Compatibility of LaFe13−x−yMnxSiyH1.6 and Eutectic Liquid GaInSn Alloy †
Abstract
:1. Introduction
2. Materials and Methods
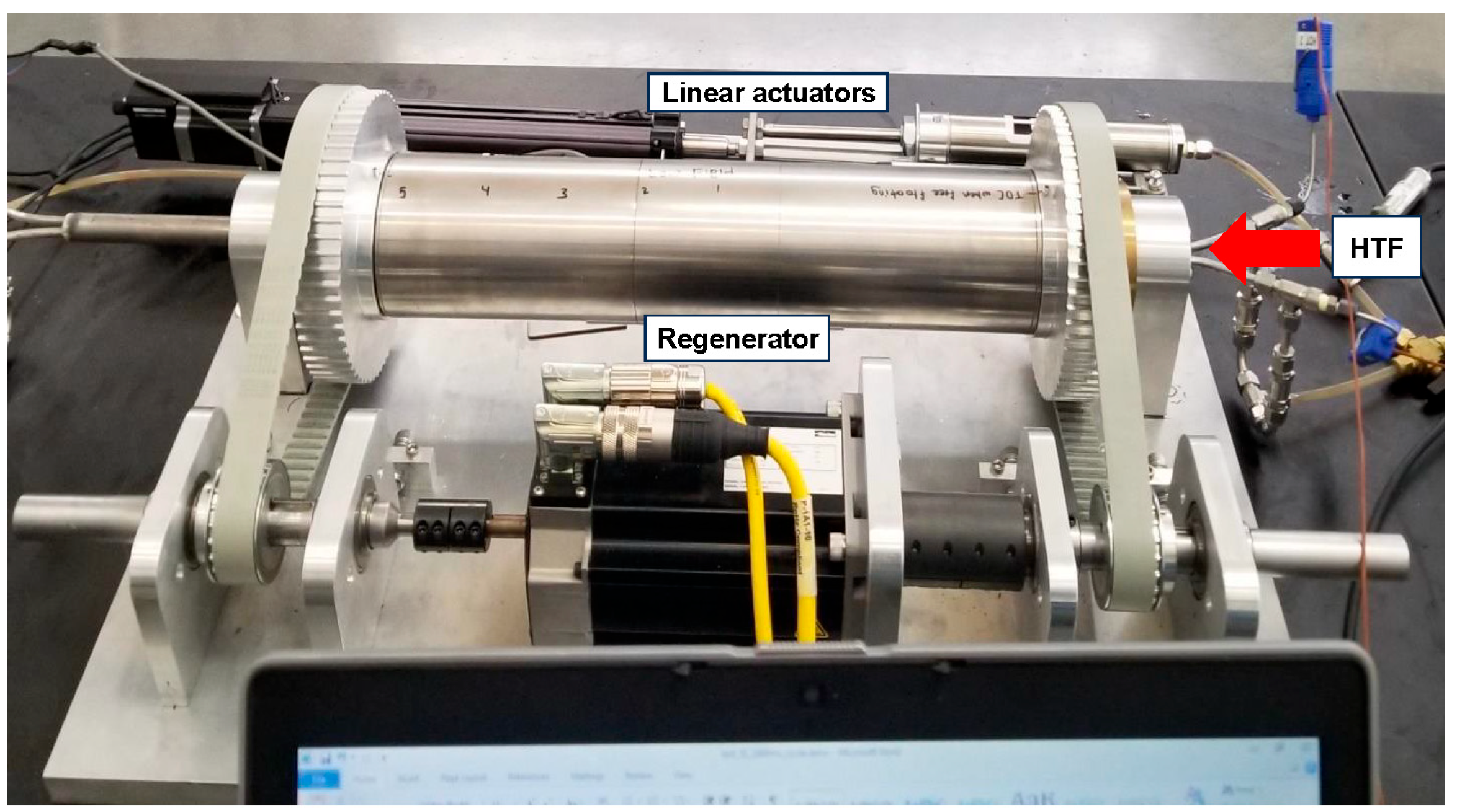
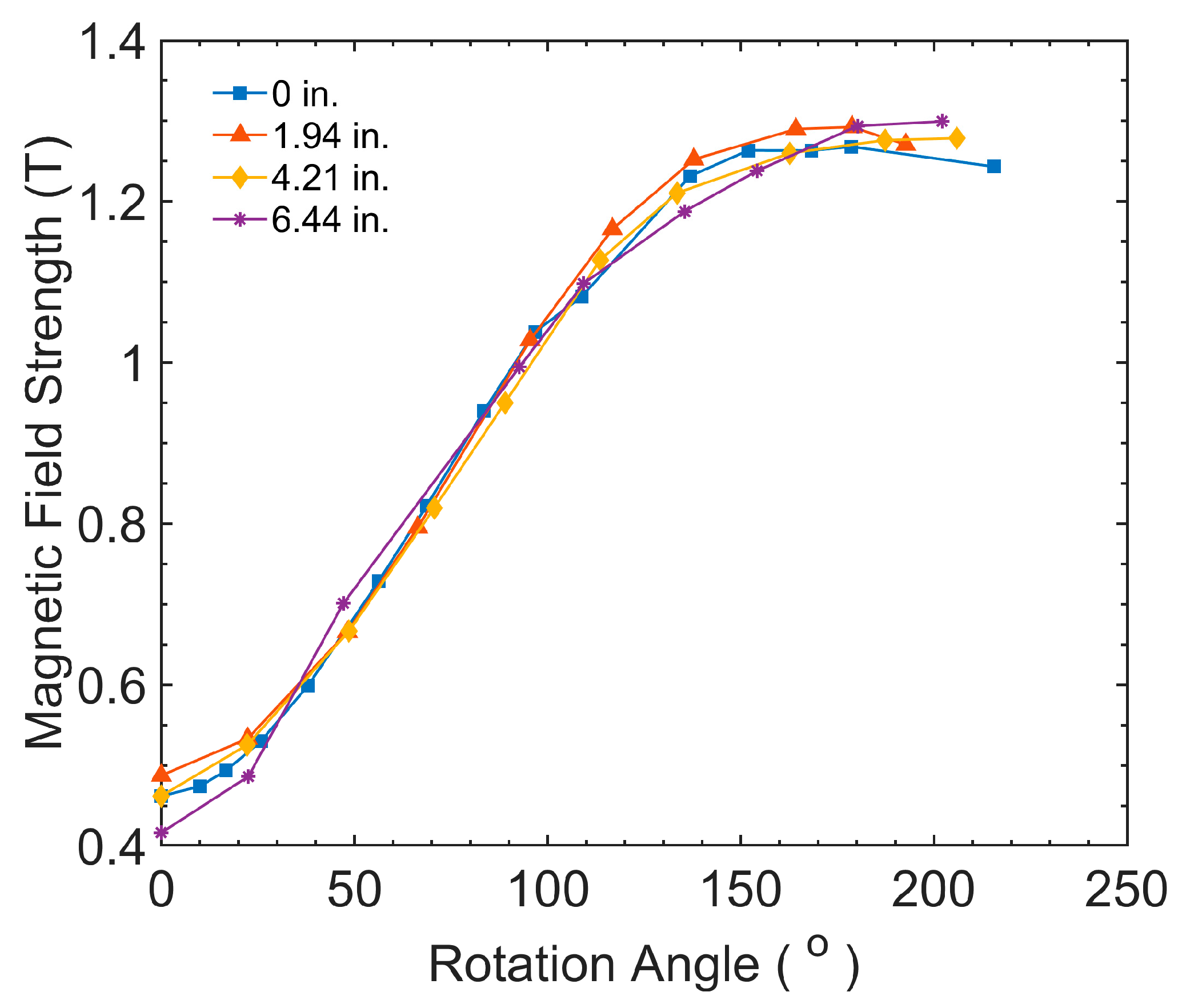
2.1. AMR Experiments
2.2. La-Fe-Si-Mn-H MCM Powder Preparation
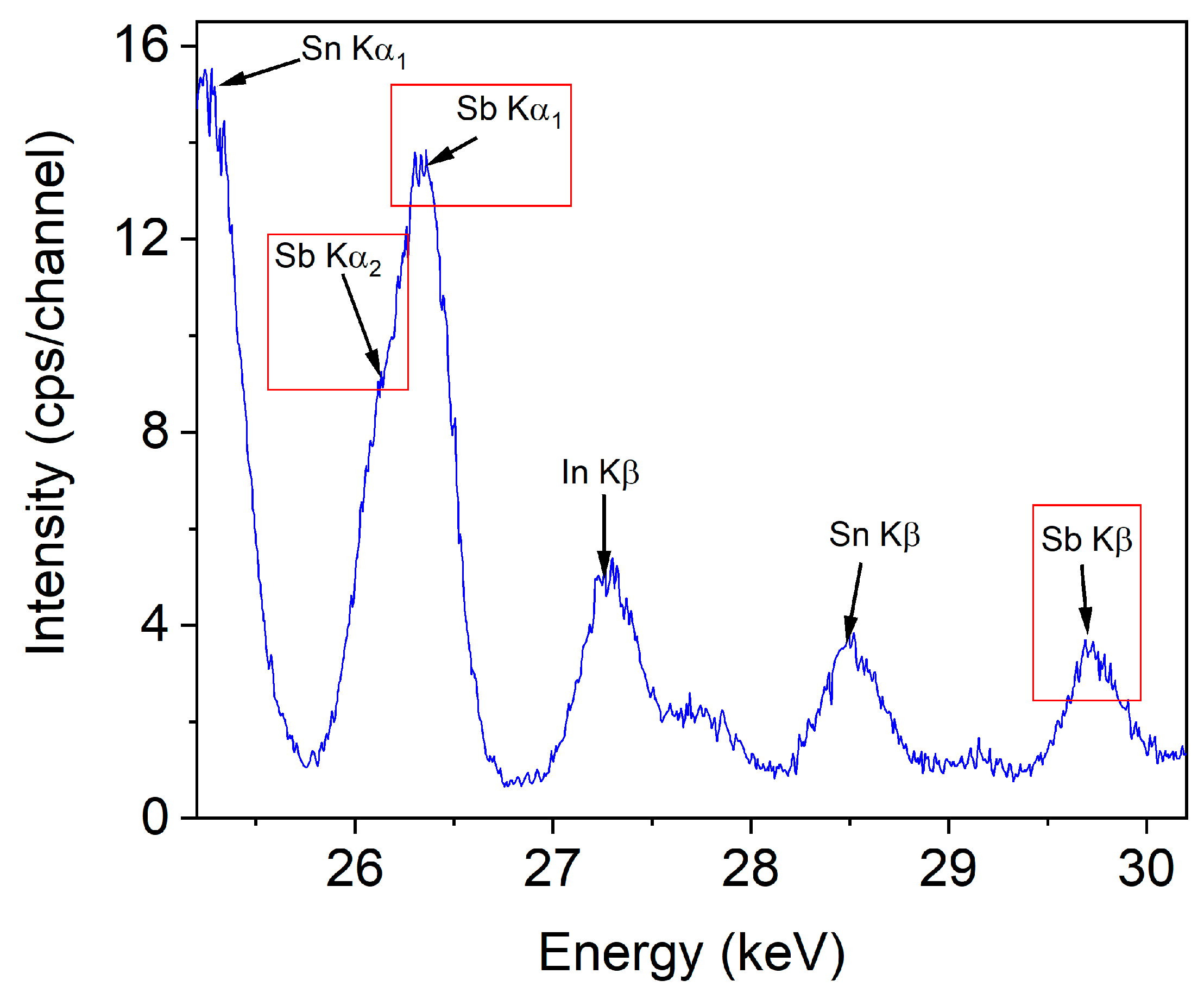
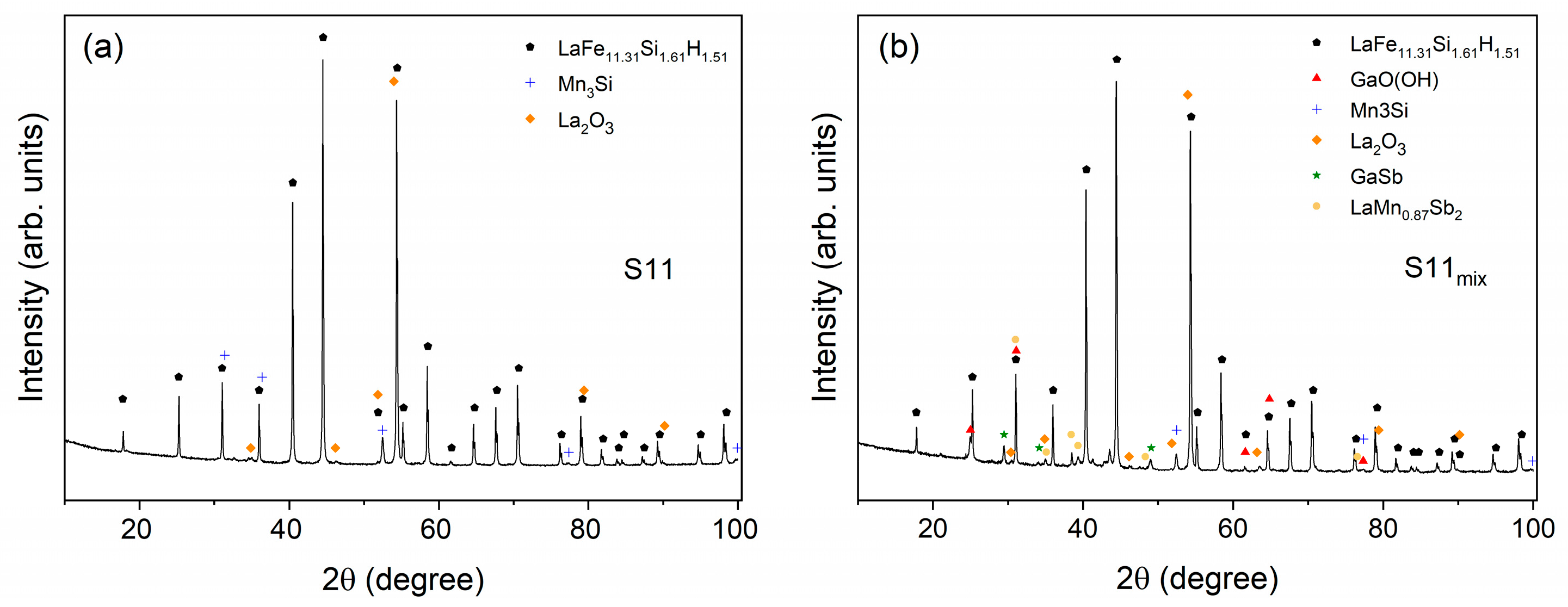
2.3. X-ray Diffraction and Fluorescence
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koshkid’ko, Y.S.; Dilmieva, E.T.; Cwik, J.; Rogacki, K.; Kowalska, D.; Kamantsev, A.P.; Koledov, V.V.; Mashirov, A.V.; Shavrov, V.G.; Valkov, V.I.; et al. Giant reversible adiabatic temperature change and isothermal heat transfer of MnAs single crystals studied by direct method in high magnetic fields. J. Alloys Compd. 2019, 798, 810–819. [Google Scholar] [CrossRef]
- Zhang, M.K.; Abdelaziz, O.; Momen, A.M.; Abu-Heiba, A. A numerical analysis of a magnetocaloric refrigerator with a 16-layer regenerator. Sci. Rep. 2017, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Pecharsky, V.K.; Gschneidner, K.A. Giant magnetocaloric effect in Gd5(Si2Ge2). Phys. Rev. Lett. 1997, 78, 4494–4497. [Google Scholar] [CrossRef]
- Barcza, A.; Katter, M.; Zellmann, V.; Russek, S.; Jacobs, S.; Zimm, C. Stability and Magnetocaloric Properties of Sintered La(Fe, Mn, Si)13Hz Alloys. IEEE Trans. Magn. 2011, 47, 3391–3394. [Google Scholar] [CrossRef]
- Hu, F.X.; Shen, B.G.; Sun, J.R.; Wang, G.J.; Cheng, Z.H. Very large magnetic entropy change near room temperature in LaFe11.2Co0.7Si1.1. Appl. Phys. Lett. 2002, 80, 826–828. [Google Scholar] [CrossRef]
- Fujita, A.; Fujieda, S.; Hasegawa, Y.; Fukamichi, K. Itinerant-electron metamagnetic transition and large magnetocaloric effects in La(FexSi1−x)13 compounds and their hydrides. Phys. Rev. B 2003, 67, 104416. [Google Scholar] [CrossRef]
- Wada, H.; Tanabe, Y. Giant magnetocaloric effect of MnAs1−xSbx. Appl. Phys. Lett. 2001, 79, 3302–3304. [Google Scholar] [CrossRef]
- Tegus, O.; Brück, E.; Buschow, K.H.J.; de Boer, F.R. Transition-metal-based magnetic refrigerants for room-temperature applications. Nature 2002, 415, 150–152. [Google Scholar] [CrossRef]
- Katter, M.; Zellmann, V.; Reppel, G.W.; Uestuener, K. Magnetocaloric Properties of La(Fe, Co, Si)13 Bulk Material Prepared by Powder Metallurgy. IEEE Trans. Magn. 2008, 44, 3044–3047. [Google Scholar] [CrossRef]
- Barclay, J.A.; Steyert, W.A. Active Magnetic Regenerator. US Patent 4,332,135, 6 January 1982. [Google Scholar]
- Gomez, J.R.; Garcia, R.F.; Catoira, A.D.; Gomez, M.R. Magnetocaloric effect: A review of the thermodynamic cycles in magnetic refrigeration. Renew. Sustain. Energ. Rev. 2013, 17, 74–82. [Google Scholar] [CrossRef]
- Kamran, M.S.; Ahmad, H.O.; Wang, H.S. Review on the developments of active magnetic regenerator refrigerators—Evaluated by performance. Renew. Sustain. Energ. Rev. 2020, 133, 16. [Google Scholar] [CrossRef]
- Manservisi, S.; Menghini, F. A CFD four parameter heat transfer turbulence model for engineering applications in heavy liquid metals. Int. J. Heat Mass Transf. 2014, 69, 312–326. [Google Scholar] [CrossRef]
- Niedermeier, K. A perspective on high-temperature heat storage using liquid metal as heat transfer fluid. Energy Storage 2023, 5, e530. [Google Scholar] [CrossRef]
- Sarvghad, M.; Ong, T.C.; Bell, S.; Rumman, R.; Maher, S.D.; Woodcock, J.W.; Will, G.; Andersson, G.; Lewis, D.A.; Steinberg, T.A. On the compatibility of liquid sodium as heat transfer fluid for advanced concentrated solar thermal energy systems. Sol. Energy Mater. Sol. Cells 2022, 246, 111897. [Google Scholar] [CrossRef]
- Liu, G.L.; Liu, J. Convective Cooling of Compact Electronic Devices Via Liquid Metals with Low Melting Points. J. Heat Transf.-Trans. Asme 2021, 143, 050801. [Google Scholar] [CrossRef]
- Wang, Q.M.; Cheng, X.M.; Li, Y.Y.; Yu, G.M.; Liu, Z. High-temperature corrosion of Sn-Bi-Zn-Ga alloys as heat transfer fluid. Rare Met. 2021, 40, 2221–2229. [Google Scholar] [CrossRef]
- Scarpa, F.; Slimani, S. Galinstan liquid metal as the heat transfer fluid in magnetic refrigeration. Appl. Therm. Eng. 2023, 232, 120971. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Lei, J.M.; Li, F.J.; Li, L.; Li, S.M.; Huang, Y.B.; Feng, K.; Tan, Q.; Lu, X.L. Investigation of the heat transfer characteristics of Galinstan liquid metal driven by electromagnetism. Appl. Therm. Eng. 2023, 230, 120776. [Google Scholar] [CrossRef]
- Wang, C.T.; Lu, Q.; Liu, Y.; Huang, H.J.; Sun, J. Progressive review of heat transfer enhancement technologies in 2010–2020. Sustain. Energy Technol. Assess. 2023, 56, 103121. [Google Scholar] [CrossRef]
- Zhang, X.D.; Yang, X.H.; Zhou, Y.X.; Rao, W.; Gao, J.Y.; Ding, Y.J.; Shu, Q.Q.; Liu, J. Experimental investigation of galinstan based minichannel cooling for high heat flux and large heat power thermal management. Energy Convers. Manag. 2019, 185, 248–258. [Google Scholar] [CrossRef]
- Zhang, R.; Hodes, M.; Lower, N.; Wilcoxon, R. Water-Based Microchannel and Galinstan-Based Minichannel Cooling beyond 1 kW/cm2 Heat Flux. IEEE Trans. Compon. Packag. Manuf. Technol. 2015, 5, 762–770. [Google Scholar] [CrossRef]
- Hodes, M.; Zhang, R.; Lam, L.S.; Wilcoxon, R.; Lower, N. On the Potential of Galinstan-Based Minichannel and Minigap Cooling. IEEE Trans. Compon. Packag. Manuf. Technol. 2014, 4, 46–56. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Diakonov, I.I.; Benezeth, P.; Gurevich, V.M.; Gavrichev, K.S.; Gorbunov, V.E.; Dandurand, J.L.; Schott, J.; Khodakovsky, I.L. Thermodynamic properties of gallium hydroxide oxide (alpha-GaOOH) at temperatures to 700 K. Eur. J. Mineral. 1997, 9, 941–951. [Google Scholar] [CrossRef]
- Kumar, V.B.; Gedanken, A.; Porat, Z.e. Facile synthesis of gallium oxide hydroxide by ultrasonic irradiation of molten gallium in water. Ultrason. Sonochem. 2015, 26, 340–344. [Google Scholar] [CrossRef] [PubMed]


| Powder | Composition | Curie Temperature (°C) |
|---|---|---|
| S1 | LaFe11.37Mn0.34Si1.29H1.6 | 19.7 |
| S2 | LaFe11.38Mn0.32Si1.30H1.6 | 22.2 |
| S3 | LaFe11.42Mn0.32Si1.27H1.6 | 23.6 |
| S4 | LaFe11.44Mn0.31Si1.25H1.6 | 26.9 |
| S5 | LaFe11.40Mn0.31Si1.29H1.6 | 28.3 |
| S6 | LaFe11.47Mn0.29Si1.24H1.6 | 30.5 |
| S7 | LaFe11.48Mn0.27Si1.24H1.6 | 32.0 |
| S8 | LaFe11.47Mn0.27Si1.27H1.6 | 33.5 |
| S9 | LaFe11.50Mn0.24Si1.26H1.6 | 36.3 |
| S10 | LaFe11.50Mn0.22Si1.27H1.6 | 37.7 |
| S11 | LaFe11.54Mn0.23Si1.23H1.6 | 41.0 |
| Powder | LaFe11.31Si1.61H1.51 I | LaFe11.31Si1.61H1.51 II | GaO(OH) | La2O3 | Mn3Si | GaSb | LaMn0.87Sb2 |
|---|---|---|---|---|---|---|---|
| S1 | 8.1 | 88.2 | 0 | 0.5 | 3.1 | 0 | 0 |
| S2 | 22.5 | 74.1 | 0 | 0.6 | 2.8 | 0 | 0 |
| S3 | 23.0 | 73.5 | 0 | 0.4 | 3.1 | 0 | 0 |
| S4 | 31.9 | 63.5 | 0 | 0.4 | 4.2 | 0 | 0 |
| S5 | 54.7 | 42.3 | 0 | 0.4 | 2.5 | 0 | 0 |
| S6 | 63.2 | 31.8 | 0 | 0.4 | 4.6 | 0 | 0 |
| S7 | 93.2 | 1.5 | 0 | 0.8 | 4.5 | 0 | 0 |
| S8 | 93.8 | 1.3 | 0 | 0.5 | 4.4 | 0 | 0 |
| S9 | 94.3 | 0.9 | 0 | 0.5 | 4.3 | 0 | 0 |
| S10 | 93.3 | 1.2 | 0 | 0.6 | 4.9 | 0 | 0 |
| S11 | 93.9 | 0.2 | 0 | 0.4 | 5.5 | 0 | 0 |
| S1mix | 55.7 | 36.7 | 4.4 | 0.6 | 2.6 | 0 | 0 |
| S2mix | 78.9 | 14.3 | 2.4 | 0.8 | 3.5 | 0 | 0 |
| S3mix | 75 | 12.2 | 9.3 | 0.4 | 3.1 | 0 | 0 |
| S4mix | 79.3 | 11 | 7.3 | 0.5 | 1.9 | 0 | 0 |
| S5mix | 90.9 | 0.3 | 6.3 | 0.5 | 2.0 | 0 | 0 |
| S6mix | 87.2 | 0.2 | 10.3 | 0.5 | 1.8 | 0 | 0 |
| S7mix | 86.5 | 1.9 | 9.1 | 0.5 | 2.0 | 0 | 0 |
| S8mix | 94.8 | 0 | 2.2 | 0.5 | 2.4 | 0 | 0 |
| S9mix | 92.1 | 0 | 4.4 | 0.5 | 2.7 | 0.3 | 0 |
| S10mix | 92.5 | 0 | 4.1 | 0.5 | 2.5 | 0.3 | 0 |
| S11mix | 87.3 | 0 | 8.3 | 0.4 | 2.5 | 0.9 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brechtl, J.; Rendall, J.; Zhang, M.; Koehler, M.R.; Nawaz, K.; Momen, A.M. Compatibility of LaFe13−x−yMnxSiyH1.6 and Eutectic Liquid GaInSn Alloy. Magnetochemistry 2024, 10, 13. https://doi.org/10.3390/magnetochemistry10020013
Brechtl J, Rendall J, Zhang M, Koehler MR, Nawaz K, Momen AM. Compatibility of LaFe13−x−yMnxSiyH1.6 and Eutectic Liquid GaInSn Alloy. Magnetochemistry. 2024; 10(2):13. https://doi.org/10.3390/magnetochemistry10020013
Chicago/Turabian StyleBrechtl, Jamieson, Joseph Rendall, Mingkan Zhang, Michael R. Koehler, Kashif Nawaz, and Ayyoub M. Momen. 2024. "Compatibility of LaFe13−x−yMnxSiyH1.6 and Eutectic Liquid GaInSn Alloy" Magnetochemistry 10, no. 2: 13. https://doi.org/10.3390/magnetochemistry10020013
APA StyleBrechtl, J., Rendall, J., Zhang, M., Koehler, M. R., Nawaz, K., & Momen, A. M. (2024). Compatibility of LaFe13−x−yMnxSiyH1.6 and Eutectic Liquid GaInSn Alloy. Magnetochemistry, 10(2), 13. https://doi.org/10.3390/magnetochemistry10020013






