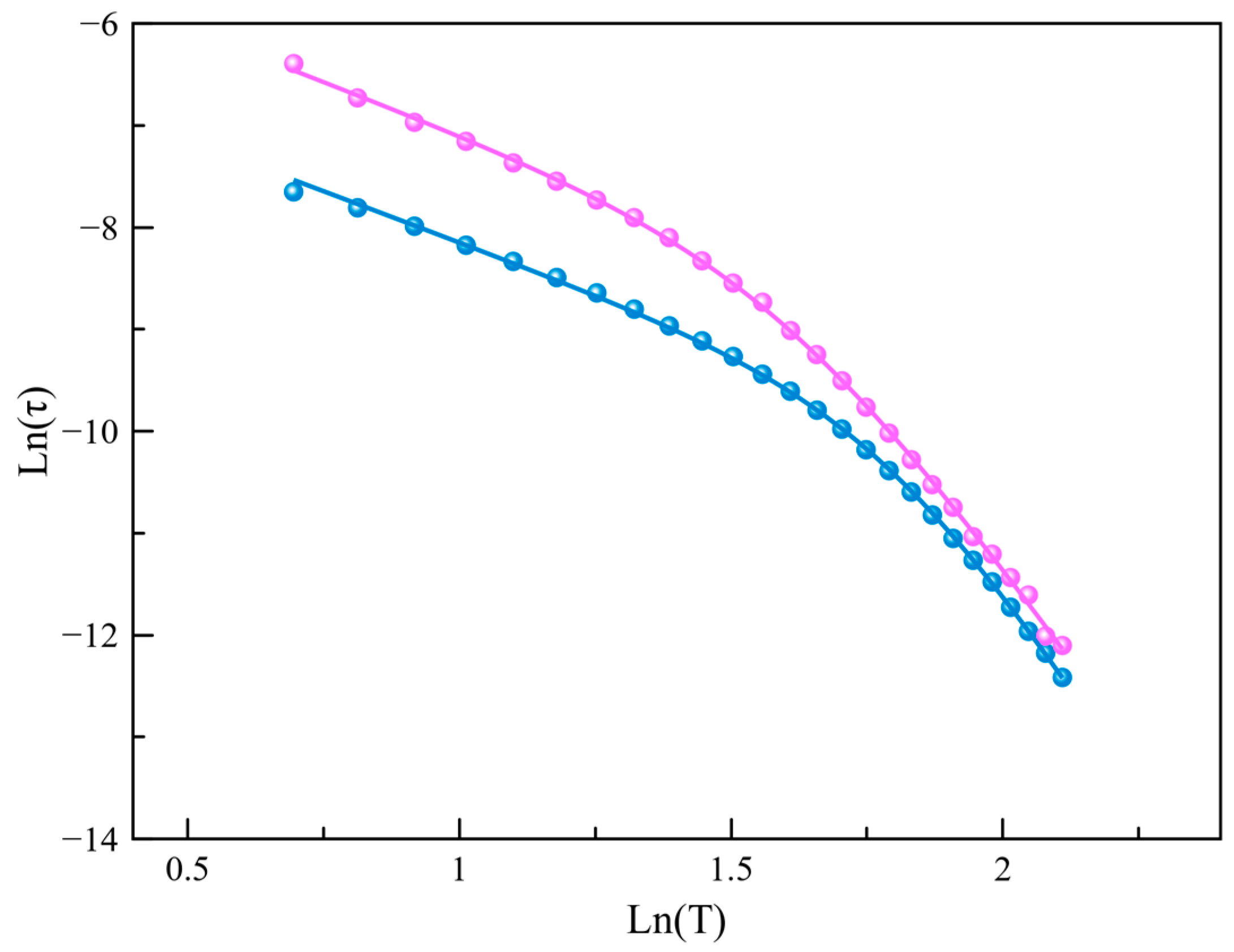
Aiming to quantify the magnetic relaxation times, a joint analysis of the χM′ and χM′′ vs. frequency (υ) data (Figure 5 and Figure S9) was performed using the generalized Debye model. Two different approaches are recommended to represent these times depending on the nature of the dominant relaxation mechanism involved. After qualitative scrutiny, we established that lnτ vs. lnT plots (Figure 7 and Figure S13) were the most appropriate for analyzing the predominant relaxation mechanisms, commonly associated with Raman or direct-like mechanisms (). Ideally, such plots should show a linear dependency with slope n. When n approaches a value close to 2 or 8, the optical or acoustic phonon-assisted Raman mechanism dominates the fastest spin reversal, while a value close to 1 indicates that the direct mechanism plays the main role. Here, we would like to note that the ideal behavior is often far away from reality in reported examples in the literature. Consequently, the confluence of various relaxation mechanisms operating at different temperatures is a common outcome. However, we aimed to keep this analysis simple and avoid overparameterization, which would eventually lead to better fitting but lack physical significance. In our case, lnτ vs. lnT plots for Co4%@1 and Co8%@1 at the two studied fields evidenced a deviation from linearity. The least-squares fit of these data yielded n values, which indicate that Raman mechanisms assisted by optical and acoustic phonons rule the relaxation of the magnetization at low and high temperatures, respectively (Table 1).
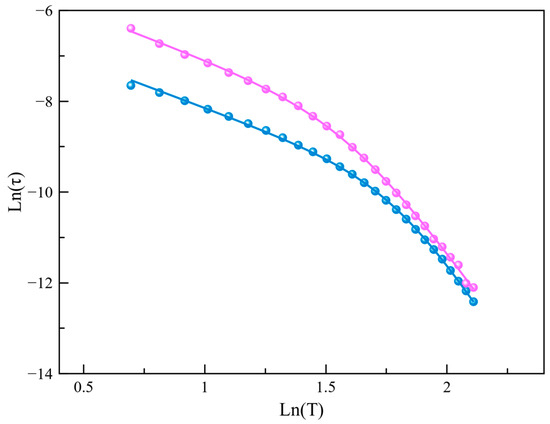
Figure 7.
The lnτ versus lnT plot for the calculated magnetic relaxation times of Co4%@1 (pink) and Co8%@1 (blue) under an applied static field of 2500 G. The solid lines represent the best-fit curves.

Table 1.
Selected parameters from the least-squares fit of the ac magnetic data of Co4%@1 and Co8%@1 a.
4. Conclusions
In this work, we applied a partial transmetalation post-synthetic strategy to achieve two new MOFs that could not be obtained by direct synthesis. Through the controlled exchange of Zn(II) with Co(II) ions in the pristine diamagnetic Zn-MOF, two MOFs with 4% and 8% Co(II) content were obtained, exhibiting SIM behavior. This underexplored strategy holds great potential to enrich the elemental composition of an MOF in a controlled manner, thereby achieving a higher degree of complexity that leads to new physicochemical properties. In particular, it serves as an excellent tool for the preparation of molecular-based magnetic materials.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/magnetochemistry10120099/s1, Figures S1–S3: SEM-EDX micrographs; Figure S4: Thermogravimetric analysis (TGA); Figures S5 and S6: Textural properties; Figures S7–S13: Magnetic dc and ac data. Table S1: Selected data for SEM-EDX; Table S2: Selected data for ICP-MS; Table S3: Selected magnetic data for Co4%@1 at different dc applied fields; Table S4: Selected magnetic data for Co8%@1 at different dc applied fields.
Author Contributions
Conceptualization, E.P., J.F.-S. and T.G.; methodology, P.E., N.M. and D.A.; formal analysis, N.M., J.F.-S. and T.G.; investigation, E.P., J.F.-S. and T.G.; data curation, J.F.-S. and N.M.; writing—original draft preparation, P.E., J.F.-S., N.M. and T.G.; writing—review and editing, J.F.-S., E.P. and T.G.; supervision, J.F.-S. and T.G.; funding acquisition, E.P. and J.F.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministero dell’Istruzione, dell’Università e della Ricerca (Italy) and the MICINN (Spain) (Project PID2022-136349OB-I00 and Excellence Unit “Maria de Maeztu” CEX2019-000919-M). D.A. also acknowledges the financial support of the European UnionNextGenerationEU under the National Recovery and Resilience Plan (NRRP) of Ministero dell’Università e della Ricerca (MUR) (Project code PE0000021, Network 4 Energy Sustainable Transition—NEST). Thanks are also extended to the “Generalitat Valenciana” (Project PROMETEO/2021/054). E.P. acknowledges the financial support of the European Research Council under the European Union’s Horizon 2020 research and innovation programme/ERC Grant Agreement No 814804, MOF-reactors. Thanks are also extended to the Ramón y Cajal Program, RYC2019-027940-I (J.F.-S.). This study forms part of the Advanced Materials programme (MFA/2022/048) and was supported by MCIN with funding from European Union NextGenerationEU (PRTR-C17.I1) and by Generalitat Valenciana.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Aromi, G.; Brechin, E.K. Synthesis of 3d Metallic Single-Molecule Magnets. In Structure and Bonding; Winpenny, R., Ed.; Springer: Berlin, Germany, 2006; Volume 122, pp. 1–67. [Google Scholar]
- AlDamen, M.A.; Clemente-Juan, J.M.; Coronado, E.; Martí-Gastaldo, C.; Gaita-Ariño, A. Mononuclear Lanthanide Single-Molecule Magnets Based on Polyoxometalates. J. Am. Chem. Soc. 2008, 130, 8874–8875. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Craig, G.A.; Murrie, M. 3d single-ion magnets. Chem. Soc. Rev. 2015, 44, 2135–2147. [Google Scholar] [CrossRef]
- Meng, Y.-S.; Jiang, S.-D.; Wang, B.-W.; Gao, S. Understanding the Magnetic Anisotropy toward Single-Ion Magnets. Acc. Chem. Res. 2016, 49, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-L.; Chen, Y.-C.; Tong, M.-L. Symmetry strategies for high performance lanthanide-based single-molecule magnets. Chem. Soc. Rev. 2018, 47, 2431–2453. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Kalita, P.; Chandrasekhar, V. Lanthanide(III)-Based Single-Ion Magnets. ACS Omega 2018, 3, 9462–9475. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E. Molecular magnetism: From chemical design to spin control in molecules, materials and devices. Nat. Rev. Mater. 2020, 5, 87–104. [Google Scholar] [CrossRef]
- Zabala-Lekuona, A.; Seco, J.M.; Colacio, E. Single-Molecule Magnets: From Mn12-ac to dysprosium metallocenes, a travel in time. Coord. Chem. Rev. 2021, 441, 213984. [Google Scholar] [CrossRef]
- Castellano, M.; Ruiz-García, R.; Cano, J.; Ferrando-Soria, J.; Pardo, E.; Fortea-Pérez, F.R.; Stiriba, S.-E.; Barros, W.P.; Stumpf, H.O.; Cañadillas-Delgado, L.; et al. Metallosupramolecular approach toward multifunctional magnetic devices for molecular spintronics. Coord. Chem. Rev. 2015, 303, 110–138. [Google Scholar] [CrossRef]
- Cornia, A.; Seneor, P. Spintronics the Molecular Way. Nat. Mater. 2017, 16, 505–506. [Google Scholar] [CrossRef]
- Gobbi, M.; Novak, M.A.; Del Barco, E. Molecular spintronics. J. Appl. Phys. 2019, 125, 240401. [Google Scholar] [CrossRef]
- Forment-Aliaga, A.; Gaita-Ariño, A. Chiral, magnetic, molecule-based materials: A chemical path toward spintronics and quantum nanodevices. J. Appl. Phys. 2022, 132, 180901. [Google Scholar] [CrossRef]
- Wang, K.; Yang, M.; Luo, J. Spintronics: Materials, Devices, and Applications; Wiley: Weinheim, Germany, 2022. [Google Scholar]
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular magnetism, quo vadis? A historical perspective from a coordination chemist viewpoint. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar] [CrossRef]
- Yuste, C.; Castellano, M.; Ferrando-Soria, J.; Stiriba, S.-E.; Marino, N.; Julve, M.; Lloret, F.; Ruiz-García, R.; Cano, J. Review: From computational design to the synthesis of molecular magnetic wires for single-molecule spintronics and quantum computing nanotechnologies. J. Coord. Chem. 2022, 75, 2359–2383. [Google Scholar] [CrossRef]
- Kumar, P.; Swain, A.; Acharya, J.; Li, Y.; Kumar, V.; Rajaraman, G.; Colacio, E.; Chandrasekhar, V. Synthesis, Structure, and Zero-Field SMM Behavior of Homometallic Dy2, Dy4, and Dy6 Complexes. Inorg. Chem. 2022, 61, 11600–11621. [Google Scholar] [CrossRef]
- Benmansour, S.; Pintado-Zaldo, C.; Martínez-Ponce, J.; Hernández-Paredes, A.; Valero-Martínez, A.; Gómez-Benmansour, M.; Gómez-García, C.J. The Versatility of Ethylene Glycol to Tune the Dimensionality and Magnetic Properties in DyIII-Anilato-Based Single-Ion Magnets. Cryst. Growth Des. 2023, 23, 1269–1280. [Google Scholar] [CrossRef]
- Little, E.J.; Mrozek, J.; Rogers, C.J.; Liu, J.; McInnes, E.J.L.; Bowen, A.M.; Ardavan, A.; Winpenny, R.E.P. Experimental realisation of multi-qubit gates using electron paramagnetic resonance. Nat. Commun. 2023, 14, 7029. [Google Scholar] [CrossRef]
- Grancha, T.; Ferrando-Soria, J.; Castellano, M.; Julve, M.; Pasán, J.; Armentano, D.; Pardo, E. Oxamato-based coordination polymers: Recent advances in multifunctional magnetic materials. Chem. Commun. 2014, 50, 7569–7585. [Google Scholar] [CrossRef]
- Estrader, M.; Salinas-Uber, J.; Barrios, L.A.; Garcia, J.; Lloyd-Williams, P.; Roubeau, O.; Teat, S.J.; Aromí, G. A Magneto-optical Molecular Device: Interplay of Spin Crossover, Luminescence, Photomagnetism, and Photochromism. Angew. Chem. Int. Ed. 2017, 56, 15622–15627. [Google Scholar] [CrossRef]
- Castellano, M.; Barros, W.P.; Ferrando-Soria, J.; Julve, M.; Lloret, F.; Pasán, J.; Ruiz-Pérez, C.; Cañadillas-Delgado, L.; Ruiz-García, R.; Cano, J. Dicopper(II) metallacyclophanes with photoswitchable oligoacene spacers: A joint experimental and computational study on molecular magnetic photoswitches. J. Coord. Chem. 2018, 71, 675–692. [Google Scholar] [CrossRef]
- Kalinke, L.H.G.; Cangussu, D.; Mon, M.; Bruno, R.; Tiburcio, E.; Lloret, F.; Armentano, D.; Pardo, E.; Ferrando-Soria, J. Metal-Organic Frameworks as Playgrounds for Reticulate Single-Molecule Magnets. Inorg. Chem. 2019, 58, 14498–14506. [Google Scholar] [CrossRef] [PubMed]
- Clérac, R.; Miyasaka, H.; Yamashita, M.; Coulon, C. Evidence for Single-Chain Magnet Behavior in a MnIII–NiII Chain Designed with High Spin Magnetic Units: A Route to High Temperature Metastable Magnets. J. Am. Chem. Soc. 2002, 124, 12837–12844. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.-C.; Liu, J.-L.; Vieru, V.; Ungur, L.; Jia, J.-H.; Chibotaru, L.F.; Lan, Y.; Wernsdorfer, W.; Gao, S.; et al. A Stable Pentagonal Bipyramidal Dy(III) Single-Ion Magnet with a Record Magnetization Reversal Barrier over 1000K. J. Am. Chem. Soc. 2016, 138, 5441–5450. [Google Scholar] [CrossRef] [PubMed]
- Escalera-Moreno, L.; Baldoví, J.J.; Gaita-Ariño, A.; Coronado, E. Exploring the High-Temperature Frontier in Molecular Nanomagnets: From Lanthanides to Actinides. Inorg. Chem. 2019, 58, 11883–11892. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Ruan, Z.-Y.; Chen, Y.-C.; Tong, M.-L. Physical stimulus and chemical modulations of bistable molecular magnetic materials. Chem. Commun. 2020, 56, 13702–13718. [Google Scholar] [CrossRef]
- McAdams, S.G.; Ariciu, A.-M.; Kostopoulos, A.K.; Walsh, J.P.S.; Tuna, F. Molecular single-ion magnets based on lanthanides and actinides: Design considerations and new advances in the context of quantum technologies. Coord. Chem. Rev. 2017, 346, 216–239. [Google Scholar] [CrossRef]
- Meihaus, K.R.; Long, J.R. Actinide-based single-molecule magnets. Dalton Trans. 2015, 44, 2517–2528. [Google Scholar] [CrossRef]
- Kindra, D.R.; Evans, W.J. Magnetic Susceptibility of Uranium Complexes. Chem. Rev. 2014, 114, 8865–8882. [Google Scholar] [CrossRef]
- Sahu, P.K.; Kharel, R.; Shome, S.; Goswami, S.; Konar, S. Understanding the unceasing evolution of Co(II) based single-ion magnets. Coord. Chem. Rev. 2023, 475, 214871. [Google Scholar] [CrossRef]
- Bamberger, H.; Albold, U.; Dubnická-Midlíková, J.; Su, C.-Y.; Deibel, N.; Hunger, D.; Hallmen, P.P.; Neugebauer, P.; Beerhues, J.; Demeshko, S.; et al. Iron(II), Cobalt(II), and Nickel(II) Complexes of Bis(sulfonamido)benzenes: Redox Properties, Large Zero-Field Splittings, and Single-Ion Magnets. Inorg. Chem. 2021, 60, 2953–2963. [Google Scholar] [CrossRef]
- Alexandru, M.-G.; Visinescu, D.; Shova, S.; Cano, J.; Moliner, N.; Lloret, F.; Julve, M. A Chain of Vertex-Sharing {CoIII2CoII2}n Squares with Single-Ion Magnet Behavior. Magnetochemistry 2023, 9, 130. [Google Scholar] [CrossRef]
- Woodall, C.; Craig, G.; Prescimone, A.; Misek, M.; Cano, J.; Faus, J.; Probert, M.R.; Parsons, S.; Moggach, S.; Martínez-Lillo, J.; et al. Pressure induced enhancement of the magnetic ordering temperature in rhenium(IV) monomers. Nat. Commun. 2016, 7, 13870. [Google Scholar] [CrossRef] [PubMed]
- Orts-Arroyo, M.; Moliner, N.; Lloret, F.; Martínez-Lillo, J. Ferromagnetic Coupling and Single-Ion Magnet Phenomenon in Mononuclear Ruthenium(III) Complexes Based on Guanine Nucleobase. Magnetochemistry 2022, 8, 93. [Google Scholar] [CrossRef]
- Vallejo, J.; Fortea-Pérez, F.R.; Pardo, E.; Benmansour, S.; Castro, I.; Krzystek, J.; Armentano, D.; Cano, J. Guest-dependent single-ion magnet behaviour in a cobalt(ii) metal–organic framework. Chem. Sci. 2016, 7, 2286–2293. [Google Scholar] [CrossRef]
- Gomez-Coca, S.; Cremades, E.; Aliaga-Alcalde, N.; Ruiz, E. Mononuclear Single-Molecule Magnets: Tailoring the Magnetic Anisotropy of First-Row Transition-Metal Complexes. J. Am. Chem. Soc. 2013, 135, 7010–7018. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Maurin, G.; Serre, C.; Cooper, A.; Férey, G. The New Age of MOFs and of Their Porous-Related Solids. Chem. Soc. Rev. 2017, 46, 3104–3107. [Google Scholar] [CrossRef]
- Freund, R.; Zaremba, O.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Dincă, M.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; et al. The Current Status of MOF and COF Applications. Angew. Chem. Int. Ed. 2021, 60, 23975–24001. [Google Scholar] [CrossRef]
- Chae, H.K.; Siberio-Pérez, D.Y.; Kim, J.; Go, Y.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A Route to High Surface Area, Porosity and Inclusion of Large Molecules in Crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef]
- Mon, M.; Bruno, R.; Ferrando-Soria, J.; Bartella, L.; Di Donna, L.; Talia, M.; Lappano, R.; Maggiolini, M.; Armentano, D.; Pardo, E. Crystallographic Snapshots of Host-Guest Interactions in Drugs@metal-Organic Frameworks: Towards Mimicking Molecular Recognition Processes. Mater. Horiz. 2018, 5, 683–690. [Google Scholar] [CrossRef]
- Fortea-Pérez, F.R.; Mon, M.; Ferrando-Soria, J.; Boronat, M.; Leyva-Pérez, A.; Corma, A.; Herrera, J.M.; Osadchii, D.; Gascon, J.; Armentano, D.; et al. The MOF-Driven Synthesis of Supported Palladium Clusters with Catalytic Activity for Carbene-Mediated Chemistry. Nat. Mater. 2017, 16, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keefee, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Grancha, T.; Mon, M.; Ferrando-Soria, J.; Gascon, J.; Seoane, B.; Ramos-Fernandez, E.V.; Armentano, D.; Pardo, E. Tuning the selectivity of light hydrocarbons in natural gas in a family of isoreticular MOFs. J. Mater. Chem. A 2017, 5, 11032–11039. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef]
- Mon, M.; Ferrando-Soria, J.; Grancha, T.; Fortea-Pérez, F.R.; Gascon, J.; Leyva-Pérez, A.; Armentano, D.; Pardo, E. Selective Gold Recovery and Catalysis in a Highly Flexible Methionine-Decorated Metal–Organic Framework. J. Am. Chem. Soc. 2016, 138, 7864–7867. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Flexible Porous Metal-Organic Frameworks for a Controlled Drug Delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef]
- Mínguez-Espallargas, G.; Coronado, E. Magnetic functionalities in MOFs: From the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef]
- Thorarinsdottir, A.E.; Harris, T.D. Metal–Organic Framework Magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef]
- Mon, M.; Bruno, R.; Ferrando-Soria, J.; Armentano, D.; Pardo, E. Metal–organic framework technologies for water remediation: Towards a sustainable ecosystem. J. Mater. Chem. A 2018, 6, 4912–4947. [Google Scholar] [CrossRef]
- Escamilla, P.; Guerra, W.D.; Leyva-Pérez, A.; Armentado, D.; Ferrando-Soria, J.; Pardo, E. Metal–organic frameworks as chemical nanoreactors for the preparation of catalytically active metal compounds. Chem. Commun. 2023, 59, 836–851. [Google Scholar] [CrossRef]
- Yuan, S.; Zou, L.; Qin, J.-S.; Li, J.; Huang, L.; Feng, L.; Wang, X.; Bosch, M.; Alsalme, A.; Cagin, T.; et al. Construction of hierarchically porous metal–organic frameworks through linker labilization. Nat. Commun. 2017, 8, 15356. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Wang, H.; Canossa, S.; Wuttke, S.; Yaghi, O.M. Pore Chemistry of Metal–Organic Frameworks. Adv. Funct. Mater. 2020, 30, 2000238. [Google Scholar] [CrossRef]
- Grancha, T.; Ferrando-Soria, J.; Zhou, H.-C.; Gascon, J.; Seoane, B.; Pasán, J.; Fabelo, O.; Julve, M.; Pardo, E. Postsynthetic Improvement of the Physical Properties in a Metal–Organic Framework through a Single Crystal to Single Crystal Transmetallation. Angew. Chem. Int. Ed. 2015, 54, 6521–6525. [Google Scholar] [CrossRef]
- Mandal, S.; Natarajan, S.; Mani, P.; Pankajakshan, A. Post-Synthetic Modification of Metal–Organic Frameworks Toward Applications. Adv. Funct. Mater. 2021, 31, 2006291. [Google Scholar] [CrossRef]
- Miyasaka, H.; Nakata, K.; Sugiura, K.; Yamashita, M.; Clérac, R. A Three-Dimensional Ferrimagnet Composed of Mixed-Valence Mn4 Clusters Linked by an {Mn[N(CN)2]6}4− Unit. Angew. Chem. Int. Ed. 2004, 43, 707–711. [Google Scholar] [CrossRef]
- Liu, K.; Li, K.; Zhang, X.; Shi, W.; Cheng, P. Constraining and Tuning the Coordination Geometry of a Lanthanide Ion in Metal–Organic Frameworks: Approach toward a Single-Molecule Magnet. Inorg. Chem. 2015, 54, 10224–10231. [Google Scholar] [CrossRef]
- Aulakh, D.; Pyser, J.B.; Zhang, X.; Yakovenko, A.A.; Dunbar, K.R.; Wriedt, M. Metal–Organic Frameworks as Platforms for the Controlled Nanostructuring of Single-Molecule Magnets. J. Am. Chem. Soc. 2015, 137, 9254–9257. [Google Scholar] [CrossRef]
- Mon, M.; Pascual-Álvarez, A.; Grancha, T.; Cano, J.; Ferrando-Soria, J.; Lloret, F.; Gascon, J.; Pasán, J.; Armentano, D.; Pardo, E. Solid-State Molecular Nanomagnet Inclusion into a Magnetic Metal–Organic Framework: Interplay of the Magnetic Properties. Chem. Eur. J. 2016, 22, 539–545. [Google Scholar] [CrossRef]
- Wakizaka, M.; Ishikawa, R.; Tanaka, H.; Gupta, S.; Takaishi, S.; Yamashita, M. Creation of a Fiel-Induced Co(II) Single-Ion Magnet by doping into a Zn(II) Diamagnetic Metal-Organic Framework. Small 2023, 19, 2301966. [Google Scholar] [CrossRef]
- Escamilla, P.; Bartella, L.; Sanz-Navarro, S.; Percoco, R.M.; Di Donna, L.; Prejanò, M.; Marino, T.; Ferrando-Soria, J.; Armentano, D.; Leyva-Pérez, A.; et al. Degradation of Penicillinic Antibiotics and β-Lactamase Enzymatic Catalysis in a Biomimetic Zn-Based Metal–Organic Framework. Chem. Eur. J. 2023, 29, e202301325. [Google Scholar] [CrossRef]
- Lloret, F.; Julve, M.; Cano, J.; Ruiz-García, R.; Pardo, E. Magnetic properties of six-coordinated high-spin cobalt(II) complexes: Theoretical background and its application. Inorg. Chim. Acta 2008, 361, 3432–3445. [Google Scholar] [CrossRef]
- Rabelo, R.; Toma, L.; Julve, M.; Lloret, F.; Pasán, J.; Cangussu, D.; Ruiz-García, R.; Cano, J. How the spin state tunes the slow magnetic relaxation field dependence in spin crossover cobalt(II) complexes. Dalton Trans. 2024, 53, 5507–5520. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange–coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).