Novel Hybrid Ferromagnetic Fe–Co/Nanodiamond Nanostructures: Influence of Carbon on Their Structural and Magnetic Properties
Abstract
:1. Introduction
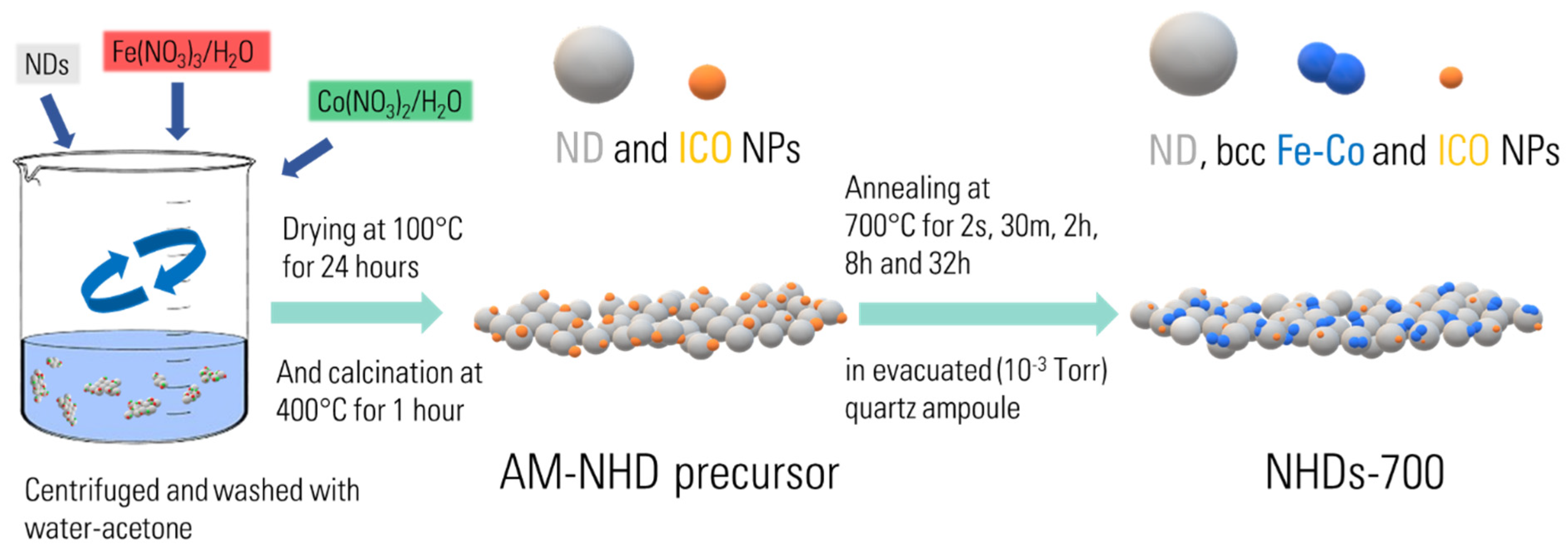
2. Experimental Section
2.1. Materials Synthesis
2.2. Materials Characterization
3. Results and Discussion
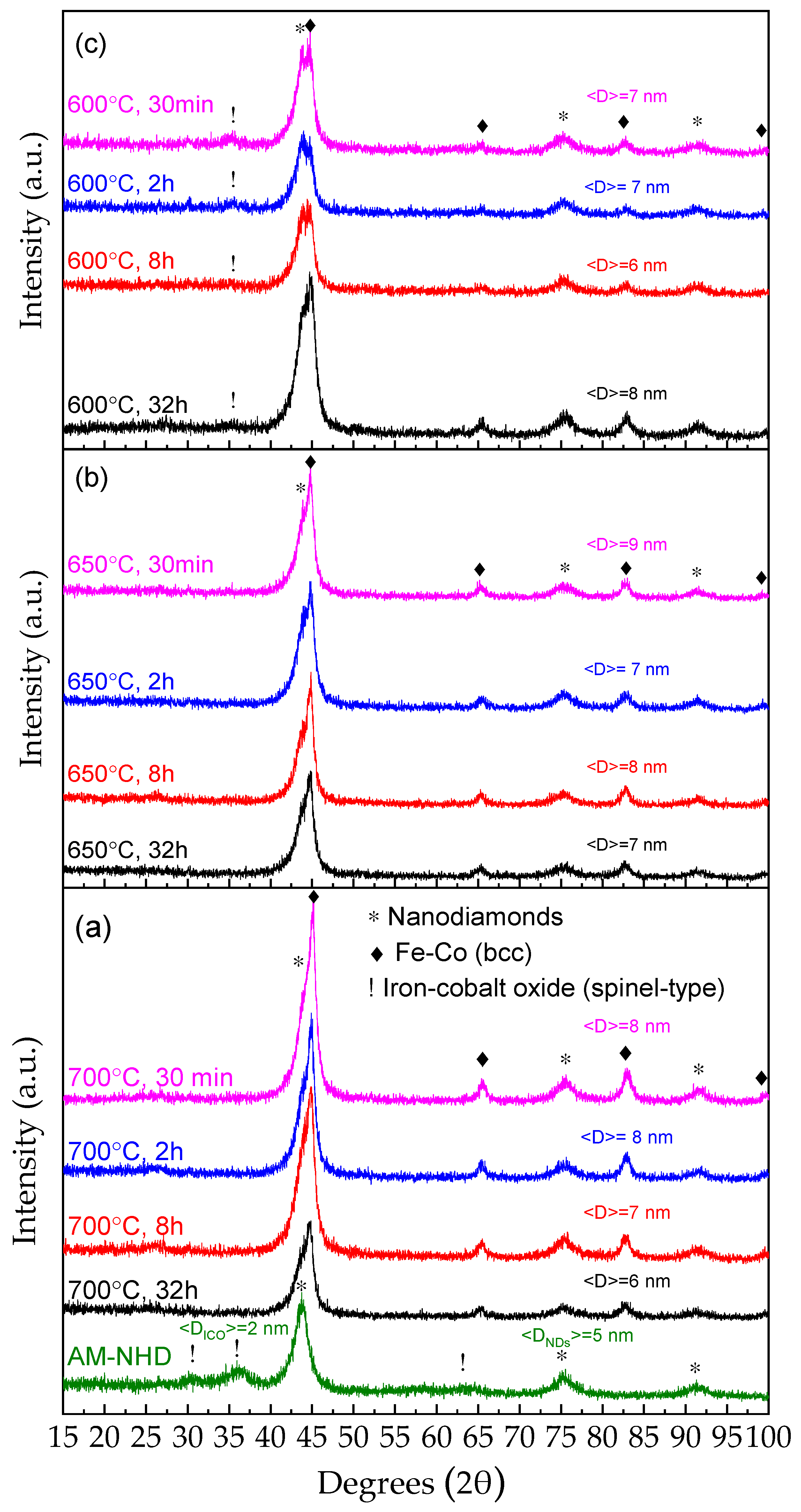
3.1. XRD
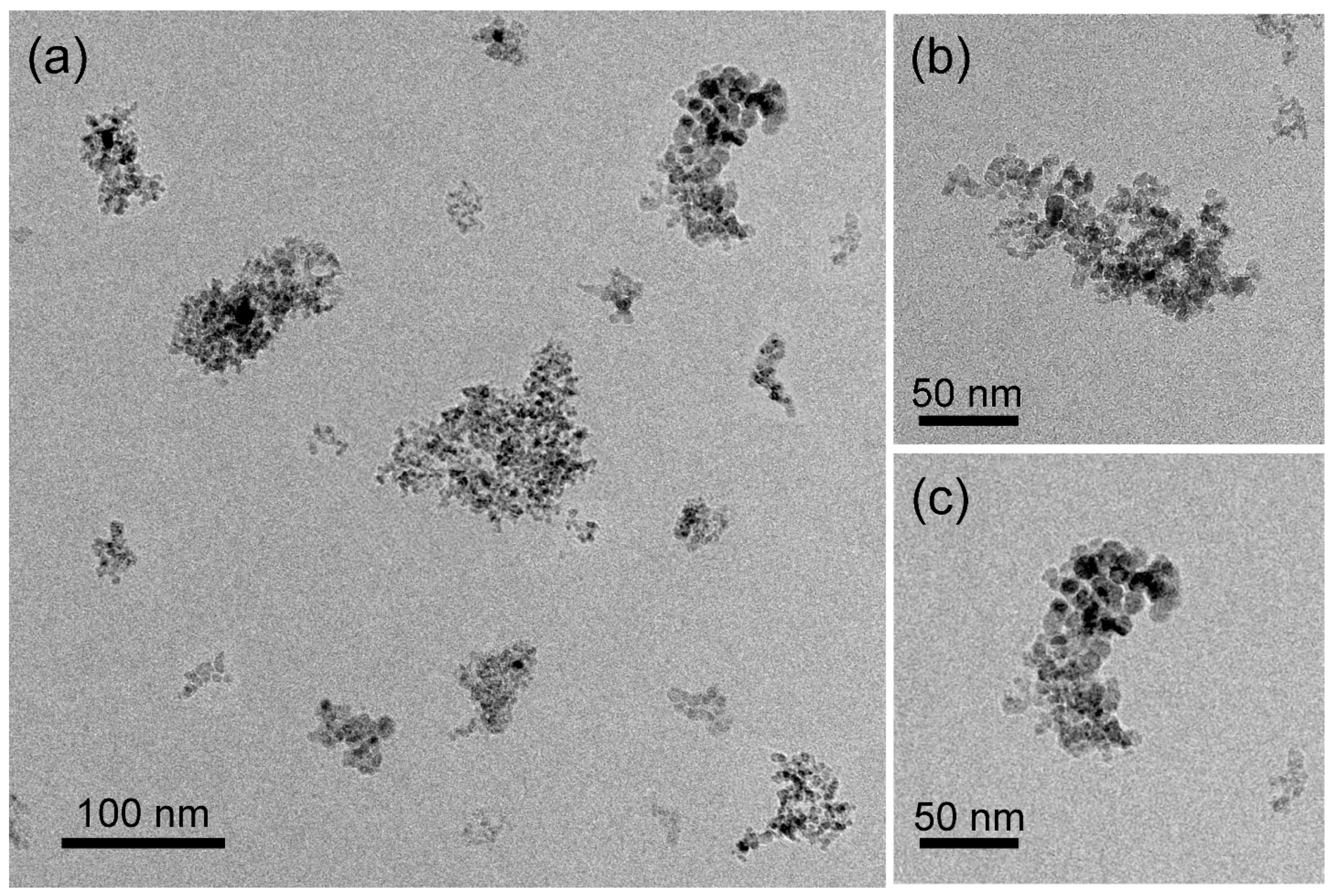
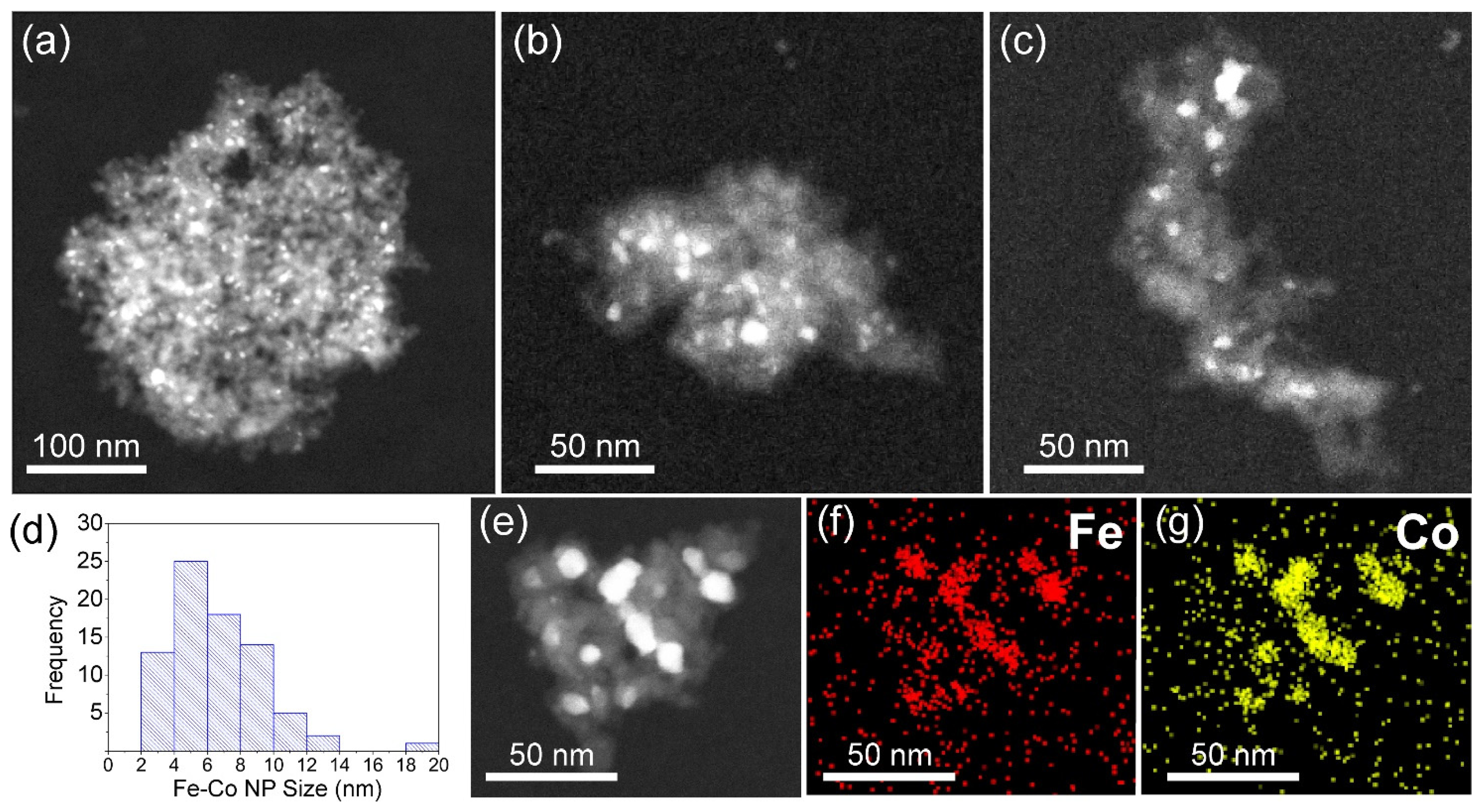
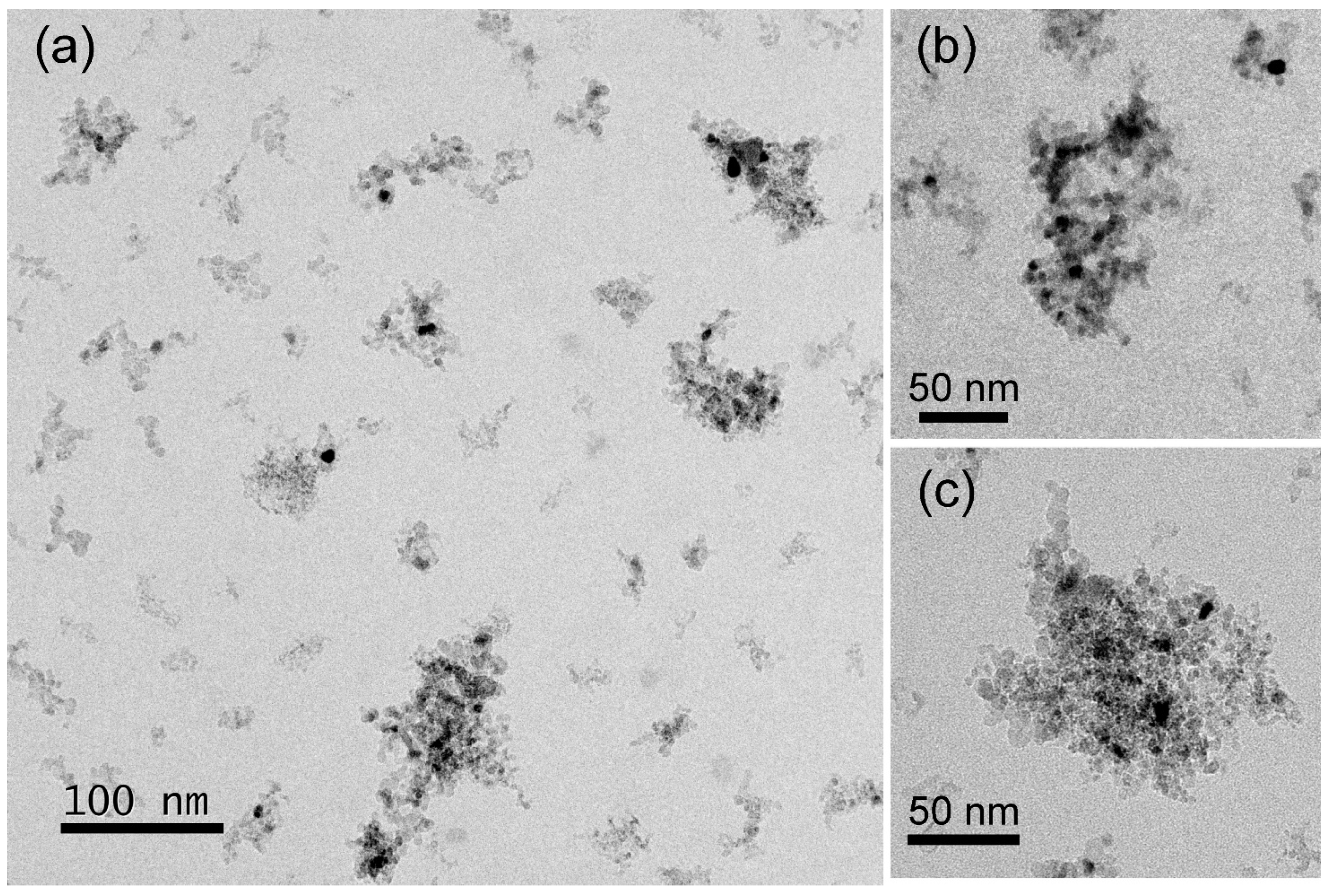
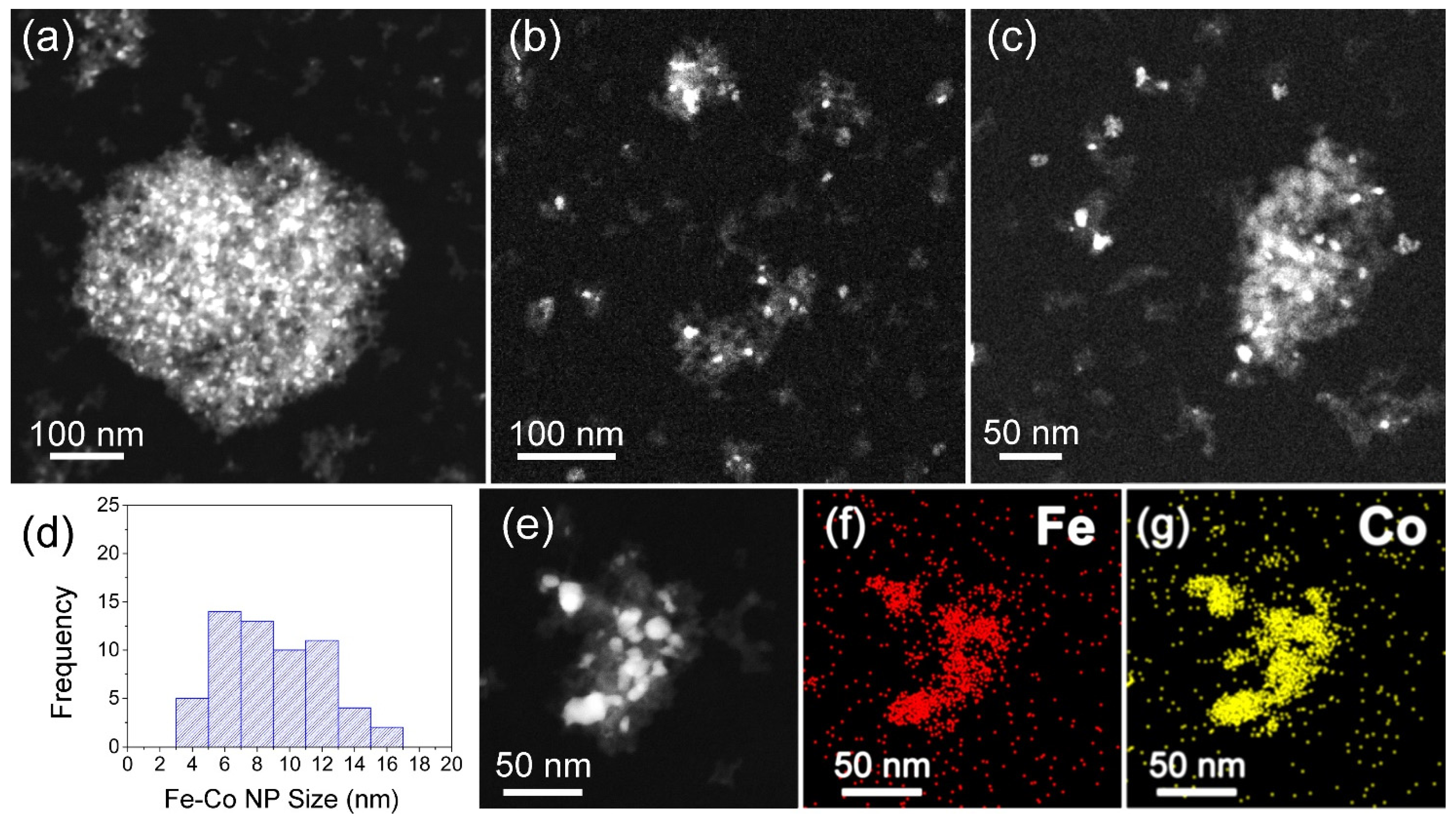
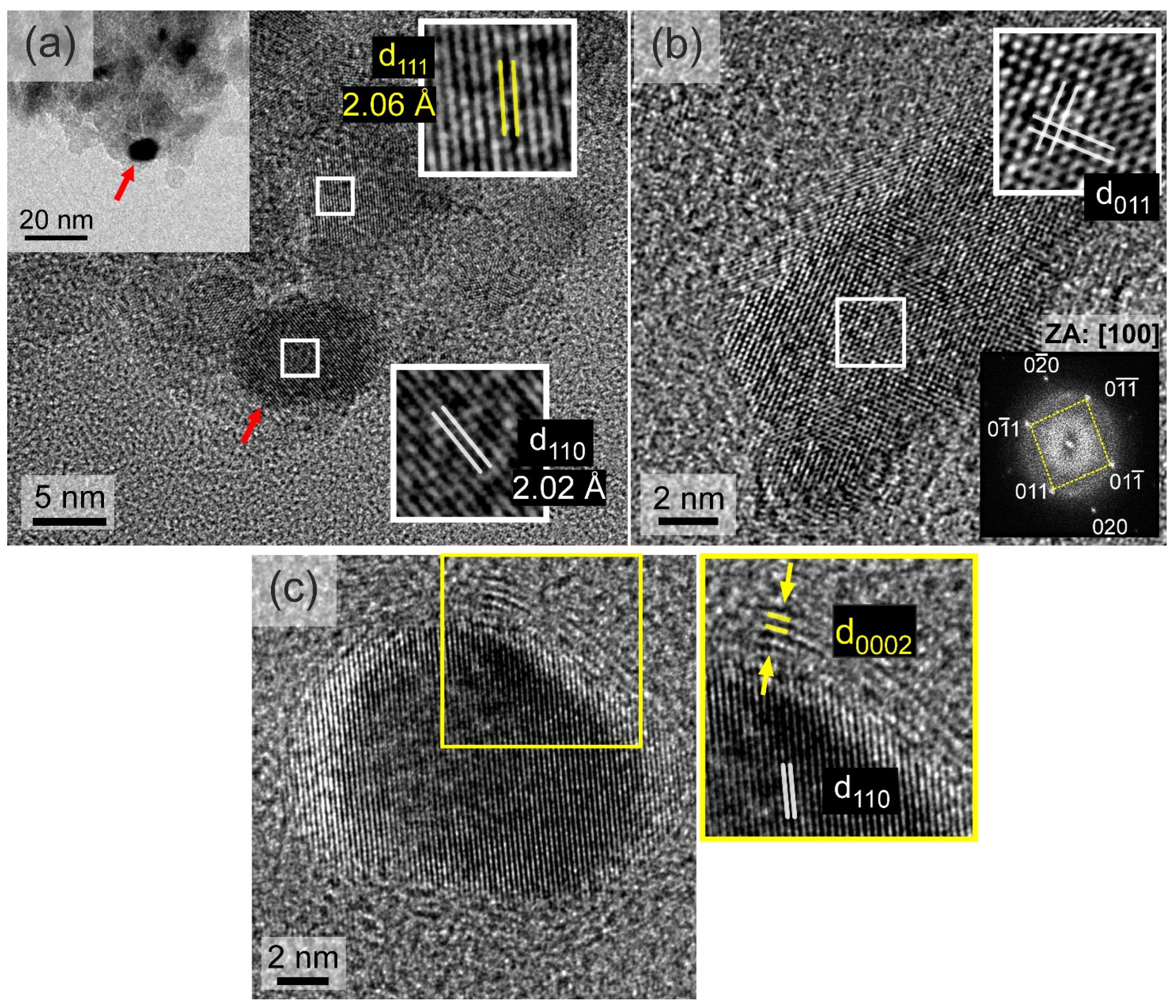
3.2. TEM, STEM, and EDS Analysis
3.3. 57Fe Mössbauer Spectroscopy
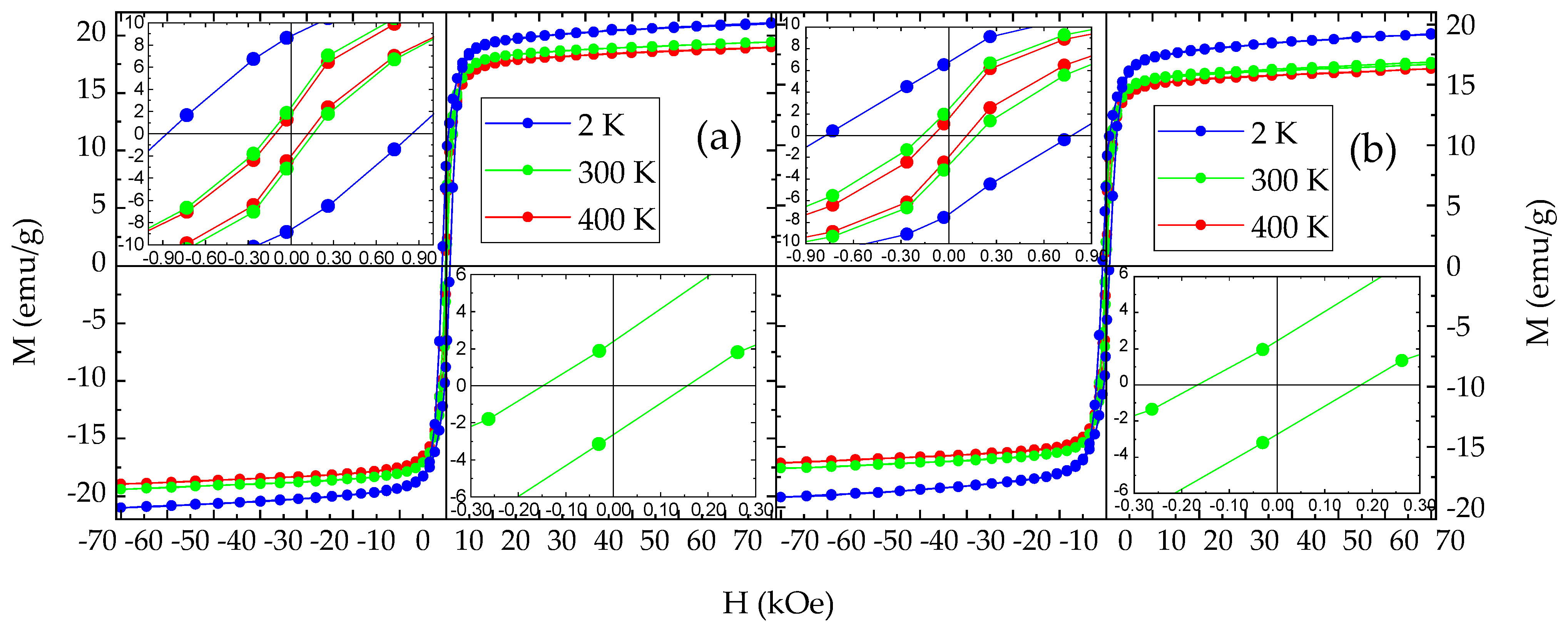
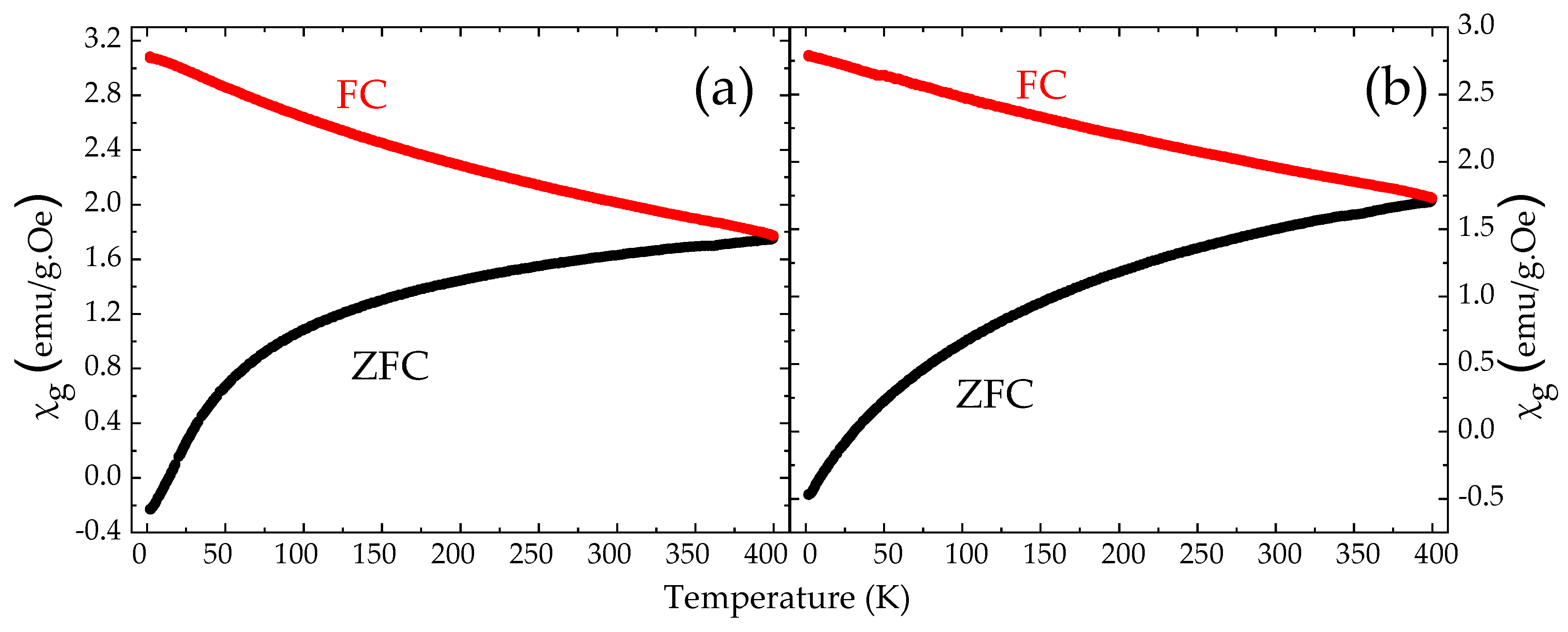
3.4. Magnetization and Magnetic Susceptibility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maze, J.R.; Stanwix, P.L.; Hodges, J.S.; Hong, S.; Taylor, J.M.; Cappellaro, P.; Jiang, L.; Dutt, M.V.G.; Togan, E.; Zibrov, A.S.; et al. Nanoscale magnetic sensing with an individual electronic spin in diamond. Nature 2008, 455, 644–647. [Google Scholar] [CrossRef]
- Purtov, K.V.; Petunin, A.I.; Burov, A.E.; Puzyr, A.P.; Bondar, V.S. Nanodiamonds as Carriers for Address Delivery of Biologically Active Substances. Nanoscale Res. Lett. 2010, 5, 631–636. [Google Scholar] [CrossRef]
- Schrand, A.M.; Hens, S.A.C.; Shenderova, O.A. Nanodiamond Particles: Properties and Perspectives for Bioapplications. Crit. Rev. Solid State Mater. Sci. 2009, 34, 18–74. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef]
- Duan, X.; Tian, W.; Zhang, H.; Sun, H.; Ao, Z.; Shao, Z.; Wang, S. sp2/sp3 Framework from Diamond Nanocrystals: A Key Bridge of Carbonaceous Structure to Carbocatalysis. ACS Catal. 2019, 9, 7494–7519. [Google Scholar] [CrossRef]
- Zeiger, M.; Jäckel, N.; Mochalin, V.N.; Presser, V. Review: Carbon onions for electrochemical energy storage. J. Mater. Chem. A 2016, 4, 3172–3196. [Google Scholar] [CrossRef]
- Coey, J.M.D. Magnetism and Magnetic Materials, 1st ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar] [CrossRef]
- Karipoth, P.; Thirumurugan, A.; Velaga, S.; Greneche, J.-M.; Joseyphus, R.J. Magnetic properties of FeCo alloy nanoparticles synthesized through instant chemical reduction. J. Appl. Phys. 2016, 120, 123906. [Google Scholar] [CrossRef]
- Jesús, F.S.-D.; Bolarín-Miró, A.M.; Escobedo, C.A.C.; Torres-Villaseñor, G.; Vera-Serna, P. Structural Analysis and Magnetic Properties of FeCo Alloys Obtained by Mechanical Alloying. J. Met. 2016, 2016, 8347063. [Google Scholar] [CrossRef]
- Chermahini, M.D.; Zandrahimi, M.; Shokrollahi, H.; Sharafi, S. The effect of milling time and composition on microstructural and magnetic properties of nanostructured Fe–Co alloys. J. Alloys Compd. 2009, 477, 45–50. [Google Scholar] [CrossRef]
- Chaubey, G.S.; Barcena, C.; Poudyal, N.; Rong, C.; Gao, J.; Sun, S.; Liu, J.P. Synthesis and Stabilization of FeCo Nanoparticles. J. Am. Chem. Soc. 2007, 129, 7214–7215. [Google Scholar] [CrossRef]
- Demayo, B.; Forester, D.W.; Spooner, S. Effects of Atomic Configurational Changes on Hyperfine Interactions in Concentrated Iron-Cobalt Alloys. J. Appl. Phys. 1970, 41, 1319–1320. [Google Scholar] [CrossRef]
- Lopes, A.M.S.; Barbosa, F.F.; Torres, M.A.M.; Pergher, S.B.C.; Braga, T.P. In situ synthesis of highly stable FeCo alloy encapsulated in carbon from ethanol. Mater. Today Commun. 2022, 32, 103900. [Google Scholar] [CrossRef]
- Lv, R.; Kang, F.; Gu, J.; Gui, X.; Wei, J.; Wang, K.; Wu, D. Carbon nanotubes filled with ferromagnetic alloy nanowires: Lightweight and wide-band microwave absorber. Appl. Phys. Lett. 2008, 93, 223105. [Google Scholar] [CrossRef]
- Ito, H.; Saito, M.; Miyamachi, T.; Komori, F.; Koganezawa, T.; Mizuguchi, M.; Kotsugi, M. Fabrication of 10-type FeCo ordered structure using a periodic Ni buffer layer. AIP Adv. 2019, 9, 045307. [Google Scholar] [CrossRef]
- Falub, C.V.; Pietambaram, S.V.; Yildirim, O.; Meduňa, M.; Caha, O.; Hida, R.; Zhao, X.; Ambrosini, J.; Rohrmann, H.; Hug, H.J. Enhanced permeability dielectric FeCo/Al2O3 multilayer thin films with tailored properties deposited by magnetron sputtering on silicon. AIP Adv. 2019, 9, 035243. [Google Scholar] [CrossRef]
- Jakobsson, A.; Şaşıoğlu, E.; Mavropoulos, P.; Ležaić, M.; Sanyal, B.; Bihlmayer, G.; Blügel, S. Tuning the Curie temperature of FeCo compounds by tetragonal distortion. Appl. Phys. Lett. 2013, 103, 102404. [Google Scholar] [CrossRef]
- Yu, R.H.; Basu, S.; Zhang, Y.; Xiao, J.Q. Magnetic domains and coercivity in FeCo soft magnetic alloys. J. Appl. Phys. 1999, 85, 6034–6036. [Google Scholar] [CrossRef]
- Song, G.; Kenney, M.; Chen, Y.-S.; Zheng, X.; Deng, Y.; Chen, Z.; Wang, S.X.; Gambhir, S.S.; Dai, H.; Rao, J. Carbon-coated FeCo nanoparticles as sensitive magnetic-particle-imaging tracers with photothermal and magnetothermal properties. Nat. Biomed. Eng. 2020, 4, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.S.; Lee, J.H.; Sun, X.; Suzuki, Y.; Mann, D.; Liu, Z.; Terashima, M.; Yang, P.C.; McConnell, M.V.; Nishimura, D.G.; et al. FeCo/graphitic-shell nanocrystals as advanced magnetic-resonance-imaging and near-infrared agents. Nat. Mater. 2006, 5, 971–976. [Google Scholar] [CrossRef]
- Hütten, A.; Sudfeld, D.; Ennen, I.; Reiss, G.; Hachmann, W.; Heinzmann, U.; Wojczykowski, K.; Jutzi, P.; Saikaly, W.; Thomas, G. New magnetic nanoparticles for biotechnology. J. Biotechnol. 2004, 112, 47–63. [Google Scholar] [CrossRef]
- Patelli, N.; Cugini, F.; Wang, D.; Sanna, S.; Solzi, M.; Hahn, H.; Pasquini, L. Structure and magnetic properties of Fe-Co alloy nanoparticles synthesized by pulsed-laser inert gas condensation. J. Alloys Compd. 2022, 890, 161863. [Google Scholar] [CrossRef]
- da Silva, R.; Carrico, A.; Filho, E.S.; da Silva, F.F.; Bufaical, L.; Soares, J.; da Costa, J.; de Araújo, J.; Morales, M. Influence of the gas atmosphere on the obtention of cobalt and iron based nanocomposites and core/shell nanoparticles by calcination in the presence of chitosan. J. Solid State Chem. 2022, 312, 123225. [Google Scholar] [CrossRef]
- Jang, M.-S.; Chang, M.S.; Kwon, Y.-T.; Yang, S.; Gwak, J.; Kwon, S.J.; Lee, J.; Song, K.; Park, C.R.; Lee, S.B.; et al. High-throughput thermal plasma synthesis of FexCo1−x nano-chained particles with unusually high permeability and their electromagnetic wave absorption properties at high frequency (1–26 GHz). Nanoscale 2021, 13, 12004–12016. [Google Scholar] [CrossRef]
- Ding, J.; Cheng, L.; Zhao, W. Self-Assembly Magnetic FeCo Nanostructures on Oxide Graphene for Enhanced Microwave Absorption. J. Electron. Mater. 2022, 51, 2856–2866. [Google Scholar] [CrossRef]
- Shokuhfar, A.; Afghahi, S.S.S. Size Controlled Synthesis of FeCo Alloy Nanoparticles and Study of the Particle Size and Distribution Effects on Magnetic Properties. Adv. Mater. Sci. Eng. 2014, 2014, 295390. [Google Scholar] [CrossRef]
- Valiev, E.; Gimaev, R.; Zverev, V.; Kamilov, K.; Pyatakov, A.; Kovalev, B.; Tishin, A. Application of the exchange-striction model for the calculation of the FeRh alloys magnetic properties. Intermetallics 2019, 108, 81–86. [Google Scholar] [CrossRef]
- Burkert, T.; Nordström, L.; Eriksson, O.; Heinonen, O. Giant Magnetic Anisotropy in Tetragonal FeCo Alloys. Phys. Rev. Lett. 2004, 93, 027203. [Google Scholar] [CrossRef] [PubMed]
- Kota, Y.; Sakuma, A. Degree of Order Dependence on Magnetocrystalline Anisotropy in Body-Centered Tetragonal FeCo Alloys. Appl. Phys. Express 2012, 5, 113002. [Google Scholar] [CrossRef]
- Odkhuu, D.; Hong, S.C. First-Principles Prediction of Possible Rare-Earth Free Permanent Magnet of Tetragonal FeCo with Enhanced Magnetic Anisotropy and Energy Product through Interstitial Nitrogen. Phys. Rev. Appl. 2019, 11, 054085. [Google Scholar] [CrossRef]
- Nishizawa, T.; Ishida, K. The Co−Fe (Cobalt−Iron) system. Bull. Alloys Phase Diagrams 1984, 5, 250–259. [Google Scholar] [CrossRef]
- Hasegawa, T.; Seki, Y. TEM-based crystal structure analysis of body-centered tetragonal structure in non-epitaxial FeCo film with added V and N. Mater. Lett. 2022, 313, 131734. [Google Scholar] [CrossRef]
- Hasegawa, T.; Niibori, T.; Takemasa, Y.; Oikawa, M. Stabilisation of tetragonal FeCo structure with high magnetic anisotropy by the addition of V and N elements. Sci. Rep. 2019, 9, 5248. [Google Scholar] [CrossRef]
- Warnicke, P.; Andersson, G.; Björck, M.; Ferré, J.; Nordblad, P. Magnetic anisotropy of tetragonal FeCo/Pt(001) superlattices. J. Phys. Condens. Matter 2007, 19, 226218. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Ishio, S. Investigation of magnetic anisotropy and magnetization process of tetragonal distorted FeCo multilayer films. Mater. Lett. 2015, 160, 238–241. [Google Scholar] [CrossRef]
- Gong, M.; Kirkeminde, A.; Wuttig, M.; Ren, S. Phase Transformation-Induced Tetragonal FeCo Nanostructures. Nano Lett. 2014, 14, 6493–6498. [Google Scholar] [CrossRef] [PubMed]
- Hume-Rothery, W. The Structures of Alloys of Iron; Elsevier: Amsterdam, The Netherlands, 1966. [Google Scholar]
- Pepperhoff, W.; Acet, M. Constitution and Magnetism of Iron and its Alloys. In Engineering Materials; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Kurdjumov, G.; Khachaturyan, A. Nature of axial ratio anomalies of the martensite lattice and mechanism of diffusionless γ → α transformation. Acta Met. 1975, 23, 1077–1088. [Google Scholar] [CrossRef]
- Gavriljuk, V.G.; Firstov, S.O.; Sirosh, V.A.; Tyshchenko, A.I.; Mogilny, G.S. Carbon Distribution in Low-Temperature Isothermal Iron-Based Martensite and Its Tetragonality. Met. I Noveishie Tekhnologii 2016, 38, 455–475. [Google Scholar] [CrossRef]
- Gavriljuk, V.; Tarasenko, A.; Tyshchenko, A. Low temperature ageing of the freshly formed Fe-C and Fe-N martensites. Scr. Mater. 2000, 43, 233–238. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y. Hidden pathway during fcc to bcc/bct transformations: Crystallographic origin of slip martensite in steels. Phys. Rev. Mater. 2018, 2, 093611. [Google Scholar] [CrossRef]
- Choo, W.; Kaplow, R. Mössbauer measurements on the aging of iron-carbon martensite. Acta Met. 1973, 21, 725–732. [Google Scholar] [CrossRef]
- Kozhitov, L.V.; Muratov, D.G.; Kostishin, V.G.; Suslyaev, V.I.; Korovin, E.Y.; Popkova, A.V. FeCo/C nanocomposites: Synthesis, magnetic and electromagnetic properties. Russ. J. Inorg. Chem. 2017, 62, 1499–1507. [Google Scholar] [CrossRef]
- Ghunaim, R.; Scholz, M.; Damm, C.; Rellinghaus, B.; Klingeler, R.; Büchner, B.; Mertig, M.; Hampel, S. Single-crystalline FeCo nanoparticle-filled carbon nanotubes: Synthesis, structural characterization and magnetic properties. Beilstein J. Nanotechnol. 2018, 9, 1024–1034. [Google Scholar] [CrossRef]
- Gielen, P.; Kaplow, R. Mossbauer effect in iron-carbon and iron-nitrogen alloys. Acta Met. 1967, 15, 49–63. [Google Scholar] [CrossRef]
- Douvalis, A.P.; Bourlinos, A.B.; Tucek, J.; Čépe, K.; Bakas, T.; Zboril, R. Development of novel FePt/nanodiamond hybrid nanostructures: L10 phase size-growth suppression and magnetic properties. J. Nanoparticle Res. 2016, 18, 115. [Google Scholar] [CrossRef]
- Ziogas, P.; Bourlinos, A.B.; Tucek, J.; Malina, O.; Douvalis, A.P. Novel Magnetic Nanohybrids: From Iron Oxide to Iron Carbide Nanoparticles Grown on Nanodiamonds. Magnetochemistry 2020, 6, 73. [Google Scholar] [CrossRef]
- Ziogas, P.; Bourlinos, A.B.; Chatzopoulou, P.; Dimitrakopulos, G.P.; Kehagias, T.; Markou, A.; Douvalis, A.P. Intriguing Prospects of a Novel Magnetic Nanohybrid Material: Ferromagnetic FeRh Nanoparticles Grown on Nanodiamonds. Metals 2022, 12, 1355. [Google Scholar] [CrossRef]
- Han, Z.; Rao, J.S.; Gangwar, L.; Namsrai, B.-E.; Pasek-Allen, J.L.; Etheridge, M.L.; Wolf, S.M.; Pruett, T.L.; Bischof, J.C.; Finger, E.B. Vitrification and nanowarming enable long-term organ cryopreservation and life-sustaining kidney transplantation in a rat model. Nat. Commun. 2023, 14, 3407. [Google Scholar] [CrossRef] [PubMed]
- Bourlinos, A.B.; Zbořil, R.; Kubala, M.; Stathi, P.; Deligiannakis, Y.; Karakassides, M.A.; Steriotis, T.A.; Stubos, A.K. Fabrication of fluorescent nanodiamond@C core–shell hybrids via mild carbonization of sodium cholate–nanodiamond complexes. J. Mater. Sci. 2011, 46, 7912–7916. [Google Scholar] [CrossRef]
- Bourlinos, A.; Simopoulos, A.; Petridis, D.; Okumura, H.; Hadjipanayis, G. Silica-Maghemite Nanocomposites. Adv. Mater. 2001, 13, 289–291. [Google Scholar] [CrossRef]
- Douvalis, A.P.; Polymeros, A.; Bakas, T. IMSG09: A57Fe-119Sn Mössbauer spectra computer fitting program with novel interactive user interface. J. Phys. Conf. Ser. 2010, 217, 012014. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurences and Uses, 1st ed.; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd. ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Lyubutin, I.S.; Gervits, E.N.; Starchikov, S.S.; Lin, C.-R.; Tseng, Y.-T.; Shih, K.-Y.; Wang, C.-C.; Chen, I.-H.; Ogarkova, Y.L.; Korotkov, N.Y. Magnetic and Mössbauer spectroscopy studies of hollow microcapsules made of silica-coated CoFe2O4 nanoparticles. Smart Mater. Struct. 2016, 25, 015022. [Google Scholar] [CrossRef]
- Hahsler, M.; Landers, J.; Nowack, T.; Salamon, S.; Zimmermann, M.; HeiBler, S.; Wende, H.; Behrens, S. Magnetic Properties and Mossbauer Spectroscopy of Fe3O4/CoFe2O4 Nanorods. Inorg. Chem. 2020, 59, 3677–3685. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Gibb, T.C. Mössbauer Spectroscopy; Springer: Dordrecht, The Netherlands, 1971. [Google Scholar]
- Gütlich, P.; Bill, E.; Trautwein, A.X. Mössbauer Spectroscopy and Transition Metal Chemistry; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Mørup, S.; Hansen, M. Superparamagnetic Particles. In Handbook of Magnetism and Advanced Magnetic Materials; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Mørup, S.; Hansen, M.F.; Frandsen, C. Magnetic interactions between nanoparticles. Beilstein J. Nanotechnol. 2010, 1, 182–190. [Google Scholar] [CrossRef]
- Kronmüller, H.; Parkin, S. (Eds.) Handbook of Magnetism and Advanced Magnetic Materials, 1st ed.; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Hamdeh, H.H.; Fultz, B.; Pearson, D.H. Mössbauer spectrometry study of the hyperfine fields and electronic structure of Fe-Co alloys. Phys. Rev. B 1989, 39, 11233–11240. [Google Scholar] [CrossRef]
- Serikov, V.V.; Kleinerman, N.M.; Golovnya, O.A. NMR and Mössbauer study of peculiarities of the structure formation in Fe–Co alloys. Phys. Met. Met. 2017, 118, 1040–1047. [Google Scholar] [CrossRef]
- Moumeni, H.; Alleg, S.; Djebbari, C.; Bentayeb, F.Z.; Grenèche, J.M. Synthesis and characterisation of nanostructured FeCo alloys. J. Mater. Sci. 2004, 39, 5441–5443. [Google Scholar] [CrossRef]
- Montano, P.A.; Seehra, M.S. Mössbauer study of the order-disorder andα−γtransitions in FeCo. Phys. Rev. B 1977, 15, 2437–2441. [Google Scholar] [CrossRef]
- Fiorani, D. (Ed.) Surface effects in magnetic nanoparticles. In Nanostructure Science and Technology; Springer: New York, NY, USA, 2005. [Google Scholar]
- Tuček, J.; Kemp, K.C.; Kim, K.S.; Zbořil, R. Iron-Oxide-Supported Nanocarbon in Lithium-Ion Batteries, Medical, Catalytic, and Environmental Applications. ACS Nano 2014, 8, 7571–7612. [Google Scholar] [CrossRef]
- Fan, X.; Gao, H.; Kou, X.; Zhang, B.; Wang, S. Synthesis of FeCo-reduced graphene oxide composite and its magnetic and adsorption properties. Mater. Res. Bull. 2015, 65, 320–324. [Google Scholar] [CrossRef]
- Shenderova, O.A.; McGuire, G.E. Science and engineering of nanodiamond particle surfaces for biological applications (Review). Biointerphases 2015, 10, 030802. [Google Scholar] [CrossRef]
- Barnard, A.S. Stability of Diamond at the Nanoscale. In Ultananocrystalline Diamond; Elsevier: Amsterdam, The Netherlands, 2012; pp. 3–52. [Google Scholar] [CrossRef]
- Petit, T.; Arnault, J.-C.; Girard, H.A.; Sennour, M.; Bergonzo, P. Early stages of surface graphitization on nanodiamond probed by x-ray photoelectron spectroscopy. Phys. Rev. B 2011, 84, 233407. [Google Scholar] [CrossRef]



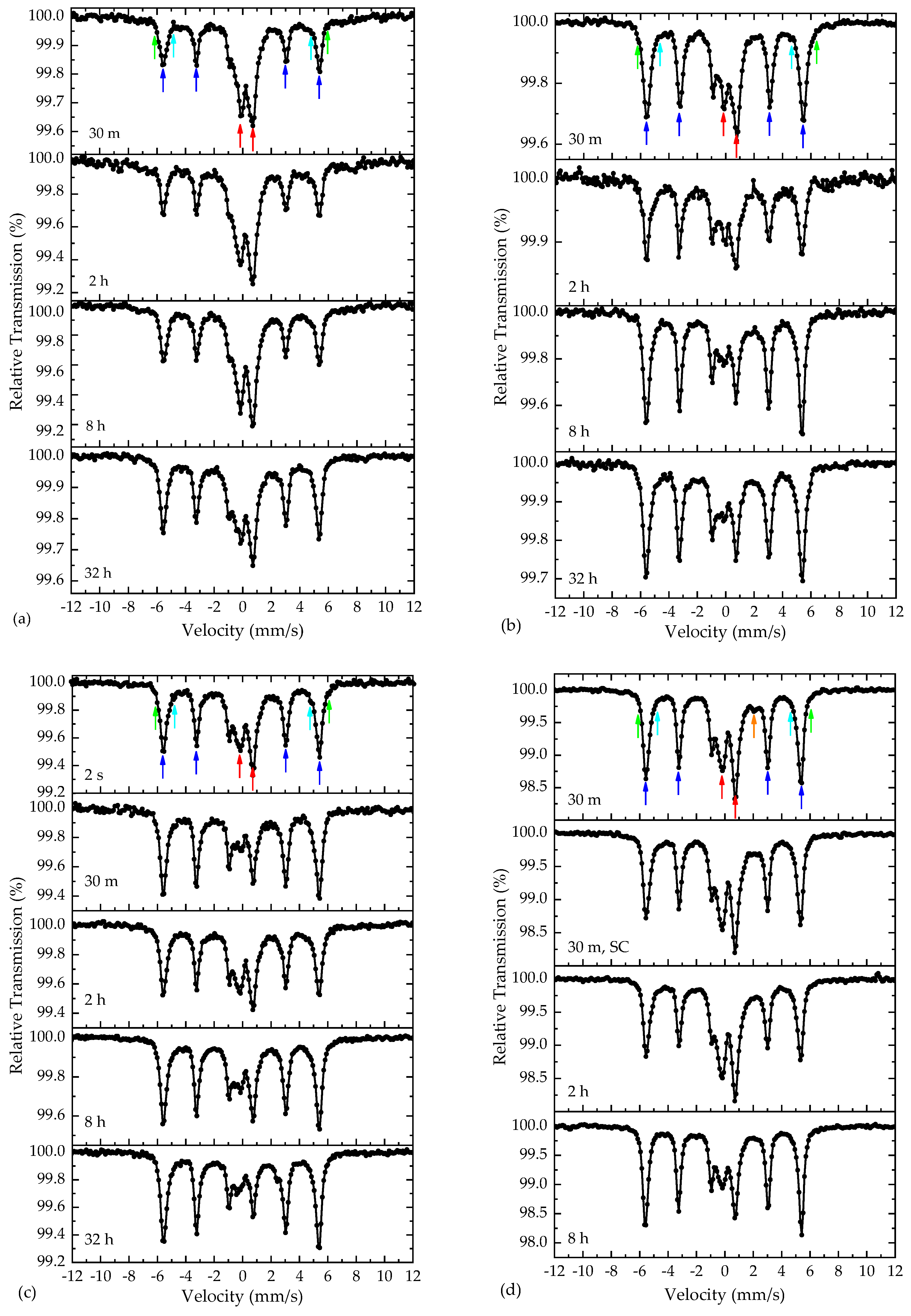
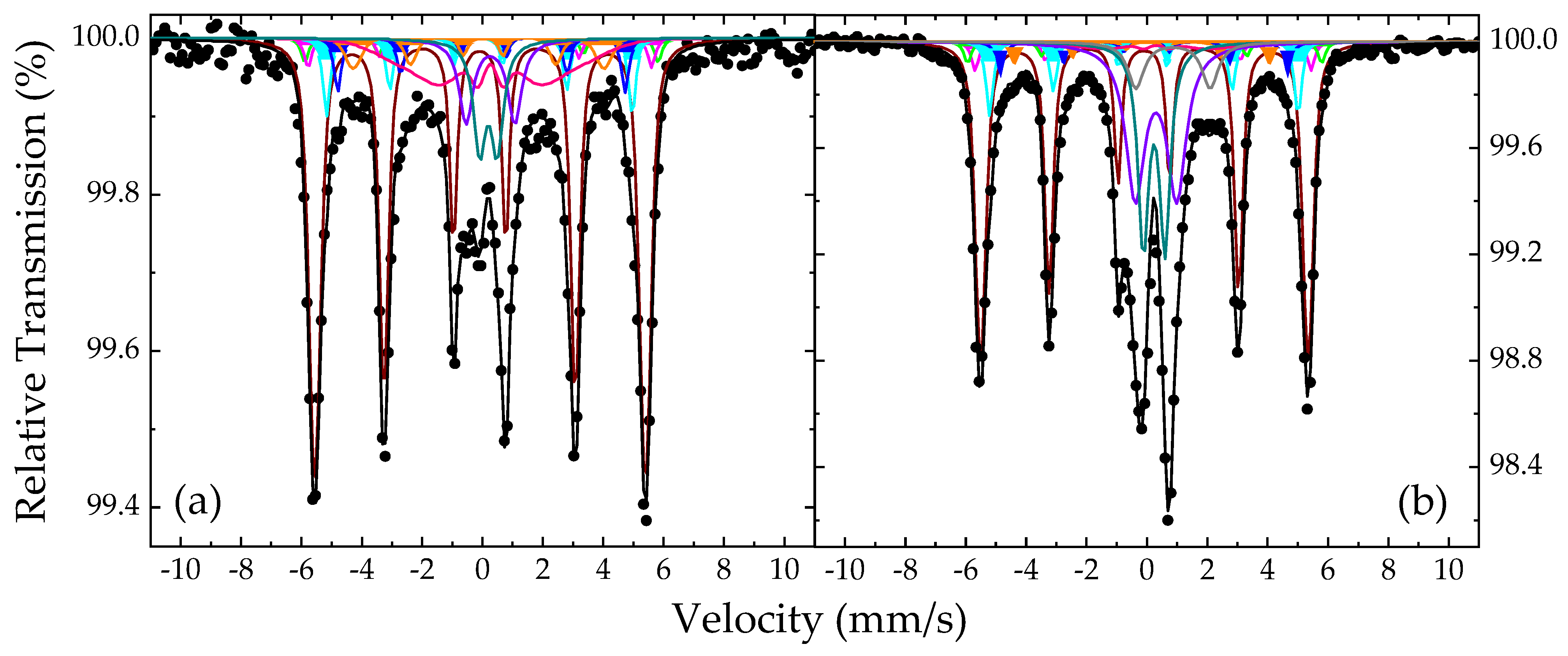
| Sample | Component | IS (mm/s) | Γ/2 (mm/s) | QS or 2ε (mm/s) | BhfC (kOe) | ΔBhf (kOe) | Area (%) | Color |
|---|---|---|---|---|---|---|---|---|
| AM-NHD precursor | SPM Fe3+ (1) | 0.33 | 0.20 | 0.65 | 0 | 0 | 57 | Dark Cayn |
| SPM Fe3+ (2) | 0.35 | 0.25 | 1.17 | 0 | 0 | 43 | Purple | |
| En-AM-NHD precursor | SPM Fe3+ (1) | 0.36 | 0.21 | 0.67 | 0 | 0 | 51 | Dark Cayn |
| SPM Fe3+ (2) | 0.37 | 0.29 | 1.17 | 0 | 0 | 31 | Purple | |
| MCOL Fe3+ (1) | 0.51 | 0.15 | 0.14 | 322 | 95 | 12 | Red | |
| MCOL Fe3+ (2) | 0.39 | 0.15 | 0.07 | 468 | 22 | 6 | Dark Yellow |
| Sample | Component | IS (mm/s) | Γ/2 (mm/s) | QS or 2ε (mm/s) | BhfC (kOe) | ΔBhf (kOe) | Area (%) | Color |
|---|---|---|---|---|---|---|---|---|
| Non-enrichedNHDs-700,30m | Fe35Co65 | 0.02 | 0.14 | 0.02 | 340 | 8 | 52 | Maroon |
| Martensitic Fe-Co (1) | 0.09 | 0.14 | 0.01 | 364 | 0 | 2 | Green | |
| Martensitic Fe-Co (2) | 0.04 | 0.14 | 0.01 | 354 | 0 | 2 | Magenta | |
| Martensitic Fe-Co (3) | 0.00 | 0.14 | 0.04 | 315 | 0 | 6 | Cyan | |
| Martensitic Fe-Co (4) | 0.13 | 0.14 | −0.08 | 296 | 0 | 5 | Blue | |
| Martensitic Fe-Co (5) | 0.08 | 0.14 | −0.15 | 259 | 13 | 5 | Orange | |
| MCOL Fe3+ | 0.28 | 0.14 | 0.00 | 155 | 65 | 14 | Pink | |
| SPM Fe3+ (1) | 0.30 | 0.22 | 0.58 | 0 | 0 | 7 | Dark Cyan | |
| SPM Fe3+ (2) | 0.34 | 0.28 | 1.58 | 0 | 0 | 7 | Purple | |
| Enriched-NHDs-700,30m withSC | Fe33Co67 | 0.02 | 0.14 | 0.02 | 337 | 7 | 41 | Maroon |
| Martensitic Fe-Co (1) | 0.09 | 0.14 | 0.01 | 365 | 0 | 2 | Green | |
| Martensitic Fe-Co (2) | 0.04 | 0.14 | 0.01 | 346 | 0 | 3 | Magenta | |
| Martensitic Fe-Co (3) | 0.05 | 0.14 | 0.04 | 318 | 0 | 7 | Cyan | |
| Martensitic Fe-Co (4) | 0.12 | 0.14 | −0.09 | 296 | 0 | 4 | Blue | |
| Martensitic Fe-Co (5) | 0.08 | 0.14 | −0.15 | 263 | 5 | 3 | Orange | |
| MCOL Fe3+ | 0.43 | 0.14 | 0.00 | 160 | 60 | 2 | Pink | |
| SPM Fe3+ (1) | 0.35 | 0.21 | 0.72 | 0 | 0 | 14 | Dark Cyan | |
| SPM Fe3+ (2) | 0.41 | 0.38 | 1.37 | 0 | 0 | 19 | Purple | |
| SPM Fe2+ | 0.97 | 0.32 | 2.46 | 0 | 0 | 5 | Grey |
| Sample | T (K) | Mmax+ (emu/g) | Mmax− (emu/g) | MR+ (emu/g) | MR− (emu/g) | HC+ (Oe) | HC− (Oe) |
|---|---|---|---|---|---|---|---|
| NHD-700,30m | 400 | 18.9 | −18.9 | 1.7 | −2.0 | 112 | −112 |
| 300 | 19.4 | −19.4 | 2.3 | −2.7 | 160 | −152 | |
| 2 | 21.1 | −21.1 | 8.7 | −8.6 | 856 | −881 | |
| En-NHD-700,30m-SC | 400 | 16.3 | −16.4 | 1.6 | −1.9 | 114 | −98 |
| 300 | 16.7 | −16.8 | 2.5 | −2.7 | 177 | −169 | |
| 2 | 19.2 | −19.2 | 6.8 | −7.2 | 781 | −776 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziogas, P.G.; Bourlinos, A.B.; Chatzopoulou, P.; Dimitrakopulos, G.P.; Markou, A.; Douvalis, A.P. Novel Hybrid Ferromagnetic Fe–Co/Nanodiamond Nanostructures: Influence of Carbon on Their Structural and Magnetic Properties. Magnetochemistry 2024, 10, 35. https://doi.org/10.3390/magnetochemistry10050035
Ziogas PG, Bourlinos AB, Chatzopoulou P, Dimitrakopulos GP, Markou A, Douvalis AP. Novel Hybrid Ferromagnetic Fe–Co/Nanodiamond Nanostructures: Influence of Carbon on Their Structural and Magnetic Properties. Magnetochemistry. 2024; 10(5):35. https://doi.org/10.3390/magnetochemistry10050035
Chicago/Turabian StyleZiogas, Panagiotis G., Athanasios B. Bourlinos, Polyxeni Chatzopoulou, George P. Dimitrakopulos, Anastasios Markou, and Alexios P. Douvalis. 2024. "Novel Hybrid Ferromagnetic Fe–Co/Nanodiamond Nanostructures: Influence of Carbon on Their Structural and Magnetic Properties" Magnetochemistry 10, no. 5: 35. https://doi.org/10.3390/magnetochemistry10050035
APA StyleZiogas, P. G., Bourlinos, A. B., Chatzopoulou, P., Dimitrakopulos, G. P., Markou, A., & Douvalis, A. P. (2024). Novel Hybrid Ferromagnetic Fe–Co/Nanodiamond Nanostructures: Influence of Carbon on Their Structural and Magnetic Properties. Magnetochemistry, 10(5), 35. https://doi.org/10.3390/magnetochemistry10050035








