Preparation and Characterization of BXFO High-Entropy Oxides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Synthesis
2.2. Characterization
3. Results and Discussion
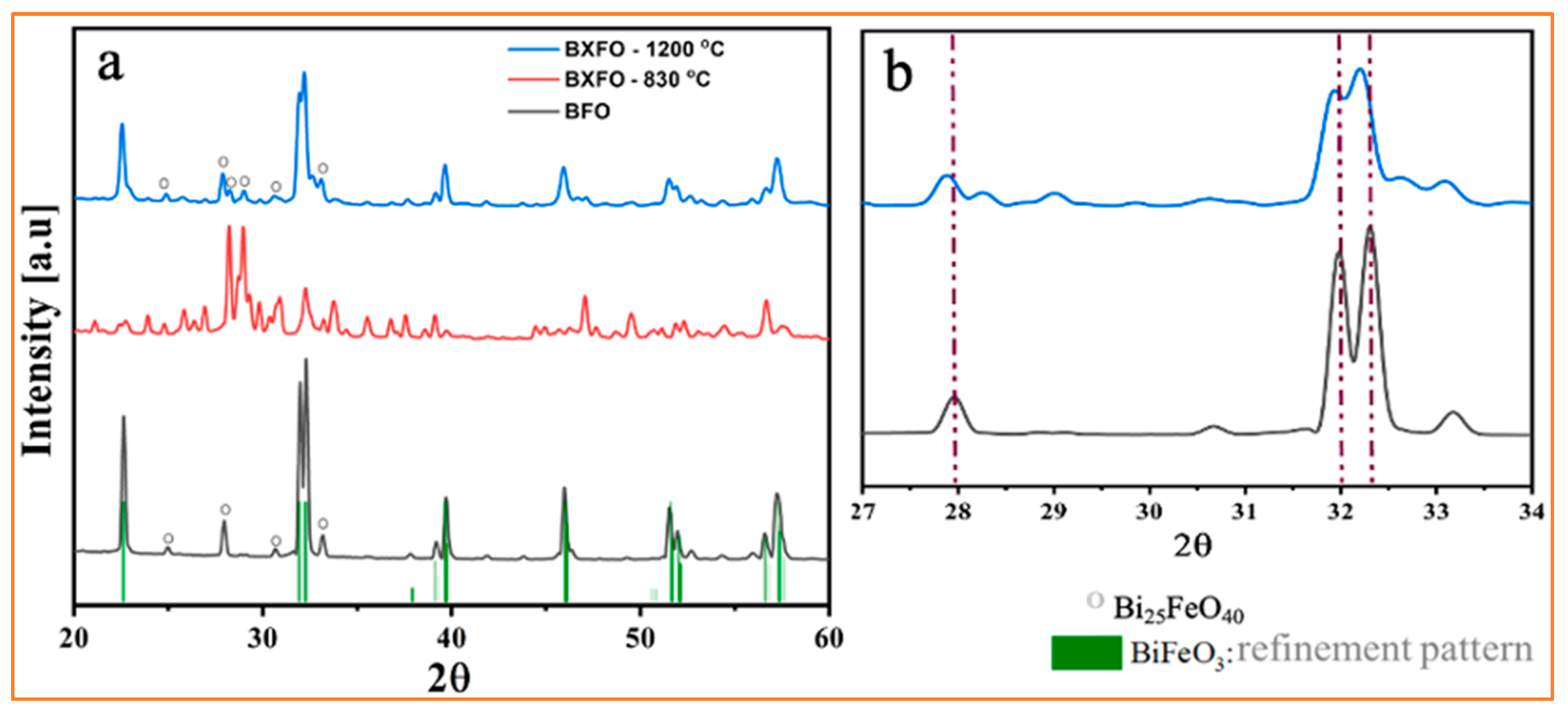
3.1. Structural Characterization
3.2. Dielectric Properties
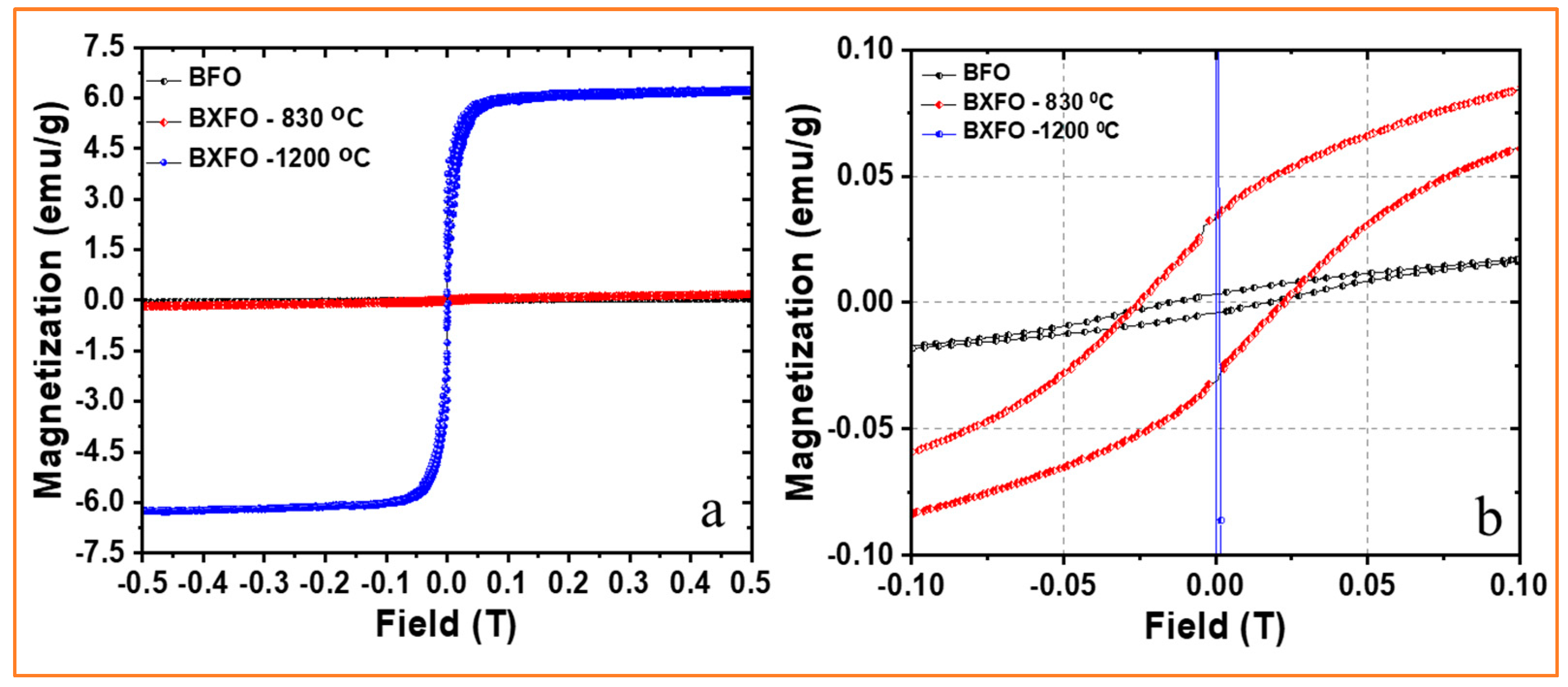
3.3. Magnetic Properties
4. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wrzesińska, A.; Khort, A.; Witkowski, M.; Szczytko, J.; Ryl, J.; Gurgul, J.; Kharitonov, D.S.; Łątka, K.; Szumiata, T.; Wypych-Puszkarz, A. Structural, electrical, and magnetic study of La-, Eu-, and Er- doped bismuth ferrite nanomaterials obtained by solution combustion synthesis. Sci. Rep. 2021, 11, 22746. [Google Scholar] [CrossRef]
- Verma, V.; Beniwal, A.; Ohlan, A.; Tripathi, R. Structural, magnetic and ferroelectric properties of Pr doped multiferroics bismuth ferrites. J. Magn. Magn. Mater. 2015, 394, 385–390. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef]
- Salian, A.; Pujar, P.; Vardhan, R.V.; Cho, H.; Kim, S.; Mandal, S. Evolution of High Dielectric Permittivity in Low-Temperature Solution Combustion-Processed Phase-Pure High Entropy Oxide (CoMnNiFeCr)O for Thin Film Transistors. ACS Appl. Electron. Mater. 2023, 5, 2608–2623. [Google Scholar] [CrossRef]
- Sarkar, A.; Wang, Q.; Schiele, A.; Chellali, M.R.; Bhattacharya, S.S.; Wang, D.; Brezesinski, T.; Hahn, H.; Velasco, L.; Breitung, B. High-Entropy Oxides: Fundamental Aspects and Electrochemical Properties. Adv. Mater. 2019, 31, 1806236. [Google Scholar] [CrossRef] [PubMed]
- Zhivulin, V.E.; Trofimov, E.A.; Zaitseva, O.V.; Sherstyuk, D.P.; Cherkasova, N.A.; Taskaev, S.V.; Vinnik, D.A.; Alekhina, Y.A.; Perov, N.S.; Naidu, K.C.B.; et al. Preparation, phase stability, and magnetization behavior of high entropy hexaferrites. IScience 2023, 26, 107077. [Google Scholar] [CrossRef]
- Wright, A.J.; Wang, Q.; Huang, C.; Nieto, A.; Chen, R.; Luo, J. From high-entropy ceramics to compositionally-complex ceramics: A case study of fluorite oxides. J. Eur. Ceram. Soc. 2020, 40, 2120–2129. [Google Scholar] [CrossRef]
- Catalan, G.; Scott, J.F. Physics and Applications of Bismuth Ferrite. Adv. Mater. 2009, 21, 2463–2485. [Google Scholar] [CrossRef]
- Yakout, S.M. Spintronics and Innovative Memory Devices: A Review on Advances in Magnetoelectric BiFeO3. J. Supercond. Nov. Magn. 2021, 34, 317–338. [Google Scholar] [CrossRef]
- Basu, S.; Hossain, S.K.M.; Chakravorty, D.; Pal, M. Enhanced magnetic properties in hydrothermally synthesized Mn-doped BiFeO3 nanoparticles. Curr. Appl. Phys. 2011, 11, 976–980. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, G.; Guo, M.; Chai, Z.; Lv, L.; Xue, M.; Ren, X.; Li, J.; Ren, H.; Xia, A. Multiferroic properties of La/Er/Mn/Co multi-doped BiFeO3 thin films. Ceram. Int. 2019, 45, 11765–11775. [Google Scholar] [CrossRef]
- Dai, W.; Li, Y.; Jia, C.; Kang, C.; Li, M.; Zhang, W. High-performance ferroelectric non-volatile memory based on La-doped BiFeO3 thin films. RSC Adv. 2020, 10, 18039–18043. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Hasan, M.K.; Munna, A.H.; Roy, D.J.; Al Hassan, M.R.; Gulshan, F. Structural, optical, and magnetic properties of compositionally complex bismuth ferrite (BiFeO3). J. Mater. Sci. Mater. Electron. 2020, 31, 19713–19727. [Google Scholar] [CrossRef]
- Suresh, P.; Hazra, B.K.; Kumar, B.R.; Chakraborty, T.; Babu, P.D.; Srinath, S. Lattice effects on the multiferroic characteristics of (La, Ho) co-substituted BiFeO3. J. Alloys Compd. 2021, 863, 158719. [Google Scholar] [CrossRef]
- Kirsch, A.; Bøjesen, E.D.; Lefeld, N.; Larsen, R.; Mathiesen, J.K.; Skjærvø, S.L.; Pittkowski, R.K.; Sheptyakov, D.; Jensen, K.M.Ø. High-Entropy Oxides in the Mullite-Type Structure. Chem. Mater. 2023, 35, 8664–8674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Robson, M.J.; Manzotti, A.; Ciucci, F. High-entropy perovskites materials for next-generation energy applications. Joule 2023, 7, 848–854. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, Y.L.; Lin, S.J.; Chen, S.K. High-Entropy Alloys—A New Era of Exploitation. Mater. Sci. Forum 2007, 560, 1–9. [Google Scholar] [CrossRef]
- Ameer, Z.; Monteduro, A.G.; Rizzato, S.; Caricato, A.P.; Martino, M.; Lekshmi, I.C.; Hazarika, A.; Choudhury, D.; Mazzotta, E.; Malitesta, C.; et al. Dielectrical performance of high-k yttrium copper titanate thin films for electronic applications. J. Mater. Sci. Mater. Electron. 2018, 29, 7090–7098. [Google Scholar] [CrossRef]
- Monteduro, A.G.; Rizzato, S.; Leo, C.; Karmakar, S.; Sirsi, F.; Leo, A.; Tasco, V.; Esposito, M.; Passaseo, A.; Caricato, A.P.; et al. Dielectric and Ferroelectric Response of Multiphase Bi-Fe-O Ceramics. Phys. Status Solidi (A) 2019, 216, 1800584. [Google Scholar] [CrossRef]
- Monteduro, A.G.; Ameer, Z.; Martino, M.; Caricato, A.P.; Tasco, V.; Lekshmi, I.C.; Rinaldi, R.; Hazarika, A.; Choudhury, D.; Sarma, D.D.; et al. Dielectric investigation of high-k yttrium copper titanate thin films. J. Mater. Chem. C Mater. 2016, 4, 1080–1087. [Google Scholar] [CrossRef]
- Monteduro, A.G.; Ameer, Z.; Rizzato, S.; Martino, M.; Caricato, A.P.; Tasco, V.; Lekshmi, I.C.; Hazarika, A.; Choudhury, D.; Sarma, D.D.; et al. Investigation of high-k yttrium copper titanate thin films as alternative gate dielectrics. J. Phys. D Appl. Phys. 2016, 49, 405303. [Google Scholar] [CrossRef]
- Zhou, S.; Pu, Y.; Zhang, Q.; Shi, R.; Guo, X.; Wang, W.; Ji, J.; Wei, T.; Ouyang, T. Microstructure and dielectric properties of high entropy Ba(Zr0.2Ti0.2Sn0.2Hf0.2Me0.2)O3 perovskite oxides. Ceram. Int. 2020, 46, 7430–7437. [Google Scholar] [CrossRef]
- Yang, B.; Liu, Y.; Lan, S.; Dou, L.; Nan, C.W.; Lin, Y.H. High-entropy design for dielectric materials: Status, challenges, and beyond. J. Appl. Phys. 2023, 133. [Google Scholar] [CrossRef]
- Fan, L.; Li, Y.; Li, J.; Xiang, Q.; Wang, X.; Wen, T.; Zhong, Z.; Liao, Y. High entropy dielectrics. Open Access J. Adv. Dielectr. 2023, 13, 2350014. [Google Scholar] [CrossRef]
- Xie, H.; Li, J.; Yang, S.; Wu, L.; Li, P.; Qi, X. Microstructures and dielectric properties of novel (La0.2Pr0.2Nd0.2Sm0.2Eu0.2)2Ce2O7 high entropy ceramics. J. Mater. Sci. Mater. Electron. 2021, 32, 27860–27870. [Google Scholar] [CrossRef]
- Suresh, P.; Srinath, S. Study of structure and magnetic properties of rare earth doped BiFeO3. Phys. B Condens. Matter 2014, 448, 281–284. [Google Scholar] [CrossRef]

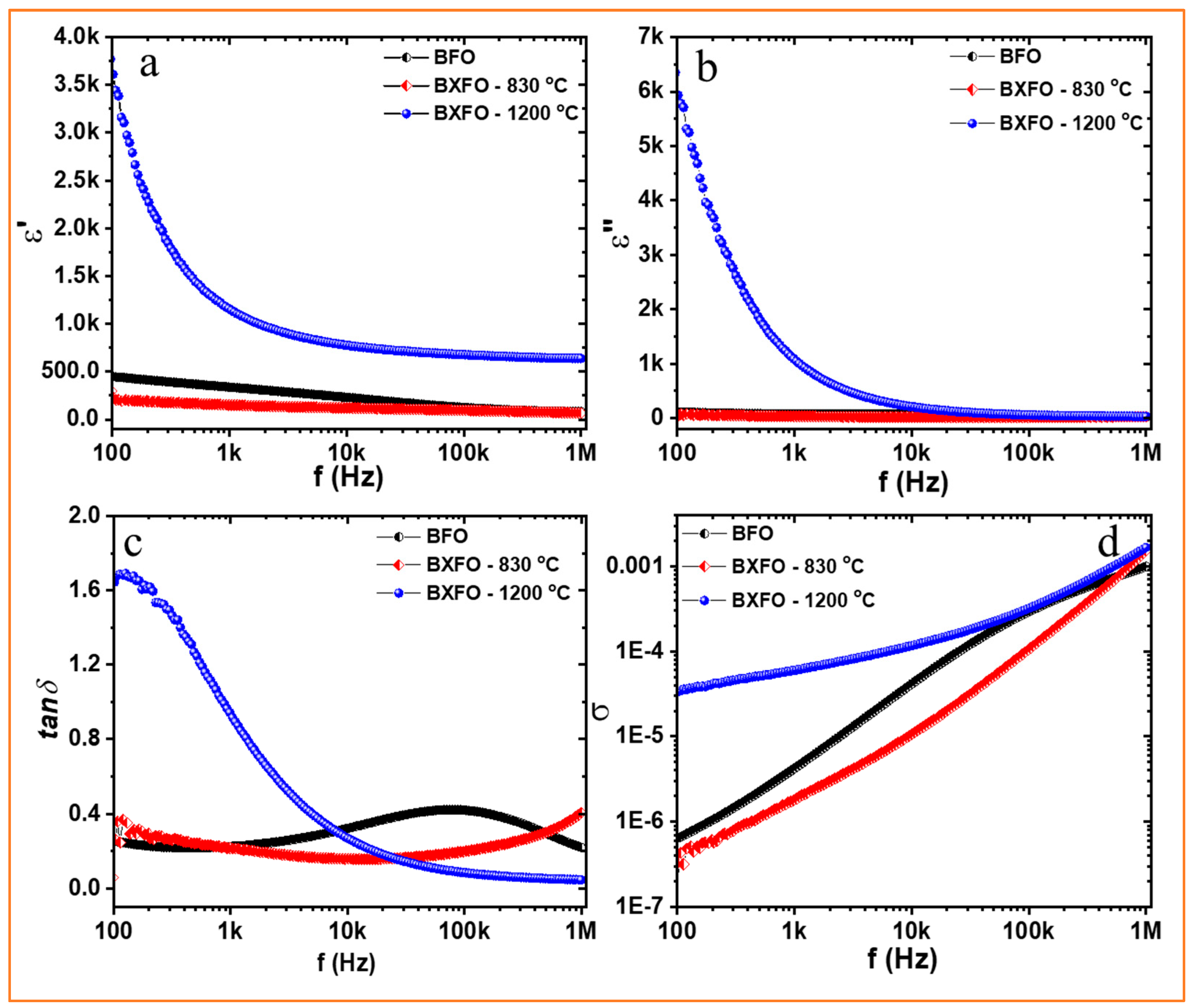
| Samples | Electric Responses at Room Temperature | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 Hz | 1 MHz | 100 Hz | 1 MHz | 100 Hz | 1 MHz | 100 Hz | 1 MHz | |||||||
| BFO | 88.7 | 11.3 | - | - | - | - | 460 | 100 | 112 | 30 | 0.3 | 0.21 | 6.5 × 10−7 | 9.9 × 10−4 |
| BXFO-830 °C | 12 | - | 49.8 | 16.1 | 11.1 | 11 | 210 | 85 | 73 | 30 | 0.3 | 0.4 | 4.3 × 10−7 | 0.002 |
| BXFO-1200°C | 72.5 | 12.9 | 14.6 | - | - | - | 3.8k | 634 | 6k | 30 | 1.7 | 0.04 | 3.3 × 10−5 | 0.002 |
| Samples | Magnetization Values | ||
|---|---|---|---|
| BFO | 0.05 | 0.003 | 0.015 |
| BXFO-830 °C | 0.176 | 0.04 | 0.02 |
| BXFO-1200 °C | 5.9 | 4.1 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, S.; Monteduro, A.G.; Rawat, R.; Rizzato, S.; Leo, A.; Khalid, S.; Maruccio, G. Preparation and Characterization of BXFO High-Entropy Oxides. Magnetochemistry 2024, 10, 60. https://doi.org/10.3390/magnetochemistry10080060
Aziz S, Monteduro AG, Rawat R, Rizzato S, Leo A, Khalid S, Maruccio G. Preparation and Characterization of BXFO High-Entropy Oxides. Magnetochemistry. 2024; 10(8):60. https://doi.org/10.3390/magnetochemistry10080060
Chicago/Turabian StyleAziz, Saba, Anna Grazia Monteduro, Ritu Rawat, Silvia Rizzato, Angelo Leo, Shahid Khalid, and Giuseppe Maruccio. 2024. "Preparation and Characterization of BXFO High-Entropy Oxides" Magnetochemistry 10, no. 8: 60. https://doi.org/10.3390/magnetochemistry10080060









